Abstract
OBJECTIVE
To estimate medical costs associated with 17 major diabetes-related complications and treatment procedures among Medicare beneficiaries aged ≥65 years with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Claims data from 100% of Medicare beneficiaries enrolled in fee-for-service plans from 2006 to 2017 were analyzed. Records with type 2 diabetes and complications were identified using ICD-9, ICD-10, and diagnosis-related group codes. The index year was the year when a person was first identified as having diabetes with an inpatient claim or an outpatient claim plus another inpatient/outpatient claim in the 2 years following the first claim in Medicare. Included individuals were followed from index years until death, discontinuation of plan coverage, or 31 December 2017. Fixed-effects regression was used to estimate the cost in years when the complication event occurred and in subsequent years. The total cost for each complication was calculated for 2017 by multiplying the complication prevalence by the cost estimate. All costs were standardized to 2017 U.S. dollars.
RESULTS
Our study included 10,982,900 beneficiaries with type 2 diabetes. Follow-up ranged from 3 to 10 years. The three costliest complications were kidney failure treated by transplant (occurring year $79,045, subsequent years $17,303), kidney failure treated by dialysis ($54,394, $38,670), and lower-extremity amputation ($38,982, $8,084). Congestive heart failure accounted for the largest share (18%) of total complication costs.
CONCLUSIONS
Costs associated with diabetes complications were substantial. Our cost estimates provide essential information needed for conducting economic evaluation of treatment and programs to prevent and delay diabetes complications in Medicare beneficiaries.
Diabetes imposes an enormous economic burden on the U.S. health care system, with estimated medical costs of $327 million in 2017 expected to be $514 million by 2025 (1). Sixty-one percent of the medical costs associated with diabetes were for persons aged ≥65 years, much of which are borne by the Medicare program (2,3). Expenditures for Medicare beneficiaries with diabetes are 1.7 times as much as for beneficiaries without diabetes (4,5).
More than one-half of total medical expenditures attributable to diabetes is for treating diabetes-related complications (2,6). Previous studies on the cost of diabetes complications focused on persons with diabetes enrolled in private health plans and aged <65 years (4,7,8). Furthermore, no studies except one by Yang et al. (9) estimated the cost of complications with low prevalence, such as kidney transplant, because of their small sample sizes. As well, all studies except Yang et al. used cross-sectional data, which could yield biased estimates due to the inability to adjust for some influential confounders. Factors such as health care–seeking behaviors, perceived health status, or ability to pay affect health service utilization and medical costs but are typically unavailable in claims data. Use of a longitudinal follow-up study design that allows adjustment for individual-level fixed effects can overcome the lack of data on confounders associated with the cross-sectional study design and improve validity and accuracy of cost estimates. In addition, the cost of diabetes complications among elderly persons with type 2 diabetes is likely to be different from the cost of diabetes complications among those aged <65 years because of differences in health insurance plans, age of the study population, and duration of diabetes.
More than 90% of diabetes cases are type 2 diabetes, which can be prevented through lifestyle changes to address risk factors. We followed cohorts of Medicare beneficiaries with type 2 diabetes to estimate the medical costs associated with all major diabetes complications and treatment procedures in this population. This information is critical for assessing the cost effectiveness of clinical and public health interventions for preventing, delaying, and managing type 2 diabetes in the Medicare population.
RESEARCH DESIGN AND METHODS
Study Population
We used 100% of Medicare claims data for 2006–2017 from the Centers for Medicare & Medicaid Services Chronic Conditions Data Warehouse (10). Medicare is the federal health insurance program for people aged ≥65 years, certain younger people with disabilities, and people with end-stage renal disease. The various parts of Medicare cover specific services: Part A covers inpatient hospital stays, care in a skilled nursing facility, hospice care, and some home health care; Part B covers certain physicians’ services, outpatient care, medical supplies, and preventive services; and Part D covers the costs of prescription drugs. These data include claims for hospital inpatient and outpatient services, physician/provider services, and services provided by home health agencies and skilled nursing facilities. We included Medicare beneficiaries with fee-for-service plans only because cost data for those with Medicare Advantage plans are not in the Medicare claims data set.
We identified our study population using a diabetes cohort database that was previously developed (11) on the basis of 100% of Medicare claims among those who enrolled in fee-for-service plans. The original cohort database used diagnostic codes (ICD-9 code 250.X or ICD-10 code E10 or E11) to identify beneficiaries with diabetes. Individuals with drug-induced diabetes, identified based on ICD-9/10 diagnostic codes, were excluded from the cohort. To be included in the study, beneficiaries must have had at least one inpatient claim for diabetes or an outpatient claim for diabetes plus another inpatient/ outpatient claim for diabetes within the 2 years after the first claim. This algorithm has been validated and used in previous studies (11,12). The cohort database required that beneficiaries be continuously enrolled in Medicare for 5 years, 2 years before and 2 years after the year when they were first identified with diabetes (i.e., the index year), to ensure the accuracy of all identified prevalence and incidence cases of diabetes. With the requirement of 2 years before the index year without diabetes for the incidence cohort, the starting age of beneficiaries included in the cohort database was 68 years. The requirement of having full medical records 2 years after the index year allowed us to have sufficient time for claims-based ascertainment of diabetes and diabetes complications. Because our focus was on type 2 diabetes, we excluded beneficiaries with type 1 diabetes using an algorithm by Zhong et al. (13). We also excluded those who did not enroll in Part D plans because their prescription drug cost data were not available. Eligible beneficiaries were followed from the index year to the year they disenrolled from Medicare, died, or 31 December 2017. Overall, 6.1% of the Medicare beneficiaries were not followed after they switched from fee-for-service plans to Medicare Advantage plans.
Outcome Variable
The outcome variable was the total annual medical cost from all payment sources (Medicare, patient out of pocket, or other health insurance plans Medicare beneficiaries may have had, e.g., group health plan, retiree coverage, Medicaid) during the follow-up period. By service category, the total cost included expenditures for inpatient and outpatient services, purchasing durable medical equipment, and services provided by a home health agency, hospice, or skilled nursing facility. All costs were adjusted to 2017 U.S. dollars using Personal Health Care expenditure deflator (14).
Diabetes Complications
We used diagnosis and procedure codes and diagnosis-related group codes (see Supplementary Table 1 for a complete list) to identify diabetes complications. We grouped the complications by type and pattern of progression and treatment, as follows: 1) Retinopathy intravitreal injections, photocoagulation, hypoglycemia, and ketoacidosis were treated as singular acute events that do not have subsequent year costs, and 2) all other complications were treated as chronic events with cost estimates in the year when the complication occurred (i.e., occurring year) and in subsequent years. For myocardial infarction (MI), stroke, and kidney failure treated by transplant, we treated years when the first event or repeated events occurred as occurring years, and years between or after acute years as subsequent years. Some beneficiaries came to the study cohorts with a previous occurrence of MI or stroke; we coded their MI and stroke status in the first year as subsequent years. Angina and revascularization were treated similarly to MI and stroke with the following modification: Whenever the beneficiary’s subsequent years coexisted with MI or MI in subsequent years, their subsequent years were recoded as zero to recognize that the presence of MI would absorb the additional cost of angina or revascularization. For nephropathy, kidney failure treated by transplant, kidney failure treated by dialysis, neuropathy, foot ulcer, lower-extremity amputation (LEA), chronic retinopathy, blindness and vision loss, and congestive heart failure (CHF), we coded the year when the first event occurred as the occurring year and all subsequent years as subsequent years.
Statistical Analysis
Because the proportion of beneficiaries with diabetes with zero total annual costs in our data set was <1%, we used a one-equation regression model as opposed to a two-part model. Unlike cross-sectional studies in which people with and without a specific complication are compared with estimates of the medical cost of the complication, the fixed-effects model uses longitudinal data to compare the medical cost of a person before and after the person developed a complication. Many factors, such as those related to health care–seeking behaviors, perceived health status, or ability to pay, affect health service utilization and medical costs but are often unavailable in claims data. Omitting these variables could lead to estimate biases. The fixed-effects regression model can inherently adjust for factors with no or missing data but that do not change over time to reduce the estimation biases (15) (Supplementary Appendix). Besides diabetes complications, we also controlled for several nondiabetes-related high-cost conditions, including cancer, HIV/AIDS, liver disease, anemia, Alzheimer disease, asthma, chronic obstructive pulmonary disease, hip fracture, arthritis, and organ transplant (excluding kidney or pancreas transplants that could result from having diabetes). A dummy variable indicating whether the beneficiary was enrolled in a Part D plan was created as an explanatory variable. Our cost estimates represented an independent assessment of a specific complication, but the total costs among beneficiaries who experienced that complication may be much higher because complications co-occur. All descriptive and regression analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute, Cary, NC).
We estimated the total cost for each complication and its share of the total complication costs in 2017. Total cost of an individual complication was calculated by multiplying the prevalence of the complication by the estimated annual cost produced from the fixed-effects regression model. The total cost associated with all complications was the sum of the costs from all 17 complications included in the study. The cost share of each complication was the cost of each complication divided by the total complication cost. To examine the heterogeneity of the cost across subgroups, we conducted subgroup analysis by sex (male/female), race/ethnicity (Black, White, Hispanic, Asian/Pacific Islander, American Indian/Alaska Native), and dual eligibility for Medicaid (yes/no). We also conducted two sensitivity analyses to examine how costs of diabetes complications would change if we included Medicare beneficiaries 1) who did not enrolled in Part D and 2) who switched to Medicare Advantage plans during the study period.
RESULTS
Our study included 8,004,804 Medicare beneficiaries with type 2 diabetes. By index year, 49.1% of the study participants were identified in 2008, and 7.7%, 7.7%, 7.5%, 7.4%, 6.7%, 7.0%, and 6.9% in 2009, 2010, 2011, 2012, 2013, 2014, and 2015, respectively. On average, beneficiaries were followed for 5.90 years (range 3–10 years). By index year, average follow-up was 7.12 years for 2008, and 7.13, 6.55, 5.86, 5.17, 4.43, 3.70, and 2.86 years for 2009, 2010, 2011, 2012, 2013, 2014, and 2015, respectively.
Table 1 shows the characteristics of the combined cohorts in beneficiaries’ index year. Beneficiaries were, on average, aged 76 years when entered into the study. More than 77% of the beneficiaries were non-Hispanic White, and 41% were men. Approximately 42% had at least one complication.
Table 1—
Demographic and clinical characteristics of Medicare beneficiaries with type 2 diabetes in the index year*
| Characteristic | Value |
|---|---|
| Beneficiaries, N** | 8,004,804 |
| Mean age at index year (years) | 75.86 |
| Sex, % | |
| Male | 41.29 |
| Female | 58.71 |
| Race/ethnicity, % | |
| Non-Hispanic White | 77.08 |
| Hispanic | 7.60 |
| Non-Hispanic Black | 10.33 |
| Asian/Pacific Islander | 3.46 |
| American Indian/Alaska Native | 0.75 |
| Unknown | 0.28 |
| Diabetes complications,† % | |
| 0 | 57.94 |
| 1 | 25.46 |
| 2 | 10.38 |
| 3 | 4.10 |
| ≥4 | 2.12 |
| Average, n | 0.68 |
| Comorbidities, % | |
| Cancer | 34.71 |
| HIV/AIDS | 0.17 |
| Organ transplant‡ | 0.06 |
Index year is the year when a person was first identified with diabetes within a Medicare fee-for-service plan.
No variance data presented because the data included all eligible patients.
Diabetes complications included retinopathy intravitreal injections, photocoagulation, hypoglycemia, ketoacidosis, nephropathy, kidney failure treated by dialysis, kidney failure treated by transplant, neuropathy, LEA, chronic retinopathy, blindness and vision loss, congestive heart failure, foot ulcer, MI, stroke, angina, and revascularization.
Organ transplant excluded kidney failure treated by transplant.
Table 2 shows the number of beneficiaries with each complication as well as the unadjusted mean annual cost of the complication during the study period. The number of beneficiaries was sufficient to generate a stable estimate of the cost for each complication. The most common complications were nephropathy (2,697,151 [33.1%]), CHF (2,197,008 [27.4%]), and stroke (2,191,709 [27.4%]); the rarest were LEA (63,358 [0.8%]), ketoacidosis (16,767 [0.2%]), and kidney failure treated by transplant (3.753 [0.1%]). Beneficiaries with kidney failure had the highest unadjusted mean annual costs ($123,013), while beneficiaries with a history of revascularization had the lowest unadjusted mean annual costs ($20,180).
Table 2—
Number of beneficiaries, average annual medical cost per person, and percentage of beneficiaries among Medicare fee-for-service population with type 2 diabetes by diabetes complication
| Complication | Beneficiaries, n | Unadjusted mean annual cost, 2017 U.S. $ | Percentage of beneficiaries |
|---|---|---|---|
| Nephropathy Subsequent years |
2,647,151 3,879,087 |
41,793 30,257 |
33.1 48.5 |
| Kidney failure treated by transplant Subsequent years |
3,753 5,839 |
123,013 44,731 |
0.1 0.1 |
| Kidney failure treated by dialysis Subsequent years |
192,824 211,024 |
110,821 85,809 |
2.4 2.6 |
| Neuropathy Subsequent years |
1,558,717 2,527,684 |
34,453f 28,238 |
19.5 31.6 |
| LEA Subsequent years |
63,358 39,575 |
103,889 41,000 |
0.8 0.5 |
| Chronic retinopathy Subsequent years |
562,539 1,131,758 |
27,306 27,817 |
7.0 14.1 |
| Blindness and vision loss Subsequent years |
504,414 603,464 |
44,082 32,789 |
6.3 7.5 |
| CHF Subsequent years |
2,197,008 3,398,735 |
48,869 33,634 |
27.4 42.5 |
| Foot ulcer Subsequent years |
1,184,493 1,531,470 |
48,555 32,947 |
14.8 19.1 |
| MI Subsequent years |
1,140,526 1,457,014 |
63,737 28,963 |
14.2 18.2 |
| Stroke Subsequent years |
2,191,709 1,537,212 |
45,903 26,822 |
27.4 19.2 |
| Angina Subsequent years |
1,205,134 862,158 |
38,618 23,368 |
15.1 10.8 |
| Revascularization Subsequent years |
1,405,376 596,626 |
43,256 20,180 |
17.6 7.5 |
| Retinopathy intravitreal injections | 347,572 | 29,476 | 4.3 |
| Photocoagulation | 373,517 | 33,493 | 4.7 |
| Hypoglycemia | 739,495 | 50,638 | 9.2 |
| Ketoacidosis | 16,767 | 55,447 | 0.2 |
The estimated annual per-person costs associated with diabetes complications after adjustment varied by condition, ranging from $0.1 to $79,860 (median $5,876) (Table 3 and Supplementary Fig. 1). The highest annual costs were associated with three microvascular complications: kidney failure treated by transplant (occurring years $79,860, subsequent years $15,568), kidney failure treated by dialysis ($54,739, $38,622), and LEA ($38,618, $7,506). The costs of other microvascular complications were all <$15,000: nephropathy ($9,576, $1,875), foot ulcer ($10,433, $2,304), blindness and vision loss ($6,511, $447), and chronic retinopathy ($682, $—0.1). For acute microvascular complications, the cost was $4,011 for retinopathy intravitreal injections and $1,558 for photocoagulation.
Table 3—
Estimated annual per-person diabetes complication costs in Medicare beneficiaries with type 2 diabetes
| Complication | Estimated costs, 2017 U.S. $ |
|---|---|
| Short-term | |
| Hypoglycemia | 9,399.2 |
| Ketoacidosis | 13,015.7 |
| Microvascular | |
| Nephropathy | 9,576.0 |
| Nephropathy history | 1,875.2 |
| Kidney failure treated by dialysis | 54,739.2 |
| Kidney failure treated by dialysis history | 38,621.6 |
| Kidney failure treated by transplant | 79,859.8 |
| Kidney failure treated by transplant history | 15,568.1 |
| Neuropathy | 4,561.9 |
| Neuropathy history | 870.7 |
| Foot ulcer | 10,433.3 |
| Foot ulcer history | 2,303.7 |
| LEA | 39,617.7 |
| LEA history | 7,506.0 |
| Chronic retinopathy | 681.8 |
| Chronic retinopathy history | (0.1) |
| Retinopathy intravitreal injections | 4,011.3 |
| Photocoagulation | 1,558.3 |
| Blindness and vision loss | 6,511.1 |
| Blindness and vision loss history | 446.9 |
| Macrovascular complications | |
| CHF | 12,476.0 |
| CHF history | 2,990.8 |
| MI | 18,981.5 |
| MI history | 1,602.5 |
| Stroke | 12,442.0 |
| Stroke history | 159.9 |
| Angina | 5,240.1 |
| Angina history | 541.7 |
| Revascularization | 11,300.6 |
| Revascularization history | 575.8 |
All annual costs of macrovascular complications were <$20,000. The cost of complications ranked in descending order were MI (occurring years $18,892, subsequent years $1,603), CHF ($12,5476, $2,991), stroke ($12,442, $160), revascularizations ($10,301, $576), and angina ($5,240, $542) (Table 3). Costs in occurring years were always higher than costs in subsequent years.
Nationally, we estimated that the total cost of complications in Medicare fee for-service beneficiaries with type 2 diabetes who were enrolled in Part D plans was $37.1 billion in 2017. CHF and stroke were the costliest complications among the 17 complications included in our study, accounting for 18% and 15% of the total, respectively (Fig. 1). Following were nephropathy (14%), revascularization (13%), MI (11%), kidney failure treated by dialysis (9%), and foot ulcer (6%); other complications each accounted for <5% of the total cost. Although kidney failure treated by transplant was the costliest per person per year, the condition accounted for only 0.21% of the total complication costs, as the number of beneficiaries who received this treatment was small. There was a similar pattern for LEA.
Figure 1—
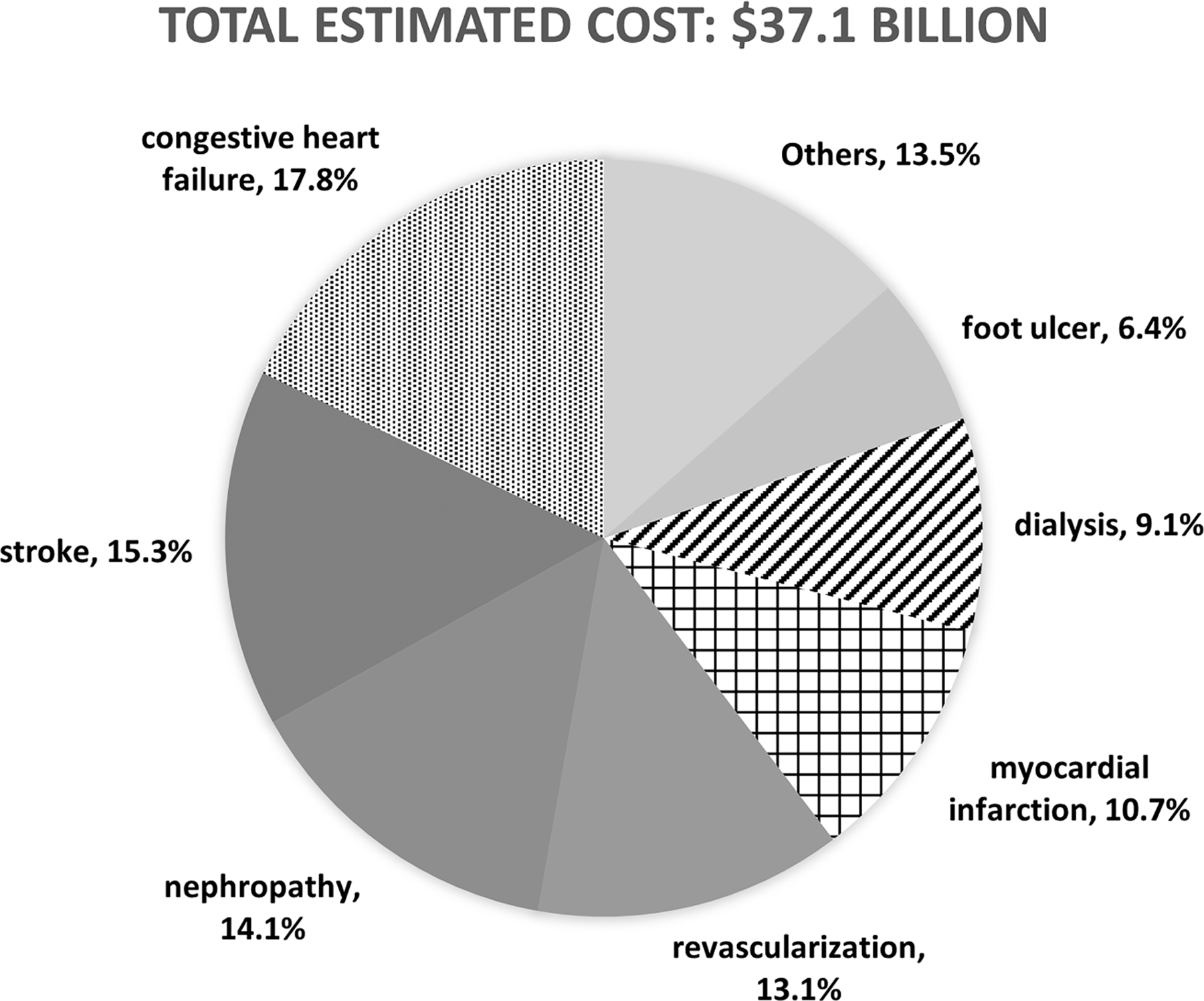
Percentage of estimated specific complication costs among total estimated complication costs in Medicare fee-for-service beneficiaries with type 2 diabetes in 2017. “Others” refers to angina, hypoglycemia, blindness and vision loss, retinopathy intravitreal injections, LEA, chronic retinopathy, ketoacidosis, kidney transplant, and photocoagulation; each complication accounted for <5% of the total.
The full results from the subgroup analysis are provided in the Supplementary Tables 2–4. Differences in annual complication costs between subgroups vary depending on the specific complication. Overall, male beneficiaries, Black or Hispanic beneficiaries, and beneficiaries with dual eligibility for Medicaid had a higher cost than their counterparts.
Results of the sensitivity analysis are presented in Supplementary Tables 5 and 6. The estimated costs became lower when those for beneficiaries without the Part D plan were included in the study. In comparison, the estimated costs changed little when those beneficiaries who switched to Medicare Advantage plans were excluded.
CONCLUSIONS
Our study presents the most comprehensive estimates on the cost of diabetes-related complications among Medicare beneficiaries aged ≥65 years with type 2 diabetes to date. Annual costs associated with complications are substantial and vary by condition. The annual perperson costs of kidney failure and LEA are especially high. When combined with prevalence of the complications, CHF and nephropathy are the costliest conditions for the overall Medicare population with type 2 diabetes. These estimates are critical for evaluating the cost effectiveness of public health programs and clinical treatments among Medicare beneficiaries with type 2 diabetes and for budgetary planning for managing these complications for the Medicare program.
Previous studies also have examined the cost of complications. Brandle et al. (16) estimated annual complication costs of acute MI, stroke, and LEA for type 2 diabetes using cross-sectional data from one managed care organization among patients aged 54–72 years. After inflating their cost estimates from 2000 to 2017 dollars, the complication costs were higher than our estimates in the occurring years as follows: $39,794 vs. $12,442 for stroke, $36,652 vs. $18,982 for MI, and $56,250 vs. $39,618 for LEA. Li et al. (17) used the same data source as Brandle et al. plus additional data from seven other managed care health plans using a cross-sectional study design. More than one-half of their population was aged <65 years. Among the complications that overlapped with ours, direct costs of complications were higher for angina ($8,468 vs. $5,240) and CHF ($16,680 vs. $12,476) and lower for LEA ($25,123 vs. $39,618). Differences in study designs (cross-sectional vs. longitudinal), ages of the populations, data sources (several managed health care organizations vs. Medicare), and number of complications included may have contributed to the differences in cost estimates between our study and these two previous studies. Besides these two studies using real-world patient data, Ward et al. (18) also estimated annual medical costs associated with diabetes complications using a microcosting approach with data from multiple sources. Their estimated annual costs were higher than ours for MI, stroke, and CHF while lower than our estimates for LEA, foot ulcer, and blindness. Studies on estimating diabetes complication costs have also been conducted in other countries (19–25). These estimates are not directly comparable to ours because of different health care systems and payment policies. However, the ranking of the complications costs seems to share some similarities. For example, the two most expensive complications were renal failure treated by dialysis or transplant and amputation. The costs associated with vision problems were the least expensive.
Ours is the second study to use a large claims database and individual fixed-effects model to estimate the annual cost of each diabetes complication. Recently, Yang et al. (9) used a large private claims database with a similar study design to examine adults aged <65 years with type 2 diabetes. Compared with our estimates, costs for these younger adults were higher for other complications. These differences could be a result of several factors, including different price indices used. Most critically, Medicare beneficiaries with type 2 diabetes have a different payment structure (26); and older adults with type 2 diabetes have different care needs due to challenges in getting the social support they need and age-related conditions and multiple comorbidities (27).
Our results show that the costs associated with treating diabetes complications in older adults are substantial. Furthermore, the cost of a complication increases considerably if that complication progresses to the advanced stage. For example, nephropathy costs $9,576 in the first year and $1,875 per year thereafter. Once the disease progresses to kidney failure, the cost increases to $54,739 if treated by dialysis and $79,860 if treated by transplant. The cost of kidney transplant could be even higher than our estimates because the costs of organ acquisition, including expenditures associated with tissue typing, crossmatching, transportation of living donors or their kidneys, and administrative costs that organizations may have aggregated at the facility level, are not reflected in the cost to Medicare. However, the cost after the first year of kidney transplant would be much lower. Managing risk factors, screening, and early treatment of diabetes complications to slow their progression may lower the cost of complications. Blood pressure control with ACE inhibitor and angiotensin receptor blocker therapies is highly effective to prevent kidney failure and to reduce costs compared with no such therapy (28). Intensive glucose control to help patients with type 2 diabetes to achieve their A1C goal has also been shown to reduce costs by delaying multiple complications and increasing time free of complications (29). A study conducted in the Netherlands found that screening for heart failure could be cost effective for the community-dwelling elderly population with diabetes (30).
Of the total costs of all diabetes-related complications in Medicare beneficiaries with type 2 diabetes in 2017, CHF, nephropathy, and stroke accounted for 47%. Lowering the risk of these three complications could therefore play a key role in reducing the cost of diabetes-related complications in this population. CHF and nephropathy could be highly correlated (31,32); better management of CHF could slow the progression of nephropathy (32) or vice versa (33). Thus, treatments, programs, and policies that target prevention, early detection, or better management of either CHF or nephropathy may lower the costs associated with diabetes-related complications in Medicare beneficiaries with type 2 diabetes.
Our cost estimates may be useful for economic evaluations of programs or policies that affect complications among Medicare beneficiaries with type 2 diabetes (34). Diabetes simulation models have been developed to estimate the long-term cost effectiveness of risk factor control of a complication, but these models require input parameters on the cost of each diabetes complication. For example, to estimate the cost effectiveness of an intervention for preventing or delaying MI, our estimated cost of $18,982 would serve as the cost associated with MI occurring and $1,602 as the subsequent annual cost until another MI developed and/or the person died. The model combines these parameters with the estimated risk of developing MI with and without the intervention each year to estimate the change in the lifetime cost associated with MI due to the intervention.
Strengths of our study include that with 11 million people with type 2 diabetes in the data set, we were able to estimate 17 diabetes complications, including those with very low prevalence. In addition, through our longitudinal design, we were able to mitigate potential estimation bias by controlling time-invariant characteristics of an individual beneficiary in the Medicare claims data.
This study has some limitations. First, acute events beyond the 2-year lookback period were not observed. It is possible that beneficiaries with previous complications would have different costs when encountering second events. However, there is evidence that cost estimates for first acute events are similar to the cost of all events (including repeated events) for MI and stroke, two of the costliest complications (35). Second, acute events may occur at the end of one calendar year and span into the beginning of the next. This may lead to an underestimation of acute costs and an overestimation of the costs of complication history in the second year. Its impact on cost estimates is unknown. Third, the algorithm to identify persons with diabetes or diabetes complications used in our study could misclassify some beneficiaries with diabetes and diabetes-related compilations. How this misspecification affected the estimated costs of diabetes-related complications is not clear. Fourth, although we accounted for inflation, the cost of complications might change over time because of factors such as technology advancement or payment policy change. For example, the cost of treating kidney failure increases with time (36). Our results might therefore underestimate costs for the complications for which treatments have become more complicated and expensive since 2006. Fifth, our study included only those enrolled Medicare beneficiaries in a fee-for-service plan and with complete prescription cost data; our cost estimates may not be directly applicable to those beneficiaries enrolled in Medicare Advantage and with no Part D drug coverage. Estimated costs associated with each complication tend to be lower for Medicare beneficiaries without Part D coverage. Sixth, we were not able to validate the algorithm we used to identify diabetes and its related complications. Although this algorithm had a good validity based on previous validation studies (37,38), it is likely that the algorithm still results in some misidentifications. More studies are needed to further refine the algorithm or to develop new algorithms to accurately identify persons with diabetes and diabetes complications based on claims data, especially in the Medicare population. Finally, fixed-effects models do not completely eliminate confounding because there are still variables that change with time that we cannot control.
In conclusion, we found that the costs associated with 17 diabetes complications and treatment procedures among Medicare beneficiaries aged ≥65 years with type 2 diabetes are substantial and vary by complication. These estimates provide the cost parameters needed for assessing the long-term health and economic consequences of interventions for preventing and managing type 2 diabetes and evaluating the economic benefits of clinical and public efforts for delaying or preventing complications in elderly Medicare beneficiaries with type 2 diabetes.
Supplementary Material
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
This article contains supplementary material online at https://doi.org/10.2337/figshare.20611344.
References
- 1.Rowley WR, Bezold C. Creating public awareness: state 2025 diabetes forecasts. Popul Health Manag 2012;15:194–200 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018;41:917–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, U.S. Department of Health and Human Services, 2014 [Google Scholar]
- 4.Krop JS, Saudek CD, Weller WE, Powe NR, Shaffer T, Anderson GF. Predicting expenditures for Medicare beneficiaries with diabetes. A prospective cohort study from 1994 to 1996. Diabetes Care 1999;22:1660–1666 [DOI] [PubMed] [Google Scholar]
- 5.Krop JS, Powe NR, Weller WE, Shaffer TJ, Saudek CD, Anderson GF. Patterns of expenditures and use of services among older adults with diabetes. Implications for the transition to capitated managed care. Diabetes Care 1998;21: 747–752 [DOI] [PubMed] [Google Scholar]
- 6.Zhuo X, Zhang P, Hoerger TJ. Lifetime direct medical costs of treating type 2 diabetes and diabetic complications. Am J Prev Med 2013;45: 253–261 [DOI] [PubMed] [Google Scholar]
- 7.Zoungas S, Woodward M, Li Q, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57:2465–2474 [DOI] [PubMed] [Google Scholar]
- 8.Wu CX, Tan WS, Toh MPHS, Heng BH. Stratifying healthcare costs using the diabetes complication severity index. J Diabetes Complications 2012;26: 107–112 [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Cintina I, Hoerger T, et al. Estimating costs of diabetes complications in people <65 years in the U.S. using panel data. J Diabetes Complications 2020;34:107735. [DOI] [PubMed] [Google Scholar]
- 10.Chronic Conditions Warehouse. Medicare Administrative Data User Guide. Baltimore, Centers for Medicare & Medicaid Services, 2019 [Google Scholar]
- 11.Andes LJ, Li Y, Srinivasan M, Benoit SR, Gregg E, Rolka DB. Diabetes prevalence and incidence among Medicare beneficiaries—United States, 2001–2015. MMWR Morb Mortal Wkly Rep 2019;68:961–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asghari S, Courteau J, Carpentier AC, Vanasse A. Optimal strategy to identify incidence of diagnostic of diabetes using administrative data. BMC Med Res Methodol 2009;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong VW, Pfaff ER, Beavers DP, et al. ; SEARCH for Diabetes in Youth Study Group. Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes 2014;15:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Department of Veterans Affairs. Measuring costs for cost-effectiveness analysis. Accessed 8 February 2022. Available from https://www.herc.research.va.gov/include/page.asp?id= measure-costs-cea [Google Scholar]
- 15.Schmidheiny K, Basel U. Panel data: fixed and random effects. Short Guide Microeconometrics 2011;7:2–7 [Google Scholar]
- 16.Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care 2003;26:2300–2304 [DOI] [PubMed] [Google Scholar]
- 17.Li R, Bilik D, Brown MB, et al. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care 2013;19: 421–430 [PMC free article] [PubMed] [Google Scholar]
- 18.Ward A, Alvarez P, Vo L, & Martin S (2014). Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012). Journal of medical economics, 17(3), 176–183. [DOI] [PubMed] [Google Scholar]
- 19.Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R. Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 2018;41: 971–978 [DOI] [PubMed] [Google Scholar]
- 20.Ray JA, Valentine WJ, Secnik K, et al. Review of the cost of diabetes complications in Australia, Canada, France, Germany, Italy and Spain. Curr Med Res Opin 2005;21:1617–1629 [DOI] [PubMed] [Google Scholar]
- 21.Clarke P, Gray A, Legood R, Briggs A, Holman R. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS study no. 65). Diabet Med 2003;20:442–450 [DOI] [PubMed] [Google Scholar]
- 22.Clarke P, Leal J, Kelman C, Smith M, Colagiuri S. Estimating the cost of complications of diabetes in Australia using administrative healthcare data. Value Health 2008;11:199–206 [DOI] [PubMed] [Google Scholar]
- 23.Clarke PM, Glasziou P, Patel A, et al. ; ADVANCE Collaborative Group. Event rates, hospital utilization, and costs associated with major complications of diabetes: a multicountry comparative analysis. PLoS Med 2010;7:e1000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen HY, Kuo S, Su PF, Wu JS, Ou HT. Health care costs associated with macrovascular, micro-vascular, and metabolic complications of type 2 diabetes across time: estimates from a population-based cohort of more than 0.8 million individuals with up to 15 years of follow-up. Diabetes Care 2020;43:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerdtham UG, Clarke P, Hayes A, Gudbjornsdottir S. Estimating the cost of diabetes mellitus-related events from inpatient admissions in Sweden using administrative hospitalization data. PharmacoEconomics 2009;27:81–90 [DOI] [PubMed] [Google Scholar]
- 26.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care 2014;37:651–658 [DOI] [PubMed] [Google Scholar]
- 27.Leung E, Wongrakpanich S, Munshi MN. Diabetes management in the elderly. Diabetes Spectr 2018;31:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel KR, Ali MK, Zhou X, et al. Cost-effectiveness of interventions to manage diabetes: has the evidence changed since 2008? Diabetes Care 2020;43:1557–1592 [DOI] [PubMed] [Google Scholar]
- 29.Gray A, Raikou M, McGuire A, et al. ; United Kingdom Prospective Diabetes Study Group. Cost effectiveness of an intensive blood glucose control policy in patients with type 2 diabetes: economic analysis alongside randomised controlled trial (UKPDS 41). BMJ 2000;320:1373–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Giessen A, Boonman-de Winter LJ, Rutten FH, et al. Cost-effectiveness of screening strategies to detect heart failure in patients with type 2 diabetes. Cardiovasc Diabetol 2016;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs 1990; 39(Suppl. 4):10–21; discussion 22–24 [DOI] [PubMed] [Google Scholar]
- 32.Silverberg D, Wexler D, Blum M, Schwartz D, Iaina A. The association between congestive heart failure and chronic renal disease. Curr Opin Nephrol Hypertens 2004;13:163–170 [DOI] [PubMed] [Google Scholar]
- 33.Silverberg DS, Wexler D, Iaina A, Schwartz D. The correction of anemia in patients with the combination of chronic kidney disease and congestive heart failure may prevent progression of both conditions. Clin Exp Nephrol 2009;13: 101–106 [DOI] [PubMed] [Google Scholar]
- 34.Pavey TG, Anokye N, Taylor AH, et al. The clinical effectiveness and cost-effectiveness of exercise referral schemes: a systematic review and economic evaluation. Health Technol Assess 2011;15:i–xii, 1–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringborg A, Yin DD, Martinell M, Stålhammar J, Lindgren P. The impact of acute myocardial infarction and stroke on health care costs in patients with type 2 diabetes in Sweden. Eur J Cardiovasc Prev Rehabil 2009;16:576–582 [DOI] [PubMed] [Google Scholar]
- 36.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2020;75(Suppl. 1): A6–A7 [DOI] [PubMed] [Google Scholar]
- 37.Lipscombe LL, Hwee J, Webster L, Shah BR, Booth GL, Tu K. Identifying diabetes cases from administrative data: a population-based validation study. BMC Health Serv Res 2018; 18:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol 1999;52: 199–207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


