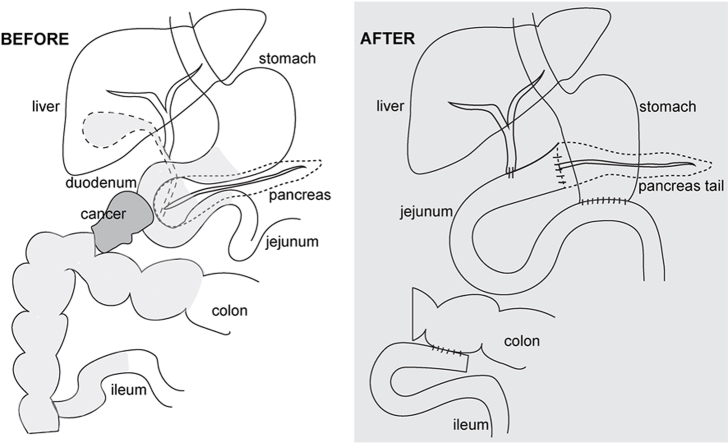
Abstract
Since ovarian cancer typically spreads intraperitoneally or via lymphatics, a retroperitoneal duodenal obstruction is a rare presentation of ovarian cancer. Such upper gastrointestinal obstruction in a young patient is diagnostically challenging and surgically difficult to address. In this case report, we describe that in an interdisciplinary approach a Whipple pancreaticoduodenectomy could be safely implemented into the interval debulking surgery to achieve complete cytoreduction. No postoperative complications were encountered. The surgical procedure was able to remove the upper gastrointestinal obstruction and thereby the need for a venting gastrostomy tube and total parenteral nutrition and thus provided good quality of life and additional lifetime.
Keywords: Duodenal stenosis, Advanced stage ovarian cancer, Interval debulking surgery, Whipple procedure
Graphical abstract
1. Introduction
Ovarian cancer remains the deadliest gynecologic cancer and the fifth leading cause of cancer-related deaths among women. The reduction in mortality from ovarian cancer over the past 40 years has been notably modest compared to other malignancies. According to the American Cancer Society, in 2023, there will be an estimated 19,710 new cases of ovarian cancer and 13,270 ovarian cancer-related deaths [1]. Most ovarian cancers are diagnosed in postmenopausal women, 10–15 % in premenopausal women and only 1 % in women <30 years of age [2]. Based on the SEER database, 7.1 % of new ovarian cancer cases are diagnosed in women ages 35–44 with a corresponding 2.1 % of all ovarian cancer deaths occurring in this age group [3]. BRCA1 and BRCA2 are genes associated with a genetic predisposition to ovarian cancer. Overall, 8–13 % of ovarian cancer cases being caused by germline mutations in BRCA1 or BRCA2 [4], but only 4 % of women ages 40 or younger with ovarian cancer will have BRCA germline mutations [5]. Ovarian cancer typically presents with non-specific symptoms, including abdominal distension, urinary symptoms, early satiety, and pelvic or abdominal discomfort which can be attributed to various gastrointestinal, urological, or other medical conditions [6]. Since ovarian cancer mainly spreads intraperitoneally or via lymphatics, obstruction of the retroperitoneal duodenum is rarely encountered.
Here, we report a high-grade serous ovarian cancer case that presented in a young patient with a malignant duodenal stenosis and required a Whipple procedure as part of the interval debulking surgery to achieve complete cytoreduction with no visible residual disease. To our knowledge, this is the first report of a Whipple procedure at the time of interval debulking surgery for ovarian cancer.Case:
A 38-year-old woman with no pertinent personal or family history initially presented with gastric reflux, nausea, vomiting over many months. Helicobacter pylori antigen testing was negative, a proton pump inhibitor was started empirically. Given the persistent symptoms, an upper endoscopy (EGD) was performed but showed no structural abnormalities. One month later, because of worsening symptoms and inability to tolerate anything by mouth, the patient was admitted to the hospital for expedited workup. Computed tomography (CT) imaging showed a pelvic mass and dilated stomach with a possible stricture of the duodenum. Cancer antigen 125 (CA-125) was elevated to 293. A biopsy of the pelvic mass was consistent with high-grade serous ovarian cancer by morphology and immunohistochemistry profile (CK7+, PAX8+, CK20-, and p53 abnormal). Next generation sequencing of the tumor biopsy showed no actionable mutations but a p53 mutation, low tumor mutational burden, and microsatellite stability. Genetic testing revealed no germline mutations. A venting gastrostomy tube was placed, total parenteral nutrition (TPN) and neoadjuvant chemotherapy with paclitaxel and carboplatin were initiated.
After 3 cycles of neoadjuvant chemotherapy, the patient presented for a second opinion to Stanford University. The CA-125 course at the time was difficult to reconstruct since it was initially not aligned with the chemotherapy cycles, however, CA-125 appeared to have decreased after three cycles of neoadjuvant chemotherapy to 47. Outside CT imaging demonstrated stable disease with possible mild improvement. Notably, the symptoms had improved, and the patient was able to tolerate soup and was passing gas and stool with the venting G-tube in place. Given the symptom improvement and the CA-125 course, interval cytoreductive surgery that would include a Whipple procedure to remove the duodenal stenosis was considered and Surgical Oncology was consulted. With all information taken into consideration and for insurance reasons, the decision was made to continue neoadjuvant chemotherapy. After additional cycles of neoadjuvant chemotherapy, her CA-125 level further decreased to 19, and repeat CT imaging showed good treatment response but a persistent mass tethering the duodenum (Fig. 1A). Six cycles of neoadjuvant chemotherapy were completed. The patient switch insurance and transferred care to Stanford University. Interval cytoreductive surgery was performed with no visible disease remaining, consistent with complete cytoreduction. The surgery consisted of a diagnostic laparoscopy, exploratory laparotomy, tumor debulking, total abdominal hysterectomy, bilateral salpingo-oophorectomy, bladder and posterior cul-de-sac peritonectomy, left pelvic lymph node dissection, Whipple pancreaticoduodenectomy and en-bloc extended right hemicolectomy with ileal descending primary ileocolonic anastomosis (Graphical Abstract). During the Whipple procedure, the terminal ileum, mid-transverse colon, and distal stomach were divided with multiple staple firings and the venting G-tube was removed. The jejunum distal of the ligament of Treitz and the gastroduodenal artery were divided, followed by the common hepatic duct and the neck of the pancreas. The superior mesenteric vein was mobilized over the vascular groove and the right gastroepiploic vein and right ileocolic pedicle were ligated. The ligament of Treitz was taken down, and the duodenojejunal flexure was moved to the right side of the abdomen. The uncinate process was dissected off the superior mesenteric artery. The Whipple specimen, including the right hemicolectomy specimen, was then removed en-bloc. Subsequently, the reconstruction was performed, including an end-to-side pancreaticojejunostomy using the proximal jejunum, end-to-side hepaticojejunostomy, gastrojejunostomy, and side-to-side ileocolonic anastomosis. Final pathology showed high-grade serous ovarian cancer infiltrating the duodenum, peripancreatic fat (1mm away from pancreatic parenchyma) and hepatic flexure of the colon. Surgery and immediate postoperative course were uncomplicated. By postoperative day nine, the patient was feeling well, tolerated full liquid diet with decreasing TPN support. The patient was discharged home without complications.
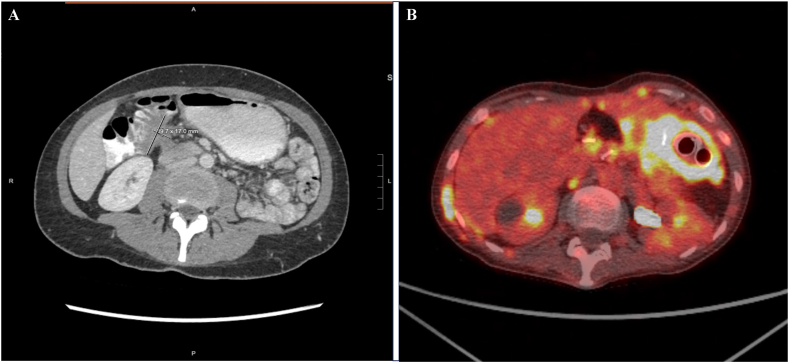
Fig. 1.
A: CT imaging before interval debulking surgery showing an approximately 4.0 × 1.7 cm right upper quadrant soft tissue lesion which tethers to the distal stomach, duodenum, and hepatic flexure and obstructs the second portion of the duodenum.
Fig. 1B: PET/CT in final weeks of life demonstrating recurrent disease with metastatic implants causing nodular thickening surrounding the stomach with 2 gastric stents in place.
The patient received three additional cycles of adjuvant chemotherapy with paclitaxel and carboplatin. Post-treatment CT imaging showed no evidence of disease. PARP inhibitor maintenance with niraparib was initiated based on PRIMA trial data [7], dose-reduced to 200 mg daily due to body weight <77 kg [8]. After one month of niraparib maintenance therapy, the PARP inhibitor was switched to olaparib due to thrombocytopenia. Repeat CT imaging after three months showed no evidence of disease. The patient felt well, was able to tolerate a regular diet, resumed work, and got married. Three months later, her CA-125 began to rise and increased abruptly to 2481. At this point, CT imaging showed progression of disease, seven months after the completion of adjuvant chemotherapy consistent with platinum-sensitive recurrence.
Systemic chemotherapy with carboplatin, pegylated liposomal doxorubicin, and bevacizumab was started. Following three cycles of this regimen, CT imaging showed a partial response. Due to the nationwide carboplatin shortage, for cycle 5, only pegylated liposomal doxorubicin and bevacizumab could be provided. Prior to cycle 6, the patient was admitted to the hospital for worsening fatigue, nausea, vomiting, and poor PO intake – all symptoms reminiscent of the initial presentation. CT imaging showed minimally worsened disease burden, however, also evidence of gastric outlet obstruction. TPN was initiated. Fluoroscopic evaluation of the upper GI tract with serial abdominal x-rays ruled out a complete obstruction but repeat EGD showed a 2–3 cm stenosis at the gastrojejunostomy, for which a gastric stent was placed. The symptoms improved immediately, and the patient was discharged home.
Three days later, the patient was re-admitted for nausea, vomiting, and abdominal pain. CT imaging demonstrated an obstruction of the afferent jejunal loop with a resultant dilation to 5.5 cm. Repeat EGD was performed with removal of the previously placed gastric stent and placement of two gastric stents: #1 from the stomach into the afferent pancreaticobiliary limb and #2 across the gastrojejunostomy stenosis into the efferent limb. Stent migration of the gastric stent #1 into the gastric lumen was corrected. The symptoms resolved postoperatively. PET/CT was performed prior to discharge home, which demonstrated extensive FDG-avid nodular thickening surrounding the stomach consistent with metastatic implants (Fig. 1B). Short time later, the patient represented with nausea, vomiting and abdominal pain. This time, CT imaging showed correctly positioned gastric stents, but a high-grade small bowel obstruction further distal to the surgical sites with a transition point in the left lower quadrant. The symptoms resolved with conservative management, and the patient tolerated full liquids. Weekly paclitaxel for platinum-refractory disease was initiated. Unfortunately, the patient had to be re-admitted a few days later for signs of intestinal obstruction. CT imaging showed again stent migration. A repeat EGD was performed, and the stents were replaced. Post-procedure, the patient developed severe abdominal pain and CT demonstrated peritoneal free fluid and air consistent with a bowel perforation at unclear location. Given the overall prognosis, the patient was counseled on best supportive care and elected for discharge home with hospice services.
2. Discussion
Complete cytoreductive surgery is paramount in the treatment of ovarian cancer. Several studies have shown that surgical cytoreduction has the greatest impact on patient's prognosis and overall survival [[9], [10], [11], [12]] with studies from Griffiths and Hacker et al. being early landmark publications [13,14]. The meta-analysis by Bristow et al. of 81 studies representing 6885 patients with advanced ovarian cancer showed that maximal surgical cytoreduction is the most important determinant of survival and each 10 % in cytoreductive effort results in 5.5 % increase in median survival time [15]. In the context of ovarian cancer cytoreductive surgery, it is exceedingly rare to include a Whipple pancreaticoduodenectomy [16] and an en-bloc extended right hemicolectomy. However, in young and otherwise healthy patients, the incorporation of these procedures into ovarian cancer cytoreduction can be considered if required to achieve complete cytoreduction. Certainly, an interdisciplinary surgical team is needed for such involved debulking surgery. In this patient, no postoperative complications were encountered, and the surgery permitted an additional year of lifetime with good quality of life. The patient was able to be free of a venting G-tube and TPN, eat normally, work regularly, and get married.
CA-125 is widely used for monitoring the disease status and efficacy of chemotherapy. Studies have shown that an early response with a CA-125 reduction of 50 % after the first cycle of neoadjuvant chemotherapy is associated with a prognostic benefit with higher likelihood of successful interval cytoreduction, progression-free survival and overall survival [17]. While the course of the CA-125 was less clear in our patient and likely showed a slower response, CA-125 normalized prior to surgery. CA-125 response in conjunction with disease response on CT imaging and the improvement of symptoms were reasons to consider the above surgery in this otherwise healthy patient.
Disease progression manifesting as re-stenosis of the upper GI tract requiring advanced stenting portends a poor prognosis. Gastrointestinal stenting is typically a palliative measure, aiming to provide relief from symptoms such as nausea, vomiting, and abdominal pain. It does not address the underlying cancer but can significantly improve a patient's quality of life at least in the short term. It is critical to consider the disease status, the patient's overall health, the extent of obstruction, and the patient's goals of care when employing shared decision making with regards to potential treatment and interventions.
3. Conclusion
Duodenal obstruction in the context of advanced-stage ovarian cancer represents an unusual scenario. A Whipple procedure can be employed in select patients to achieve complete cytoreduction to no visible disease and can help provide good quality of life.
Conflict of interest/disclosure statement
None.
Funding
Stanford Cancer Institute, Grant# 1252052-100-WXDJA.
Prior presentation
None.
Patient consent
Written informed consent was obtained from the patient/patient family for publication of this case report including the clinical data and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Data availability statement
The data that has been used is confidential.
CRediT authorship contribution statement
Sahana Somasegar: Writing – original draft, Investigation, Formal analysis, Data curation. Lauren Tostrud: Writing – review & editing, Investigation, Data curation. George Poultsides: Writing – review & editing, Methodology, Conceptualization. Malte Renz: Writing – review & editing, Visualization, Supervision, Resources, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.(NCI), N.C.I. Cancer stat facts: ovarian cancer. Surveillance, Epidemiology, and End Results Program (SEER) 2023 https://seer.cancer.gov/statfacts/html/ovary.html Available from: [Google Scholar]
- 2.Berek J.S., Renz M., Friedlander M.L., Bast R.C. In: Holland-frei: Cancer Medicine. Bast R.C., Byrd J.C., Crice C., Hawk E., Khuri F.R., Pollock R., Tsimberidou A.M., Willett C., Willman C., editors. Wiley; New Jersey: 2023. Epithelial ovarian, fallopian tube, and peritoneal cancer; pp. 1311–1328. [Google Scholar]
- 3.Goff B.A., et al. Ovarian carcinoma diagnosis. Cancer. 2000;89(10):2068–2075. doi: 10.1002/1097-0142(20001115)89:10<2068::aid-cncr6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Ramus S.J., Gayther S.A. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 2009;3(2):138–150. doi: 10.1016/j.molonc.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risch H.A., et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am. J. Hum. Genet. 2001;68(3):700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olson S.H., et al. Symptoms of ovarian cancer. Obstet. Gynecol. 2001;98(2):212–217. doi: 10.1016/s0029-7844(01)01457-0. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Martin A., et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N. Engl. J. Med. 2019;381(25):2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 8.Berek J.S., et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 2018;29(8):1784–1792. doi: 10.1093/annonc/mdy181. [DOI] [PubMed] [Google Scholar]
- 9.Winter W.E., 3rd, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2007;25(24):3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 10.Chang S.J., Bristow R.E. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: redefining 'optimal' residual disease. Gynecol. Oncol. 2012;125(2):483–492. doi: 10.1016/j.ygyno.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 11.du Bois A., et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton C.A., et al. Clinicopathologic characteristics associated with long-term survival in advanced epithelial ovarian cancer: an NRG Oncology/Gynecologic Oncology Group ancillary data study. Gynecol. Oncol. 2018;148(2):275–280. doi: 10.1016/j.ygyno.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths C.T. vol. 42. Natl Cancer Inst Monogr; 1975. pp. 101–104. (Surgical Resection of Tumor Bulk in the Primary Treatment of Ovarian Carcinoma). [PubMed] [Google Scholar]
- 14.Hacker N.F., et al. Primary cytoreductive surgery for epithelial ovarian cancer. Obstet. Gynecol. 1983;61(4):413–420. [PubMed] [Google Scholar]
- 15.Bristow R.E., et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 16.Beissel J.M., et al. Pancreaticoduodenectomy in optimal primary cytoreduction of epithelial ovarian cancer: a case report and review of the literature. Gynecol Oncol Rep. 2014;10:25–27. doi: 10.1016/j.gore.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee M., et al. Clinical significance of CA125 level after the first cycle of chemotherapy on survival of patients with advanced ovarian cancer. Yonsei Med. J. 2016;57(3):580–587. doi: 10.3349/ymj.2016.57.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.