Abstract
Introduction
Chronic short sleep duration (i.e., <7 h sleep daily) could reduce the brain's ability to attenuate toxin and protein accumulation, which may contribute to Parkinson's disease (PD). The purpose of this study was to characterize the relationship between self-reported sleep duration from adolescence to adulthood and the age of diagnosis in people with PD. A secondary purpose was to characterize the interaction between sleep duration and physical activity through the lifespan on the age of PD diagnosis.
Methods
A secondary data analysis was performed using the Fox Insight data set. Multiple regression analysis was used to determine the age range that sleep duration best predicted the age of diagnosis of PD. Hierarchical linear multiple regression was performed to assess if self-reported sleep duration, physical activity, and their interaction predicted the age of diagnosis for PD, after accounting for sociodemographic factors.
Results
Both sleep (p < 0.001) and physical activity time (p = 0.013) significantly predicted the of age of onset of PD. In contrast, there was no evidence to support an interaction of sleep by physical activity on the age of diagnosis of PD. Sleep duration at 46–55 years maintained significance after controlling for education, income, race, ethnicity, and sex (p < 0.001). Weekly duration of time spent performing moderate-intensity physical activity was added as an input variable.
Conclusion
Sleep duration significantly predicts the age of diagnosis of PD, with shorter sleep duration associated with a younger age of diagnosis of PD.
Keywords: Neurodegeneration, Short sleep duration, Diagnosis age, Physical activity
Highlights
-
•
Sleep duration in middle-aged adults is a key predictive variable on the age of diagnosis of Parkinson's disease.
-
•
There is no interaction effect between sleep duration and physical activity on the age of diagnosis of Parkinson's disease.
-
•
Moderate-intensity physical activity in middle-aged adults is a predicting variable on the age of diagnosis of PD.
1. Introduction
Parkinson's disease (PD) is a neurodegenerative disease in which apoptosis of dopaminergic neurons results in motor and nonmotor symptoms that can affect an individual's daily living [1]. PD is characterized by the degeneration of dopaminergic neurons in the brain [2]. One nonmotor symptom, sleep disturbance, may be caused by alterations in brain chemistry and various medications, including those prescribed specifically for PD [2]. Indeed, approximately 75 % of people with PD report sleep disturbances [3]. Interestingly, sleep disorders may occur before the clinical manifestations of PD occur [4]; however, the relationship between disordered sleep and the pathophysiology of PD is not clearly understood.
According to a consensus statement from the American Academy of Sleep Medicine and the Sleep Research Society, normal sleep duration for an adult is 7–9 h of sleep each night [5]. Any sleep duration outside of this recommended amount could have deleterious effects on health [5,6]. Chronic short sleep duration (i.e., less than 7 h sleep nightly) could reduce the brain's ability to attenuate toxin and protein accumulation in the brain [6]. One of these protein aggregates that greatly affects people with PD is alpha synuclein, an unfolded neuronal protein that disrupts protein homeostasis and elicits neuronal death [2]. Another important neurotransmitter in PD pathophysiology is γ‐aminobutyric acid (GABA), an inhibitory neurotransmitter in the central nervous system [7]. GABA neurons aid in the sleep cycle, and persons who chronically sleep shorter durations have reduced GABA levels in the brain [7]. GABA neurons are also reduced in those with PD, and is believed to be the cause of sleep disorders in this population. Another important link between PD and sleep disorders is orexin, a neuropeptide expressed in the central nervous system that is responsible for modulating circadian rhythm [8]. Orexin, similar to GABA, is reduced in persons with PD [8]. The reduction of orexin increases the degeneration of dopaminergic neurons in the brain, contributing to sleep disorders [8].
To treat sleep-related issues and other symptoms of PD, exercise can be a cost-effective and robust option [9,10]. Chronic aerobic and resistance exercise can improve sleep patterns in persons with PD [11]. Indeed, 3 months of resistance exercise training may improve sleep quality, and reduce sleep disturbances, for those with PD [12]. Additionally, a combination of aerobic and resistance exercise programming, twice per week for 6 months, may improve nighttime sleep patterns in persons with PD [13]. For those without PD, long-term exercise training may reduce the risk for developing the disease [10]. However, it is not clear if chronic exercise can mitigate the negative effects of sleep deprivation, and how these factors relate to the diagnosis of PD. The purpose of this study was therefore to characterize the relationship between self-reported sleep duration from adolescence to adulthood and the age of disease diagnosis in people with PD. A second purpose of this study was to quantify the relationship between altered chronic sleep duration and amount of moderate-intensity physical activity (MPA) throughout the lifespan with the age of diagnosis of PD.
2. Methods
A secondary data analysis was performed on a sample of data collected through the Fox Insight data set, an ongoing investigation updated monthly consisting of questionnaires collected longitudinally over several years [14]. Fox Insight is a clinical study that includes data collected on patients with PD and caretakers, households, and any other person closely related to someone with PD. The goal of this longitudinal study is to explore self-reported patient outcomes and genetic data in relation to PD. The study officially began in April 2017, and is ongoing. The research team requested access to the data for this study in February 2023 and received access the same month. To gain access, an application (including registration, justification for access, and contact information) to request database access was completed by the researchers on foxden.michaeljfox.org on February 24, 2023. An email was sent by the organization confirming the request and access was granted on the same day. Data analysis was completed November 2023. In the study, participants complete self-reported questionnaires online upon initial enrollment prompted via email and through the Fox Insight dashboard created for all participants. Questionnaires include data on disease state and symptoms, performance of activities of daily living, medical history, and family history. Eligibility for the study includes persons with a diagnosis of PD or those who are family to, live with, care for, or have a personal relationship with, a person with PD. Additionally, multiple one-time questionnaires relating health and disease characteristics, and several longitudinal questionnaires investigating symptoms and daily living for people with PD, are administered.
2.1. Participants and procedure
For the purposes of this study, only one-time questionnaires that participants completed upon initial enrollment in the Fox Insight study were analyzed. Although data is collected on participants with and without self-reported PD, only those who reported a diagnosis of PD were included for the present study. Upon registration of the Fox Insight study, participants are asked “Do you currently have a diagnosis of Parkinson's disease, or Parkinsonism, by a physician or other health care professional (most recent PD diagnosis)?” Those who answered “Yes” were included for analysis. Examples of collected data include participant demographics, medical information, cognitive and physical experiences, and PD symptoms, when applicable. Those who answered all questions were included in the analysis.
2.2. Primary independent variables: sleep and physical activity
Sleep and physical activity (PA) data were collected using the Physical Activity and Sleep questionnaire (PD-RFQ-Us), an environmental exposure questionnaire developed by Fox Insight to assess PA and sleep through each decade. Within this questionnaire, participants are asked detailed questions regarding lifestyle, personal habits, living and work environments, medication, and healthy history from adolescence to adulthood [14,15]. Specifically, data analyzed include self-reported sleep habits and physical activity across 7 age ranges: 12–17, 18–25, 26–35, 36–45, 46–55, 56–65, and 66+ years. Participants respond to questions regarding sleep, moderate PA, and vigorous PA: “From [decade], in a typical week how many hours of sleep did you engage in?” Responses for average weekly sleep are recorded on a 6-point scale (1 = <5 h, 2 = 5–6 h, 3 = 6–7 h, 4 = 7–8 h, 5 = >8 h, 6 = don't know). A similar structure is used to assess average weekly PA (1 = <1 h, 2 = 1–4 h, 3 = 5–10 h, 4 = >10 h/week, 5 = don't know, 6 = prefer not to answer). To provide guidance on PA behaviors, participants are asked to consider brief descriptions of PA when answering the survey questions. For example, MPA is described as activities producing moderate increases in breathing and heart rate. Participants are instructed to consider activities at work, home, and hobbies when responding to each question.
2.3. Primary dependent variable: age of diagnosis
Age of diagnosis of PD is calculated using a two-step process. First, participants who indicate they received a diagnosis of PD are also asked to provide the “Age when were first diagnosed with Parkinson's disease (to the best of your memory),” recorded as the month and year of diagnosis. Next, age of diagnosis in years is calculated by taking the difference from their date of birth to the reported date of diagnosis.
2.4. Demographic covariates
For the current study, sociodemographic information was collected for sex, race, Hispanic ethnicity, education level, and household income.
2.5. Data analysis
First, multiple regression analysis was used to determine at which age range sleep duration best predicted the age of diagnosis of PD. Next, hierarchical linear multiple regression was performed to assess the extent to which self-reported sleep duration, PA variables, and their interaction predicted the age of diagnosis for PD after accounting for sociodemographic factors. Specifically, three blocks were sequentially tested. The initial block (block 0) consisted of the following covariates: 1) age, 2) race, 3) Hispanic ethnicity, 4) education level, and 5) household income. In blocks one, two, and three, sleep duration for ages 46–55 years, time spent performing moderate PA for ages 46–55 years, and their interaction were added to the regression equations, respectively. Interest was paid to the predictive power of each variable (standardized beta coefficient, β), as well as the amount of additional variance that sleep duration (across different decades) was able to explain in the age of diagnosis of PD (R2 change).
2.6. Data access and ethics
Access for data was requested and received February 2023. Data was downloaded in June 2023. All data analysis was completed in October 2023. Fox Insight retrieved institutional review approval through the New England Institutional Review Board (IRB: 120,160,179; Legacy IRB#: 14–236, Sponsor Protocol Number: 1, Study Title: Fox Insight). De-identified data is public and available for download from https://doi.org/10.25549/bxya-6133.
3. Results
3.1. Enrollment statistics
A total of 38,485 people with PD were enrolled in the Fox Insight study at the time of analysis. Data was obtained from persons reporting a diagnosis of PD and who completed the Environmental Exposure Questionnaires, resulting in a total of 2306 participants included in the present analysis.
3.2. Descriptive statistics
Participants age upon study analysis was 65.5 ± 8.2 years, and the mean age of PD diagnosis was 60.1 ± 9.2 years. Of the total sample, 50.8 % (1171) were male, with 4 missing data points for sex (n = 2302). The majority of participants tended to be higher income (61.6 %), college educated (76.6 %), and identify as non-Hispanic (96.7 %). Demographic statistics are provided in Table 1.
Table 1.
Participant demographics.
| Demographic Variable | Descriptive Statistics |
|---|---|
| Participants (n) | 2306 |
| Age (years) | 65.5 ± 8.2 |
| Age of PD diagnosis (years) | 60.1 ± 9.2 |
| Sex; n (%) | |
| Male | 1171 (50.8 %) |
| Female | 1131 (49.0 %) |
| Education; n (%) | |
| Less than college | 535 (23.2 %) |
| College degree | 1762 (76.6 %) |
| Household Income; n (%) | |
| < $50,000 | 555 (24.1 %) |
| ≥ $50,000 | 1418 (61.6 %) |
| Race/Ethnicity; n (%) | |
| White/Caucasian | 2253 (97.7 %) |
| Non-white* | 47 (2.0 %) |
| Hispanic | 76 (3.3 %) |
Age and age of PD diagnosis reported as mean ± SD. *Non-white includes African American, American Indian or Alaska Native, Asian, and Native Hawaiian or Pacific Islander.
3.2.1. Linear regression
A multiple linear regression model was used to predict the effect of sleep duration across the lifespan on the age of diagnosis of PD. Sleep duration predicted the age of diagnosis of PD (R2 = 0.068, p < 0.001). Within the model, only sleep duration in middle-aged years of life (i.e., 46–55 years) significantly predicted the age of diagnosis of PD (β = 0.246, p < 0.001), in such that those who slept longer had higher ages of diagnosis of PD. Alternatively, shorter sleep duration in middle-aged years was associated with a younger age of diagnosis of PD.
3.2.2. Hierarchical linear regression analyses: primary outcome variables
A linear regression model was used to predict the age of diagnosis of PD based on sleep duration (Table 2). The initial block accounted for 1.9 % of the variance of age of diagnosis, primarily driven by sex (β = −0.055, p = 0.027), education (β = 0.091, p < 0.001), and Hispanic ethnicity (β = −0.043, p = 0.045). Specifically, men, higher education, and Hispanic ethnicity were significantly associated with an earlier age of diagnosis for PD.
Table 2.
Regression models reporting unstandardized coefficients (B), standard errors (SE), and standardized coefficients (β) for sleep duration and physical activity scores.
| Variable | Block 0 |
Block 1 |
Block 2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | B | SE | β | B | SE | β | |
| R2 | 0.019b | 0.072 | 0.75 | ||||||
| R2 Change | 0.053b | 0.004a | |||||||
| Sex | −1.017 | 0.387 | −0.055a | −0.985 | 0.376 | −0.053a | −0.918 | 0.376 | −0.050a |
| Education | 0.541 | 0.127 | 0.091b | 0.423 | 0.124 | 0.071b | 0.425 | 0.124 | 0.072b |
| Income | −0.018 | 0.117 | −0.003 | 0.005 | 0.114 | 0.001 | −0.009 | 0.114 | −0.002 |
| Race (W) | 2.436 | 2.708 | 0.036 | 1.594 | 2.636 | 0.024 | 1.934 | 2.630 | 0.029 |
| Hispanic | −2.239 | 1.118 | −0.043a | −1.686 | 1.089 | −0.032 | −1.673 | 1.088 | −0.032 |
| Sleep Duration | 2.087 | 0.184 | 0.231b | 2.594 | 0.374 | 0.287b | |||
| MPA | 1.288 | 0.519 | 0.144a | ||||||
| Sleep x MPA | −0.251 | 0.144 | −0.126 | ||||||
W, White. MPA, moderate intensity physical activity. Sleep and MPA are measured as the number of average hours per week.
p < 0.05.
p < 0.001.
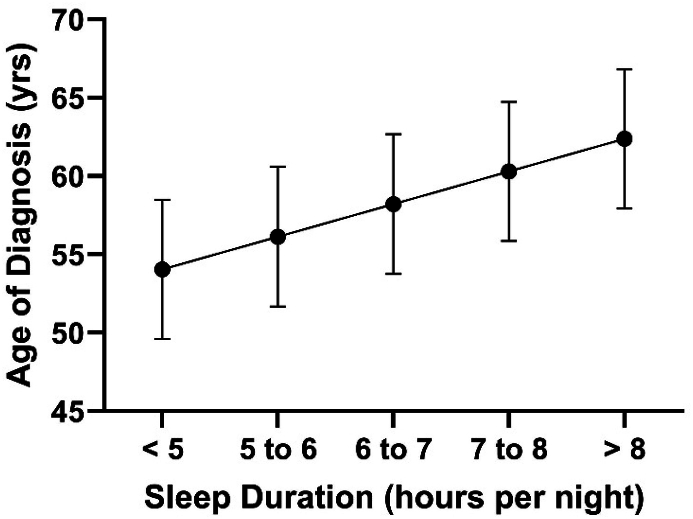
For the next block, the addition of sleep duration from 46 to 55 years significantly increased the amount of variance explaining the age of diagnosis of PD (R2 Change = 0.053, β = 0.231, p < 0.001). Sleep duration from 46 to 55 years was the only significant predictor in this model (p < 0.001, Fig. 1) and Hispanic ethnicity no longer contributed as a significant factor (β = −0.032, p > 0.05).
Fig. 1.
Relationship between sleep duration during 46–55 years of age, and age of diagnosis of Parkinson's disease after controlling for sex, education, income, race, and Hispanic ethnicity. Regression line is significant at p < 0.05. Error bars represent standard error.
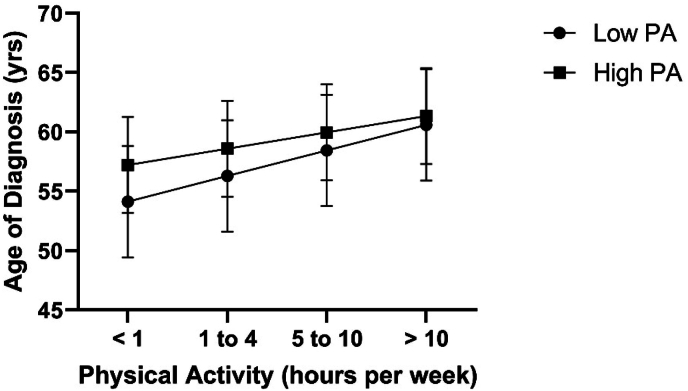
The final block accounted for 0.4 % of the variance in the age of diagnosis of PD (R2 Change = 0.004, p = 0.013). In addition to sleep duration, MPA average weekly duration from 46 to 55 years significantly predicted the age of diagnosis of PD (β = 0.144, p = 0.013, Fig. 2). The interaction between MPA and sleep duration was non-significant (Table 2).
Fig. 2.
Relationship between weekly hours of physical activity during 46–55 years of age, and age of diagnosis of Parkinson's disease as a function of physical activity after controlling for sex, education, income, race, and Hispanic ethnicity. Regression lines between high and low physical activity groups do not significantly differ from one another. Error bars represent standard error.
4. Discussion
The goal of this study was to examine the extent to which sleep patterns and PA habits across the lifespan predict age of diagnosis of PD. The results support the hypothesis that chronic short duration sleep in middle age (i.e., 46–55 years) may lead to a younger age of diagnosis of PD, even after controlling for relevant sociodemographic factors. Furthermore, given the established bidirectional relationship between PA and sleep, the extent to which higher levels of PA may potentially buffer the negative effects of chronic short duration sleep on age of diagnosis of PD was tested. While PA significantly predicted the age of diagnosis of PD, there was no evidence to support an interaction with sleep.
According to the findings of this study, adults with PD, who on average slept less than the recommended hours of sleep per night between the ages of 46–55 years, had an earlier age of diagnosis of PD compared to those who met this recommendation. Moreover, this effect persisted after accounting for sociodemographic factors (i.e., sex, education, income, race) that have been associated with age of diagnosis of PD [[16], [17], [18]]. This finding can most likely be explained by the deleterious effects of sleep deprivation on the brain. Dopamine concentrations, which are decreased in those with PD, are reduced by approximately 10 % each decade from early adulthood [19]. Importantly, dopamine plays a role in the sleep-wake cycle [20,21]. In mice models, sleep deprivation remodels dopaminergic receptors, specifically reducing dopamine's affinity to bind to D1 receptors [21]. D1 receptors are responsible for motor behavior and cognition, and are believed to play a critical role in motor and cognitive impairment in those with PD [22]. While knowledge regarding the effect of sleep duration on dopamine is limited, there is evidence that sleep deprivation alters brain chemistry, which could affect the areas of the brain affected in the pathophysiology of PD. Chronic sleep disturbance increases neuroinflammation, alpha synucleinopathies, and atherosclerosis of the vasculature in the brain, impairs nocturnal brain oxygenation, and alters protein homeostasis, all of which contribute to PD [23,24]. Disrupted sleep, accompanied by age-related reductions in dopamine, could therefore elicit shortened sleep duration and lead to a younger age of diagnosis of PD.
Sleep duration was categorized and measured as a specific subset of sleep disorders that could be prevalent in people prior to a diagnosis of PD. Inadequate sleep duration is associated with many sleep disorders, including REM sleep behavior disorder (RBD), which may be prodromal of PD [25,26]. With RBD, an imbalance of the excitatory and inhibitory neuronal pathways occurs [24,27], and a strong correlation between RBD and alpha-syuncleinopathies exist [28]. Among those with RBD, an 82.4 % increase in the risk of developing neurodegenerative diseases exists after 10.5 years. This risk increases to 96.6 % after 14 years [29]. These results align with the findings in the study, and emphasizes the importance of sleep health during middle-aged years of life. While RBD was not analyzed as a factor in the current study, sleep duration was characterized from adolescence to the diagnosis of PD, with short sleep duration associated with a younger age of diagnosis of PD.
PA predicted the age of diagnosis in such that higher scores (longer weekly duration) of PA increased the age of diagnosis of PD. In other words, more PA weekly delayed the diagnosis of PD. PA could mitigate the deleterious effects of chronic short sleep duration. Chronic sleep deprivation can increase the unfolded protein response, which is characteristic in the brain of those with PD [19]. However, persons who are physically active and possess chronic abnormal sleep duration have diminished risk factors associated with inadequate sleep duration [20]. Chronic exercise training decreases oxidative stress and inflammation, and exercise can improve endoplasmic reticulum stress that acts to attenuate protein misfolding [30,31]. Following exercise, lactate from skeletal muscle activity can stimulate the release of irisin in the brain [32]. Irisin is a hormone that increases brain-derived neurotrophic factor (BDNF), which helps increase slow-wave sleep, improving overall sleep quality [32]. Chronic exercise can increase circulating levels of irisin in healthy individuals and in individuals with metabolic disease [32]. Irisin is emerging as a potential treatment option for PD due to decreased alpha-synuclein aggregation and dopamine loss [32,33], which could further explain the positive effects of exercise on sleep quality. According to the results of the current study, PA predicts age of diagnosis in middle-aged adults, but an interaction between sleep duration and PA was not observed. Both chronic alterations in sleep duration and reduced levels of PA increase disease risk and mortality. In healthy older adults, rates of sleep disturbances and lower levels of PA are inversely proportional [34]. However, increased levels of PA concurrent with poor sleep quality may be neuroprotective [35]. More specifically, the risk of developing PD is highest among those who possess poor sleep quality and low levels of PA, when compared to only those who exhibit higher levels of PA [35]. This contradicts our findings that there was no interaction between PA and sleep duration. This could be due to the uniqueness of the present study, in that sleep patterns and PA were observed through the lifespan, from adolescence to age of PD diagnosis. Furthermore, this is the first study to observe sleep duration as a predicting factor on the age of diagnosis of PD as early as 20 years prior to diagnosis (i.e., in middle-aged years of life). Also, PA questionnaires were administered at baseline and in a 2–7 year follow-up period in a previous study [35]. In the present study, PA data was collected from recall throughout the lifespan. These factors may be the reason that the results of the current study do not support an interaction of PA with sleep. Nonetheless, according to the results of the current study, both sleep duration and the amount of PA have an effect on the age of diagnosis of PD.
4.1. Limitations
Though insight into the relationship between sleep duration and PD was elucidated in the current study, there are several limitations. First, this is a secondary data analysis of an ongoing study, and thus all analyses of the present study are derived from self-reported questionnaires and recall through the lifespan. Participants were asked to select a range of hours for sleep duration and PA from adolescence to current age based on recall. The participants included in this analysis have a diagnosis of PD and are older adults, which could contribute to inaccurate recall due to prolonged time between age ranges and reduced cognition. Moreover, a recall bias may exist where reporting sleep duration earlier in life may be more prone to error than sleep in middle age. This bias could potentially explain why sleep duration in this more recent period accounted for the most variance in age of diagnosis of PD. Additionally, the sample of participants in the current study was primarily White, potentially reducing the generalizability to other racial backgrounds. Furthermore, although racial and ethnic differences were accounted for statistically, the effects of these adjustments may be limited given relative homogeneity of our sample. However, the highest prevalence of PD has been reported among White populations [18], which could explain this homogeneity in our sample. In the future, evidence from a diverse sample should be explored to determine the effects of sleep duration and PA on the age of diagnosis of PD.
5. Conclusion
According to the results of this study, sleep duration significantly predicts the age of diagnosis of PD, with shorter sleep duration associated with a younger age of diagnosis of PD.
This study is unique in that sleep duration through the lifespan and its influence on PD was observed. An advantage of the current study is the large sample size, increasing power to detect significant relationships in our variables. Additionally, sociodemographic variables that influence the diagnosis of PD were accounted for in the current study, thus increasing the credibility of our statistical findings. Sleep is a unique mammalian phenomenon necessary for normal body function, and sleep disturbances may be expressed in, or prodromic to, chronic diseases. This study is the first to investigate chronic sleep duration through the lifespan on the age of diagnosis of PD. Future research is needed in further exploring the effects of exercise on age of diagnosis of PD concurrently with chronic sleep deprivation to determine the benefits of PA on disease risk prevention.
CRediT authorship contribution statement
Cayla E. Clark: Writing – review & editing, Writing – original draft, Resources, Methodology, Formal analysis, Conceptualization. Joshua Gold: Writing – review & editing, Supervision, Methodology, Formal analysis. B. Rhett Rigby: Writing – review & editing, Supervision, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The Fox Insight Study (FI) is funded by The Michael J. Fox Foundation for Parkinson's Research. We would like to thank the Parkinson's community for participating in this study to make this research possible.
Appendix A.
Data used in the preparation of this article were obtained from the Fox Insight database.
(https://foxinsight-info.michaeljfox.org/insight/explore/insight.jsp) on February 24, 2023. For up-to-date information on the study, visit https://foxinsight-info.michaeljfox.org/insight/explore/insight.jsp.
References
- 1.Parkinson’s Disease . NIH National Institute on Aging; April 14, 2022. Causes, symptoms, and treatments.https://www.nia.nih.gov/health/parkinsons-disease/parkinsons-disease-causes-symptoms-and-treatments#treatments [Google Scholar]
- 2.Stefanis L. α-Synuclein in Parkinson's disease. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sleep Disorders Parkinson's foundation. https://www.parkinson.org/understanding-parkinsons/non-movement-symptoms/sleep-disorders#:∼:text=Some/20PD/20medications/20can/20disrupt,and/20overall/20quality/20of/20life
- 4.Xiao X., Rui Y., Jin Y., Chen M. Relationship of sleep disorder with neurodegenerative and psychiatric diseases: an updated review. Neurochem Res. 2024;49:568–582. doi: 10.1007/s11064-023-04086-5. [DOI] [PubMed] [Google Scholar]
- 5.Watson N.F., Badr M.S., Belenky G., et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38:843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai X.Y., Chen C., Manohar S., Husain M. Impact of sleep duration on executive function and brain structure. Commun Biol. 2022;5:201. doi: 10.1038/s42003-022-03123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Kuraishy H.M., Al-Gareeb A.I., Albuhadily A.K., et al. Sleep disorders cause Parkinson's disease or the reverse is true: good GABA good night. CNS Neurosci Ther. 2024;30(3) doi: 10.1111/cns.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Xue Y., Liu M.F., Wang Y., Chen L. Orexin and Parkinson's disease: a protective neuropeptide with therapeutic potential. Neurochem Int. 2020;138 doi: 10.1016/j.neuint.2020.104754. [DOI] [PubMed] [Google Scholar]
- 9.Fayyaz M., Jaffery S.S., Anwer F., Zil-E-Ali A., Anjum I. The effect of physical activity in Parkinson's disease: a mini-review. Cureus. 2018;10 doi: 10.7759/cureus.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X., Fu Z., Le W. Exercise and Parkinson's disease. Int Rev Neurobiol. 2019;147:45–74. doi: 10.1016/bs.irn.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Shafiq M.A., Singh J., Khan Z.A., Neary J.P., Bardutz H.A. Effect of exercise on sleep quality in Parkinson's disease: a mini review. BMC Neurol. 2024;24:49. doi: 10.1186/s12883-024-03548-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva-Batista C., de Brito L.C., Corcos D.M., et al. Resistance training improves sleep quality in subjects with moderate Parkinson's disease. J. Strength Cond. Res. 2017;31:2270–2277. doi: 10.1519/JSC.0000000000001685. [DOI] [PubMed] [Google Scholar]
- 13.Coe S., Franssen M., Collett J., et al. Physical activity, fatigue, and sleep in people with Parkinson's disease: a secondary per protocol analysis from an intervention trial. Parkinsons Dis. 2018;2018 doi: 10.1155/2018/1517807. Published 2018 Sep. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox insight study data. 2019. [DOI]
- 15.Smolensky L., Amondikar N., Crawford K., et al. Fox Insight collects online, longitudinal patient-reported outcomes and genetic data on Parkinson's disease. Sci Data. 2020;7:67. doi: 10.1038/s41597-020-0401-2. Published 2020 Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi J., Tian J., Fan Y., et al. Intelligence, education level, and risk of Parkinson's disease in European populations: a Mendelian randomization study. Front Genet. 2022;13 doi: 10.3389/fgene.2022.963163. Published 2022 Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Najafi F., Mansournia M.A., Abdollahpour I., Rohani M., Vahid F., Nedjat S. Association between socioeconomic status and Parkinson's disease: findings from a large incident case-control study. BMJ Neurol Open. 2023;5(1) doi: 10.1136/bmjno-2022-000386. Published 2023 Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ben-Joseph A., Marshall C.R., Lees A.J., Noyce A.J. Ethnic variation in the manifestation of Parkinson's disease: a narrative review. J Parkinsons Dis. 2020;10(1):31–45. doi: 10.3233/JPD-191763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J., Kim H.J. Normal aging induces changes in the brain and neurodegeneration progress: review of the structural, biochemical, metabolic, cellular, and molecular changes. Front Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.931536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizrahi-Kliger A.D., Feldmann L.K., Kühn A.A., Bergman H. Etiologies of insomnia in Parkinson's disease - lessons from human studies and animal models. Exp Neurol. 2022;350 doi: 10.1016/j.expneurol.2022.113976. [DOI] [PubMed] [Google Scholar]
- 21.Lim M.M., Xu J., Holtzman D.M., Mach R.H. Sleep deprivation differentially affects dopamine receptor subtypes in mouse striatum. Neuroreport. 2011;22:489–493. doi: 10.1097/WNR.0b013e32834846a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones-Tabah J., Mohammad H., Paulus E.G., Clarke P.B.S., Hébert T.E. The signaling and pharmacology of the dopamine D1 receptor. Front Cell Neurosci. 2022;15 doi: 10.3389/fncel.2021.806618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabia S., Fayosse A., Dumurgier J., et al. Association of sleep duration in middle and old age with incidence of dementia. Nat Commun. 2021;12:2289. doi: 10.1038/s41467-021-22354-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thangaleela S., Sivamaruthi B.S., Kesika P., et al. Neurological insights into sleep disorders in Parkinson's disease. Brain Sci. 2023;13:1202. doi: 10.3390/brainsci13081202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag A., Horsfall L., Walters K., Noyce A., Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2015;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 26.Al-Qassabi A., Fereshtehnejad S.M., Postuma R.B. Sleep disturbances in the prodromal stage of Parkinson disease. Curr Treat Options Neurol. 2017;19:22. doi: 10.1007/s11940-017-0458-1. [DOI] [PubMed] [Google Scholar]
- 27.Bohnen N.I., Hu M.T.M. Sleep disturbance as potential risk and progression factor for Parkinson's disease. J Parkinsons Dis. 2019;9:603–614. doi: 10.3233/JPD-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sobreira-Neto M.A., Stelzer F.G., Gitaí L.L.G., Alves R.C., Eckeli A.L., Schenck C.H. REM sleep behavior disorder: update on diagnosis and management. Arq. Neuropsiquiatr. 2023;81:1179–1194. doi: 10.1055/s-0043-1777111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galbiati A., Verga L., Giora E., Zucconi M., Ferini-Strambi L. The risk of neurodegeneration in REM sleep behavior disorder: a systematic review and meta-analysis of longitudinal studies. Sleep Med Rev. 2019;43:37–46. doi: 10.1016/j.smrv.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Estébanez B., de Paz J.A., Cuevas M.J., González-Gallego J. Endoplasmic reticulum unfolded protein response, aging and exercise: an update. Front Physiol. 2018;9:1744. doi: 10.3389/fphys.2018.01744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X., van Egmond L.T., Cedernaes J., Benedict C. The role of exercise-induced peripheral factors in sleep regulation. Mol. Metab. 2020;42 doi: 10.1016/j.molmet.2020.101096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parada-Sánchez S.G., Macias-Cervantes M.H., Pérez-Vázquez V., Vargas-Ortiz K. The effects of different types of exercise on circulating irisin levels in healthy individuals and in people with overweight, metabolic syndrome and type 2 diabetes. Physiol Res. 2022;71:457–475. doi: 10.33549/physiolres.934896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avgerinos K.I., Liu J., Dalamaga M. Could exercise hormone irisin be a therapeutic agent against Parkinson's and other neurodegenerative diseases? Metabol. Open. 2023;17 doi: 10.1016/j.metop.2023.100233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong X., Qi W., Xing F., et al. Association of abnormal sleep duration and sleep disturbance with physical activity in older adults: between- and within-person effects. J Am Med Dir Assoc. 2024;25:368–374. doi: 10.1016/j.jamda.2023.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Chen L.H., Sun S.Y., Li G., et al. Physical activity and sleep pattern in relation to incident Parkinson's disease: a cohort study. Int. J. Behav. Nutr. Phys. Act. 2024;21:17. doi: 10.1186/s12966-024-01568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]