Abstract
Diarrheas are common risks faced by piglets during the weaning period. This study investigated the alleviating effects of artificial parasin I protein (API) on growth performance and intestinal health of weaned pigs upon enterotoxigenic Escherichia coli (ETEC) challenge. Sixty piglets were randomly divided into five groups and fed a basal diet (CON) or basal diet supplemented with API at 0, 750, and 1500 mg/kg or antibiotics for 5 weeks. On d 15 and 25, piglets were challenged with ETEC K88 except for the CON group. Before the ETEC challenge (d 1–14), dietary API supplementation improved growth performance, and 750 mg API increased (P < 0.05) the average daily gain (ADG), decreased (P < 0.05) feed to gain ratio (F/G) and diarrhea index of weaned piglets. ETEC challenge (during d 15–35) reduced growth performance and increased (P < 0.01) the F/G, diarrhea rate, and diarrhea index. This event was accompanied by the numerically increased malondialdehyde (MDA) levels in serum and ileum, the decreased (P < 0.05) zonula-occludens-1 (ZO-1) and interleukin-6 (IL-6) in the ileum, and the increased (P = 0.04) secretory immunoglobulin A (sIgA) protein in the ileum. Artificial parasin I protein supplementation alleviated the negative impact of ETEC. The 750 mg/kg API inclusion elevated (P < 0.05) ADG and decreased (P < 0.05) F/G. Two levels of API decreased (P < 0.01) the diarrhea rate and diarrhea index. Meanwhile, API inclusion decreased (P < 0.01) the crypt depth in the jejunum, elevated (P < 0.05) villus height in the duodenum and villus height to crypt depth ratio in the duodenum and ileum, up-regulated (P < 0.05) ZO-1 gene, and down-regulated (P < 0.05) mucin-2 gene in the jejunum, and 1500 mg/kg API decreased (P < 0.01) sIgA level and down-regulated (P < 0.05) IL-1β gene in the ileum. Furthermore, 750 mg/kg API elevated (P < 0.01) Bifidobacteria population and acetic acid concentrations in the cecal chyme. In conclusion, API supplementation alleviates the negative impact of ETEC on growth performance and intestinal health, thus can be applied as an antibiotic alternative in weaned piglets.
Keywords: Artificial parasin I protein, Growth performance, Intestinal barrier, Diarrhea, Weaned piglet
1. Introduction
Weaning is a highly stressful period for piglets and can accompany severe diarrhea and growth check. Diarrhea induced by weaning stress (e.g., dietary changes, adapting to a new environment, and histological changes in the small intestine) not only negatively affects the immune system's response but also causes intestinal dysfunction (Boudry et al., 2004; Kick et al., 2012; Spreeuwenberg et al., 2001). Weaning stress also increases the risk of piglets suffering from pathogenic bacteria. Enterotoxigenic Escherichia coli (ETEC) is the most common etiological agent responsible for inducing severe diarrhea in weaned piglets by colonizing the small intestine via fimbriae and producing various toxins (Traserra et al., 2023; Wang et al., 2019). Among the pathogenic E. coli strains isolated from weaned pigs, F4 (K88) and F18 are commonly observed mucosal types (García et al., 2020). During the initial weeks of life, the expression of F4 receptors gradually increases in the mucosa, which renders it more valuable for studying as a challenged model of weaned piglets (Conway et al., 1990; Li et al., 2015; Xu et al., 2020). Enterotoxigenic Escherichia coli infection decreases the villus length/crypt depth ratio in the ileum (Gao et al., 2013), reduces the mRNA expression of zonula-occludens-1 (ZO-1) in the ileum (Zhu et al., 2023), and aggravates the intestinal microbiota dysbiosis (Bin et al., 2018). Traditionally, antibiotics are the most powerful substances that prevent and treat bacterial infections in animal husbandry. However, the abuse of antibiotics and the adverse effects of antibiotic resistance on human and animal health have been increasingly recognized as the primary concern (Casey et al., 2023; Young et al., 2022). Therefore, there is a critical necessity to develop novel, safe, and efficient antibiotic substitutes to improve the growth and intestinal health of weaned piglets.
Antimicrobial peptides (AMPs), derived from microorganisms, plants, or animals, have demonstrated a wide range of effectiveness against bacteria, fungi, and viruses. They are less likely to induce drug resistance compared with other antimicrobial agents (Wang et al., 2018). Generally, AMPs exhibit a net positive charge and a high ratio of hydrophobic amino acids, allowing them to interact directly with the bacterial membrane or cell wall, preventing the induction of antibiotic resistance (Zhang and Gallo, 2016). Parasin I (PI), isolated from the skin mucus of wounded catfish, is a potent 19-residue linear antimicrobial peptide (Cho et al., 2002). The study reports that the minimal inhibitory concentrations (MICs) of PI against the microorganism (e.g., Bacillus subtilis and E. coli) were 1 to 4 μg/mL, indicating it is one of the most potent antimicrobial peptides (Park et al., 1998). However, a notable problem with applying AMPs is the high costs of extraction and purification in the animal feed industry. Thus, our lab expressed a fusion protein containing parasin I peptide, successfully over-expressed it in the yeast expression system, and named it as artificial parasin I protein (API) (Zhao et al., 2015). Previous studies have shown that dietary API reduces diarrhea and mortality rates in rabbits by decreasing the abundance of E. coli in the cecum (Zhang et al., 2022) and improves intestinal epithelium functions and digestive enzyme activity to strengthen intestinal health in broilers (Peng et al., 2022). However, whether API supplementation can be effective in weaned piglets under stress conditions remains unclear. Therefore, in this study, we developed an ETEC K88-challenged weaned piglet model to evaluate the potential alleviating effects of API on growth performance, intestinal barrier function, and intestinal microbiota.
2. Materials and methods
2.1. Ethics statement and preparation of API
The experiment and all animal procedures were reviewed and approved by the Animal Care and Use Committee of the Laboratory Animal Center of Sichuan Agricultural University, China (Ethics Approval Number: SCAUAC202110-2).
The API used in this study was developed by our laboratory. It was prepared using a 50-L fermenter (Shanghai Baoxing Bio-engineering Equipment Co. Ltd, Shanghai, China) containing basal salt medium, induced at 29 °C for 72 h. The fresh yeast fermentation broth was mixed with the adsorbent at a ratio of 1 to 2 (wt:wt) and dried in a ventilated area. Each kilogram of product provided about 8 g API protein, which was ultimately used in animal experiments.
2.2. Animal, diet, and experimental design
A total of 60 castrated piglets (Duroc × Landrace × Yorkshire) were weaned at 28 d old with an average body weight of 7.25 ± 0.78 kg. These piglets were randomly assigned into five groups with six replicates of two pigs per pen. They were fed a basal diet (CON) or a basal diet supplemented with API at 0 (ECON), 750 (E750API), 1500 (E1500API) mg/kg or antibiotics containing 10 mg/kg colistin sulphate and 10 mg/kg enrofloxacin (EANT) for 5 weeks. On d 15 and 25, the CON group was orally infused with 100 mL Luria–Bertani (LB) culture medium, whereas the other groups were orally administered with 100 mL LB culture medium containing ETEC K88 strain at a concentration of 1 × 109 CFU/mL. All piglets were kept in a nursery room at a 25 to 28 °C temperature range and had free access to feed and clean water. The diarrhea scoring of each piglet was visually accessed at 08:00 and 16:00 every day according to the assessment of the pig's fecal score (Table S1). The basal diet (Table 1) was designed to fulfill the precise nutrient specifications of pigs in accordance with the NRC (2012).
Table 1.
Experiment basal diet composition and nutrient level (%, as air-dry basis)1.
| Ingredients | Content | Nutrient levels | Content |
|---|---|---|---|
| Corn | 32.98 | Calculated nutrient level | |
| Extruded corn | 27.00 | Digestible energy, MJ/kg | 14.77 |
| Fish meal | 3.00 | SID lysine | 1.29 |
| Soy protein concentrate | 7.00 | SID methionine | 0.46 |
| Whey powder | 8.00 | SID methionine + cysteine | 0.71 |
| Extruded soybean meal | 6.00 | SID threonine | 0.76 |
| Soybean meal | 11.00 | SID tryptophan | 0.21 |
| Soybean oil | 2.00 | Analyzed nutrient level | |
| Limestone | 0.50 | Crude protein | 19.79 |
| Dicalcium phosphate | 0.87 | Calcium | 0.91 |
| L-threonine, 98.5% | 0.18 | Total phosphorus | 0.72 |
| L-lysine HCl, 98.5% | 0.52 | ||
| DL-methionine | 0.18 | ||
| L-tryptophan, 98% | 0.02 | ||
| Salt | 0.30 | ||
| Choline chloride | 0.10 | ||
| Vitamin premix2 | 0.05 | ||
| Mineral premix3 | 0.30 | ||
| Total | 100.00 | ||
SID = standardized ileal digestible.
All essential nutrient requirements in the diets met or exceeded NRC (2012) recommendations for piglets.
The multi-vitamin premix provided the following per kilogram of diet: vitamin A, 9000 IU; vitamin D3, 3000 IU; vitamin E, 20 IU; vitamin K3, 3.0 mg; vitamin B1, 1.5 mg; vitamin B2, 4.0 mg; vitamin B6, 3.0 mg; vitamin B12, 0.2 mg; niacin, 30.0 mg; pantothenic acid,15.0 mg; folic acid, 0.75 mg; biotin, 0.1 mg.
The mineral premix provided the following per kilogram of diet: Fe (FeSO4·7H2O), 100 mg; Cu (CuSO4·5H2O), 6 mg; Zn (ZnSO4·H2O), 100 mg; Mn (MnSO4·H2O), 4 mg; I (KI), 0.14 mg; Se (Na2SeO3), 0.3 mg.
2.3. Feed chemical analyses
The feed samples were collected using the tetrad method and then crushed until all of them passed through a 0.42-mm (40 mesh) sieve (Zhang and Lu, 2019). The crushed material was collected and mixed. The feed chemical analyses were conducted following the methodology outlined in AOAC (2005), with three replicates for each treatment group. The crude protein was determined by fully automatic Kjeldahl nitrogen determination (KjeltecTM 8420, FOSS, Denmark) and calculated using the factor 6.25 of nitrogen. Calcium and toal phosphorus were determined using a graphite furnace atomic absorption spectrometer (novAA400, Analytik Jena AG, Germany). Digestible energy (DE) and amino acid nutrient levels were calculated from the diet composition at the estimated requirement with reference to the NRC (2012).
2.4. Growth performance and diarrhea index
Piglets were weighed at d 1, 14, and 35, and the feed consumption per pen was measured weekly to determine average daily gain (ADG), average daily feed intake (ADFI), and feed to gain ratio (F/G). The occurrence of diarrhea was assessed by diarrhea index and diarrhea incidence. A fecal score of 2 or 3 is considered clinical diarrhea.
2.5. Sample collection
At the end of the trial, pigs in each group were weighed after an overnight fast of 8 h. Subsequently, six pigs approaching the average body weight in each group were chosen and administered intravenous sodium pentobarbital (200 mg/kg BW) for anesthesia. The middle portions of the duodenum, jejunum, and ileum were isolated, delicately rinsed with pre-cold 0.9% physiological saline, and fixed in a 4% paraformaldehyde solution for subsequent morphological analysis. Simultaneously, the intestinal mucosa was scraped from the remaining intestinal segments, promptly frozen in liquid nitrogen and stored at −80 °C until further analysis. Additionally, the chyme from the middle region of the cecum was collected, placed in liquid nitrogen without delay, and preserved at −80 °C for subsequent microbial analysis.
2.6. Serum biochemical analyses
The serum concentrations of total protein (TP), albumin (ALB), and urea, as well as the activity of alkaline phosphatase (ALP), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by automatic biochemical radiometer (3100, HITACHI, Tokyo, Japan) through corresponding commercial kits (Sichuan Maker Biotechnology Co., Ltd, Chengdu, Sichuan, China).
2.7. Antioxidant enzyme analyses
The total antioxidant capacity (T-AOC), activities of total superoxide dismutase (T-SOD), and glutathione peroxidase (GSH-Px) in serum and intestinal mucosa were determined by using the corresponding assay kits (no. A005-1-2, A001-1-1, A015-1-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China). Malondialdehyde (MDA) concentration was also determined by an assay kit (A003-1-2, Nanjing Jiancheng Bioengineering Institute, China). Protein concentration in the samples was determined by a bicinchoninic acid (BCA) protein assay kit (AR0197, Boster Biological Technology Co., Ltd, Wuhan, Hubei, China). Optical density (OD) values were analyzed using an enzyme marker (Model 680, Bio-Rad, CA, USA). Each measurement was performed three times to ensure accuracy.
2.8. Histomorphometric measurements of intestinal tissue
Four intestinal samples (n = 4) were randomly selected from each group and prepared using paraffin embedding techniques. The samples were cut into sections measuring 5 μm in thickness. After that, they were treated with hematoxylin and eosin (H&E) staining and a neutral resin was applied to seal the samples for preservation. The crypt depth and villus height were determined at 10× magnification using a digital trinocular microscope camera microscope (Leica Biosystems, Wetzlar, Germany) and measured by Image-Pro plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Measurements included villus height (VH), crypt depth (CD), and villus height to crypt depth ratio (VH/CD). A minimum of 10 properly oriented intact villi were analyzed in triplicate for each piglet.
2.9. Secretory immunoglobulin A (sIgA) in intestinal mucosal
The concentrations of sIgA in the intestinal mucosa were determined using commercially available porcine Enzyme-Linked Immunosorbent Assay (ELISA) kits (no. NN-36234O1, Jiangsu Meimian Industrial Co., Ltd, Yancheng, Jiangsu, China) according to the provided instructions. The protein concentration was determined using the bicinchoninic acid (BCA) protein assay kit (no. AR0197, Boster Biological Technology Co., Ltd., Wuhan, Hubei, China). All procedures were performed in accordance with the protocols outlined in the respective kit manuals.
2.10. Caecal microbiological analysis
Total DNA from the caecal chyme (200 mg) was extracted using the Stool DNA Kit (no. D4015-01, Omega Bio-Tek, USA) in accordance with the manufacturer's instructions. Total bacteria were identified in a final volume of 25 μL using the HiScript III RT SuperMix kit (no. R323, Nanjing Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China). The AceQ Universal U+ Probe Master Mix V2 kit (no. Q513-02, Nanjing Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China) was used for the detection of Lactobacillus, E. coli, and Bifidobacteria. All reaction procedures concerning the kit recommendations were performed by conventional PCR on the Opticon DNA Engine (Bio-Rad, CA, USA). The primers and probe sequences of bacteria are listed in Table S2. Cycling threshold (Ct) values and baseline settings were established by the automated analysis settings, and the copy number of the target group for each reaction was calculated from the standard curve, which was generated from a standard plasmid constructed from plasmid DNA at 10-fold serial dilution (1 × 101 to 1 × 109 copies/μL).
2.11. Metabolite concentrations in cecal chyme
The concentrations of short-chain fatty acids were determined using a gas chromatograph system (VARIAN CP-3800, Varian, CA, USA) following the method (Franklin et al., 2002). In brief, 1000 mg of the cecal chyme was centrifuged at 12,000 × g at 4 °C for 10 min. Then, 1 mL of the resulting supernatant was combined with 0.2 mL of metaphosphoric acid and incubated at room temperature for 30 min. After centrifugation to remove any precipitate, 1 μL of the sample was immediately applied to the analysis using a flame ionization detector and an oven temperature range of 100 to 150 °C.
2.12. The real-time qPCR analyses
Total RNA from jejunum and ileum mucous was extracted using RNAiso Plus (no. 9108, Takara, Dalian, Liaoning, China) according to the manufacturer's instructions. The quality of the total RNA was examined by agarose gel electrophoresis and the concentration was detected using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, MA, USA). The cDNA was synthesized by reverse transcription of total eligible RNA using the HiScript III RT SuperMix kit (no. R323, Nanjing Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China). The real-time qPCR was carried out with ChamQ Universal SYBR qPCR Master Mix (no. Q711, Nanjing Vazyme Biotech Co., Ltd., Nanjing, Jiangsu, China) on the QuantStudio 5 Flex system (Applied Biosystems, USA). The final volume of the reaction mixture was 10 μL, and two replicates per gene were performed. The primers of tight junction-related genes (zonula-occludens-1[ZO-1], zonula-occludens-2 [ZO-2], and occludin [OCLN]), mucoproteins gens (mucin-1 [MUC-1] and mucin-2 [MUC-2]), cytokines genes (interleukin-1β [IL-1β], interleukin-6 [IL-6], interleukin-8 [IL-8], and interleukin-10 [IL-10]), and reference genes (β-actin and glyceraldehyde-3-phosphate dehydrogenase [GAPDH]) were designed with Primer Express 3.0 (Applied Biosystem, CA, USA). The detailed primer sequences can be found in Table S3. The target genes were corrected for the geometric mean of the Ct values of the reference genes and the relative mRNA expression was calculated using the 2−ΔΔCt method.
2.13. Statistical analysis
Statistical analysis was performed using SPSS 27.0 software. The diarrhea rate was analyzed using the chi-square test. The CON and ECON groups were tested using independent samples t-tests to evaluate the response to the ETEC challenge. The ETEC-challenged groups (ECON, E750API, E1500API, and EANT) were analyzed by one-way ANOVA. When the one-way ANOVA P-value was less than 0.05, Duncan's multiple range test was performed to determine whether there were differences between each ETEC-challenged group. Results were presented as mean ± standard error of the mean (SEM). Differences between treatments were considered highly significant at P < 0.01, significant at P < 0.05, and trending at 0.05 ≤ P < 0.10.
3. Results
3.1. API supplementation improved growth performance of weaned piglets upon ETEC challenge
As shown in Table 2, before the ETEC challenge (d 1–14), 750 mg/kg API dietary inclusion increased (P < 0.05) the ADG, decreased (P < 0.05) the F/G and diarrhea index, and 1500 mg/kg API and antibiotics supplementation showed numerically positive effects on those parameters. API and antibiotics supplementation also numerically elevated the ADFI of piglets during d 1–14.
Table 2.
Effects of API supplementation on the growth performance of ETEC-challenged piglets.
| Item | Treatments1 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | ECON | EANT | E750API | E1500API | P12 | P23 | ||
| BW, kg | ||||||||
| d 1 | 7.25 | 7.26 | 7.22 | 7.26 | 7.27 | 0.157 | 0.98 | 0.99 |
| d 14 | 9.11 | 9.22 | 9.66 | 9.94 | 9.69 | 0.167 | 0.84 | 0.52 |
| d 35 | 18.97 | 17.93 | 19.57 | 19.66 | 19.04 | 0.295 | 0.36 | 0.13 |
| ADG, g/d | ||||||||
| d 1–14 | 133.00 | 139.82b | 174.05ab | 191.58a | 172.65ab | 6.609 | 0.47 | 0.03 |
| d 15–35 | 469.74 | 414.48b | 472.32a | 463.20a | 445.26ab | 8.026 | 0.11 | 0.04 |
| ADFI, g/d | ||||||||
| d 1–14 | 264.69 | 262.94 | 299.91 | 304.61 | 293.53 | 7.181 | 0.93 | 0.16 |
| d 15–35 | 685.79 | 656.94 | 689.29 | 695.52 | 671.47 | 10.940 | 0.53 | 0.62 |
| F/G, g/g | ||||||||
| d 1–14 | 1.99 | 1.88a | 1.74ab | 1.60b | 1.73ab | 0.037 | 0.22 | 0.04 |
| d 15–35 | 1.46 | 1.59∗∗a | 1.46b | 1.50b | 1.51ab | 0.015 | <0.01 | 0.02 |
| Diarrhea rate, % | ||||||||
| d 1–14 | 16.67 | 14.29 | 12.50 | 11.31 | 13.69 | 0.981 | 0.57 | 0.74 |
| d 15–35 | 20.00 | 47.08∗∗a | 35.83b | 35.00b | 39.17b | 1.421 | <0.01 | <0.01 |
| Diarrhea index | ||||||||
| d 1–14 | 0.59 | 0.53a | 0.45ab | 0.33b | 0.40ab | 0.026 | 0.51 | 0.04 |
| d 15–35 | 0.49 | 1.29∗∗a | 0.83b | 0.92b | 0.87b | 0.057 | <0.01 | <0.01 |
API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; F/G = feed to gain ratio.
Mean and total SEM are listed in separate columns (n = 6).
CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC.
P1 is the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01).
P2 is the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Values with different lowercase letter superscripts within a row mean significant differences (P < 0.05).
Compared with the CON group, the ETEC challenge (d 15–35) reduced growth performance, as reflected by elevated (P < 0.01) F/G, increased (P < 0.01) diarrhea rate and diarrhea index, and numerically decreased ADG. Compared with the ECON group, 750 mg/kg API and antibiotics dietary inclusion elevated ADG (P < 0.05) and decreased (P < 0.05) F/G, whereas dietary 1500 mg/kg API inclusion also exhibited numerically positive effects on ADG and F/G of piglets under ETEC challenge. Moreover, two levels of API and antibiotics supplementation decreased (P < 0.01) the diarrhea rate and diarrhea index (d 15–35). API and antibiotics supplementation showed no effects on ADFI in piglets suffered with ETEC.
3.2. Effects of API supplementation on serum biochemical indicators
As presented in Table 3, the ETEC challenge elevated (P = 0.03) the concentrations of TP, increased (P ;< 0.01) the activity of AST, and tended to elevate (P = 0.07) the urea concentrations in serum when compared with the CON group. API and antibiotics supplementation moderately alleviated the higher serum AST activity induced by the ETEC challenge, especially 1500 mg/kg API and antibiotics inclusion decreased (P < 0.05) serum AST activity, and 750 mg/kg API supplementation exhibited numerically positive effects. Dietary API and antibiotics supplementation showed no effects on serum TP and urea levels in piglets upon ETEC challenge. ETEC challenge and API inclusion exhibited no impact on serum ALB concentration, ALT and ALP activities.
Table 3.
Effects of API supplementation on serum biochemical indexes of ETEC-challenged piglets.
| Item | Treatments1 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | ECON | EANT | E750API | E1500API | P12 | P23 | ||
| TP, g/L | 47.15 | 51.07∗ | 50.47 | 50.97 | 50.47 | 0.726 | 0.03 | 0.99 |
| ALB, g/L | 26.48 | 28.97 | 28.86 | 29.29 | 28.30 | 0.523 | 0.19 | 0.94 |
| ALT, U/L | 35.50 | 48.37 | 55.97 | 47.89 | 53.58 | 3.294 | 0.24 | 0.80 |
| AST, U/L | 28.59 | 54.28∗∗a | 35.64b | 41.32ab | 33.21b | 2.665 | <0.01 | 0.01 |
| ALP, U/L | 171.67 | 166.33 | 198.33 | 173.67 | 177.50 | 7.373 | 0.80 | 0.48 |
| Urea, mmol/L | 1.83 | 2.67 | 1.87 | 2.22 | 2.69 | 0.149 | 0.07 | 0.15 |
API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; TP = total protein; ALB = albumin; ALT = alanine transaminase; AST = aspartate transaminase; ALP = alkaline phosphatase. Mean and total SEM are listed in separate columns (n = 6).
CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC.
P1 is the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01).
P2 is the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Values with different lowercase letter superscripts within a row mean significant differences (P < 0.05).
3.3. API supplementation enhanced serum and intestinal antioxidant capacity
The results of the antioxidant capacity in the serum, jejunum, and ileum of ETEC-challenged pigs are shown in Fig. 1. ETEC challenge tended to increase (P = 0.09) serum content of MDA (Fig. 1A), whereas exhibited no impact on T-AOC and the activitives of GSH-Px and T-SOD. Two levels of API and antibiotics addition decreased (P < 0.01) serum content of MDA in piglets upon ETEC challenge. ETEC challenge also affected antioxidant capacity in the gut, which tended to increase (P = 0.08) the content of MDA in the ileum (Fig. 1B) and elevated (P = 0.02) activity of GSH-Px in the jejunum (Fig. 1D). While ETEC challenge exhibited no effects on T-AOC and the activity of T-SOD (Fig. 1F and H). Compared with the ECON group, two levels of dietary API and antibiotics supplementation decreased (P < 0.05) MDA content in the jejunum and ileum, except that 750 mg/kg API only showed a numerically reduced MDA content in the jejunum (Fig. 1B). Antibiotics and dietary 1500 mg/kg API addition also elevated (P < 0.05) T-AOC in the jejunum (Fig. 1F), increased (P < 0.05) T-AOC and activities of GSH-Px and T-SOD in the ileum (Fig. 1D, F, and H), and 1500 mg/kg dietary API group showed better improvement on antioxidant capacity.
Fig. 1.
Effects of API supplementation on serum and intestinal antioxidant capacity of ETEC-challenged piglets. (A-B) MDA content in serum, jejunum and ileum; (C-D) GSH-Px activity in serum, jejunum and ileum; (E-F) T-AOC activity in serum, jejunum and ileum; (G-H) T-SOD activity in serum, jejunum and ileum. API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; MDA = malondialdehyde; T-AOC = total antioxidant capacity; T-SOD = total superoxide dismutase; GSH-Px = glutathione peroxidase. CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC. Results were expressed as mean and total SEM (n = 6). P1 was the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01). P2 was the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Different lowercase letters mean a significant difference between the ETEC-challenged groups (P < 0.05).
3.4. API supplementation improved intestinal morphology
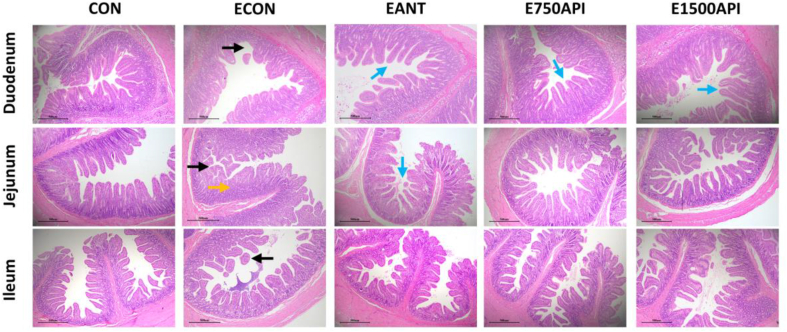
As shown in Fig. 2 and Table 4, the ETEC challenge impaired the intestinal morphology, as reflected by the shedding of jejunal and ileal villus, the deeper (P = 0.02) CD in the jejunum, the numerically decreased VH in the duodenum, and the numerically decreased VH/CD in the duodenum and ileum when compared with the CON group. Dietary 750 and 1500 mg/kg API inclusion decreased (P < 0.01) the CD and tended to increase (P = 0.08) VH/CD ratio in the jejunum. Two levels of API also elevated (P < 0.05) VH in the duodenum and VH/CD ratio in the duodenum and ileum. Besides, the antibiotics inclusion increased (P < 0.05) the VH in the jejunum, whereas 750 mg/kg API supplementation showed numerically positive effects.
Fig. 2.
Effects of API supplementation on intestinal morphology of ETEC-challenged piglets (H&E; magnification 40×). API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC. The yellow arrows indicate a significant increase in crypt depth of piglets challenged with ETEC. The black arrows point to partial breakage of intestinal villus. The blue arrows show the local enhancement of the intestinal villus in piglets upon ETEC challenge by API or antibiotics.
Table 4.
Effects of API supplementation on the morphology of the small intestine of ETEC-challenged piglets.
| Item | Treatments1 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | ECON | EANT | E750API | E1500API | P12 | P23 | ||
| Duodenum | ||||||||
| VH, μm | 420.88 | 357.22b | 452.21a | 434.34a | 447.85a | 14.351 | 0.17 | 0.04 |
| CD, μm | 332.82 | 414.61 | 368.25 | 292.33 | 311.49 | 21.927 | 0.30 | 0.18 |
| VH/CD | 1.33 | 0.98b | 1.29ab | 1.55a | 1.48a | 0.078 | 0.13 | 0.02 |
| Jejunum | ||||||||
| VH, μm | 317.34 | 333.99b | 450.21a | 398.00ab | 370.40b | 14.795 | 0.61 | 0.02 |
| CD, μm | 259.61 | 307.58∗a | 331.44a | 257.81b | 261.48b | 10.331 | 0.02 | <0.01 |
| VH/CD | 1.28 | 1.18 | 1.43 | 1.61 | 1.49 | 0.061 | 0.48 | 0.08 |
| Ileum | ||||||||
| VH, μm | 265.05 | 251.90 | 285.98 | 336.89 | 297.65 | 14.825 | 0.74 | 0.25 |
| CD, μm | 254.73 | 287.69 | 220.59 | 241.55 | 192.82 | 14.730 | 0.42 | 0.13 |
| VH/CD | 1.16 | 0.94b | 1.44a | 1.53a | 1.66a | 0.082 | 0.21 | <0.01 |
API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; VH = villus height; CD = crypt depth; VH/CD = the ratio of villus height to crypt depth.
Mean and total SEM are listed in separate columns (n = 4).
CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC.
P1 is the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01).
P2 is the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Values with different lowercase letter superscripts within a row mean significant differences (P < 0.05).
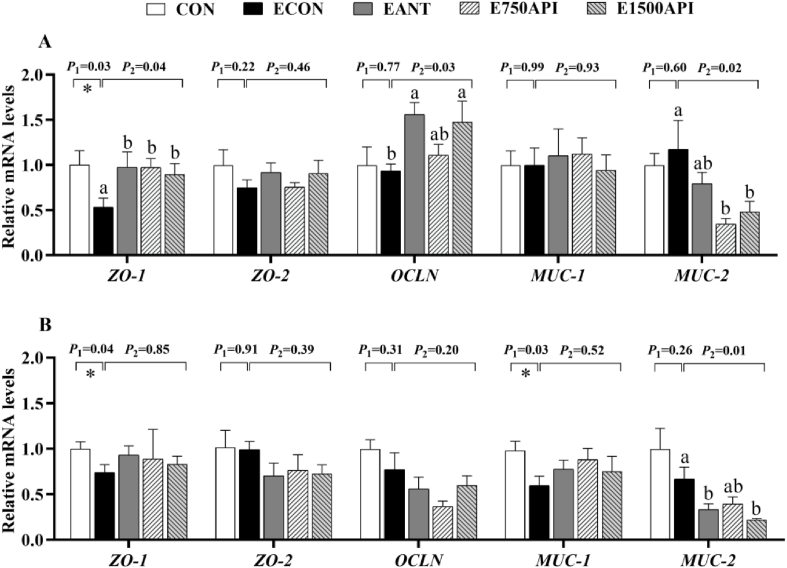
3.5. Effects of API supplementation on critical genes related to intestinal barrier functions
The results for critical genes associated with intestinal barrier function in the jejunum and ileum are shown in Fig. 3. As shown in Fig. 3A, the ETEC challenge down-regulated (P = 0.03) the mRNA levels of ZO-1, whereas exhibited no impact on the expression of ZO-2, OCLN, MUC-1 and MUC-2 in the jejunum. Compared with the ECON group, two levels of API and antibiotics inclusion up-regulated (P < 0.05) ZO-1, and 1500 mg/kg API and antibiotics elevated (P < 0.05) the expression level of OCLN, whereas 750 mg/kg API only showed numerically positive effects. Both two levels of API addition down-regulated (P < 0.05) MUC-2 in the jejunum. As shown in Fig. 3B, ETEC challenge down-regulated (P < 0.05) ZO-1 and MUC-1 whereas exhibiting no impact on ZO-2, OCLN, and MUC-2 in the ileum. Although there was no statistical difference, API and antibiotics inclusion groups showed a higher mRNA abundance of ZO-1 and MUC-1 in ETEC-challenged piglets. Two levels of dietary API and antibiotics supplementation down-regulated (P < 0.05) MUC-2, except that 750 mg/kg API only showed a numerically reduced expression of MUC-2. Dietary API and antibiotics exhibited no impact on ZO-2 and OCLN mRNA levels in the ileum upon ETEC challenge.
Fig. 3.
Effects of API supplementation on the expression of intestinal barrier functions-related genes of ETEC-challenged piglets. (A) The mRNA expression of intestinal barrier functions-related genes in jejunum. (B) The mRNA expression of intestinal barrier functions-related genes in the ileum. API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; ZO-1 = zonula-occludens-1; ZO-2 = zonula-occludens-2; OCLN = occludin; MUC-1 = mucin-1; MUC-2 = mucin-2. CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC. Results were expressed as mean and total SEM (n = 6). P1 was the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01). P2 was the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Different lowercase letters mean a significant difference between the ETEC-challenged groups (P < 0.05).
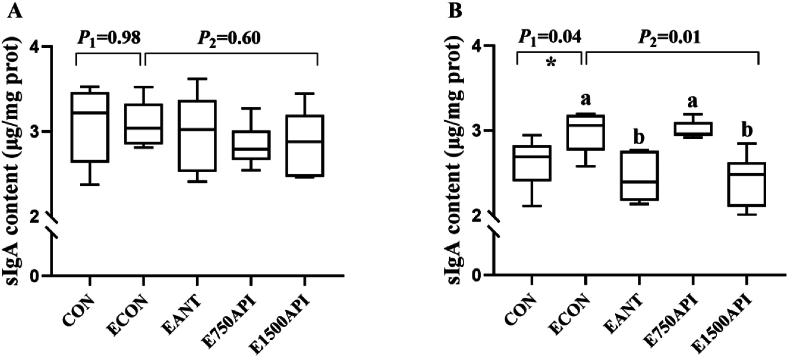
3.6. Effects of API supplementation on intestinal sIgA protein content and expression of immune-related genes
The results of sIgA protein content are presented in Fig. 4. ETEC challenge and API inclusion exhibited no impact on the content of sIgA in the jejunum (Fig. 4A). Although ETEC challenge elevated (P = 0.04) the content of sIgA in the ileum, API and antibiotics supplementations moderately alleviated the higher sIgA content induced by ETEC challenge, especially 1500 mg/kg API decreased (P < 0.05) the sIgA content (Fig. 4B).
Fig. 4.
Effects of API supplementation on intestine sIgA content of ETEC-challenged piglets. (A) The sIgA content in jejunum. (B) The sIgA content in ileum. API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; sIgA = secretory immunoglobulin A; CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC. Results were expressed as mean and total SEM (n = 6). P1 was the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01). P2 was the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Different lowercase letters mean a significant difference between the ETEC-challenged groups (P < 0.05).
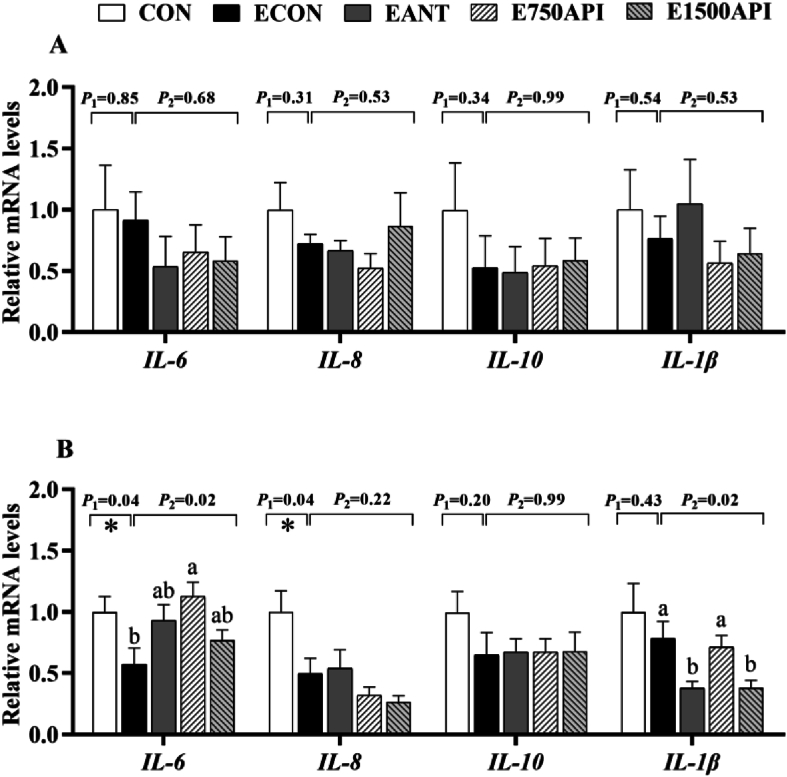
The results of immune-related genes in the intestinal mucosa are presented in Fig. 5. As shown in Fig. 5A, ETEC challenge and API supplementation did not affect the mRNA expression levels of IL-6, IL-8, IL-10 and IL-1β in the jejunum compared with the CON group. ETEC challenge suppressed the expression of immune-related genes in the ileum, especially down-regulated (P = 0.04) IL-6 and IL-8 expression levels, whereas exhibiting no impact on the expression levels of IL-10 and IL-1β (Fig. 5B). Relative to the ECON group, dietary supplementation with API altered the expression of immune-related genes in the ileum of ETEC-challenged piglets. It shows that 750 mg/kg API up-regulated (P < 0.05) IL-6 gene, and 1500 mg/kg API and antibiotics supplementations showed numerical elevation of IL-6 expression level. Furthermore, 1500 mg/kg API and antibiotics down-regulated (P < 0.05) mRNA expression level of the IL-1β. However, API and antibiotics inclusion exhibited no impact on the expression levels of IL-8 and IL-10.
Fig. 5.
Effects of API supplementation on the expression of intestinal immune-related genes of ETEC-challenged piglets. (A) The mRNA expression of intestinal immune-related genes in the jejunum. (B) The mRNA expression of intestinal immune-related genes in the ileum. API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-1β = interleukin-1β; CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC. Results were expressed as mean and total SEM (n = 6). P1 was the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01). P2 was the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Different lowercase letters mean a significant difference between the ETEC-challenged groups (P < 0.05).
3.7. API supplementation improved microbial populations and metabolites in cecal chyme
AMPs exert their biological functions mainly through antibacterial capacity and API contains a functional fragment of parasin I. Therefore, the intestinal microbial populations and their metabolites in cecal chyme were further investigated. As shown in Table 5, the ETEC challenge tended to increase (P = 0.07) the population of E. coli, numerically decreased the abundance of Bifidobacteria, and had no impact on the population of total bacteria and Lactobacillus in cecal chyme. Although there was no statistical difference, two levels of API and antibiotics inclusion numerically decreased the population of E. coli in the cecum of piglets upon ETEC challenge. Antibiotics inclusion showed no impact on the population of Bifidobacteria and Lactobacillus, whereas dietary API inclusion increased the Bifidobacteria population, with 750 mg/kg API showing the highest (P < 0.05) Bifidobacteria levels in the cecum. Most strangely, dietary supplementation with 750 mg/kg API reduced (P < 0.05) the abundance of Lactobacillus in the cecum of piglets upon ETEC challenge.
Table 5.
Effects of API supplementation on cecal microbial populations and its metabolites of ETEC-challenged piglets.
| Item | Treatments1 |
SEM |
P-value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | ECON | EANT | E750API | E1500API | P12 | P23 | ||
| Microbial populations, lg (CFU/g) | ||||||||
| Total bacteria | 10.92 | 10.87 | 10.74 | 10.65 | 10.57 | 0.087 | 0.85 | 0.67 |
| Escherichia coli | 7.35 | 8.37 | 7.75 | 7.47 | 7.42 | 0.196 | 0.07 | 0.35 |
| Bifidobacterial | 6.37 | 5.85b | 5.57b | 6.95a | 6.18ab | 0.188 | 0.35 | 0.04 |
| Lactobacillus | 5.18 | 5.15a | 5.31a | 4.24b | 5.43a | 0.156 | 0.92 | 0.01 |
| VFA, mg/g | ||||||||
| Acetic acid | 3.24 | 3.13b | 3.33b | 3.80ab | 4.23a | 0.146 | 0.82 | 0.03 |
| Propionic acid | 1.36 | 1.61 | 1.82 | 1.63 | 1.82 | 0.087 | 0.34 | 0.75 |
| Butyric acid | 0.21 | 0.16 | 0.16 | 0.19 | 0.23 | 0.011 | 0.18 | 0.08 |
API = artificial parasin I protein; ETEC = enterotoxigenic Escherichia coli; VFA = volatile fatty acids. Mean and total SEM are listed in separate columns (n = 6).
CON = basal diet; ECON = basal diet + ETEC; EANT = basal diet + antibiotics + ETEC; E750API = basal diet + 750 mg/kg API + ETEC; E1500API = basal diet + 1500 mg/kg API + ETEC.
P1 is the t-test P-value between CON and ECON group (∗P < 0.05, ∗∗P < 0.01).
P2 is the ANOVA P-value among the ETEC-challenged groups (ECON vs. EANT vs. E750API vs. E1500API). Values with different lowercase letter superscripts within a row mean significant differences (P < 0.05).
ETEC challenge exhibited no impact on the concentrations of acetic acid, propionic acid, and butyric acid in the chyme of cecum, although the value of butyric acid was numerically lower compared with the CON groups. Two levels of dietary API addition elevated the concentrations of acetic acid, with the 1500 mg/kg API group showing the higher (P < 0.05) acetic acid concentration in the cecal chyme. The 1500 mg/kg API supplementation also tended to elevate (P = 0.08) the butyric acid concentrations in the cecal chyme.
4. Discussion
In the present study, dietary API supplementation improved the growth performance of weaned piglets during 1 to 14 d, especially 750 mg/kg API increased ADG, decreased F/G ratio, and diarrhea index. Previous research has shown the beneficial impact of API on intestinal health in rabbits (Zhang et al., 2022) as well as broilers under normal conditions (Peng et al., 2022; Tang et al., 2023; Yin et al., 2023). To further assess the potential alleviating effects of API on intestinal health under stress conditions, piglets in the current study were twice challenged with ETEC by oral gavage on d 15 and 25. ETEC infection induces inflammatory response and compromises intestinal barrier function, resulting in diarrhea and growth retardation (Wang et al., 2020; Zhang et al., 2020; Zhen et al., 2022). Our study showed that the ETEC challenge increased the diarrhea rates, average diarrhea index, F/G ratio and the specific marker sIgA in the ileum of piglets, implying the exhibition of adverse effects on the piglets. As antimicrobial peptides improve the growth performance of animals primarily based on their antimicrobial activity, and API is a bioengineering protein developed from antimicrobial peptides PI. Zhang et al. (2021) suggests that piglets injected with antimicrobial peptides (WK3) improved ADG and ADFI upon ETEC challenge. Similarly, in this study, API elevated the ADG and reduced the F/G ratio in the ETEC-challenged pigs, which shared similar effects of antibiotics. ETEC-induced diarrhea is often a cause of reduced growth performance in weaned pigs. Cecropin treatment decreases the incidence of diarrhea by 47.6% in ETEC-challenged piglets (Wu et al., 2012). In the present study, both API and antibiotics inclusion decreased the ETEC-induced diarrhea rates and diarrhea index. As PI exhibits vigorous antimicrobial activities after releasing from corresponding fusion proteins (Zhao et al., 2015). Thus, the favorable effects of API on ETEC-induced diarrhea could plausibly be attributed to its robust antimicrobial activity.
Serum biochemical indicators are critical biomarkers of tissue damage and serve as potential diagnostic markers for some pathological conditions (Mendes et al., 2019; Tothova et al., 2018). TP reflects protein synthesis and metabolism in the body, dehydration leads to plasma reduction and increased TP levels. AST and urea primarily indicate the health statuses of the liver and kidney, respectively. In this study, the ETEC challenge elevated the activity of AST and the concentrations of TP and urea in serum, which further confirmed that the ETEC challenge leads to severe diarrhea and impairs the health of piglets. Dietary supplementation with antimicrobial peptides (fmbJ) decreases the serum AST activity (He et al., 2014). In this study, API supplementation moderately alleviated the higher serum AST activity and urea level induced by ETEC, suggesting the alleviation effect of API on ETEC-challenged piglets. MDA serves as a standard marker for assessing the extent of oxidative stress (Niedernhofer et al., 2003). ETEC challenge leads to an imbalanced redox state in piglets, increasing the MDA levels in jejunum (Xiong et al., 2019). AMPs can enhance the antioxidant capacity and decrease serum MDA content in fish models (Su et al., 2019). Similarly, in our current study, the ETEC challenge tended to increase MDA content in serum and ileum, whereas API addition decreased MDA levels in these samples. Antioxidant enzymes are essential components of the antioxidant defense system of animals, capable of neutralizing toxic oxygen products and promoting the redox balance. Injecting WK3 reduces the MDA concentrations and increases the SOD activity in the jejunum of ETEC-challenged piglets (Zhang et al., 2021). In this study, the relatively high dose of API elevated the activity of antioxidant enzymes (e.g., GSH-Px, T-SOD), and T-AOC in the ileum of challenged piglets. These results indicated that API enhanced antioxidant capacity in piglets upon ETEC challenge.
The typical feature of piglet diarrhea is the alteration of intestinal morphology, mainly reflected by the VH, CD and VH/CD ratio. The intestinal villus, as an essential part of the digestive tract, is highly associated with the digestive capacity of the small intestine (Marion et al., 2002). The crypt is in the invagination of the intestinal wall and its hyperplasia indicates the deterioration of intestinal morphology (Hu et al., 2013). ETEC challenge induces deleterious alterations in the morphology of intestines, characterized by diminished VH, heightened CD, and reduced VH/CD ratio in the small intestine of piglets (Duan et al., 2022; Sun et al., 2021). The administration of AMPs exhibits positive impact on the intestinal morphology of animals challenged with E. coli (Daneshmand et al., 2019; Yu et al., 2020; Zhang et al., 2021). Similarly to earlier findings, API decreased the CD in the jejunum and elevated the VH in the duodenum of ETEC-challenged piglets in the current study. Moreover, the VH/CD ratio is a well-known criterion used to evaluate intestinal health, and both doses of API supplementation increased the VH/CD ratio in the ileum, suggesting the beneficial effects of API on the intestinal morphology of ETEC-challenged piglets.
Intestinal health also depends on the intestinal mucosal mechanical barrier, which consists of the intestinal epithelial cells, the tight junctions (TJs) between the intestinal epithelial cells, and the mucus layer covering the surface of the intestinal epithelial cell (Johansson et al., 2013). MUC-2, a critical component of the mucous, is responsible for intestinal barrier protection and disease prevention (Liu et al., 2020). Pigs challenged with ETEC show the up-regulation MUC-2 during the peak of infection, whereas they show decreased expression during recovery periods (Kim et al., 2019). Similarly to the previous study, the ETEC challenge exhibited no impact on the MUC-2 level, whereas two levels of dietary API down-regulated MUC-2 in the jejunum and ileum. TJs, such as OCLN, ZO-1 and claudins, largely contribute to the integrity of the mucosal mechanical barrier (Zihni et al., 2016). ETEC infection decreases the mRNA expression levels of ZO-1 in the ileum, leading to compromised intestinal barrier integrity of weaned piglets (Zhu et al., 2023). AMPs, including porcine beta-defensin 2 and Cathelicidin-WA, have been demonstrated to modulate the expression of ZO-1, OCLN, and claudins (Han et al., 2015; Yi et al., 2016). Similarly, ETEC challenge down-regulated the expression of ZO-1 in the jejunum and ileum, whereas appropriate API supplementation up-regulated the expression of ZO-1 and OCLN in the jejunum. A study on IPEC-J2 cells shows that biogenic Microcin J25 protects intestinal epithelial integrity by directly killing pathogens (Yu et al., 2018). Hence, the improved barrier function may be partly attributed to the antimicrobial activity of API. However, this protective effect needs further investigation. In general, dietary inclusion with API improved the morphology barrier function of the intestine, which may partly explain why piglets supplemented with API alleviated the severe diarrhea caused by ETEC challenges.
The function of the intestinal immune barrier mainly depends on the immunogenic substances, such as sIgA and cytokines. sIgA provides the first line of defense in protecting the intestinal epithelium from enteropathogenic bacteria and enteric toxins (Apter et al., 1993; Hutchings et al., 2004). As a representative biomarker for the ETEC challenge, sIgA improves mucosal defense by resisting bacterial proteolytic enzymes and binding to antigens (Peterson et al., 2007). As a defensive response, the adhesion of pathogenic bacteria in the gut leads to higher sIgA levels (Gao et al., 2013; Liu et al., 2019; Sun et al., 2015). In this study, the ETEC challenge increased sIgA levels in the ileum, whereas API inclusion especially 1,500 mg/kg API prevented the increasing levels of sIgA. Reducing the content of sIgA by nicotinic acid compromised the ETEC K88 infection, which is associated with the increase of endogenous AMPs (Zhen et al., 2022). Previous study shows that AMPs (GW-Q4) completely inhibit ETEC adhesion onto intestinal porcine epithelial cell-1 (Wu et al., 2021). Hence, decreasing sIgA levels in the ileum may contribute to inhibiting ETEC adhesion in the intestine, thus alleviating the negative effect of ETEC on gut health. API addition also affected the expression of immune-related genes in the gut. Pro-inflammatory cytokines, such as IL-6 and IL-1β, are critical in mediating the host immune response after being infected by pathogenic microorganisms (Franchi et al., 2012; Guo et al., 2021). The effects of ETEC on intestinal immunity remain controversial. ETEC infection can elicit intestinal immune responses and increase IL-6 and IL-8 expression (McLamb et al., 2013). However, the ETEC challenge has also been found to down-regulate the expression of IL-1β and IL-6 in the ileal mucosa of piglets (Li et al., 2022). Whether the pro-inflammatory cytokines are increased or decreased, the ETEC challenge compromises the immune function of the small intestine. In the present study, the decreased expression of IL-6 and IL-8 in the ileum indicated the negative regulatory effects of ETEC on immune function. API supplementation recovered the expression of IL-6 and decreased the expression of IL-1β in our study. Various types of AMPs exhibit distinct immunomodulatory activities, including the regulation of cytokine expression (Jeon et al., 2017). Hence, our results suggested that API can modulate ETEC-induced abnormal immune function in the small intestine. Notably, the regulation of IL-1β by API followed a similar trend to the sIgA levels. Previous studies have found that IL-1β not only transports sIgA within the intestinal but also promotes sIgA production (Jung et al., 2015; Moon et al., 2014). Hence, the down-regulation of IL-1β by dietary API may contribute to the lower sIgA production in the ileal mucosa, thus ameliorating the gut immune response after infected by ETEC.
Intestinal flora homeostasis plays a crucial role in intestinal health. ETEC infection disrupts the intestinal microbial structure and function, thus causing gut microbiota dysbiosis in piglets (Bin et al., 2018). AMPs, secreted by animal intestinal epithelial cells, beneficially modulate gut microbiota by directly killing pathogenic bacteria (Abdelgawad et al., 2023). Meanwhile, dietary supplementation with AMPs also improves the gut microbiota composition of the animal (Yoon et al., 2013). In this study, dietary API supplementation also effectively meliorated the microflora population of cecal flora. Two levels of API numerically decreased the abundance of E. coli in the cecal of ETEC-challenged pigs. The 750 mg/kg API increased the population of Bifidobacterium, which showed better effects than the antibiotics inclusion. Antibiotics can shift the gut microbiota to long-term alternative dysbiotic states, which may promote the development and aggravation of the disease (Jo et al., 2021; Pi et al., 2019). Hence, it is reasonable that API is more effective in improving caecal microbial populations than antibiotics under pathogenic microorganism infection. Interestingly, the inclusion of 750 mg/kg API reduced the abundance of Lactobacillus in cecal upon ETEC challenge, which is probably related to the selectivity of AMPs for bacteria (Xiong et al., 2017). Thus, API may be more favorable for improving Bifidobacterium; however, further studies are needed. Intestinal microorganisms can produce various short-chain fatty acids from undigestible carbohydrates, essential in improving intestinal defense and enhancing gut development (Diao et al., 2017; Fukuda et al., 2011). In this study, 1500 mg/kg API elevated the concentration of acetic acid and numerically increased butyric acid in the cecal chyme of ETEC-challenged pigs, thus may contribute to strengthening the ability of intestinal defense against pathogenic bacteria and improving intestinal development.
5. Conclusion
In the present study, the ETEC challenge induces diarrhea, impairs intestinal morphology, causes intestinal mucosal barrier dysfunction, and reduces the growth performance of piglets. API dietary inclusion partly alleviates the negative impact of the ETEC challenge on growth performance and intestinal health of weaned piglets, which is reflected by the reduction of diarrhea incidence, improvement of intestinal morphology, and enhancement of intestinal barrier function and microbial populations. Dietary supplementation with 750 mg/kg API has a better effect on growth performance.
Author contributions
Congzhi Zou and Hua Zhao: conceived and designed the trial; Congzhi Zou, Shenggang Yin and Wanxin Zhao: were involved in the animal experiments, analysis, and data collection; Congzhi Zou: ran all the experiments with help from Wanxin Zhao, Shenggang Yin, Xiaoyu Xiang, Jiayong Tang, Gang Jia, Lianqiang Che, Guangmang Liu, Gang Tian, Xiaoling Chen, Jingyi Cai, and Bo Kang; Congzhi Zou and Hua Zhao: wrote the manuscript; and Hua Zhao: had primary responsibility for the final content. All authors read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that might inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported partly by the Sichuan Science and Technology Program (No. 2021ZDZX0009, 2021ZDZX0011), the Special Research Funding for Discipline Construction in Sichuan Agricultural University (No. 03570126), and the Sichuan Longda Animal Husbandry Science and Technology Co., Ltd (No. 2122319014).
Footnotes
Peer review under the responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2024.04.015.
Appendix Supplementary data
The following is the Supplementary data to this article:
References
- Abdelgawad A., Nicola T., Martin I., Halloran B.A., Tanaka K., Adegboye C.Y., Jain P., Ren C., Lal C.V., Ambalavanan N., O'Connell A.E., Jilling T., Willis K.A. Antimicrobial peptides modulate lung injury by altering the intestinal microbiota. Microbiome. 2023;11(1):226. doi: 10.1186/s40168-023-01673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC . 18th ed. Association of Official Analytical Chemists International; Gaithersburg, MD: 2005. Official methods of analysis. [Google Scholar]
- Apter F.M., Lencer W.I., Finkelstein R.A., Mekalanos J.J., Neutra M.R. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun. 1993;61(12):5271–5278. doi: 10.1128/iai.61.12.5271-5278.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin P., Tang Z.Y., Liu S.J., Chen S., Xia Y.Y., Liu J.Q., Wu H., Zhu G.Q. Intestinal microbiota mediates enterotoxigenic Escherichia coli-induced diarrhea in piglets. BMC Vet Res. 2018;14(1):385. doi: 10.1186/s12917-018-1704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudry G., Peron V., Le Huerou-Luron I., Lalles J.P., Sève B. Weaning induces both transient and long-lasting modifications of absorptive, secretory, and barrier properties of piglet intestine. J Nutr. 2004;134:2256–2262. doi: 10.1093/jn/134.9.2256. [DOI] [PubMed] [Google Scholar]
- Casey J.A., Tartof S.Y., Davis M.F., Nachman K.E., Price L., Liu C., Yu K., Gupta V., Innes G.K., Tseng H.F., Do V., Pressman A.R., Rudolph K.E. Impact of a statewide livestock antibiotic use policy on resistance in human urine Escherichia coli isolates: a synthetic control analysis. Environ Health Perspect. 2023;131(2) doi: 10.1289/EHP11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway P.L., Welin A., Cohen P.S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990;58(10):3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H., Park I.Y., Kim H.S., Lee W.T., Kim M.S., Kim S.C. Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J. 2002;16(3):429–431. doi: 10.1096/fj.01-0736fje. [DOI] [PubMed] [Google Scholar]
- Daneshmand A., Kermanshahi H., Sekhavati M.H., Javadmanesh A., Ahmadian M. Antimicrobial peptide, cLF36, affects performance and intestinal morphology, microflora, junctional proteins, and immune cells in broilers challenged with E. coli. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-50511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Jiao A.R., Yu B., He J., Yu J., Zheng P., Huang Z.Q., Luo Y.H., Luo J.Q., Mao X.B., Chen D.W. Stimulation of intestinal growth with distal ileal infusion of short-chain fatty acid: a reevaluation in a pig model. RSC Adv. 2017;49(7):30792–30806. [Google Scholar]
- Duan Q.M., Chen D.W., Yu B., Huang Z.Q., Luo Y.H., Zheng P., Mao X.B., Yu J., Luo J.Q., Yan H., He J. Effect of sialyllactose on growth performance and intestinal epithelium functions in weaned pigs challenged by enterotoxigenic Escherichia Coli. J Anim Sci Biotechnol. 2022;13(1):30. doi: 10.1186/s40104-022-00673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L., Kamada N., Nakamura Y., Burberry A., Kuffa P., Suzuki S., Shaw M.H., Kim Y.G., Núñez G. NLRC4-driven production of IL-1β discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat Immunol. 2012;13(5):449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M.A., Mathew A.G., Vickers J.R., Clift R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs 24 days of age. J Anim Sci. 2002;80(11):2904–2910. doi: 10.2527/2002.80112904x. [DOI] [PubMed] [Google Scholar]
- Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K., Tobe T., Clarke J.M., Topping D.L., Suzuki T., Taylor T.D., Itoh K., Kikuchi J., Morita H., Hattori M., Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- Gao Y., Han F., Huang X., Rong Y., Yi H., Wang Y. Changes in gut microbial populations, intestinal morphology, expression of tight junction proteins, and cytokine production between two pig breeds after challenge with Escherichia coli K88: a comparative study. J Anim Sci. 2013;91(12):5614–5625. doi: 10.2527/jas.2013-6528. [DOI] [PubMed] [Google Scholar]
- García V., Gambino M., Pedersen K., Haugegaard S., Olsen J.E., Herrero-Fresno A. F4- and F18-positive enterotoxigenic escherichia coli isolates from diarrhea of postweaning pigs: genomic characterization. Appl Environ Microbiol. 2020;86(23):e01913–e01920. doi: 10.1128/AEM.01913-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Wang B., Wang T., Gao L., Yang Z.J., Wang F.F., Shang H.W., Hua R., Xu J.D. Biological characteristics of IL-6 and related intestinal diseases. Int J Biol Sci. 2021;17(1):204–219. doi: 10.7150/ijbs.51362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F.F., Zhang H.W., Xia X., Xiong H.T., Song D.G., Zong X., Wang Y.Z. Porcine beta-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. J Immunol. 2015;194(4):1882–1893. doi: 10.4049/jimmunol.1402300. [DOI] [PubMed] [Google Scholar]
- He Y.J., Liu B., Xie J., Ge X.P., Xu P., Lu Y., Lu F.X., Lu Z.X. Effects of antibacterial peptide extracted from Bacillus subtilis fmbJ on the growth, physiological response and disease resistance of megalobrama amblycephala. Isr J Aquacult Bamidgeh. 2014;66:1–10. [Google Scholar]
- Hu C.H., Xiao K., Luan Z.S., Song J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J Anim Sci. 2013;91(3):1094–1101. doi: 10.2527/jas.2012-5796. [DOI] [PubMed] [Google Scholar]
- Hutchings A.B., Helander A., Silvey K.J., Chandran K., Lucas W.T., Nibert M.L., Neutra M.R. Secretory immunoglobulin A antibodies against the sigma1 outer capsid protein of reovirus type 1 Lang prevent infection of mouse Peyer's patches. J Virol. 2004;78(2):947–957. doi: 10.1128/JVI.78.2.947-957.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D., Jacob B., Kwak C., Kim Y. Short antimicrobial peptides exhibiting antibacterial and anti-inflammatory activities derived from the N-Terminal Helix of papiliocin. Bull Kor Chem Soc. 2017;38(11):1260–1268. [Google Scholar]
- Jo H.E., Kwon M.S., Whon T.W., Kim D.W., Yun M., Lee J., Shin M.Y., Kim S.H., Choi H.J. Alteration of gut microbiota after antibiotic exposure in finishing swine. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.596002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M.E., Sjövall H., Hansson G.C. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10(6):352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung Y., Wen T., Mingler M.K., Caldwell J.M., Wang Y.H., Chaplin D.D., Lee E.H., Jang M.H., Woo S.Y., Seoh J.Y., Miyasaka M., Rothenberg M.E. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8(4):930–942. doi: 10.1038/mi.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kick A.R., Tompkins M.B., Flowers W.L., Whisnant C.S., Almond G.W. Effects of stress associated with weaning on the adaptive immune system in pigs. J Anim Sci. 2012;90(2):649–656. doi: 10.2527/jas.2010-3470. [DOI] [PubMed] [Google Scholar]
- Kim K., He Y.J., Xiong X., Ehrlich A., Li X.D., Raybould H., Atwill E.R., Maga E.A., Jorgensen J., Liu Y.H. Dietary supplementation of Bacillus subtilis influenced intestinal health of weaned pigs experimentally infected with a pathogenic E. coli. J Anim Sci Biotechnol. 2019;10(4):969–980. [Google Scholar]
- Li H., Zhao P., Lei Y., Li T., Kim I. Response to an Escherichia coli K88 oral challenge and productivity of weanling pigs receiving a dietary nucleotides supplement. J Anim Sci Biotechnol. 2015;6:49. doi: 10.1186/s40104-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bao X., Yang F., Tian J., Su W., Yin J., Yao K., Li T., Yin Y. Ornithine Alpha-Ketoglutarate alleviates inflammation via regulating ileal mucosa microbiota and metabolites in enterotoxigenic Escherichia coli-infected pigs. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.862498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.D., Song M.H., Yun W., Lee J.H., Kim H.B., Cho J.H. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poultry Sci. 2019;98(5):2026–2033. doi: 10.3382/ps/pey575. [DOI] [PubMed] [Google Scholar]
- Liu Y., Yu X.J., Zhao J.X., Zhang H., Zhai Q.X., Chen W. The role of MUC2 mucin in intestinal homeostasis and the impact of dietary components on MUC2 expression. Int J Biol Macromol. 2020;164:884–891. doi: 10.1016/j.ijbiomac.2020.07.191. [DOI] [PubMed] [Google Scholar]
- Marion J., Biernat M., Thomas F., Savary G., Le Breton Y., Zabielski R., Le Huerou-Luron I., Le Dividich J. Small intestine growth and morphometry in piglets weaned at 7 days of age effects of level of energy intake. Reprod Nutr Dev. 2002;42(4):339–354. doi: 10.1051/rnd:2002030. [DOI] [PubMed] [Google Scholar]
- McLamb B.L., Gibson A.J., Overman E.L., Stahl C., Moeser A.J. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLoS One. 2013;8 doi: 10.1371/journal.pone.0059838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes R.S., Oliveira M.V., Padilha G.A., Rocha N.N., Santos C.L., Maia L.A., Fernandes M.V.S., Cruz F.F., Olsen P.C., Capelozzi V.L., de Abreu M.G., Pelosi P., Rocco P.R.M., Silva P.L. Effects of crystalloid, hyper-oncotic albumin, and iso-oncotic albumin on lung and kidney damage in experimental acute lung injury. Respir Res. 2019;20(1):155. doi: 10.1186/s12931-019-1115-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C., VanDussen K.L., Miyoshi H., Stappenbeck T.S. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014;7(4):818–828. doi: 10.1038/mi.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedernhofer L.J., Daniels J.S., Rouzer C.A., Greene R.E., Marnett L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J Biol Chem. 2003;278(33):31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 11th revised edition. The National Academy Press; Washington, DC: 2012. Nutrient requirements of swine. [Google Scholar]
- Park I.Y., Park C.B., Kim M.S., Kim S.C. Parasin I, an antimicrobial peptide derived from histone H2A in the catfish, parasilurus asotus. FEBS Lett. 1998;437(3):258–262. doi: 10.1016/s0014-5793(98)01238-1. [DOI] [PubMed] [Google Scholar]
- Peng C.F., Zhao H., Tang Y.T., Zhao W.X. Effects of bioengineering protein API on growth performance and gut health of broilers. J SICHUAN Agric Univ. 2022;40(5):676–682. [In chinese] [Google Scholar]
- Peterson D.A., McNulty N.P., Guruge J.L., Gordon J.I. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2(5):328–339. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Pi Y., Gao K., Peng Y., Mu C.L., Zhu W.Y. Antibiotic-induced alterations of the gut microbiota and microbial fermentation in protein parallel the changes in host nitrogen metabolism of growing pigs. Animal. 2019;13(2):262–272. doi: 10.1017/S1751731118001416. [DOI] [PubMed] [Google Scholar]
- Spreeuwenberg M.A.M., Verdonk J.M.A.J., Gaskins H.R., Verstegen M.W.A. Small intestine epithelial barrier function is compromised in pigs with low feed intake at weaning. J Nutr. 2001;131:1520–1527. doi: 10.1093/jn/131.5.1520. [DOI] [PubMed] [Google Scholar]
- Su Y.L., Chen G., Chen L.S., Li J.Z., Wang G., He J.Y., Zhan T.Y., Li Y.W., Yan M.T., Huang Y.H., Qin Q.W., Dan X.M., Sun H.Y. Effects of antimicrobial peptides on serum biochemical parameters, antioxidant activity and non-specific immune responses in Epinephelus coioides. Fish Shellfish Immunol. 2019;86:1081–1087. doi: 10.1016/j.fsi.2018.12.056. [DOI] [PubMed] [Google Scholar]
- Sun Q.J., Liu D., Guo S.S., Chen Y.X., Guo Y.M. Effects of dietary essential oil and enzyme supplementation on growth performance and gut health of broilers challenged by Clostridium perfringens. Anim Feed Sci Technol. 2015;207:234–244. [Google Scholar]
- Sun Y., Duarte M.E., Kim S.W. Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim Nutr. 2021;7(2):326–333. doi: 10.1016/j.aninu.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.T., Yin S.G., Peng C.F., Tang J.Y., Jia G., Che L.Q., Liu G.M., Tian G., Chen X.L., Cai J.Y., Kang B., Zhao H. Compound bioengineering protein supplementation improves intestinal health and growth performance of broilers. Poultry Sci. 2023;102(11):1–10. doi: 10.1016/j.psj.2023.103037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tothova C., Nagy O., Nagyova V., Kovac G. Serum protein electrophoretic pattern in dairy cows during the periparturient period. J Appl Anim Res. 2018;46(1):33–38. [Google Scholar]
- Traserra S., Casabella-Ramón S., Vergara P., Jimenez M. E. coli infection disrupts the epithelial barrier and activates intrinsic neurosecretory reflexes in the pig colon. Front Physiol. 2023;2(14) doi: 10.3389/fphys.2023.1170822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Oman T.J., Zhang R., De Gonzalo C.V.G., Zhang Q., van der Donk W.A. The glycosyltransferase involved in thurandacin biosynthesis catalyzes both O- and S-Glycosylation. ACS Chem Biol. 2018;13:1270–1278. doi: 10.1021/ja411159k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Liu Y., Tang H., Yu Y., Zhang Q. ITGB5 plays a key role in Escherichia coli F4ac-induced diarrhea in piglets. Front Immunol. 2019;10:2834. doi: 10.3389/fimmu.2019.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang W.W., Wang R.F., Hao X.R., Duan Y.X., Meng Z.Q., An X.P., Qi J.W. Dietary fermented soybean meal inclusion improves growth performance and ileal barrier function of the weaned piglets challenged by enterotoxigenic Escherichia coli K88. Anim Feed Sci Technol. 2020;268 [Google Scholar]
- Wu K.C., Hua K.F., Yu Y.H., Cheng Y.H., Cheng T.T., Huang Y.K., Chang H.W., Chen W.J. Antibacterial and antibiofilm activities of novel antimicrobial peptides against multidrug-resistant enterotoxigenic escherichia coli. Int J Mol. 2021;22(8):3926. doi: 10.3390/ijms22083926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Zhang F., Huang Z., Liu H., Xie C., Zhang J., Thacker P.A., Qiao S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides. 2012;35(2):225–230. doi: 10.1016/j.peptides.2012.03.030. [DOI] [PubMed] [Google Scholar]
- Xiong M., Bao Y., Xu X., Wang H., Han Z., Wang Z., Liu Y., Huang S., Song Z., Chen J., Peek R.M., Jr., Yin L., Chen L.F., Cheng J. Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides. Proc Natl Acad Sci USA. 2017;114(48):12675–12680. doi: 10.1073/pnas.1710408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., Huang J., Li X.Y., Zhang Z., Jin M.L., Wang J., Xu Y.W., Wang Z.L. Icariin and its phosphorylated derivatives alleviate intestinal epithelial barrier disruption caused by enterotoxigenic Escherichia coli through modulate p38 MAPK in vivo and in vitro. FASEB J. 2019;34(1):1783–1801. doi: 10.1096/fj.201902265R. [DOI] [PubMed] [Google Scholar]
- Xu Y., Lahaye L., He Z., Zhang J., Yang C., Piao X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+) Anim Nutr. 2020;6(3):269–277. doi: 10.1016/j.aninu.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi H.B., Zhang L., Gan Z.S., Xiong H.T., Yu C.H., Du H.H., Wang Y.Z. High therapeutic efficacy of Cathelicidin-WA against postweaning diarrhea via inhibiting inflammation and enhancing epithelial barrier in the intestine. Sci Rep. 2016;6:1–12. doi: 10.1038/srep25679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin S.G., Su L.Z., Shao Q.J., Fan Z.Y., Tang J.Y., Jia G., Liu G.M., Tian G., Chen X.L., Cai J.Y., Kang B., Zhao H. Compound bioengineering protein improves growth performance and intestinal health in broiler chickens under high-temperature conditions. J Anim Sci. 2023;101:1–11. doi: 10.1093/jas/skad370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C.C.W., Karmacharya D., Bista M., Sharma A.N., Goldstein T., Mazet J.A.K., Johnson C.K. Antibiotic resistance genes of public health importance in livestock and humans in an informal urban community in Nepal. Sci Rep. 2022;12(1) doi: 10.1038/s41598-022-14781-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J.H., Ingale S.L., Kim J.S., Kim K.H., Lohakare J., Park Y.K., Park J.C., Kwon I.K., Chae B.J. Effects of dietary supplementation with antimicrobial peptide-P5 on growth performance, apparent total tract digestibility, faecal and intestinal microflora and intestinal morphology of weanling pigs. J Sci Food Agric. 2013;93(3):587–592. doi: 10.1002/jsfa.5840. [DOI] [PubMed] [Google Scholar]
- Yu H., Wang Y., Zeng X., Cai S., Wang G., Liu L., Huang S., Li N., Liu H., Ding X., Song Q., Qiao S. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020;34(1):1018–1037. doi: 10.1096/fj.201901717R. [DOI] [PubMed] [Google Scholar]
- Yu H.T., Ding X.L., Shang L.J., Zeng X.F., Liu H.B., Li N., Huang S., Wang Y.M., Wang G., Cai S., Chen M.X., Levesque C.L., Johnston L.J., Qiao S.Y. Protective ability of biogenic antimicrobial peptide microcin J25 against enterotoxigenic Escherichia Coli-induced intestinal epithelial dysfunction and inflammatory responses IPEC-J2 cells. Front Cell Infect Microbiol. 2018;8:1–13. doi: 10.3389/fcimb.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.H., Lu J.X. China Agriculture Press; Beijing: 2019. Feed quality detection and nutritional value assessment technology. [Google Scholar]
- Zhang L., Tang J.Y., Jia G., Tian G. Effects of bioengineering protein API on growth performance and intestinal health of meat rabbits. Chinese J Anim Nutr. 2022;34(7):4696–4703. [In chinese] [Google Scholar]
- Zhang L.C., Guo T., Zhan N., Sun T.T., Shan A.S. Effects of the antimicrobial peptide WK3 on diarrhea, growth performance and intestinal health of weaned piglets challenged with enterotoxigenic Escherichia coli K88. Food Nutr Res. 2021;65:1–9. doi: 10.29219/fnr.v65.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.J., Gallo R.L. Antimicrobial peptides. Curr Biol. 2016;26(1):R14–R19. doi: 10.1016/j.cub.2015.11.017. [DOI] [PubMed] [Google Scholar]
- Zhang M., Hou G., Hu P., Feng D., Wang J., Zhu W. Nano chitosan–zinc complex improves the growth performance and antioxidant capacity of the small intestine in weaned piglets. Br J Nutr. 2020;126(6):801–812. doi: 10.1017/S0007114520004766. [DOI] [PubMed] [Google Scholar]
- Zhao H., Tang J., Cao L., Jia G., Long D., Liu G., Chen X., Cai J., Shang H. Characterization of bioactive recombinant antimicrobial peptide parasin I fused with human lysozyme expressed in the yeast Pichia pastoris system. Enzym Microb Technol. 2015;77:61–67. doi: 10.1016/j.enzmictec.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Zhen R., Feng J., He D., Chen Y., Chen T., Cai W., Xiong Y., Qiu Y., Jiang Z., Wang L., Yi H. Effects of niacin on resistance to enterotoxigenic Escherichia coli infection in weaned piglets. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.865311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Le M., He Z., Bai Y., Yang J., Ye J., et al. Dietary berberine supplementation improves growth performance and alleviates gut injury in weaned piglets by modulating ileal microbiota and metabolites. Food Funct. 2023;14(9):4143–4162. doi: 10.1039/d3fo01044a. [DOI] [PubMed] [Google Scholar]
- Zihni C., Mills C., Matter K., Balda M.S. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17(9):564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.