Abstract
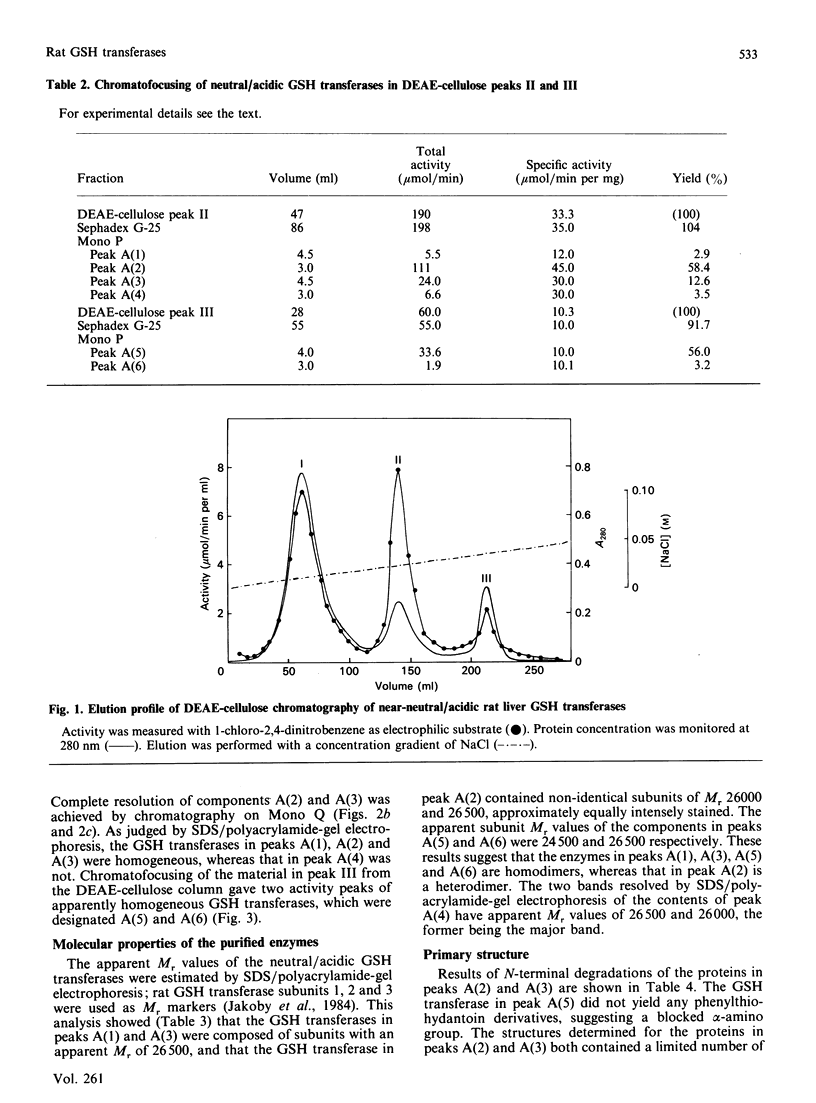
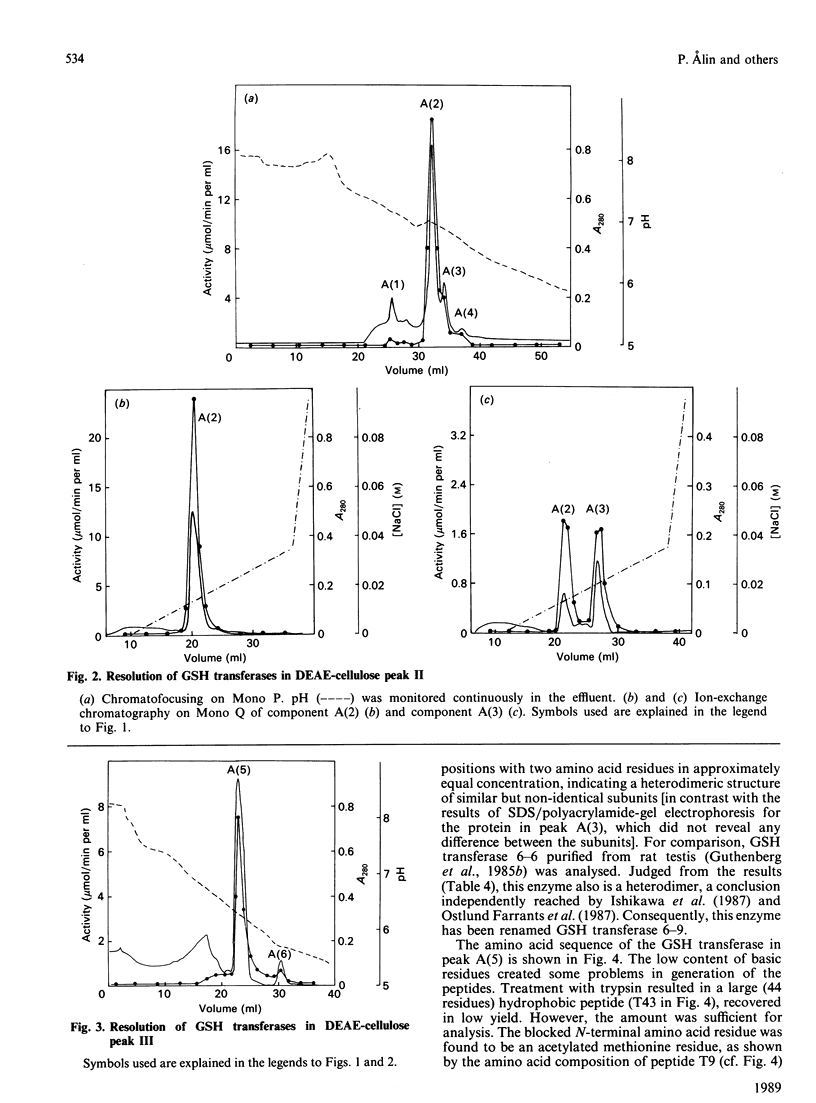
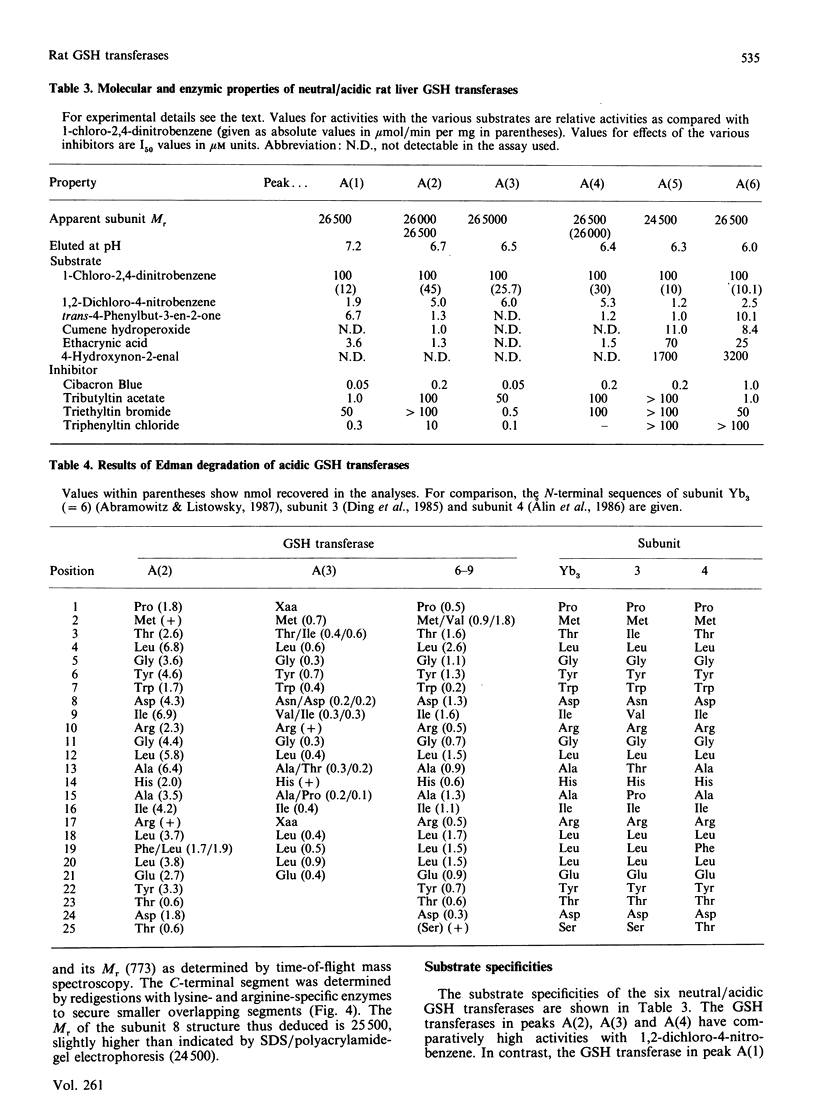
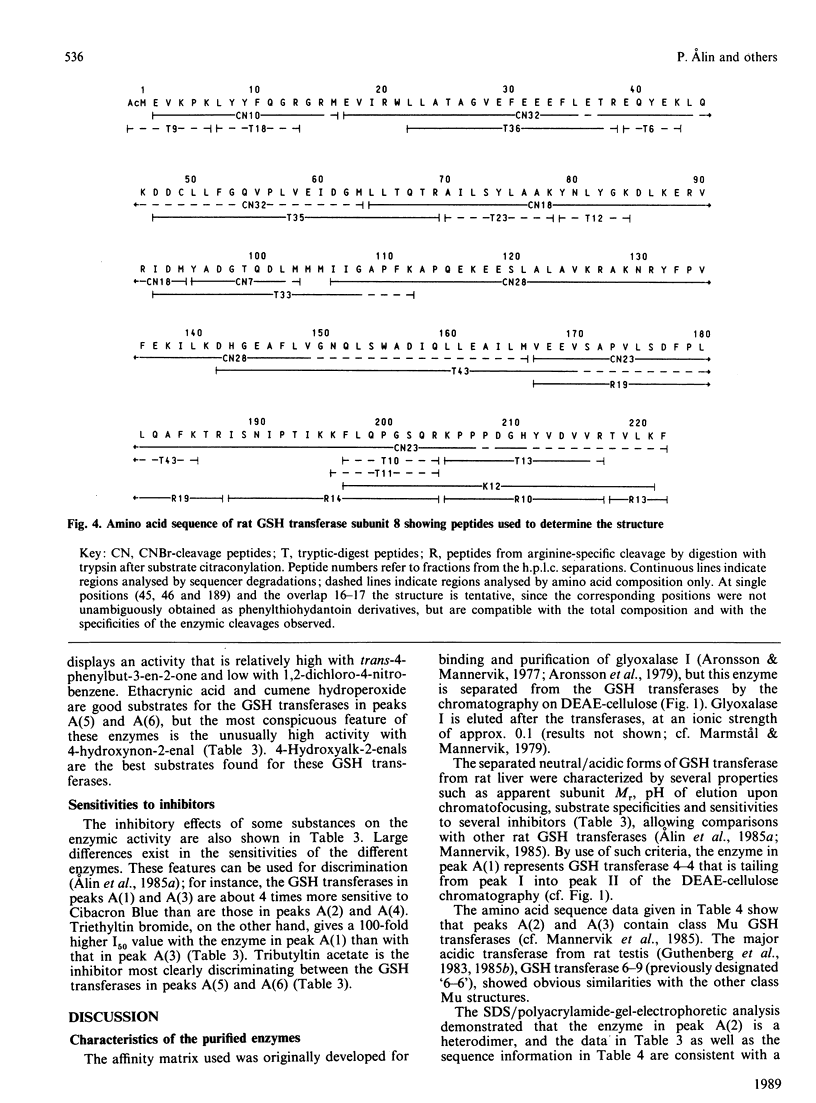
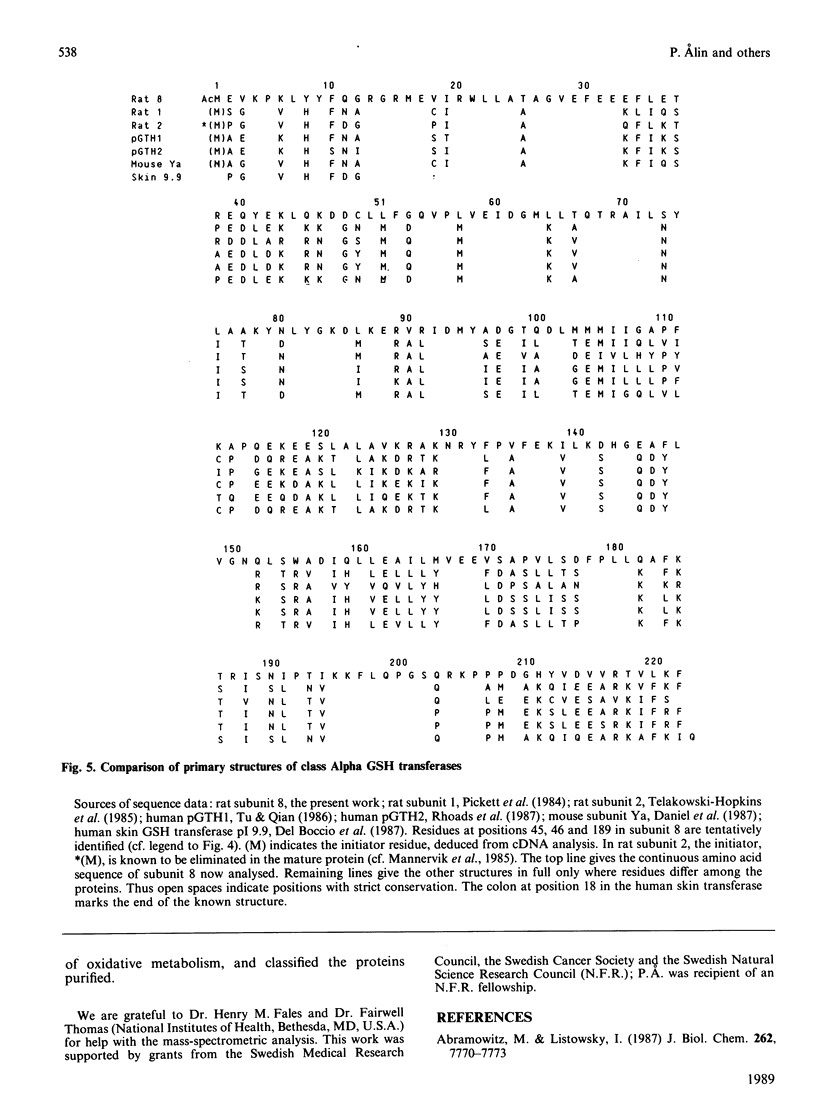
Six GSH transferases with neutral/acidic isoelectric points were purified from the cytosol fraction of rat liver. Four transferases are class Mu enzymes related to the previously characterized GSH transferases 3-3, 4-4 and 6-6, as judged by structural and enzymic properties. Two additional GSH transferases are distinguished by high specific activities with 4-hydroxyalk-2-enals, toxic products of lipid peroxidation. The most abundant of these two enzymes, GSH transferase 8-8, a class Alpha enzyme, has earlier been identified in rat lung and kidney. The amino acid sequence of subunit 8 was determined and showed a typical class Alpha GSH transferase structure including an N-acetylated N-terminal methionine residue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Alin P., Jensson H., Guthenberg C., Danielson U. H., Tahir M. K., Mannervik B. Purification of major basic glutathione transferase isoenzymes from rat liver by use of affinity chromatography and fast protein liquid chromatofocusing. Anal Biochem. 1985 May 1;146(2):313–320. doi: 10.1016/0003-2697(85)90545-7. [DOI] [PubMed] [Google Scholar]
- Alin P., Mannervik B., Jörnvall H. Cytosolic rat liver glutathione transferase 4-4. Primary structure of the protein reveals extensive differences between homologous glutathione transferases of classes alpha and mu. Eur J Biochem. 1986 Apr 15;156(2):343–350. doi: 10.1111/j.1432-1033.1986.tb09588.x. [DOI] [PubMed] [Google Scholar]
- Alin P., Mannervik B., Jörnvall H. Structural evidence for three different types of glutathione transferase in human tissues. FEBS Lett. 1985 Mar 25;182(2):319–322. doi: 10.1016/0014-5793(85)80324-0. [DOI] [PubMed] [Google Scholar]
- Aronsson A. C., Mannervik B. Characterization of glyoxalase I purified from pig erythrocytes by affinity chromatography. Biochem J. 1977 Sep 1;165(3):503–509. doi: 10.1042/bj1650503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronsson A. C., Tibbelin G., Mannervik B. Purification of glyoxalase I from human erythrocytes by the use of affinity chromatography and separation of the three isoenzymes. Anal Biochem. 1979 Jan 15;92(2):390–393. doi: 10.1016/0003-2697(79)90676-6. [DOI] [PubMed] [Google Scholar]
- Askelöf P., Guthenberg C., Jakobson I., Mannervik B. Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J. 1975 Jun;147(3):513–522. doi: 10.1042/bj1470513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Booth J., Boyland E., Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961 Jun;79(3):516–524. doi: 10.1042/bj0790516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederlund E., Lindqvist Y., Söderlund G., Brändén C. I., Jörnvall H. Primary structure of glycolate oxidase from spinach. Eur J Biochem. 1988 May 2;173(3):523–530. doi: 10.1111/j.1432-1033.1988.tb14029.x. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Daniel V., Sharon R., Tichauer Y., Sarid S. Mouse glutathione S-transferase Ya subunit: gene structure and sequence. DNA. 1987 Aug;6(4):317–324. doi: 10.1089/dna.1987.6.317. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Esterbauer H., Mannervik B. Structure-activity relationships of 4-hydroxyalkenals in the conjugation catalysed by mammalian glutathione transferases. Biochem J. 1987 Nov 1;247(3):707–713. doi: 10.1042/bj2470707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Boccio G., Di Ilio C., Alin P., Jörnvall H., Mannervik B. Identification of a novel glutathione transferase in human skin homologous with class alpha glutathione transferase 2-2 in the rat. Biochem J. 1987 May 15;244(1):21–25. doi: 10.1042/bj2440021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Flinta C., Persson B., Jörnvall H., von Heijne G. Sequence determinants of cytosolic N-terminal protein processing. Eur J Biochem. 1986 Jan 2;154(1):193–196. doi: 10.1111/j.1432-1033.1986.tb09378.x. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Alin P., Mannervik B. Glutathione transferase from rat testis. Methods Enzymol. 1985;113:507–510. doi: 10.1016/s0076-6879(85)13067-3. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Astrand I. M., Alin P., Mannervik B. Glutathione transferases in rat testis. Acta Chem Scand B. 1983;37(3):261–262. [PubMed] [Google Scholar]
- Guthenberg C., Jensson H., Nyström L., Osterlund E., Tahir M. K., Mannervik B. Isoenzymes of glutathione transferase in rat kidney cytosol. Biochem J. 1985 Sep 15;230(3):609–615. doi: 10.1042/bj2300609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Carboxypeptidase from yeast. Large scale preparation and the application to COOH-terminal analysis of peptides and proteins. J Biol Chem. 1973 Apr 10;248(7):2296–2302. [PubMed] [Google Scholar]
- Hayes J. D., Chalmers J. Bile acid inhibition of basic and neutral glutathione S-transferases in rat liver. Biochem J. 1983 Dec 1;215(3):581–588. doi: 10.1042/bj2150581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Clarkson G. H. Purification and characterization of three forms of glutathione S-transferase A. A comparative study of the major YaYa-, YbYb- and YcYc-containing glutathione S-transferases. Biochem J. 1982 Dec 1;207(3):459–470. doi: 10.1042/bj2070459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Inhibition of hepatic and extrahepatic glutathione S-transferases by primary and secondary bile acids. Biochem J. 1986 Jan 15;233(2):407–415. doi: 10.1042/bj2330407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. Biochem J. 1984 Dec 15;224(3):839–852. doi: 10.1042/bj2240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and physical characterization of glutathione S-transferase K. Differential use of S-hexylglutathione and glutathione affinity matrices to isolate a novel glutathione S-transferase from rat liver. Biochem J. 1986 Feb 1;233(3):789–798. doi: 10.1042/bj2330789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Rat liver glutathione S-transferases. A study of the structure of the basic YbYb-containing enzymes. Biochem J. 1983 Sep 1;213(3):625–633. doi: 10.1042/bj2130625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yamada H., Kaziro Y., Mizumoto K. Messenger RNA guanylyltransferase from Saccharomyces cerevisiae. Large scale purification, subunit functions, and subcellular localization. J Biol Chem. 1987 Feb 15;262(5):1989–1995. [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Jensson H., Guthenberg C., Alin P., Mannervik B. Rat glutathione transferase 8-8, an enzyme efficiently detoxifying 4-hydroxyalk-2-enals. FEBS Lett. 1986 Jul 28;203(2):207–209. doi: 10.1016/0014-5793(86)80743-8. [DOI] [PubMed] [Google Scholar]
- Kaiser R., Holmquist B., Hempel J., Vallee B. L., Jörnvall H. Class III human liver alcohol dehydrogenase: a novel structural type equidistantly related to the class I and class II enzymes. Biochemistry. 1988 Feb 23;27(4):1132–1140. doi: 10.1021/bi00404a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence R. A., Burk R. F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Axelsson K., Larson K. Thioltransferase. Methods Enzymol. 1981;77:281–285. doi: 10.1016/s0076-6879(81)77038-1. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Marmstål E., Mannervik B. Purification, characterization and kinetic studies of glyoxalase I from rat liver. Biochim Biophys Acta. 1979 Feb 9;566(2):362–370. doi: 10.1016/0005-2744(79)90040-8. [DOI] [PubMed] [Google Scholar]
- Ostlund Farrants A. K., Meyer D. J., Coles B., Southan C., Aitken A., Johnson P. J., Ketterer B. The separation of glutathione transferase subunits by using reverse-phase high-pressure liquid chromatography. Biochem J. 1987 Jul 15;245(2):423–428. doi: 10.1042/bj2450423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Robertson I. G., Jensson H., Guthenberg C., Tahir M. K., Jernström B., Mannervik B. Differences in the occurrence of glutathione transferase isoenzymes in rat lung and liver. Biochem Biophys Res Commun. 1985 Feb 28;127(1):80–86. doi: 10.1016/s0006-291x(85)80128-5. [DOI] [PubMed] [Google Scholar]
- Tahir M. K., Ozer N., Mannervik B. Isoenzymes of glutathione transferase in rat small intestine. Biochem J. 1988 Aug 1;253(3):759–764. doi: 10.1042/bj2530759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rothkopf G. S., Pickett C. B. Structural analysis of a rat liver glutathione S-transferase Ya gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9393–9397. doi: 10.1073/pnas.83.24.9393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C. P., Qian B. Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA. Biochem Biophys Res Commun. 1986 Nov 26;141(1):229–237. doi: 10.1016/s0006-291x(86)80358-8. [DOI] [PubMed] [Google Scholar]


