Abstract
Background
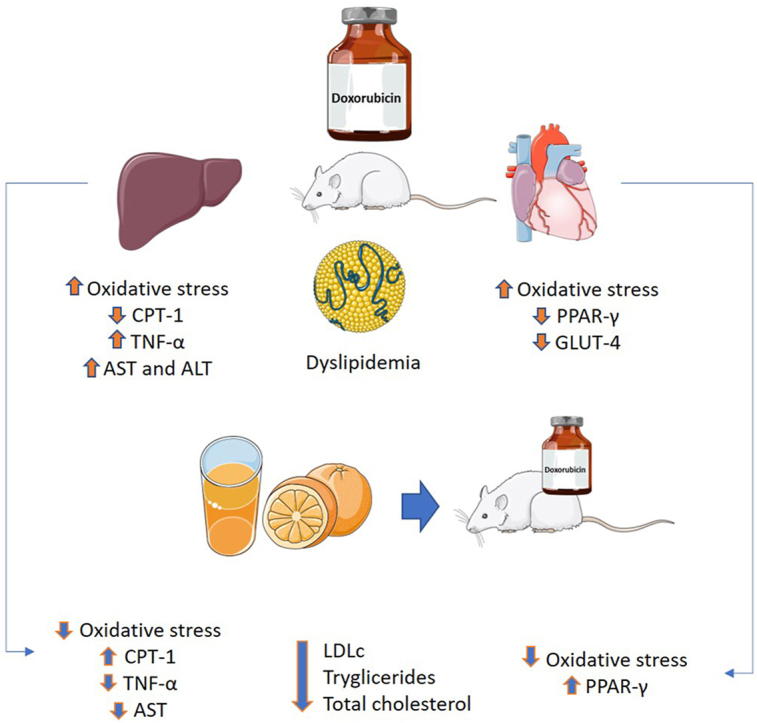
Doxorubicin (DOX) is a highly effective chemotherapy drug widely used to treat cancer, but its use is limited due to multisystemic toxicity. Lipid metabolism is also affected by doxorubicin. Orange juice can reduce dyslipidemia in other clinical situations and has already been shown to attenuate cardiotoxicity. Our aim is to evaluate the effects of Pera orange juice (Citrus sinensis L. Osbeck) on mitigating lipid metabolism imbalance, metabolic pathways, and DOX induced cytotoxic effects in the heart and liver.
Methods
Twenty-four male Wistar rats were allocated into 3 groups: Control (C); DOX (D); and DOX plus Pera orange juice (DOJ). DOJ received orange juice for 4 weeks, while C and D received water. At the end of each week, D and DOJ groups received 4 mg/kg/week DOX, intraperitoneal. At the end of 4 weeks animals were submitted to echocardiography and euthanasia.
Results
Animals treated with DOX decreased water intake and lost weight over time. At echocardiography, DOX treated rats presented morphologic alterations in the heart. DOX increased aspartate aminotransferase (AST), alanine aminotransferase (ALT), total cholesterol, high density lipoprotein (HDL), low-density lipoprotein (LDL), and triglycerides. It also reduced superoxide dismutase (SOD) activity, increased protein carbonylation in the heart and dihydroethidium (DHE) expression in the liver, decreased glucose transporter type 4 (GLUT4) and the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ1) in the heart, and reduced carnitine palmitoyltransferase I (CPT1) in the liver.
Conclusion
DOX caused dyslipidemia, liver and cardiac toxicity by increasing oxidative stress, and altered energy metabolic parameters in both organs. Despite not improving changes in left ventricular morphology, orange juice did attenuate oxidative stress and mitigate the metabolic effects of DOX.
Keywords: Doxorubicin, Cardiotoxicity, Hepatotoxicity, Energy metabolism, Dyslipidemia, Oxidative stress
Graphical abstract
1. Introduction
Doxorubicin (DOX) is an anthracycline class chemotherapeutic drug widely used in treating solid cancers such as breast, lung and prostate, and hematological cancers such as acute myeloblastic and lymphoblastic leukemia [1]. Despite good results in cancer treatment, DOX induces many collateral effects. Since DOX selectivity for tumor cells is not perfect, it has a cytotoxic effect intrinsically associated with reactive oxygen species production in mitochondria [2,3]. There is a high affinity between DOX and mitochondria due to cardiolipin, a phospholipid of the inner mitochondrial membrane. Inside mitochondria, DOX is converted into semiquinone that reacts with O2 producing superoxide and H2O2, one of the most important reactive oxygen species (ROS). These ROS interact with several molecules that lead to mitochondrial dysfunction, releasing pro-apoptotic signals [4,5].
Following mitochondrial dysfunction, DOX can induce an imbalance in the cellular energy metabolism of several tissues. The energy metabolism is defined as the process by which living cells acquire energy from carbohydrates, lipids and proteins to perform its basic functions [6]. In previous studies, DOX decreased the myocardial mitochondrial ATPases in rats treated acutely and chronically with the drug [7], reduced the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) in mice [8] and the glucose transporter type 4 (GLUT4) in skeletal muscle of rats [9], as well as the carnitine palmitoyltransferase I enzyme (CPT1) [10], which are all essential components of lipid and glucose metabolism.
The PPARγ are ligand-dependent transcription factors that regulate the transcription of various genes involved in energy metabolism, adipogenesis and insulin sensitivity [11]. GLUT4 is a transporter that allows glucose to enter the cell across the membrane and represents approximately 70 % of the total glucose transporters of the body and are controlled by PPARγ [12]. CPT1 is an enzyme localized in the outer mitochondria membrane, that regulates the transport of fatty acids, derived from lipids, to the cytosol, where the energy generation takes place.
The decreased ability to transport and oxidize blood fatty acids [13,14] in the liver and in the heart caused by DOX could be negative, especially for the heart, which, under physiological conditions, has fatty acids as the main substrate for ATP production [[15], [16], [17], [18]].
The liver plays a central role in lipid metabolism, serving as the center for lipoprotein uptake, formation, and export to the circulation [19]. Additionally, DOX causes an increase in blood triglycerides (TG) and in low-density lipid cholesterol (LDL), causing dyslipidemia. Taken together, these changes in energy metabolism can lead to multiorgan dysfunction, such as cardiac dysfunction.
Chronic heart failure is the limiting side effect for using more effective doses of DOX in treating tumors. Therefore, pharmacology is constantly looking for strategies to increase DOX selectivity for cancer cells or decrease the side effects of its treatment. Since DOX side effects are associated with ROS production, several studies have obtained promising results investigating the effects of natural compounds with antioxidant properties [20,21].
Studies on humans have shown that orange juice consumption can reduce dyslipidemia, showing a reduction in total cholesterol (TC), LDL, TG and an increase in high-density lipid cholesterol (HDL) in normolipidemic and hyperlipidemic patients [[22], [23], [24], [25]]. This reduction in total and LDL cholesterol can be related to hesperidin and naringenin, flavonoids found in citric fruits that may modulate lipid metabolism [[26], [27], [28]]. These flavonoids attenuated cardiotoxicity in pre-clinical studies. Also, Vitamin C administration improved dyslipidemia caused by DOX [28][]. However, studies involving the consumption of whole orange juice and not just one of its components are scarce in the literature.
We previously observed that orange juice intake improved the activity of enzymes related to lipid and glucose cellular metabolism [29], which could impact lipid serum profile; however, the exact pathways involved in the cellular metabolism were not extensively evaluated previously.
Therefore, the aim of this study was to evaluate the effect of Pera orange juice (Citrus sinensis L. Osbeck) consumption as a strategy to mitigate changes in the metabolic pathways, lipid metabolism imbalance and cytotoxic side effects induced by DOX in the heart and liver of rats.
2. Material and methods
2.1. Experimental protocol
Twenty-four 200g and 2-month-old male Wistar rats were kept in individual cages under controlled conditions with a 12-h light/dark cycle and free access to standard chow. Rats were randomly divided in three groups of 8 animals: Controls (C); DOX (D); and DOX plus Pera orange juice (DOJ). DOJ animals received orange juice only ad libitum for 4 weeks. C and D animals received water only for the same period. All liquids and chow were replaced every 24 h and daily intake recorded. Body weight was recorded every week.
D and DOJ animals received intraperitoneal injections (IP) of DOX (4 mg/kg) at the end of each week, totaling 16 mg/kg DOX per animal [[30], [31], [32]]. Group C animals received the same volume of sterile saline IP. Animals were anesthetized and submitted to echocardiography 48 h after last injection. They were subsequently euthanized with thiopental sodium (120 mg/kg, IP) and heart, liver and blood were collected. Blood was centrifugated and resulting serum stored at −80 °C. Heart and liver were weighed, the left ventricle was stored at −80 °C, the liver was fixed with alcohol (70 %) and then embedded in paraffin.
Prior approval was obtained from our local ethics committee (protocol number CEUA 1306/2019) which complies with the Brazilian National Council standards for the Control of Animal Experimentation. Pera orange juice was donated by Fundecitrus (Foundation for Citriculture Protection, Araraquara, Brazil), and all juice was from the same production batch. The juice remained frozen at −80 °C until use. The main flavonoids found in the orange juice used in this study had been previously reported by our group [29].
2.2. Echocardiography
Animals were lightly anesthetized with ketamine (50 mg/kg, IP) and xylazine (10 mg/kg, IP). Echocardiogram was performed using Vivid S6 (General Electric Medical Systems, Boston, MA, USA) with a 5.0–11.5 MHz multifrequency transductor by the same examiner blinded to animal groups. The following structural variables were obtained by bidimensional analysis: posterior wall (PWT) and interventricular septum thickness (IST), left atrium (LAD), and left ventricle systolic (LVSD) and diastolic diameters (LVDD). The following functional variables were obtained using transmittal Doppler: peak initial diastolic filling velocity (E wave), peak late diastolic filling velocity (A wave), and isovolumetric relaxation time (IRT). We additionally performed tissue Doppler to evaluate systolic (S′), initial (E′), and late (A’) mitral ring displacement in the lateral and septal wall. Left ventricular shortening fraction (SF = (LVDD-LVSD)/LVDD) and E/A ratio were used as functional parameters for systolic and diastolic function, respectively [33].
2.3. Biomarkers of hepatotoxicity and dyslipidemia
Alanine aminotransferase (ALT) and serine aminotransferase (AST) enzyme levels were used to evaluate hepatic toxicity. Metabolic disturbance was evaluated by measuring glucose, TG, TC, and HDL levels in serum samples. All assays were performed by colorimetric enzymatic methods (Labtest, Belo Horizonte, Brazil) in a spectrophotometric analyzer. LDL values were calculated by the Friedewald formula: LDL = CT-HDL- (TG/5) [34].
2.4. Oxidative stress
Liver samples were cut (8 μm) and placed onto gelatin-coated histological slides. Dihydroethidium (DHE) solution (5 μM DHE + 0.05 % DMSO) was applied to slides. Superoxide content in liver was measured by fluorescence of 2-OH-ethidium using a fluorescence microscope with a red excitation filter and quantified by ImageJ software through mean fluorescence per unit area [35]. Left ventricle tissue was homogenized in sodium phosphate buffer (pH 7.4, 0.01 M) and centrifuged (800×g/10 min 4 °C). Supernatant protein content was determined using the Bradford colorimetric method (Bio-Rad, Hercules, CA, USA). Superoxide dismutase (SOD) and Catalase activity, and protein carbonylation were measured by spectrophotometric assay, as previous described [36].
2.5. Western blot
We quantified PPAR-γ (peroxisome proliferator-activated receptor gamma), CPT-1 (carnitine palmitoyl transferase 1), GLUT-4 (glucose transporter type 4), and TNF-α (tumor necrosis factor alpha) in left ventricle tissue. Samples were homogenized in extraction buffer (NaCl 1M, Triton 1 %, Sodium deoxycholate 0.5 %, SDS 0.1 %, Tris base, proteinase inhibitor 1 %, Sodium orthovanadate 1 %, NaF 1M-1%) using a bead homogenizer (Bullet Blender® homogenizer, model BBX24, USA). Samples were then centrifuged (800×g/10 min 4 °C) and supernatant protein content determined by the Bradford colorimetric method (Bio-Rad, Hercules, CA, USA). Homogenates were diluted in Laemmli Buffer (100 °C/5 min) and placed in 10 % SDS–PAGE gel (Tris - HCL 240 mM pH 8.9, polyacrylamide 4 %, glycerol, APS and TEMED) for electrophoretic separation [37]. After the electrophoresis run, proteins were transferred to nitrocellulose membranes by a mini-transblot system (Bio-Rad, Hercules, CA, USA). Membranes were blocked with 5 % Blotting-Grade Blocker (Bio-Rad, Hercules, CA, USA) and subsequently incubated overnight with anti-GLUT-4 (sc-53566, 65 kDa, 1:200 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), anti-PPAR-γ (sc-7273, 54/57 kDa, 1:500 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), anti-TNF-α (sc-52746, 26 kDa, 1:500 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), anti-CPT1 (sc-393070, 86/90–94 kDa, 1:300 dilution, Santa Cruz Biotechnology, Dallas, TX, USA), or anti-GAPDH (sc-32233, 37 kDa, 1:40000 dilution, Santa Cruz Biotechnology, Dallas, TX, USA). Membranes were then incubated with HRP-conjugated secondary antibodies for 90 min. Protein immunodetection was performed by chemiluminescence using Immobilon® Classico Western HRP Substrate (Millipore, USA). Protein signal was recorded by ImageQuant™ LAS 4000 (GE Healthcare, USA) and quantified by ImageJ software. We analyzed 6 samples per group and performed inter experiment controls using the same control sample in each electrophoresis run. Normalization was performed by mean expression of control samples in each blotting and by GAPDH expression.
2.6. Immunohistochemistry
Liver samples were cut (8 μm) and placed onto silane-coated slides. Slides were then subjected to antigen retrieval in 0.01 M citrate buffer pH 6.0 (120 °C/20 min) in an electric pressure cooker. Subsequently, slides were washed in peroxidase blockade solution (5 % H2O2 in PBS) for 30 min, washed in non-specific antigen blockade solution (Bio-Rad, Hercules, CA, USA) for 30 min, and incubated with anti-PPAR-γ (sc-7273, 54/57 kDa, 1:100 dilution, Santa Cruz Biotechnology, Dallas, TX, USA) or anti-TNF-α (sc-52746, 26 kDa, 1:100 dilution, Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 4 °C. Slides were then incubated with a one-step horseradish peroxidase (HRP)-polymer (Bio-Rad, Hercules, CA, USA) for 20 min. DAB chromogen (Bio-Rad, Hercules, CA, USA) was added to react with polymer, and slides were stained with hematoxylin and eosin. Semiquantitative analysis consisted of calculating the immunolabelling index (%) for PPAR-γ. Qualitative TNF-α analysis consisted of counting the number of positive cells in the field. Images were obtained using Olympus cellSens software (Olympus Corporation, Shinjuku, Japan).
2.7. Statistical analysis
Variables with normal distribution were presented as means ± standard deviations. Variables with non-normal distribution were presented as medians and quartiles. Normality was tested by Shapiro-Wilk. Analysis was performed by one-way ANOVA followed by the Tukey test. We considered a statistical significance of 5 % for all analyses.
3. RESULTS
3.1. Body weight and liquid consumption
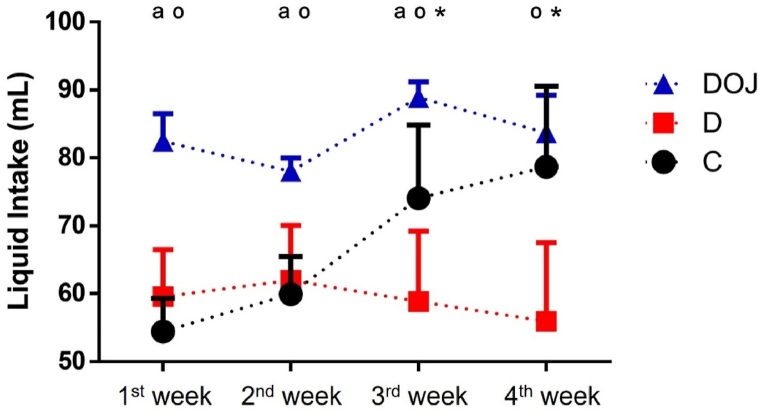
Throughout most of the protocol, animals that drank orange juice had a higher liquid intake than animals that received only water (Fig. 1). DOX administration was responsible for a decrease in water intake (Fig. 1) and weight loss over time (Table 1) as initial body weight was similar between groups. When normalized by body weight neither treatment modified LV or liver mass (Table 1).
Fig. 1.
– Liquid consumption.
Values are expressed as average consumption of liquid in seven days for all animals in each group (n = 8 rats/group). Animals treated with orange juice had a higher liquid intake. Groups: Circle, control; square, doxorubicin; triangle, doxorubicin + orange juice. One-way ANOVA was applied considering p < 0.05; a p < 0.0001 DOJ vs C group; o p < 0.0001 DOJ vs D group; and * p < 0.0001 C vs D group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
– Body and organ weights.
| C (n = 8) | D (n = 8) | DOJ (n = 8) | |
|---|---|---|---|
| BW (g) | 369 ± 69 | 239 ± 34**** | 227 ± 42**** |
| Liver (g) | 14.9 ± 3.93 | 10.1 ± 1.85*** | 9.04 ± 1.34**** |
| LV (g) | 0.67 ± 0.13 | 0.44 ± 0.12**** | 0.44 ± 0.07**** |
| Liver/BW | 3.98 ± 0.37 | 4.23 ± 0.45 | 4.03 ± 0.49 |
| LV/BW | 1.81 ± 0.10 | 1.84 ± 0.41 | 1.94 ± 0.19 |
BW, body weight at the end of experiment; LV, left ventricle; LV/BW, left ventricle weight corrected by body weight. Groups: C - control; D - doxorubicin; DOJ - doxorubicin + orange juice. One-way ANOVA was applied considering p < 0.05; ***p < 0.001 and ****p < 0.0001 vs C group.
3.2. Echocardiogram
When normalized to body weight, DOX increased the diameter of all chambers, confirming that DOX induced morphological alterations in the heart. In addition, DOX elevated PWT and LVMI, meaning that it induced heart hypertrophy. Complementary treatment with POJ either conjugated or not conjugated with DOX, did not modify any of these effects. We did not observe any influence from treatments on functional parameters (Table 2).
Table 2.
– Echocardiographic parameters.
| C (n = 8) | D (n = 8) | DOJ (n = 8) | |
|---|---|---|---|
| PWT/BW (mm/kg) | 3.43 ± 0.42 | 4.80 ± 0.61* | 5.15 ± 0.66* |
| LAD/BW (mm/kg) | 15.1 ± 2.45 | 21.6 ± 3.42**** | 22.9 ± 3.44**** |
| LVSD/BW (mm/kg) | 8.97 ± 1.40 | 13.0 ± 3.42** | 14.8 ± 3.24**** |
| LVDD/BW (mm/kg) | 20.0 ± 2.62 | 28.3 ± 3.94**** | 30.3 ± 3.82**** |
| E/A ratio | 1.45 ± 0.23 | 1.49 ± 0.55 | 1.70 ± 0.38 |
| IRT (ms) | 23.8 ± 0.72 | 27.7 ± 2.92 | 28.2 ± 2.73 |
| S’ (m/s) | 3.56 ± 0.10 | 3.27 ± 0.11 | 3.37 ± 0.16 |
| E’ (m/s) | 3.51 ± 0.20 | 3.41 ± 0.59 | 3.63 ± 0.48 |
| A’ (m/s) | 4.74 ± 0.28 | 3.31 ± 0.52 | 3.24 ± 0.31 |
| FS (%) | 54.9 ± 5.29 | 54.2 ± 9.94 | 51.3 ± 8.39 |
Groups had no difference in heart rate (not shown). Structural parameters: PWT/BW, posterior wall thickness normalized by body weight; LAD/BW, left atrium diameter normalized by body weight; LVSD/BW, left ventricle systolic diameter normalized by body weight; LVDD/BW, left ventricle diastolic diameter normalized by body weight. Functional parameters: IRT - isovolumetric relaxation time; S′- systolic displacement of the mitral ring in the lateral and septal wall; E′- initial displacement of the mitral ring in the lateral and septal wall; A’ - late displacement of the mitral ring in the lateral and septal wall. Groups: C, control; D, doxorubicin; DOJ, doxorubicin + orange juice. One-way ANOVA was applied considering p < 0.05; *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs C group.
3.3. Biomarkers of hepatotoxicity and dyslipidemia
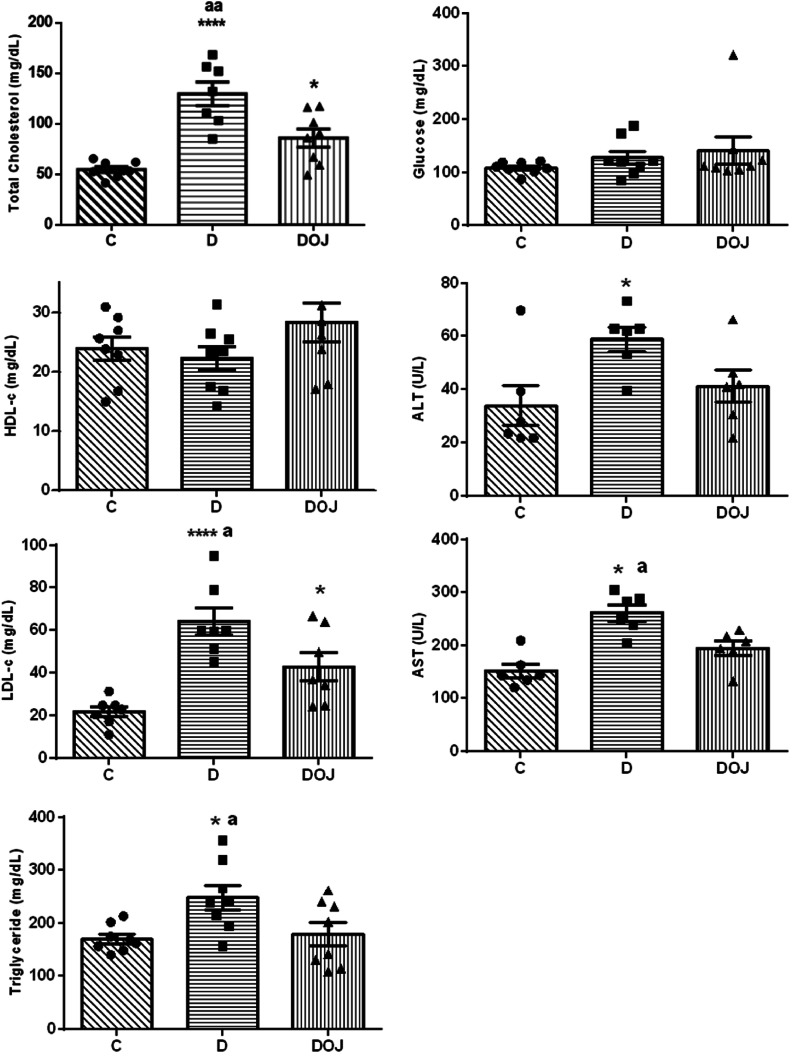
These results are shown in Fig. 2. DOX treatment led to an increase in TC, LDL fraction, and TG, indicating that DOX causes dyslipidemia. Pera orange juice consumption reduced TC, LDL, and TG compared to Group D. Neither DOX or POJ administration was capable of changing glucose and HDL. As expected, DOX raised serum ALT and AST levels, indicating that it causes significant damage to the liver and possibly to mitochondria. Orange juice consumption protected the liver against injury as we observed decreased AST in the DOJ compared to D.
Fig. 2.
– Biomarkers of hepatotoxicity and dyslipidemia
LDLc - low density lipoprotein cholesterol; HDLc - high density lipoprotein cholesterol; AST - Aspartate transaminase; ALT - Aspartate transaminase. Groups: C, control; D, doxorubicin; DOJ, doxorubicin + orange juice. One-way ANOVA was applied considering p < 0.05; ap<0.05 and aap<0.01 vs DOJ, and *p < 0.05 and ****p < 0.0001 vs C.
3.4. Oxidative stress
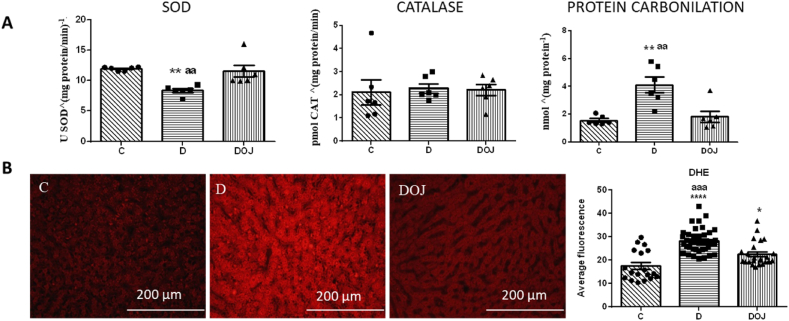
DOX treatment reduced SOD enzyme antioxidant activity in cardiac tissue without affecting CAT activity. We also observed increased protein carbonylation in the heart, a marker for oxidative damage to cellular proteins. As DOX reduced oxidative defense and increased oxidation products, we assumed that DOX caused an oxidative stress state in the heart. When orange juice was administered together with DOX (DOJ group), we observed an increase in SOD activity and a decrease in protein carbonylation in the heart, suggesting attenuation of oxidative damage in this tissue. In the liver, we observed an increase in DHE markers of DOX treated rats. As DHE reacts with superoxide it means that the liver was in a high oxidative stress state. Also, orange juice administration attenuated hepatic oxidative stress, as there was a decrease in DHE markers in DOJ compared to D (Fig. 3).
Fig. 3.
– Oxidative stress parameters
A: heart parameters; B: hepatic parameters using dihydroethidium (DHE) technique. SOD: superoxide dismutase. Groups: C, control; D, doxorubicin; DOJ, doxorubicin + orange juice. One-way ANOVA was applied considering p < 0.05; *p < 0.05 and **p < 0.01 and ****p < 0.0001 vs C, and ap<0.05 and aap<0.01 and aaap<0.001 vs DOJ. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.5. Protein expression by Western blot
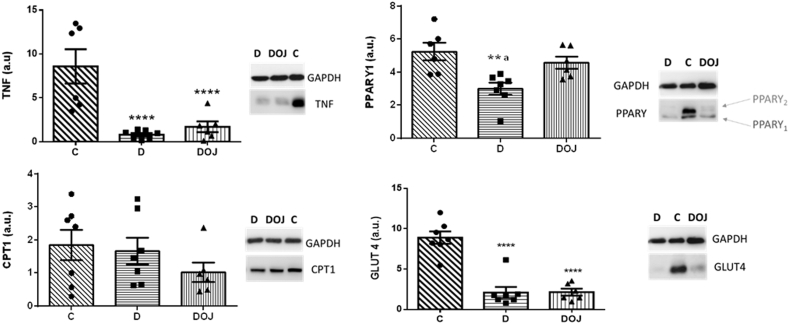
In the heart, DOX reduced GLUT4 and PPARγ1 expression, which suggests changes in physiological cellular energy metabolism. DOX also decreased TNF-α expression in myocardial tissue. Pera orange juice partially restored PPARγ1 expression but had no effect on TNF-α or GLUT4 expression. We observed no differences in CPT1 expression in heart tissue (Fig. 4).
Fig. 4.
– Heart protein expression evaluated by Western blot
Groups: C, control; D, doxorubicin; DOJ, doxorubicin + orange juice; OJ, orange juice. TNF-α - tumor necrosis factor alpha; CPT-1 - carnitine palmitoyl transferase 1; PPAR-γ -peroxisome proliferator-activated receptor gamma; GLUT-4 - glucose transporter type 4. One-way ANOVA was applied considering p < 0.05. **p < 0.01, ***p < 0.001 and ****p < 0.0001 vs C, and ap<0.05 vs DOJ.
3.6. Immunohistochemistry
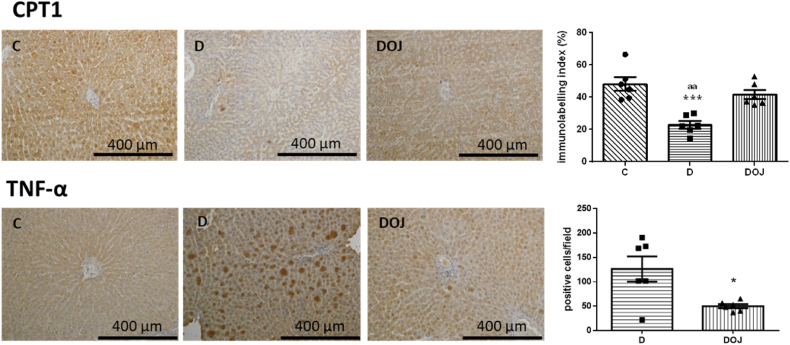
In the liver, DOX decreased CPT1 marking and increased TNF-α positive cells. In contrast, the DOJ group exhibited decreased TNF-α positive cells and increased in CPT1 marking compared to the D group (Fig. 5). Taken together, these results suggest that orange juice attenuates the changes in metabolic and inflammatory parameters in the liver.
Fig. 5.
– Liver immunohistochemistry for CPT-1 (carnitine palmitoyl transferase 1) and TNF-α (tumor necrosis factor alpha). Groups: C, control; D, doxorubicin; DOJ, doxorubicin + orange juice. ***p < 0.001 vs C and aap<0.01 vs DOJ. The imaging program was not able to detect immune labeling in Group C for TNF-α, we therefore compared only D and DOJ groups with a t-test. Where * represents p < 0.05 compared to the D group. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
DOX induced cardiac and hepatic damage, changes in heart and liver oxidative markers, modified parameters related to cellular metabolic control in both organs, and induced dyslipidemia. Orange juice administration mitigated dyslipidemia, decreased oxidative stress, and modulated the expression of proteins involved in cellular metabolism.
Lipid control is modulated by several factors, including transcriptional factors such as PPAR-γ. DOX induced PPAR-γ down-regulation, with subsequent changes in lipid and glucose metabolism, as this factor affects the transcription of proteins related to lipid transport to the myocyte, (GLUT-4 and lipoprotein lipase) inhibiting adipogenesis [13,14]. Also, DOX may induce changes in CPT-1, responsible for lipid transport and essential for β-oxidation [14,38]. In fact, we observed a decrease in GLUT-4 and PPAR-γ1 expression in the left ventricle, and reduced CPT-1 in the liver of animals treated with DOX.
Considering that 70 % of the energy used by myocardial metabolism comes from lipid oxidation, the heart is more susceptible to injury when lipid metabolism is altered [39]. Recently, Ribeiro et al., showed that DOX decreased β-hydroxy acyl-CoA dehydrogenase activity and increased phosphofructokinase activity, associated with lipid and glucose metabolism, respectively. In this study, orange juice intake improved the activity of both these enzymes, but the molecular mechanisms behind the effects were not studied [29].
Previous studies have shown that orange juice consumption can improve dyslipidemia in human and in some experimental studies [[22], [23], [24],28,40,41]. To the best of our knowledge, this is the first study to evaluate the effect of orange juice consumption on dyslipidemia caused by DOX. We also observed that this beneficial effect was combined with improved CPT-1 expression in the liver and improved PPAR-γ1 expression in the heart.
Mitochondrial mechanisms are also related to DOX-induced metabolic changes. DOX can decrease activity of the enzymes involved in Krebs cycle and the electron transport chain [[7], [29]], compromising cellular energy generation. DOX has a high affinity to mitochondria due to high cardiolipin content in its membrane, which leads to misfunction of the electron transport chain and increased reactive oxygen species (ROS) formation [[42], [43], [44]]. An antioxidant defense and ROS imbalance culminates in a state known as oxidative stress. Superoxide is one of the main ROS produced by mitochondria. These ROS can react with DNA, lipids and proteins, generating intermediate products such as protein carbonylation. In our study, we observed increased protein carbonylation in the heart and increased superoxide species in the liver, shown by the DHE marker. As expected and previously shown by other authors [[29], [45], [46], [47], [48], [49], [50], [51]], orange juice consumption was able to decrease both parameters in our study.
One important aspect is the fact that we did not observe effects any from orange juice on cardiac morphology or function. Cardiac remodeling is an adaptive process that can pass through aggression, genetic modulation, and cellular and molecular changes, which result in morphological and function changes. We believe that experimental time could impact these results.
We observed decreased serum AST concentrations in animals treated with DOX and orange juice. This could represent an improvement in liver damage, as AST is abundant in hepatocytes [[52], [53], [54]]. However, mitochondria also have a large amount of AST, and the improved AST in our study may be a marker of decreased mitochondrial injury [55]. Favoring this hypothesis is the fact that orange juice consumption in our study did not improve serum ALT content.
DOX is metabolized by CYP450, an enzyme abundant in hepatocytes near blood vessels [56]. Our study showed more pronounced TNF-α marking in areas near blood vessels in the liver, suggesting an increase in hepatic inflammation induced by DOX. Interestingly, orange juice decreased TNF-α marking in the liver, which agrees with previous studies showing the anti-inflammatory properties of orange juice [[57], [58], [59]]. Specifically in the DOX-induced toxicity model, administration of naringenin, a flavonoid present in orange juice, also decreased TNF-α and hepatic inflammation [27]. Unexpectedly, we also observed decreased TNF-α in heart tissue of DOX treated rats as several studies have described increased TNF-α in DOX-induced cardiotoxicity [[60], [61], [62], [63]]; however, most of them used ELISA to determine TNF-α concentration instead of Western blot analysis.
Our study has some limitations. We did not perform all the analysis in heart and liver due to technical problems in the collection of the organs. Also, we did not evaluate the complete pathway involved in the energy metabolism regulation. Further studies should be performed to investigate the contribution of each compound of orange juice in the beneficial effects observed in the present study.
Taken together, our results suggest that orange juice could be an interesting strategy in mitigating DOX induced multiorgan toxicity. The potential to use orange juice in translational studies are excellent since it is low cost, palatable, and widely available throughout the world.
5. Conclusion
As hypothesized, DOX induced dyslipidemia, increased oxidative stress in the liver and heart and induced changes in metabolism related cellular markers in both organs, characterizing the toxicity of this chemotherapy drug. Even though orange juice administration did not impact left ventricular morphology, it attenuated dyslipidemia, oxidative stress and metabolic marker changes caused by DOX. Orange juice consumption may be an effective strategy for mitigating DOX induced toxicity.
Funding
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for the scholarship (Process n. 2021/09517-2) and financial support (Process n. 2022/15954-9).
Authors contribution
Conceptualization: BFP, SARP; Data curation: LAMZ, SARP, BFP; Formal analysis: RPC, APDR, BFP, MFM, LAMZ, SARP, BFP; Funding acquisition: BFP; Methodology: RPC, APDR, MGM, ASSF, CRT, NFF, SGZ; Project administration: BFP; Writing - original draft: RPC, CRT; Writing - review & editing: MFM, LAMZ, SARP, BFP; Read and approved final version: RPC, APDR, MGM, ASSF, CRT, NFF, SGZ, MFM, LAMZ, SARP, BFP.
Data availability statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
CRediT authorship contribution statement
Ronny Peterson Cabral: Writing – original draft, Methodology, Formal analysis. Ana Paula Dantas Ribeiro: Methodology, Formal analysis. Marina Gaiato Monte: Methodology. Anderson Seiji Soares Fujimori: Methodology, Formal analysis. Carolina Rodrigues Tonon: Writing – original draft, Formal analysis. Natalia Fernanda Ferreira: Methodology. Silmeia Garcia Zanatti: Methodology. Marcos Ferreira Minicucci: Writing – review & editing, Formal analysis. Leonardo Antonio Mamede Zornoff: Writing – review & editing, Formal analysis, Data curation. Sergio Alberto Rupp de Paiva: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Bertha Furlan Polegato: Writing – review & editing, Project administration, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We thank Colin Knaggs for the English language editing.
References
- 1.Mattioli R., Ilari A., Colotti B., Mosca L., Fazi F., Colotti G. Doxorubicin and other anthracyclines in cancers: activity, chemoresistance and its overcoming. Mol Aspects Med. 2023;93 doi: 10.1016/j.mam.2023.101205. [DOI] [PubMed] [Google Scholar]
- 2.Pugazhendhi A., Edison T.N.J.I., Velmurugan B.K., Jacob J.A., Karuppusamy I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26–30. doi: 10.1016/j.lfs.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 3.Varela-López A., Battino M., Navarro-Hortal M.D., Giampieri F., Forbes-Hernández T.Y., Romero-Márquez J.M., et al. An update on the mechanisms related to cell death and toxicity of doxorubicin and the protective role of nutrients. Food Chem. Toxicol. 2019;134 doi: 10.1016/j.fct.2019.110834. [DOI] [PubMed] [Google Scholar]
- 4.Kong C.Y., Guo Z., Song P., Zhang X., Yuan Y.P., Teng T., et al. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int. J. Biol. Sci. 2022;18(2):760–770. doi: 10.7150/ijbs.65258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawat P.S., Jaiswal A., Khurana A., Bhatti J.S., Navik U. Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021;139 doi: 10.1016/j.biopha.2021.111708. [DOI] [PubMed] [Google Scholar]
- 6.Rigoulet M., Bouchez C.L., Paumard P., Ransac S., Cuvellier S., Duvezin-Caubet S., et al. Cell energy metabolism: an update. Biochim. Biophys. Acta Bioenerg. 2020;1861(11) doi: 10.1016/j.bbabio.2020.148276. [DOI] [PubMed] [Google Scholar]
- 7.Wu R., Wang H.L., Yu H.L., Cui X.H., Xu M.T., Xu X., et al. Doxorubicin toxicity changes myocardial energy metabolism in rats. Chem. Biol. Interact. 2016;244:149–158. doi: 10.1016/j.cbi.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Yan J., Xu S.C., Kong C.Y., Zhou X.Y., Bian Z.Y., Yan L., et al. Piperine alleviates doxorubicin-induced cardiotoxicity via activating PPAR- γ in mice. PPAR Res. 2019;2019:1–11. doi: 10.1155/2019/2601408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lima Junior E.A., Yamashita A.S., Pimentel G.D., De Sousa L.G.O., Santos R.V.T., Gonçalves C.L., et al. Doxorubicin caused severe hyperglycaemia and insulin resistance, mediated by inhibition in AMPk signalling in skeletal muscle. J Cachexia Sarcopenia Muscle. 2016;7(5):615–625. doi: 10.1002/jcsm.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling G., Wang X., Tan N., Cao J., Li W., Zhang Y., et al. Mechanisms and drug intervention for doxorubicin-induced cardiotoxicity based on mitochondrial bioenergetics. Camara A, organizador. Oxid. Med. Cell. Longev. 2022;2022:1–16. doi: 10.1155/2022/7176282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho M.V.D., Gonçalves-de-Albuquerque C.F., Silva A.R. PPAR gamma: from definition to molecular targets and therapy of lung diseases. Int. J. Mol. Sci. 2021;22(2):805. doi: 10.3390/ijms22020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang T., Wang J., Hu X., Huang X., Chen G.X. Current understanding of glucose transporter 4 expression and functional mechanisms. World J. Biol. Chem. 2020;11(3):76–98. doi: 10.4331/wjbc.v11.i3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takano H., Komuro I. Roles of peroxisome proliferator-activated receptor γ in cardiovascular disease. J Diabetes Complications. 2002;16(1):108–114. doi: 10.1016/s1056-8727(01)00203-3. [DOI] [PubMed] [Google Scholar]
- 14.Renu K.K.B.S., Parthiban S., S S., George A.P.B.T.P., et al. Elevated lipolysis in adipose tissue by doxorubicin via PPARα activation associated with hepatic steatosis and insulin resistance. Eur. J. Pharmacol. 2019;843:162–176. doi: 10.1016/j.ejphar.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T., Sano M. Deranged myocardial fatty acid metabolism in heart failure. Int. J. Mol. Sci. 2022;23(2):996. doi: 10.3390/ijms23020996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopaschuk G.D., Ussher J.R., Folmes C.D.L., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiol Rev. janeiro de. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 17.Chennuru A., Saleem M.T.S. Antioxidant, lipid lowering, and membrane stabilization effect of sesamol against doxorubicin-induced cardiomyopathy in experimental rats. BioMed Res. Int. 2013;2013:1–5. doi: 10.1155/2013/934239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olorundare O.E., Adeneye A.A., Akinsola A.O., Sanni D.A., Koketsu M., Mukhtar H. Clerodendrum volubile ethanol leaf extract: a potential antidote to doxorubicin-induced cardiotoxicity in rats. J. Toxicol. 2020;2020:1–17. doi: 10.1155/2020/8859716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Qiu Y., Liu Y., Huang N., Hua C., Wang Q., et al. Citronellal ameliorates doxorubicin‐induced hepatotoxicity via antioxidative stress, antiapoptosis, and proangiogenesis in rats. J. Biochem. Mol. Toxicol. 2021;35(2) doi: 10.1002/jbt.22639. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Liu X.J., Chen J.B., Cao J.P., Li X., Sun C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022;62(14):3833–3854. doi: 10.1080/10408398.2020.1870035. [DOI] [PubMed] [Google Scholar]
- 21.Aptekmann N.P., Cesar T.B. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013;12(1):119. doi: 10.1186/1476-511X-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cesar T.B., Aptekmann N.P., Araujo M.P., Vinagre C.C., Maranhão R.C. Orange juice decreases low-density lipoprotein cholesterol in hypercholesterolemic subjects and improves lipid transfer to high-density lipoprotein in normal and hypercholesterolemic subjects. Nutr. Res. 2010;30(10):689–694. doi: 10.1016/j.nutres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Kurowska E.M., Borradaile N.M., Spence J.D., Carroll K.K. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr. Res. 2000;20(1):121–129. doi: 10.1016/S0271-5317(99)00144-X. [DOI] [Google Scholar]
- 24.Simpson E.J., Mendis B., Macdonald I.A. Orange juice consumption and its effect on blood lipid profile and indices of the metabolic syndrome; a randomised, controlled trial in an at-risk population. Food Funct. 2016;7(4):1884–1891. doi: 10.1039/c6fo00039h. [DOI] [PubMed] [Google Scholar]
- 25.Bok S.H., Lee S.H., Park Y.B., Bae K.H., Son K.H., Jeong T.S., et al. Plasma and hepatic cholesterol and hepatic activities of 3-Hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999;129(6):1182–1185. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox L.J., Borradaile N.M., de Dreu L.E., Huff M.W. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J. Lipid Res. 2001;42(5):725–734. doi: 10.1016/S0022-2275(20)31634-5. [DOI] [PubMed] [Google Scholar]
- 27.Escudero-López B., Cerrillo I., Ortega Á., Martín F., Fernández-Pachón M.S. Effect of acute intake of fermented orange juice on fasting and postprandial glucose metabolism, plasma lipids and antioxidant status in healthy human. Foods. 2022;11(9):1256. doi: 10.3390/foods11091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesan N., Venkatesan P., Karthikeyan J., Arumugam V. Protection by taurine against adriamycin-induced proteinuria and hyperlipidemia in rats. Exp Biol Med. 1997;215(2):158–164. doi: 10.3181/00379727-215-44122. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro A.P.D., Pereira A.G., Todo M.C., Fujimori A.S.S., Dos Santos P.P., Dantas D., et al. Pera orange (Citrus sinensis) and Moro orange (Citrus sinensis (L.) Osbeck) juices attenuate left ventricular dysfunction and oxidative stress and improve myocardial energy metabolism in acute doxorubicin-induced cardiotoxicity in rats. Nutrition. 2021:91–92. doi: 10.1016/j.nut.2021.111350. 111350. [DOI] [PubMed] [Google Scholar]
- 30.Mohamad E.A., Ahmed S.M., Masoud M.A., Mohamed F.A., Mohammed H.S. Cardioprotective potential of moringa oleifera leaf extract loaded niosomes nanoparticles - against doxorubicin toxicity in rats. Curr Pharm Biotechnol. 2024 doi: 10.2174/0113892010303097240605105013. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Yarana C., Siwaponanan P., Maneechote C., Khuanjing T., Ongnok B., Prathumsap N., et al. Extracellular vesicles released after doxorubicin treatment in rats protect cardiomyocytes from oxidative damage and induce pro-inflammatory gene expression in macrophages. Int. J. Mol. Sci. 2022;23(21) doi: 10.3390/ijms232113465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun X., Song Y., Xie Y., Han J., Chen F., Sun Y., et al. Shenlijia attenuates doxorubicin-induced chronic heart failure by inhibiting cardiac fibrosis. Xue YB, organizador. Evid Based Complement Alternat Med. 2021;2021:1–13. doi: 10.1155/2021/6659676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez P.F., Okoshi K., Zornoff L.A.M., Oliveira S.A., Campos D.H.S., Lima A.R.R., et al. Echocardiographic detection of congestive heart failure in postinfarction rats. J. Appl. Physiol. 2011;111(2):543–551. doi: 10.1152/japplphysiol.01154.2010. [DOI] [PubMed] [Google Scholar]
- 34.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. PMID: 4337382. [PubMed] [Google Scholar]
- 35.Kurumbail R.G., et al. In: Neumann D., Viollet B., editors. vol. 1732. Humana Press; New York, NY: 2018. Biophysical interactions of direct AMPK activators. (AMPK. Methods in Molecular Biology). [DOI] [PubMed] [Google Scholar]
- 36.Garcia J.L., Vileigas D.F., Gregolin C.S., Costa M.R., Francisqueti-Ferron F.V., Ferron A.J.T., et al. Rice (Oryza sativa L.) bran preserves cardiac function by modulating pro-inflammatory cytokines and redox state in the myocardium from obese rats. Eur. J. Nutr. 2022;61(2):901–913. doi: 10.1007/s00394-021-02691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dantas D., Pereira A.G., Fujimori A.S.S., Ribeiro A.P.D., De Almeida Silva C.C.V., Monte M.G., et al. Doxycycline attenuates doxorubicin-induced cardiotoxicity by improving myocardial energy metabolism in rats. J Cardiovasc Dev Dis. 2022;9(8):254. doi: 10.3390/jcdd9080254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renu K., Vinayagam S., Madhyastha H., Madhyastha R., Maruyama M., Suman S., et al. Exploring the pattern of metabolic alterations causing energy imbalance via PPARα dysregulation in cardiac muscle during doxorubicin treatment. Cardiovasc. Toxicol. 2022;22(5):436–461. doi: 10.1007/s12012-022-09725-x. [DOI] [PubMed] [Google Scholar]
- 39.Tokarska-Schlattner M., Wallimann T., Schlattner U. Alterations in myocardial energy metabolism induced by the anti-cancer drug doxorubicin. C R Biol. 2006;329(9):657–668. doi: 10.1016/j.crvi.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 40.Santos K.G.D., Yoshinaga M.Y., Glezer I., Chaves-Filho A.D.B., Santana A.A.D., Kovacs C., et al. Orange juice intake by obese and insulin-resistant subjects lowers specific plasma triglycerides: a randomized clinical trial. Clin Nutr ESPEN. 2022;51:336–344. doi: 10.1016/j.clnesp.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 41.Lima A.C.D., Cecatti C., Fidélix M.P., Adorno M.A.T., Sakamoto I.K., Cesar T.B., et al. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: controlled clinical trials. J. Med. Food. 2019;22(2):202–210. doi: 10.1089/jmf.2018.0080. [DOI] [PubMed] [Google Scholar]
- 42.Pointon A.V., Walker T.M., Phillips K.M., Luo J., Riley J., Zhang S.D., et al. Doxorubicin in vivo rapidly alters expression and translation of myocardial electron transport chain genes, leads to ATP loss and caspase 3 activation. Melov S, organizador. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marcillat O., Zhang Y., Davies K.J.A. Oxidative and non-oxidative mechanisms in the inactivation of cardiac mitochondrial electron transport chain components by doxorubicin. Biochem. J. 1989;259(1):181–189. doi: 10.1042/bj2590181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirone L., D'Ambrosio L., Forte M., Genovese R., Schiavon S., Spinosa G., et al. Mitochondria and doxorubicin-induced cardiomyopathy: a complex interplay. Cells. 2022;11(13):2000. doi: 10.3390/cells11132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Constans J., Bennetau-Pelissero C., Martin J.F., Rock E., Mazur A., Bedel A., et al. Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men. Clin Nutr. 2015;34(6):1093–1100. doi: 10.1016/j.clnu.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 46.De Oliveira Caland R.B., Cadavid C.O.M., Carmona L., Peña L., De Paula Oliveira R. Pasteurized orange juice rich in carotenoids protects Caenorhabditis elegans against oxidative stress and β -amyloid toxicity through direct and indirect mechanisms. Oxid. Med. Cell. Longev. 2019;2019:1–13. doi: 10.1155/2019/5046280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangel-Huerta O.D., Aguilera C.M., Martin M.V., Soto M.J., Rico M.C., Vallejo F., et al. Normal or high polyphenol concentration in orange juice affects antioxidant activity, blood pressure, and body weight in obese or overweight adults. J. Nutr. 2015;145(8):1808–1816. doi: 10.3945/jn.115.213660. [DOI] [PubMed] [Google Scholar]
- 48.Deyhim F., Lopez E., Gonzalez J., Garcia M., Patil B.S. Citrus juice modulates antioxidant enzymes and lipid profiles in orchidectomized rats. J. Med. Food. 2006;9(3):422–426. doi: 10.1089/jmf.2006.9.422. [DOI] [PubMed] [Google Scholar]
- 49.Haidari F., Ali Keshavarz S., Reza Rashidi M., Mohammad Shahi M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009;45(3):285–291. doi: 10.3164/jcbn.09-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dourado G.K.Z.S., Cesar T.B. Investigation of cytokines, oxidative stress, metabolic, and inflammatory biomarkers after orange juice consumption by normal and overweight subjects. Food Nutr. Res. 2015;59(1) doi: 10.3402/fnr.v59.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miles E.A., Calder P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: a narrative review. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.712608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalender Y., Yel M., Kalender S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. Toxicology. 2005;209(1):39–45. doi: 10.1016/j.tox.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 53.AlAsmari A.F., Alharbi M., Alqahtani F., Alasmari F., AlSwayyed M., Alzarea S.I., et al. Diosmin alleviates doxorubicin-induced liver injury via modulation of oxidative stress-mediated hepatic inflammation and apoptosis via NfkB and mapk pathway: a preclinical study. Antioxidants. 2021;10(12):1998. doi: 10.3390/antiox10121998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasanna P.L., Renu K., Valsala Gopalakrishnan A. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020;250 doi: 10.1016/j.lfs.2020.117599. [DOI] [PubMed] [Google Scholar]
- 55.Vuppalanchi Raj, Chalasani Naga. In: Practical Hepatic Pathology: A Diagnostic Approach. Saxena Romil., editor. W.B. Saunders; 2011. 5 - laboratory tests in liver disease; pp. 55–62. 9780443068034. [DOI] [Google Scholar]
- 56.Barata I.S., Gomes B.C., Rodrigues A.S., Rueff J., Kranendonk M., Esteves F. The complex dynamic of phase I drug metabolism in the early stages of doxorubicin resistance in breast cancer cells. Genes. 2022;13(11):1977. doi: 10.3390/genes13111977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cara K.C., Beauchesne A.R., Wallace T.C., Chung M. Effects of 100% orange juice on markers of inflammation and oxidation in healthy and at-risk adult populations: a scoping review, systematic review, and meta-analysis. Adv. Nutr. 2022;13(1):116–137. doi: 10.1093/advances/nmab101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coelho R.C.L.A., Hermsdorff H.H.M., Bressan J. Anti-inflammatory properties of orange juice: possible favorable molecular and metabolic effects. Plant Foods Hum Nutr. 2013;68(1):1–10. doi: 10.1007/s11130-013-0343-3. [DOI] [PubMed] [Google Scholar]
- 59.Rocha D.M.U.P., Lopes L.L., Da Silva A., Oliveira L.L., Bressan J., Hermsdorff H.H.M. Orange juice modulates proinflammatory cytokines after high-fat saturated meal consumption. Food Funct. 2017;8(12):4396–4403. doi: 10.1039/c7fo01139c. [DOI] [PubMed] [Google Scholar]
- 60.Ibrahim Fouad G., Ahmed K.A. Curcumin ameliorates doxorubicin-induced cardiotoxicity and hepatotoxicity via suppressing oxidative stress and modulating iNOS, NF-κB, and TNF-α in rats. Cardiovasc. Toxicol. 2022;22(2):152–166. doi: 10.1007/s12012-021-09710-w. [DOI] [PubMed] [Google Scholar]
- 61.Gilliam L.A.A., Moylan J.S., Ferreira L.F., Reid M.B. TNF/TNFR1 signaling mediates doxorubicin-induced diaphragm weakness. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;300(2):L225–L231. doi: 10.1152/ajplung.00264.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Birks E.J., Burton P.B.J., Owen V., Mullen A.J., Hunt D., Banner N.R., et al. Elevated tumor necrosis factor- and interleukin-6 in myocardium and serum of malfunctioning donor hearts. Circulation. 2000;102(Supplement 3) doi: 10.1161/01.cir.102.suppl_3.iii-352. III-352-III–358. [DOI] [PubMed] [Google Scholar]
- 63.Alyasiry E., Janabi A., Hadi N. Dipyridamole ameliorates doxorubicin-induced cardiotoxicity. J Med Life. 2022;15(9):1184–1190. doi: 10.25122/jml-2021-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.