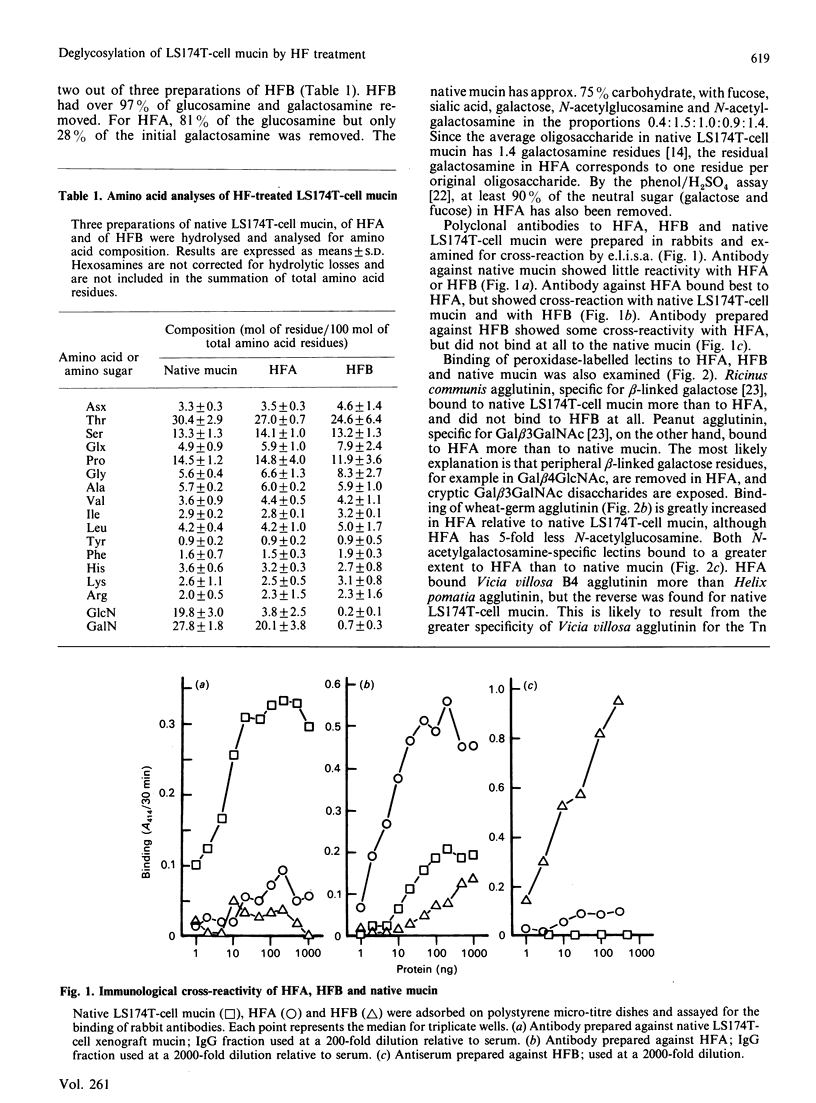
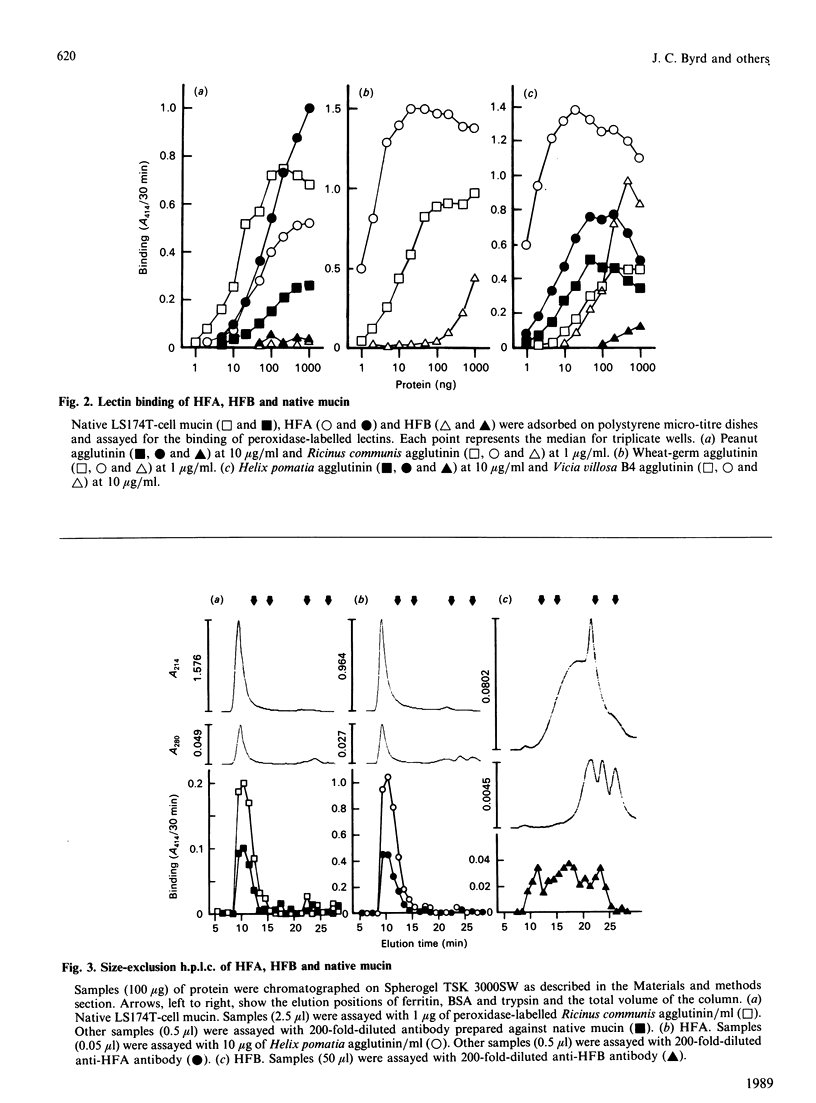
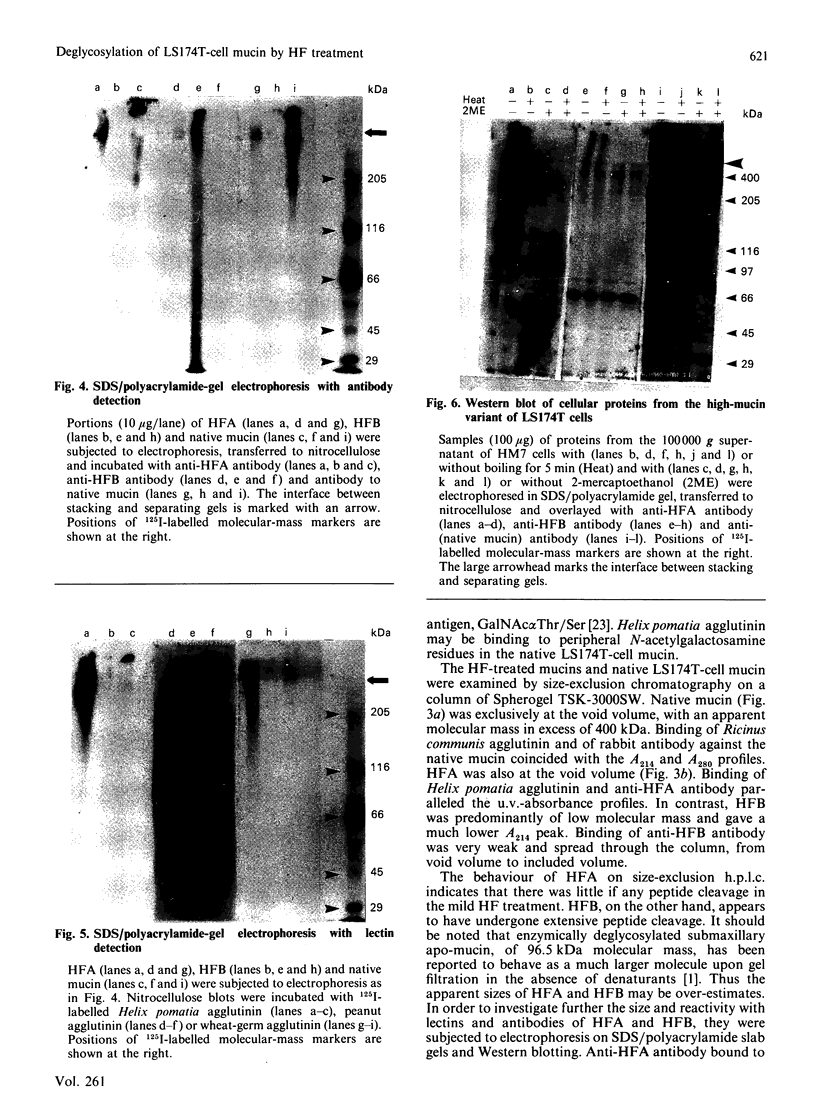
Abstract
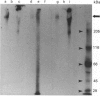
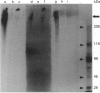
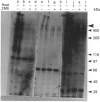
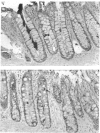
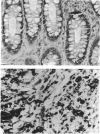
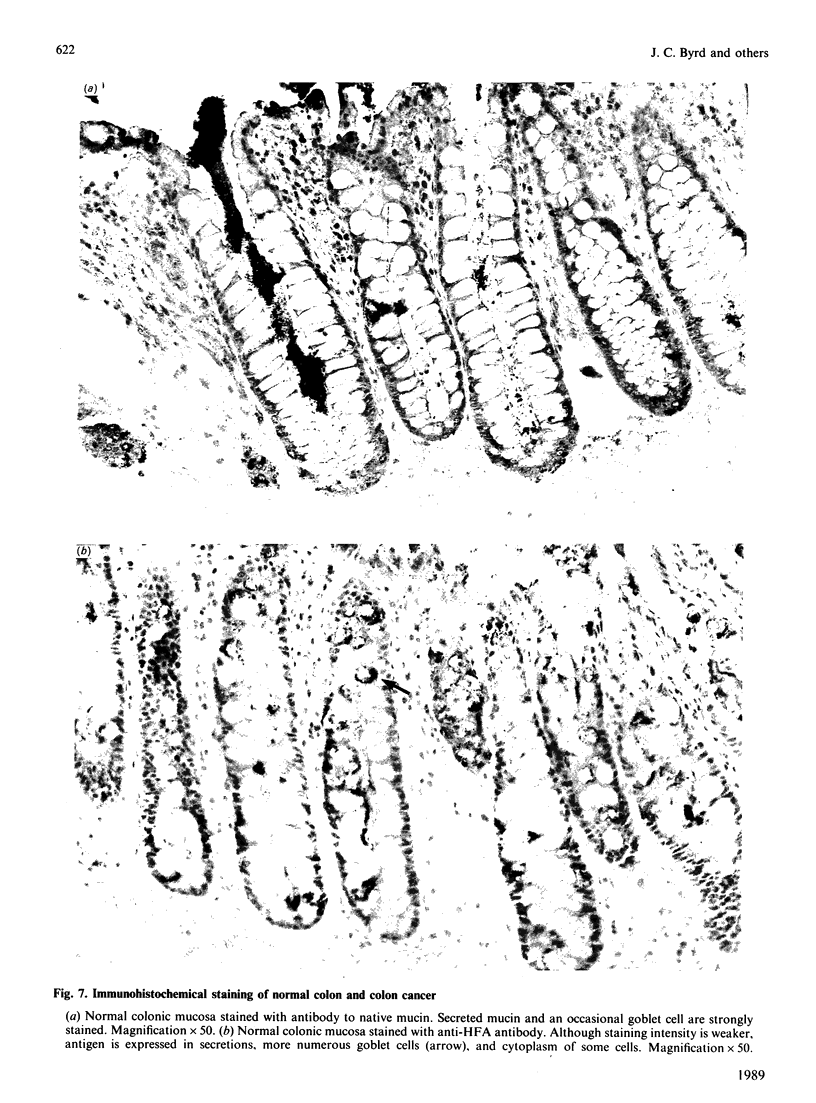
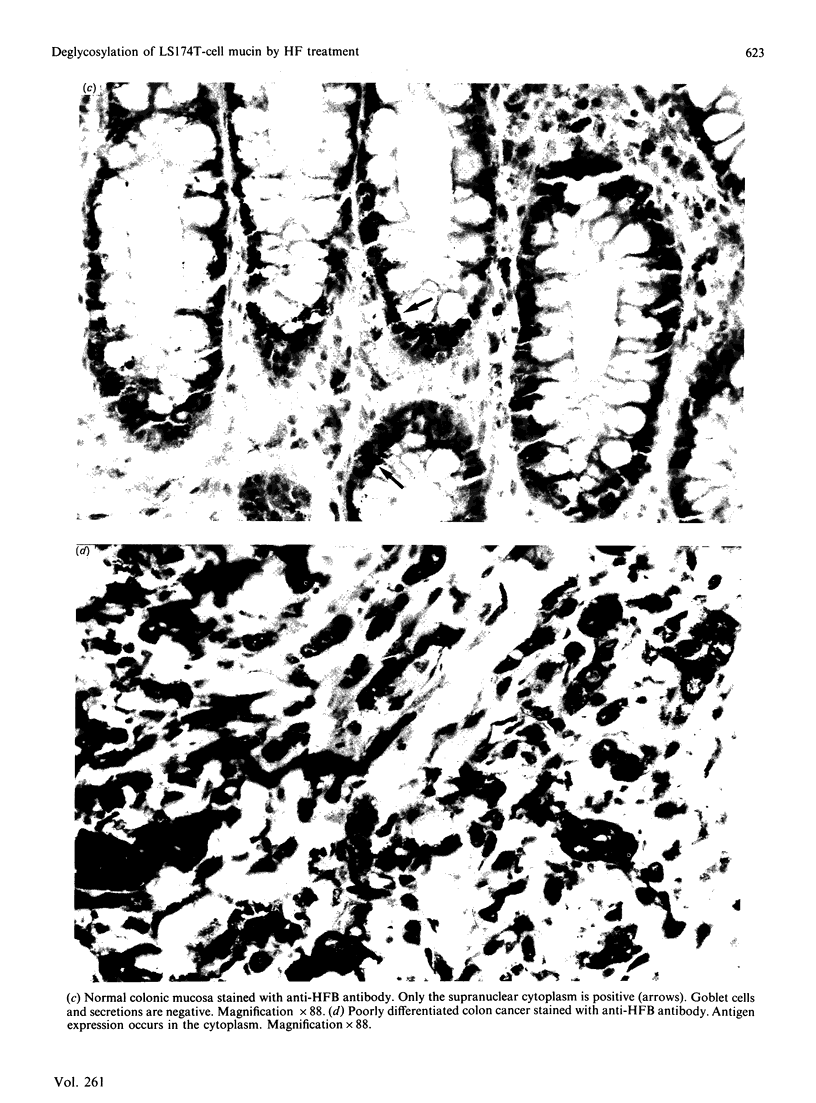
Mucin from xenografts of LS174T human colon cancer cells was treated with anhydrous HF for 1 h at 0 degree C to give a product (HFA) with over 80% of the glucosamine and hexose removed, but retaining some galactosamine, and for 3 h at room temperature to give a product (HFB) devoid of carbohydrate. Rabbit antibodies against HFA bound to HFA much more than to HFB, and bound to native mucin to an intermediate extent. Antibodies to HFB bound to HFB more than to HFA, and did not bind to native mucin. Both HFA and native mucin bound a number of lectins, but HFB did not. By SDS/polyacrylamide-gel electrophoresis and size-exclusion h.p.l.c., native mucin and HFA are of apparent molecular mass greater than 400 kDa, whereas HFB is heterogeneous and of low molecular mass. On Western blots, antibody to HFA detected both high-molecular-mass mucin and a 90 kDa protein in homogenates of LS174T cells. Antibody to HFB detected a major 70 kDa band as well as higher-molecular-mass species. In tissue sections of normal colon and colon cancers, antibody to HFA showed both cytoplasmic and extracellular staining, whereas antibody to HFB generally stained only cytoplasmic antigens. These results indicate that anti-HFB antibody is specific for apo-mucin, whereas anti-HFA antibody is specific for GalNAc-apo-mucin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhavanandan V. P., Hegarty J. D. Identification of the mucin core protein by cell-free translation of messenger RNA from bovine submaxillary glands. J Biol Chem. 1987 Apr 25;262(12):5913–5917. [PubMed] [Google Scholar]
- Burchell J., Gendler S., Taylor-Papadimitriou J., Girling A., Lewis A., Millis R., Lamport D. Development and characterization of breast cancer reactive monoclonal antibodies directed to the core protein of the human milk mucin. Cancer Res. 1987 Oct 15;47(20):5476–5482. [PubMed] [Google Scholar]
- Byrd J. C., Nardelli J., Siddiqui B., Kim Y. S. Isolation and characterization of colon cancer mucin from xenografts of LS174T cells. Cancer Res. 1988 Dec 1;48(23):6678–6685. [PubMed] [Google Scholar]
- Eckhardt A. E., Timpte C. S., Abernethy J. L., Toumadje A., Johnson W. C., Jr, Hill R. L. Structural properties of porcine submaxillary gland apomucin. J Biol Chem. 1987 Aug 15;262(23):11339–11344. [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Fauconnet M., Rochemont J. A single-column amino acid analysis method which resolves hexosamines and several cysteine derivatives. Anal Biochem. 1978 Dec;91(2):403–409. doi: 10.1016/0003-2697(78)90525-0. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hirohashi S., Clausen H., Yamada T., Shimosato Y., Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: its identification as Tn antigen. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentjens T., van de Kamp A., Spee-Brand R., Strous G. J. Biosynthesis, processing and secretion of mucus glycoprotein in the rat stomach. Biochim Biophys Acta. 1986 Jul 11;887(2):133–141. doi: 10.1016/0167-4889(86)90047-9. [DOI] [PubMed] [Google Scholar]
- Kuan S. F., Byrd J. C., Basbaum C. B., Kim Y. S. Characterization of quantitative mucin variants from a human colon cancer cell line. Cancer Res. 1987 Nov 1;47(21):5715–5724. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Biochemical characterization of the component parts of intestinal mucin from patients with cystic fibrosis. Biochem J. 1984 Dec 1;224(2):345–354. doi: 10.1042/bj2240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Potier M., Forstner G. G., Forstner J. F. Radiation inactivation of human intestinal mucin: determination of the size of the functional antigenic unit. Biochim Biophys Acta. 1986 Apr 11;881(2):248–257. doi: 10.1016/0304-4165(86)90011-5. [DOI] [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Houvenaghel M. C., Tramu G., Lamblin G., Roussel P. Action of trifluoromethanesulfonic acid on highly glycosylated regions of human bronchial mucins. Carbohydr Res. 1986 Aug 15;151:7–19. doi: 10.1016/s0008-6215(00)90325-2. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Lamport D. T. Anhydrous hydrogen fluoride deglycosylates glycoproteins. Anal Biochem. 1977 Oct;82(2):289–309. doi: 10.1016/0003-2697(77)90165-8. [DOI] [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Sage H., Brown C. F., Kaufman B. Effects of deglycosylation on the architecture of ovine submaxillary mucin glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3167–3172. [PubMed] [Google Scholar]
- Shi Z. R., Itzkowitz S. H., Kim Y. S. A comparison of three immunoperoxidase techniques for antigen detection in colorectal carcinoma tissues. J Histochem Cytochem. 1988 Mar;36(3):317–322. doi: 10.1177/36.3.3278057. [DOI] [PubMed] [Google Scholar]
- Slomiany A., Liau Y. H., Takagi A., Laszewicz W., Slomiany B. L. Characterization of mucus glycoprotein fatty acyltransferase from gastric mucosa. J Biol Chem. 1984 Nov 10;259(21):13304–13308. [PubMed] [Google Scholar]
- Timpte C. S., Eckhardt A. E., Abernethy J. L., Hill R. L. Porcine submaxillary gland apomucin contains tandemly repeated, identical sequences of 81 residues. J Biol Chem. 1988 Jan 15;263(2):1081–1088. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward H. D., Ringler N. J., Selvakumar R., Simet I. M., Bhavanandan V. P., Davidson E. A. Deglycosylation studies on tracheal mucin glycoproteins. Biochemistry. 1987 Aug 25;26(17):5315–5322. doi: 10.1021/bi00391a015. [DOI] [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Boland C. R., Kim Y. D., Tomita J. T., Palekar A., Bennington J. L., Trump B. F., Kim Y. S. Comparison of T-antigen expression in normal, premalignant, and malignant human colonic tissue using lectin and antibody immunohistochemistry. Cancer Res. 1986 Sep;46(9):4841–4847. [PubMed] [Google Scholar]
- Yuan M., Itzkowitz S. H., Palekar A., Shamsuddin A. M., Phelps P. C., Trump B. F., Kim Y. S. Distribution of blood group antigens A, B, H, Lewisa, and Lewisb in human normal, fetal, and malignant colonic tissue. Cancer Res. 1985 Sep;45(9):4499–4511. [PubMed] [Google Scholar]