Abstract
Left ventricular thrombus (LVT) is a severe consequence that typically follows acute myocardial infarction (MI) and can occur in nonischemic cardiomyopathies. In patients who have experienced an ST-segment elevation acute myocardial infarction (STEMI), LVT is seen up to 15% of the time; for patients without an ischemic cardiomyopathy, it is only 2% to 36% of the time. According to Virchow's triad, the cornerstone of LVT formation includes endothelial injury, blood stasis, and hypercoagulability. However, LVT increases morbidity and mortality in patients with both ischemic and nonischemic cardiomyopathies by increasing the risk of stroke or systemic embolism. Studies on nonischemic etiology are limited, and the majority of LVT case series concentrate on ischemic cardiomyopathies. We present this case with the nonischemic cardiomyopathies caused by LVT. Specifically, the patient underwent coronary artery assessment using photon-counting computed tomography, which is among the most advanced systems worldwide.
Keywords: Left ventricular thrombus, Nonischemic cardiomyopathies, Dilated cardiomyopathy, Glomerulonephritis, Photon-counting computed tomography
Introduction
Left ventricular thrombus seems to be a serious adverse effect of a number of conditions. The primary cause of LVT is ischemic heart disease [1]. Other causes include dilated cardiomyopathy (DCM), hypertrophic obstructive cardiomyopathy, takotsubo cardiomyopathy, and hypercoagulation diseases [2,3]. Three factors contribute to the formation of LVT: endothelial injury, blood stasis, and hypercoagulability. There are variations in the pathophysiology of LVT formation. For example, endocardial injury and inflammation may be predominant factors in ischemic heart disease, while blood stasis may be the dominant factor in DCM, and hypercoagulability is a major mechanism in hypercoagulation diseases (such as autoimmune diseases, immobile conditions, etc.) [3]. LVT increases the increases the risk of mortality, stroke, cardiovascular events, and systemic embolism [2,4].
Nowadays, there are many available techniques to diagnose LVT. The standard technology for detecting and monitoring LVT is transthoracic echocardiography (TTE) [5,6]. Small intercostal spaces, large body size, chest deformities, or lung disease might make TTE difficult for patients, which can lead to poor acoustic windows as well as the inability to detect LVT [5,6]. The high sensitivity and specificity diagnosis and evaluation of LVT is still cardiac magnetic resonance imaging (CMR) [7]. Using gadolinium in a CMR scan can help find abnormal structures inside the ventricles and make it easier to see the differences between healthy heart tissue and scarred myocardium [7]. Few studies have used cardiac computed tomography (CT) to identify lung vein thrombosis (LVT). The diagnosis of LVT using computed tomography (CT) remains invalidated. Contrast-enhanced coronary CT angiography has a lot of potential for widespread use in finding LVT because it is quick, cheap, and has a very high spatial resolution (<1 mm) [7].
Besides diagnosing LVT through imaging techniques, identifying the causes of LVT formation is crucial not only for treatment but also for prevention. Acute myocardial infarction (AMI) is the most common cause in ischemic cardiomyopathies [1]. Furthermore, nonischemic cardiomyopathies (such as cardiomyopathy diseases, takotsubo cardiomyopathy, or hypercoagulation diseases) contribute to this complication [2,8,9]. Finding coronary arteries with digital subtraction angiography (DSA) or Coronary Computed Tomographic Angiography (CCTA) can differentiate between the 2 disease groups. CCTA is a noninvasive method for assessing coronary arteries, and photon-counting cardiac computed tomography represents the most advanced system for transforming coronary artery morphological assessment due to its high spatial resolution and spectral imaging capabilities [10,11].
Case presentation
A 38-year-old Vietnamese male was admitted to the Department of Internal Medicine due to orthopnea. His medical history included hypertension and heavy alcohol consumption. There was no significant family history. He left untreated leg swelling, unstable blood pressure, and worsening orthopnea 1 month before admission. During the examination, he exhibited dyspnea and edema in the lower limbs. His vital signs indicated a high blood pressure of 190/100 mmHg, a fast regular heart rate of 110 bpm, oxygen saturation of 96% in room air, and a respiratory rate of 25 breaths per minute. During the chest examination, we detected signs of congestive heart failure and an abnormal murmur.
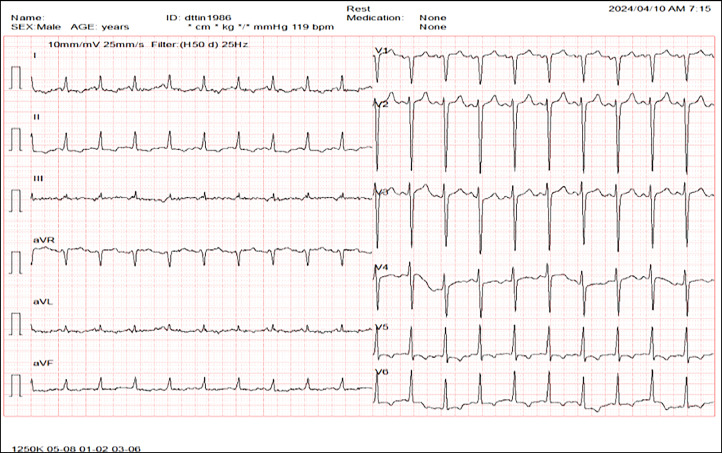
We conducted numerous subclinical tests and image analyses for diagnosis. The results of the complete blood count, renal function (eGFR: 86.62 mL/minute/1.73 m²), and liver function were within normal ranges. The N-terminal pro B-type natriuretic peptide level was 31837.8 pg/mL, and the high-sensitivity cardiac troponin level was approximately 439.5 pg/mL. Proteinuria in a 24-hour sample was measured in grams and with erythrocytes. The electrocardiogram showed rhythm with tachycardia at 120 bpm and ST-segment depression in V5-V6, D1, aVL, D2, and aVF (Fig. 1).
Fig. 1.
Electrocardiogram: Sinus rhythm, 120 bpm heartbeat, and ST-segment depression in V5-V6, D1, aVL, D2, and aVF.
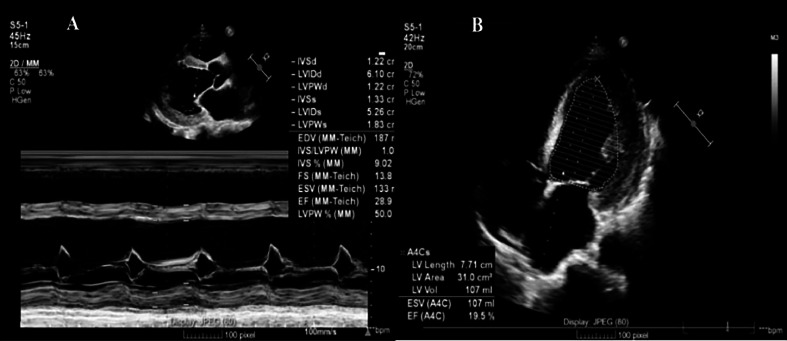
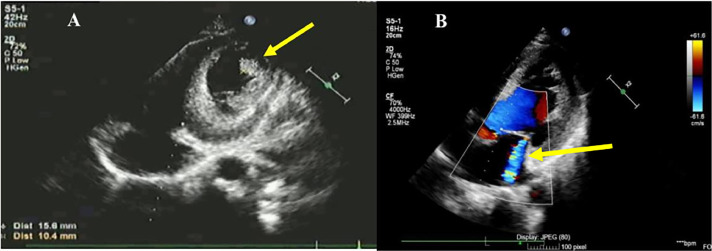
The transthoracic echocardiogram (TTE) revealed a decreased left ventricular function with an ejection fraction (EF) of 19% (Simpson method), severe global hypokinetic left ventricle, mitral valve, and aortic valve regurgitation (Fig. 2). Notably, there was a dilated left heart chamber, and an apical LVT was identified (Fig. 3).
Fig. 2.
Transthoracic echocardiogram (TTE): (A) Left ventricular ejection fraction by Teicholz method. (B) Left ventricular ejection fraction using the Simpson method.
Fig. 3.
Transthoracic echocardiogram (TTE): (A) Left ventricular thrombus 18 × 19 mm (yellow-arrow). (B) Mitral valve regurgitation (yellow arrow).
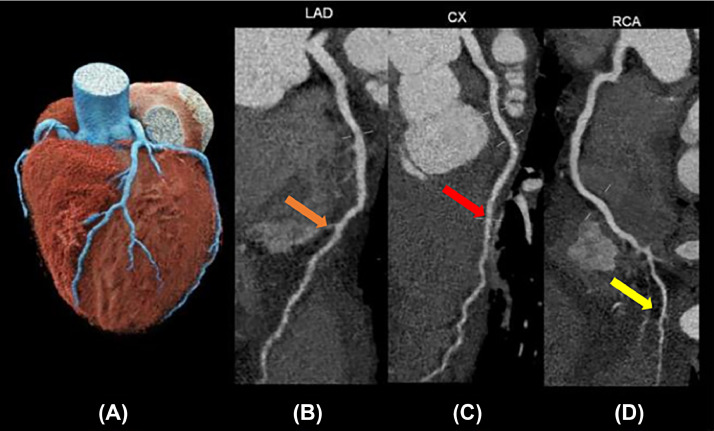
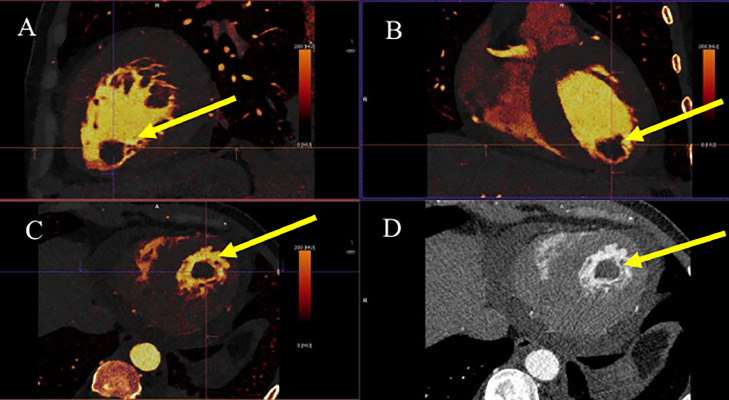
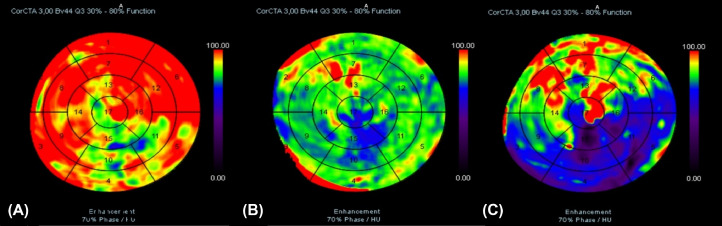
We found that the male patient had heart failure with a reduced ejection fraction (19%), glomerulonephritis, high blood pressure, LVT, and rising high-sensitivity cardiac troponin levels. The patient underwent cardiac computed tomography to assess coronary arteries. The aim was to differentiate between acute myocardial infarction and dilated cardiomyopathy. The imaging revealed the noncalcified plaques on the walls of coronary arteries (Fig. 4). Additionally, a mass without iodine concentration, which was suggested for thrombi, was witnessed in the intracardiac (Fig. 5). The perfusion imaging showed an abnormal blood flow in the left ventricle on the polarmap (Fig. 6).
Fig. 4.
Coronary artery system in photon-counting computed tomography: (A) VRT image. (B-D) show MPR of RCA (orange arrow), LAD (red arrow), and LCX branches (yellow arrow) with noncalcification plaques.
Fig. 5.
Intracardiac thrombi at the left ventricle: (A-C) show no iodine contribution to the thrombus (yellow arrow). (D) shows thrombi on multiplanar reformation or reconstruction (MPR) imaging (yellow arrow).
Fig. 6.
Perfusion map (semiquantitative analysis feature): The color on the map shows the ability of myocardial first-pass enhancement based on the Hounsfield unit. The color changed from dark to red, indicating a lack of enhancement to a normal state. (A) Normal blood flow case (red area); (B) showed the decrease of the patient's myocardial blood flow was from slight to medium in most of the 17-segment region of the left ventricle in comparison with (A) and (C). (C) Right coronary artery (RCA) occlusion showed the significant decrease of myocardial blood flow, focusing on some regions including 3, 4, 5, 9, 10, 11, 15, 16.
With all the information provided above, the final diagnosis in this patient was DCM-caused heart failure with reduced ejection fraction (HFrEF 19%), glomerulonephritis, hypertension, and left ventricular thrombus. This patient was prescribed medication including combined valsartan and sacubitril 100 mg twice a day, furosemide 20 mg, spironolactone 50 mg, dapagliflozin 10 mg, digoxin 0.25 mg half a pill per day, and rivaroxaban 20 mg.
Discussion
Dilated cardiomyopathy (DCM) is defined as when the left ventricle (LV) is bigger and systolic dysfunction happens in parts of the heart that can't be explained by other health problems like hypertension, valve diseases, or coronary artery disease [8,9]. In athletes, LV dilation can occur with normal ejection fraction (EF) or due to other environmental factors; this is not a cardiomyopathy but may indicate an early stage of DCM. DCM typically manifests as heart failure, characterized by symptoms such as edema, orthopnea, and paroxysmal nocturnal dyspnea. It can also manifest as low cardiac output, characterized by fatigue and dyspnea during exertion, arrhythmias, conduction system disease, and thromboembolic disease, which can result in stroke [10]. The diagnosis of DCM is confirmed by the presence of both components, including left ventricular enlargement; systolic dysfunction (an ejection fraction less than 50% is usually estimated from a two-dimensional echocardiogram, other noninvasive cardiac studies (computed tomography or MRI) or from a left ventricular angiogram); and fractional shortening, which is another clinical measure of systolic function. A fractional shortening less than 25% indicates systolic dysfunction [[8], [9], [10]].
This patient required hospitalization due to pulmonary edema. His symptoms included shortness of breath, swelling in his lower limbs, a swollen jugular vein, and wet rales in his lungs. An increase in N-terminal pro-B-type natriuretic peptide, a lower ejection fraction (LVEF 19%), and a dilated left ventricle were also seen on an echocardiogram. The patient also showed an increase in high-sensitivity cardiac troponin and ST-segment depression in V5-V6, D1, aVL, D2, and aVF. Therefore, our initial diagnosis was acute coronary syndrome, which led to pulmonary edema. However, there were various reasons for the elevated cardiac troponin levels in this patient, including dilated cardiomyopathy (he had enough criteria of disease), tachycardia, and severe heart failure. Consequently, an assessment of the coronary artery system allowed to distinguish between acute myocardial infarction and dilated cardiomyopathy.
Cardiac computed tomography (CT) is a popular tool for evaluating cardiovascular structures. It has become the guideline's recommended first-line imaging test for various cardiac diseases based on widespread clinical research [12]. Coronary CT angiography (CCTA) is recommended as a first-line test for stable chest pain by the American College of Cardiology/American Heart Association guidelines of 2012 and European Society of Cardiology guidelines of 2019 [11,13]. Cardiac CT using dual-energy techniques benefits from improved image quality, which enhances the diagnostic value of these scans, especially on photon-counting CT [14]. The advanced detectors in PCCT scanners enable them to have higher dose efficiency and be smaller. Thus, PCCT leads to the overall growth of diagnostic abilities and confidence. Intracardiac masses such as lipomas, fibromas, myxomas, and thrombi are found in less than 0.1% of autopsy studies [15,16]. CT scans give a very detailed picture of the heart's volume and can show how a heart tumor is connected to nearby heart parts like valves, chambers, arteries, myocardium, or epicardium. Dual energy computed tomography's material composition capabilities are useful for differentiating between cardiac masses based on iodine concentrations. In our case, a patient was admitted to the hospital with orthopnea and an increase in high-sensitivity cardiac troponin. It's suitable for CCTA indication to evaluate coronary arteries, cardiac structure, and perfusion imaging. The standard 17-myocardial segment model of LV based on the distribution is suggested by the AHA for all cardiac imaging to analyze the regional left ventricular function and perfusion. In ischemic conditions involving coronary arteries, the abnormal blood flow is only seen on the map if the stenosis is over 50% [17]. In cases of coronary occlusion, the decrease in myocardial blood flow is seen on the map that corresponds with the individual region. The slight stenosis of all coronary arteries but the hypoperfusion were seen at all 17-myocardial segment models of LV in this patient. It's suggested for a disease that involves myocardial disease. The imaging revealed an abnormal myocardial perfusion and intracardiac thrombi. That suggests that myocardial lesions are systematic diseases.
The patient had foamy urine; proteinuria in 24 hours was 1536 grams and 200 erythrocytes, so we suspected the accompanying condition was glomerulonephritis. Therefore, we tested the Streptococcus pyogenes ASO concentration, and the result was 374 UI/mL (the normal value is over 200 UI/mL). When the immune system hurts the basement membrane, mesangium, or capillary endothelium, it's called glomerularonephritis. This could lead to blood in the urine, protein in the urine, or azotemia [18]. As a common illustration of secondary glomerulonephritis caused by streptococcal infection is poststreptococcal glomerulonephritis (PSGN). The majority of glomerulonephritis types are progressive disorders. It could worsen and cause tubulointerstitial fibrosis, which gets worse over time without treatment, and chronic glomerulonephritis, which affects the glomeruli. This makes the glomerular filtration rate go down. It allows uremic toxins to be stored, which in turn affects the development of cardiac disorders that are associated with end-stage renal disease (ESRD) and chronic kidney disease (CKD). Hematuria (pink or cola-colored urine from red blood cells in the urine), proteinuria (foamy or bubbly urine from too much protein in the urine), high blood pressure, edema (swelling in the face, hands, feet, and abdomen from holding on to water), decreased urine output, nausea and vomiting, muscle cramps, and tiredness were all symptoms of glomerulonephritis [18].
The incidence of LVT peaks at 15% in patients with ST-segment elevation MI (STEMI) and ranges approximately from 2% to 36% in nonischemic cardiomyopathies patients [1,19]. Depending on thrombus morphology and the time of follow-up, LV thrombus is associated with up to 22% risk of embolism and 37% risk of major adverse cardiovascular events (MACEs) [19]. In this case, a normal coronary artery system with poor left ventricular function and blood stasis might be the main reason for left ventricular thrombus formation, which was a key factor in DCM. On the other hand, hypercoagulation is the main cause of glomerular diseases [3]. In DCM, reduced left ventricular ejection fraction and an enlarged left ventricle have been proven to be risk factors for blood stasis and LVT formation. Moreover, defective endothelial function and hypercoagulation have been demonstrated in heart failure. The myocardial inflammation is related to the significant increase in platelet activation. Hypercoagulation is linked to 4 main factors in glomerular diseases: nephrotic syndrome, vasculitis (inflammation of the vascular wall), medications (corticosteroids and cyclosporine A), and nephrotic syndrome. Hypercoagulation is caused by an imbalance between the anticoagulation factors: a decrease in proteins C, S, and antithrombin III, as well as an increase in plasma coagulation factors. Vasculitis, also known as vascular wall inflammation, can happen in both the systemic and renal vascullitis types. It changes the structure of the vessel wall, damages endothelial cells, makes the body stop working properly, and starts the clotting cascade. Moreover, high platelet counts are observed in acute and chronic inflammation. In this patient, rivaroxaban was one of the direct oral anticoagulants (DOACs) that was used to treat LVT. The 2022 American Heart Association (AHA) guidelines provide minimal guidance on anticoagulation for treating LV thrombus in DCM [19]. Vitamin K antagonist (VKA) therapy is limited by several disadvantages. For instance, interactions with food or medications, varying efficacy as a result of genetic polymorphisms, and the necessity of consistent anticoagulation monitoring pose significant challenges. DOACs are advocated for similar or even better effectiveness, fewer interactions, and convenience of use [20]. The next plans for him include genetic testing and family screening, as well as primary and secondary sudden cardiac death prevention, which have been described in the 2023 European Society of Cardiology (ESC) Guidelines for the Management of Cardiomyopathies [21].
Conclusion
Left ventricular thrombus is a dangerous complication following many conditions. Despite medical advancements and the decreasing incidence of LVT, this complication still leads to stroke or other systemic embolisms. Coronary angiography distinguishes between ischemic and nonischemic cardiomyopathies, while photon-counting cardiac computed tomography is a compelling technology due to its ability to capture detailed coronary arteries with high definition. DOACs are advocated for their similar or even better effectiveness in treating left ventricular thrombus.
Ethics approval and consent to participate
Ethical approval was not necessary for the preparation of this article. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. Informed consent for patient information to be published in this article was obtained.
Financial support and sponsorship
Nil.
Author contributions
Trang Thi Thao Pham and Thang Minh Le contributed equally to this article as co-frst authors. Trang Thi Thao Pham, Thang Minh Le, and Khanh Duong Nguyen contributed to write original draft. Trang Thi Thao Pham, Cuong Manh Nguyen, Cuong Chi Tran, Anh Duong Quoc Nguyen, and Cuong Manh Nguyen contributed to collect and interpret the imaging. Trang Thi Thao Pham, Cuong Manh Nguyen, Chinh Duc Nguyen, Cuong Manh Nguyen, and Tran Tran Nguyen made substantial contributions to collect patient data and clinical data analysis. All authors have read, revised, and approved the final published version of the manuscript. All authors were responsible for submission of our study for publication.
Patient consent
The authors certify that they have obtained all appropriate patient consent forms.
Footnotes
Competing Interests: The authors declare that they have no competing interests here were no conflicts of interest.
Acknowledgments: We would like to acknowledge our colleagues at Can Tho S.I.S General Hospital and Can Tho University of Medicine and Pharmacy for their wonderful collaboration.
References
- 1.Camaj A, Fuster V, Giustino G, Bienstock S W, Sternheim D, Mehran R, et al. Left ventricular thrombus following acute myocardial infarction: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(10):1010–1022. doi: 10.1016/j.jacc.2022.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Cruz Rodriguez J B, Okajima K, Greenberg B H. Management of left ventricular thrombus: a narrative review. Ann Transl Med. 2021;9(6):520. doi: 10.21037/atm-20-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehana E M, Shawky A M, Abdelrahman H S. Insights on the left ventricular thrombus in patients with ischemic dilated cardiomyopathy. Egyptian J Radiol Nucl Med. 2021;52(1):246. doi: 10.1186/s43055-021-00628-5. [DOI] [Google Scholar]
- 4.Romero C, Achury J, Ortiz-Pereira M. Stroke and intracardiac thrombus: a case series. Neurol Perspect. 2023;3(3):1–5. doi: 10.1016/j.neurop.2022.08.002. [DOI] [Google Scholar]
- 5.Talle M A, Buba F, Anjorin C O. Prevalence and aetiology of left ventricular thrombus in patients undergoing transthoracic echocardiography at the university of maiduguri teaching hospital. Adv Med. 2014;2014 doi: 10.1155/2014/731936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, et al. Guidelines for the use of echocardiography in the evaluation of a cardiac source of embolism. J Am Soc Echocardiogr. 2016;29(1):1–42. doi: 10.1016/j.echo.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14(8):721–740. doi: 10.1093/ehjci/jet123. [DOI] [PubMed] [Google Scholar]
- 8.Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE, et al. Dilated cardiomyopathy. Nat Rev Dis Primers. 2019;5(1):32. doi: 10.1038/s41572-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertini M, Balla C, Pavasini R, Boriani G, et al. Efficacy of cardiac resynchronization therapy in patients with isolated ventricular noncompaction with dilated cardiomyopathy: a systematic review of the literature. J Cardiovasc Med (Hagerstown) 2018;19(7):324–328. doi: 10.2459/jcm.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 10.Huang L, Zhao X, Wang J, Liang L, Tian P, Chen Y, et al. Clinical profile, treatment, and prognosis of left ventricular thrombus in dilated cardiomyopathy. Clin Appl Thromb Hemost. 2023;29:1–11. doi: 10.1177/10760296231179683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC) Eur Heart J. 2019;41(3):407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 12.Tran AV, Nguyen NM, Ngo TH, Tran BLT, Pham PT, Phan HMT, Nguyen PM, et al. Diagnostic performance of 128-slice computed tomography angiography in patients with suspected coronary artery disease. J Taibah Univ Med Sci. 2023;18(6):1599–1607. doi: 10.1016/j.jtumed.2023.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126(25):e354–e471. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 14.Trang PT, Cuong TC, Tha TT, Dil MH, Cuong NM, Tin DN, et al. A Complicated case report of coronary artery fistula. Med Arch. 2023;77(6):489–492. doi: 10.5455/medarh.2023.77.489-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam K Y, Dickens P, Chan A C. Tumors of the heart. A 20-year experience with a review of 12,485 consecutive autopsies. Arch Pathol Lab Med. 1993;117(10):1027–1031. https://pubmed.ncbi.nlm.nih.gov/8215825 [PubMed] [Google Scholar]
- 16.Reynen K. Frequency of primary tumors of the heart. Am J Cardiol. 1996;77(1):107. doi: 10.1016/s0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- 17.Seitun S, Castiglione Morelli M, Budaj I, Boccalini S, Galletto Pregliasco A, Valbusa A, et al. Stress computed tomography myocardial perfusion imaging: a new topic in cardiology. Rev Esp Cardiol (Engl Ed) 2016;69(2):188–200. doi: 10.1016/j.rec.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Ali A A, Zoltowska D, Sattiraju S. Missov E ,et al. Cardioembolic stroke in the setting of multiple left ventricular thrombi. BMJ Case Rep. 2021;14(5) doi: 10.1136/bcr-2021-242195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Li LC, Li YX, Gui C, Yang LH, et al. Development and validation of a risk model for intracardiac thrombosis in patients with dilated cardiomyopathy: a retrospective study. Sci Rep. 2024;14(1):1431. doi: 10.1038/s41598-024-51745-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, He L, Quan X, Liang Y, et al. A meta-analysis comparing different oral anticoagulation for the treatment of ventricular thrombus. RCM. 2022;23(7):1–12. doi: 10.31083/j.rcm2307243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbelo E, Protonotarios A, Gimeno J R, Arbustini E, Barriales-Villa R, Basso C, et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur Heart J. 2023;44(37):3503–3626. doi: 10.1093/eurheartj/ehad194. [DOI] [PubMed] [Google Scholar]