Abstract
Assessing and predicting quality of groundwater is crucial in managing groundwater availability effectively. In the current study, groundwater quality was thoroughly appraised using various indexing methods, including the drinking water quality index (DWQI), pollution index of heavy metals (HPI), pollution index (PI), metal index (MI), degree of contamination (Cd), and risk indicators, like hazard quotient (HQ) and total hazard indicator (HI). The assessments were augmented through multivariate analytical techniques, models based on recurrent neural networks (RNNs), and integration of geographic information system (GIS) technology. The analysis measured physicochemical parameters across 48 groundwater wells from El-Menoufia region, revealing distinct water types influenced by ion exchange, rock-water interactions, and silicate weathering. Notably, the groundwater showed elevated levels of certain metals, particularly manganese (Mn) and lead (Pb), exceeding the drinking water limits. The DWQI deemed the bulk of the tested samples suitable for consumption, assigning them to the "good" category, whereas a small number were considered inferior quality. The HPI, MI, and Cd indices indicated significant pollution in the central study region. The PI revealed that Pb, Mn, and Fe were significant contributors to water pollution, falling between classes IV (strongly affected) and V (seriously affected). HQ and HI analyses identified the central area of the study as particularly prone to metal contamination, signifying a high risk to children via oral and dermal routes and to adults through oral exposure alone (non-carcinogenic risk). The adults had no health risks due to dermal contact. Finally, the RNN simulation model effectively predicted the health and water quality indices in training and testing series. For instance, the RNN model excelled in predicting the DWQI, with three key parameters being crucial. The model demonstrated an excellent fit on the training set, achieving an R2 of 1.00 with a very low root mean of squared error (RMSE) of 0.01. However, on the testing set, the model's performance slightly decreased, showing an R2 of 0.96 and an RMSE of 2.73. Regarding HPI, the RNN model performed exceptionally well as the primary predictor, with R2 values of 1.00 (RMSE = 0.01) and 0.93 (RMSE = 27.35) for the training and testing sets, respectively. This study provides a unique perspective for improving the integration of various techniques to gain a more comprehensive understanding of groundwater quality and its associated health risks, with a strong focus on feature selection strategies to enhance model accuracy and interpretability.
Keywords: Human health risk, Hydrogeochemistry, Multivariate analysis, RNN, GIS
Graphical abstract
Highlights
-
•
Water Quality Assessment using DWQI, HPI, PI, MI, Cd, HQ, and HI, along with advanced modeling techniques.
-
•
Wells reveal distinct water types influenced by ion exchange, rock-water interactions, and silicate weathering processes.
-
•
Elevated Metal Levels particularly manganese (Mn) and lead (Pb), exceeding drinking water limits.
-
•
Health Risks and Predictive Modeling due to metal exposure, with accurate predictions using RNN models.
1. Introduction
Groundwater and surface water are the principal drinking water supply in arid and semiarid regions globally, although its quality is deteriorating owing to water scarcity [[1], [2], [3],[3], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12]]. The interaction between rocks and water leads to various geochemical processes that influence the hydrochemical parameters of groundwater [13]. Consequently, scientists worldwide have recently focused on understanding the fundamental processes that operate these ions and investigating the groundwater contamination impacts on environmental and health [[14], [15], [16]]. In recent years, numerous studies have been conducted to examine the hydrogeochemical characteristics and water quality, including the influence of the physical and chemical properties of groundwater, both natural and human-induced contaminants. Regrettably, as society has advanced, water quality has significantly degraded owing to various natural and human activities, impacting the hydrogeological environment and public health [[17], [18], [19], [20], [21]].
Heavy metals are toxic and detrimental to human health and ecosystems when they exceed specified thresholds [22]. Heavy metals, in particular, can degrade groundwater quality, affect human health and endanger natural ecosystems [23]. Some heavy metals, such as copper (Cu), manganese (Mn), nickel (Ni), zinc (Zn), and chromium (Cr), are required in small amounts for metabolism. However, they are toxic and hazardous to human health at high concentrations [24,25]. Other metals, such as cadmium (Cd) and mercury, are toxic even at low levels [26,27]. Regularly assessing heavy metals distribution, amounts, and potential health risks can help to regulate and prevent these negative impacts.
Research has found that high levels of pollutants in groundwater significantly harm human health and ecosystem components [28,29]. High level of Fe in water can induce joint pain, liver damage, and exhaustion [30]. In contrast, high contaminated water with Mn metal can develop serious neurological abnormalities and increase newborn mortality [31]. Elevated groundwater SO42− levels in Assiut District negatively impact human skin, hair, and eyes [32]. Heavy metal poisoning of water poses a non-cancerous health danger to residents of India's Chotanagpur Plateau periphery [33]. Groundwater with high anion and cation concentrations can be considered natural or artificial. Anthropogenic factors such as agricultural fertilization and industrial effluent discharge contribute to high NO3− concentrations [22], while natural factors, such as high evaporation and minerals weathering cause high groundwater F− concentrations [34]. Understanding the geochemical progressions that drive the evolution of water chemistry is critical for precisely identifying the origins of variables controlling their distribution. Many previous research investigations have primarily performed on the sources and influences of elements level enrichment in water, simulation of geochemical progressions, and effects of water level changes on the evolution of groundwater properties [24,[33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]. The hydrodynamic conditions, stratigraphy, and circulation of water conditions all contribute to the horizontal zonation of water quality or characteristics [36,38,43]. Continuous monitoring and evaluation of trace metal concentrations in groundwater are critical for ensuring the safe use of groundwater while protecting human and environmental health.
Examining and analyzing trace metal pollution in groundwater requires various approaches and tactics for evaluating PTEs contamination, determining the source of trace metals and undertaking risk assessments. However, monitoring groundwater quality can be difficult because of the complex and dynamic nature of its physical, chemical, and regulatory characteristics [29,44]. Qualitative and quantitative studies on trace metal contamination levels in groundwater present unique challenges. To overcome these problems, various trace metal-based evaluation indicators are commonly used, including HPI and HEI. These indices help to understand the total level of PTEs pollution in groundwater by simultaneously taking numerous metal characteristics into account. The appraisal of WQIs is widely acknowledged to be a highly effective procedure for appropriateness of water determination for specific purposes. These WQIs are numerical values derived from the physical, biological, and chemical characteristics of groundwater [37,39,45]. Utilizing water quality indicators, specifically DWQI, HPI, MI, Cd, PI, and HI, for classifying water quality proves to be more effective than conventional techniques that compare measured parameters predetermined water quality standards [26,46,47]. Additionally, machine learning techniques such as recurrent neural network models were used to examine how well groundwater worked in determining whether drinking was appropriate and what health risks were involved. Strategies that select features based on model analyses, for example, can identify characteristics with potent discriminative and forecasting prowess [48]. Such a strategy amplifies the model efficacy by discarding redundant characteristics and mitigating overfitting while maintaining the initial feature depiction, thus enhancing understandability [49]. The importance of feature selection techniques is increasing in predictive modeling [50]. Various studies have scrutinized different tactics to reduce data dimensionality.
Egypt, in particular, has witnessed a substantial rise in water demand in recent decades because of the increasing population and increased agricultural productivity. The El-Menoufia governorate, located in Egypt's Middle Delta, lies within a semi-arid region with a population of approximately 4,366,000 as of January 2018. The primary Nile Delta aquifer, consisting of Quaternary sediments, extends across the entire Nile Delta [51]. The aquifer, classified as semi-confined due to its thin clay layer, receives regular recharge from irrigation water infiltration. Given the study's location in northern Egypt, its connectivity to the surface water of the Nile River, and the prevalence of agricultural and industrial activities, continuous monitoring of water quality is essential for assessing health risks. In addition, hydrogeological and hydrochemical properties of aquifers is crucial for enhancing management practices and protecting groundwater resources. Regular evaluation of water quality from the point of origin to the end of use is essential for creating extensive databases that detail the properties of water, which in turn contributes to reducing health risks[[52], [53], [54]].
The current study aims to: (i) Determine the geochemical characteristics of groundwater by analyzing physicochemical parameters and metals to assess the quality based on DWQI and pollution indicators (PIs), providing a comprehensive overview of water quality, (ii) estimate the suitability of water for drinking using statistical analysis and machine learning (ML) models and assess associated health risks, with a particular focus on feature selection strategies for enhanced model performance, and (iii) Utilize a comprehensive framework that incorporates physicochemical characteristics, selected WQIs, multivariate modeling, ML models, and GIS approaches, ensuring a robust, precise, and efficient approach to assess and manage water resources.
2. Material and method
2.1. Site description
One of Egypt's Middle Delta governorates is El-Menoufia. The study area was in the semi-arid zone. The entire area is 2543 km2 (981.99 square miles). The study area was located to the southern reaches of the Nile Delta. Longitudes 30.99°E and latitudes 30.52°N define this region (Fig. 1). Menoufia has a population of approximately 4 366,000 people (as of January 2018). Summers are often hot (37–40 °C), whereas winters are cold (12–20 °C). During December and February, rainfall throughout the year; and the mean annual precipitation within the delta fluctuates between 25 mm/yr in the southern region and 200 mm/yr in the north of the Delta.
Fig. 1.
Map of the study area and investigated groundwater points.
2.2. Hydrogeological settings
The study area is covered by Quaternary deposits, which are divided into two units. The upper unit is Holocene, and consists of silt and clay. Its thickness varied from 5 m to more than 20 m. The lower unit assigned to the Pleistocene comprises sand and gravel with clay lenses. The thickness of this aquifer varies from 450 to 600 m and is underlined by Pliocene clay. The transmissivity of the aquifer varied from 3000 to 5000 m2/day. The hydraulic conductivity ranged from 50 to 100 m/day [55]. The effective porosity differs from 15 % to 18 %, and the storage coefficient ranges between 0.01 and 0.001 [48]. Groundwater levels in the Pleistocene aquifer ranged from 8 m in the south to 2 m in the north. The flow moved from the southeast to northwest. The aquifer is continuously supplied by the infiltration of excess irrigation water and seepage from the leading network of canals and drains or directly from the Damietta branch. The recharge from rainfall was negligible. The aquifer is discharged through natural outflow drains and the Rosetta branch, with groundwater extracted from the existing wells for water supply and cultivation [55].
2.3. Sampling and analyses
In April 2022, 48 samples were collected from boreholes in the investigated region (El-Menoufia region). The location or position of each sampling points was determined using GPS 315 (Fig. 1). Samples of groundwater were collected and placed in polyethylene bottles at each sampling location. Subsequently, filtration of water through a filter paper (0.45 μm). For the analysis of heavy and trace metals, the collected samples were adjusted to a pH level below two by introducing concentrated HNO3 as a preservative. This pH adjustment was performed to prepare samples for subsequent analyses. Once the bottles had been adequately filled, they were immediately hermetically secured and transferred to a refrigeration unit regulated at specific temperature (4 °C). In this research, 22 parameters were examined, including pH, TDS, electrical conductivity (EC), as well as the concentrations of K+, Na+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, CO32−, NO3−, and the trace elements Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn. These analyses were conducted using established and standardized analytical methods [56]. TDS, pH, and EC were directly measured on-site with an accurate salinity multi-parameter analyzer, specifically Hanna HI 9811-5. Ca2+, Mg2+, Cl−, HCO3−, and CO32− were determined by volumetric titration. The PTEs, including Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn, were measured via an inductively coupled plasma mass spectrometer (ICP-MS) manufactured in Waltham, USA. An ultraviolet/visible spectrophotometer was utilized to analyse Na+, K+, SO42−, and NO3−. These analytical procedures conformed the guidelines outlined in the APHA (American Public Health Association) standards [56].
2.4. Assurance and control of quality analysis
Water analysis adhered to the standards outlined by the American Public Health Association [57]. All instruments were meticulously standardized using deionize water and buffer solution prior to analysis. Several assurance procedures were employed throughout the water examination process.
The analytical methods were validated through instrument calibration, assessments of accuracy, and predictability evaluations. Charging balance errors (CBE) were assessed during field observations and further validated in the laboratory. Each sample was analyzed in triplicate, and the average values were reported. Anion-cation balance errors were analyzed using Equation (1), based on neutrality principles, which posits that total cations should be equal the total anions. For all examined samples, the CBE was maintained within the range of acceptance (±5 %).
| (1) |
In addition, the accuracy of the process was carefully validated through examinations using CRM and blank analysis. This method guaranteed the precision and dependability of the findings obtained in assessing water quality. The different metal contents in the sample solutions were obtained using a curve for calibration. To calibrate the device, a 50-mL (mL) intermediate standard was employed as an operational standard for toxic metals. When the correlation coefficient could be more than 0.999, it indicated that the relationship was strong. The measured amount of every metal in the collected sample was determined using interpolation of calibration curves. Every examination was conducted in triplicate.
2.5. Multivariate statistical analysis
A reliable data mining approach for pattern detection inside homogenous groups or clusters of instances (variables) is cluster analysis (CA) [19]. This method involves building a type of binary data tree out of successively comparable groups of points. The developing point clusters should then exhibit both high inter- and intra-cluster heterogeneity [38]. Various methods are employed to create and merge consistent sets of water sample data into meaningful groups. The clustering process is performed by applying Ward's linkage requirement, and the outcomes are visually depicted in the structure a dendrogram and a two-dimensional diagram.
Principal Component Analysis, which is commonly used for exploratory purposes to transform high-dimensional data into a lower-dimensional representation. Often, numerous correlated variables within the initial dataset can be effectively condensed into a smaller group of unrelated variables, referred to as PC or axes [37]. These variables are made up of the original correlated variables and the lists of coefficients termed eigenvectors, which are linearly independent (orthogonal) variables (called weightings). The first PC defines the largest proportion of variation in the dataset, while subsequent PCs explain the remaining fraction of variance. The PCs are formed in a progressive array of components with a declining contribution to total variance [38]. The KMO (Kaiser-Meyer-Olkin) and Bartlett's tests were employed to assess the appropriateness of the data for factor analysis, specifically evaluating the adequacy of the sample for each variable in the model. A KMO value between 0.5 and 0.8 is considered acceptable, while a value below 0.5 suggests that the data may not be suitable for factor analysis [44].
2.6. Hydrochemical characteristics and interpolation
The hydrochemical evaluation, mechanism controlling water chemistry, hydrochemical facies, and source of ions was determined using several graphical and statistical methods, including ionic ratio, chloro alkaline index, and Piper and Gibbs diagrams. Diagrammes software and Excel sheets were used for visualization.
Using ArcGIS version 10.5 software, the spatial analysis of the 10 chemical parameters utilized in the WQI was completed. The spatial analytical instrument used the inverse distance-weighted (IDW) methodology to execute the data interpolation process. The distribution of water quality was mapped using the IDW method, which estimates unknown values based on the premise that values at sample sites closer to the unsampled point are more comparable to one another than at points farther away [58].
2.7. Indexing approaches
Calculations of the WQIs, including the DWQI, HPI, MI, Cd, and PI, were performed using the measured amounts of diverse chemical constituents. These indices facilitate a comprehensive assessment of aquatic purity, incorporating the detection and concentration of metallic substances and their influence on water quality [[59], [60], [61], [62]].
2.7.1. Water quality index (DWQI)
The DWQI, a mathematically derived index, is widely recognized as the most influential metric for understanding the comprehensive quality of investigated samples intended for drinking. It was recognized by applying the arithmetic weight method [63], as represented by equation (2).
| (2) |
The index of sub-quality for every parameter, denoted as Qi, was calculated according to the groundwater concentration (Ci) and the standard (Si) for each groundwater parameter's drinking water value, following the WHO guidelines [64]. The weight unit for each parameter, represented as Wi, was utilized for the calculation (Eq. (3)). These calculations encompass 12 physicochemical parameters, denoted as 'n = 12′, and are expressed by equation (4).
| (3) |
| (4) |
wi f of every variable was computed using equation (5) according to the suggested criteria (World Health Organization, 2008).
| (5) |
K is the ratio of a constant.
To calculate the DWQI, it's essential to assign weights (wi) to each groundwater parameter and then determine the quality rating range (Qi) and the relative weight (Wi). Accordingly, the Wi values were assigned to parameters such as pH, EC, TDS, K+, Na+, Cl−, Ca2+, Mg2+, SO42−, HCO3−, CO32−, and NO3−. The weights (wi) were calculated utilizing Eq. (5). The assignment of weightings corresponded with each parameter's significance concerning the aggregate quality metric for potable water.Tabulated inclusions for the established norms, corresponding weight factors (wi), and their proportional magnitudes (Wi) for the parameters under examination are disclosed in Table 1, Table 2s.
Table 1.
Evaluation of statistical data on assessed physicochemical properties.
| Parameters | pH | EC | TDS | K+ | Na+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | CO32− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Minimum value | 7.50 | 190.0 | 161.0 | 1.0 | 8.0 | 10.94 | 8.0 | 24.63 | 12.0 | 0.00 | 0.00 |
| Mean value | 8.10 | 547.5 | 356.5 | 3.5 | 34.0 | 29.16 | 58.0 | 54.18 | 75.8 | 141.83 | 15.00 |
| Maximum value | 8.60 | 1829.0 | 929.8 | 10.0 | 150.0 | 60.75 | 120.0 | 172.38 | 355.0 | 405.65 | 60.00 |
| St Dev | 0.22 | 307.9 | 169.1 | 1.7 | 27.06 | 10.66 | 26.89 | 33.93 | 62.36 | 98.57 | 17.06 |
| (CV, %) | 2.73 | 56.25 | 47.4 | 50.5 | 79.58 | 36.57 | 46.36 | 62.62 | 82.27 | 69.5 | 113.74 |
| WHO-2017 | 6.5–8.5 | 1500.0 | 500 | 12 | 200 | 50 | 75 | 250 | 250 | 120 | 350 |
|
Parameters |
NO3− |
Al |
Cd |
Cr |
Cu |
Fe |
Mn |
Ni |
Pb |
V |
Zn |
| Min | 4.43 | 0.00 | 0.0006 | 0.00 | 0.0001 | 0.0024 | 0.0011 | 0.00 | 0.0005 | 0.00 | 0.00 |
| Mean | 39.85 | 0.02 | 0.00 | 0.01 | 0.01 | 0.17 | 0.15 | 0.00 | 0.01 | 0.01 | 0.01 |
| Max | 132.84 | 0.27 | 0.00 | 0.03 | 0.02 | 2.73 | 2.46 | 0.02 | 0.21 | 0.03 | 1.06 |
| St Dev | 32.94 | 0.07 | 0.00 | 0.01 | 0 | 0.53 | 0.59 | 0.00 | 0.05 | 0.01 | 0.21 |
| (CV, %) | 82.67 | 455.77 | 31.27 | 70.06 | 57.49 | 318.11 | 384.81 | 129.4 | 566.64 | 42.33 | 1740.67 |
| WHO (2017) | 50.0 | 0.2 | 0.003 | 0.05 | 0.05 | 0.3 | 0.1 | 0.02 | 0.01 | 0.05 | 3.0 |
*Note: All units are in milligrams per liter (mg/L), except pH, which has no units and electrical conductivity (μS/cm); St Dev for standard deviation.
Table 2.
The loading factors between the measured attributes.
| Parameter | F1 | F 2 | F 3 |
|---|---|---|---|
| TDS | 0.989 | −0.004 | −0.096 |
| K+ | 0.679 | 0.307 | 0.185 |
| Na+ | 0.888 | 0.078 | 0.143 |
| Mg2+ | 0.775 | −0.067 | 0.127 |
| Ca2+ | 0.876 | −0.127 | −0.294 |
| Cl− | 0.808 | −0.055 | −0.212 |
| SO42- | 0.712 | 0.209 | −0.298 |
| HCO3− | 0.719 | −0.509 | 0.201 |
| CO32- | 0.191 | 0.869 | 0.123 |
| NO3− | 0.243 | −0.058 | 0.893 |
2.7.2. Pollution indices (PIs)
The PIs, which include HPI, MI, Cd, and PI, were determined based on the concentrations of metals, namely Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn. The entire water quality was assessed using a toxicity index, HPI, calculated by assigning numerical weights to metals (Table 2s). The HPI values serve as an indicator of the combined influence of these metals on the overall water quality, considering the suggested standard or limits guidelines (Si) for each metal, such as Al, Cd, Fe, Mn, Cr, Cu, Ni, Pb, V, and Zn. The computation of the HPI value was performed using equation (6), a methodology that has been employed in previous research studies [65,66].
| (6) |
In the formula presented, the terms Wi and Qi denote the specific weights and indices related to various metals, specifically Al, Cd, Cr, Cu, Ni, Pb, Fe, Mn, V, and Zn. The symbol 'n' indicates the aggregate number of metals being analyzed, which is 10 in the given scenario. The classifications of HPI based on threshold value 100 are grounded in benchmarks set forth by prior investigative work [26,61,67].
As per equation (7), the MI indicator was employed to assess negative effect of PTEs on health. However, MI provides a rapid means to gauge the overall or general quality of water, particularly its negative impact on health [63].
| (7) |
Within this framework, the symbol Hc represents the quantification of each metallic element identified within the collected aqueous specimen, Hmax signifies the uppermost allowable threshold for metallic content, and the subscript 'i' refers to the sample's ordinal position.
The assessment involved calculating the contamination factors for individual metals that surpassed the acceptable thresholds to determine the contamination levels in groundwater. As defined in previous studies, these contamination factors are quantified as Cd values and can be derived using equations (8), (9)).
| (8) |
| (9) |
Cfi is the contamination parameter for metals, CAi denotes the value of analysis for metals, CNi denotes the permitted level for every metal, and CNi is computed using the highest acceptable concentration (MAC).
The impact of metal pollution was assessed through PI values. These values represent the individual contaminating influence of metal and classified into five groups (as shown in Table 3s). Equation (10) determines this classification.
| (10) |
In the equation, "Ci" represents the metal concentration, while "Si" denotes the level of each specific metal present in aquatic systems [63].
Table 3.
Correlation findings between the measured metals and factors.
| Metal | F 1 | F 2 | F 3 | F 4 | F 5 | F 6 | F 7 |
|---|---|---|---|---|---|---|---|
| Fe | 0.088 | −0.658 | −0.391 | −0.165 | 0.173 | −0.307 | 0.369 |
| Mn | 0.468 | −0.301 | −0.602 | 0.403 | 0.122 | −0.067 | −0.170 |
| Al | −0.222 | −0.670 | 0.465 | 0.026 | −0.123 | 0.121 | 0.299 |
| Cd | 0.019 | 0.034 | −0.136 | −0.133 | −0.884 | −0.281 | 0.186 |
| Cr | −0.176 | 0.087 | 0.342 | 0.745 | −0.205 | −0.267 | −0.061 |
| Cu | −0.619 | −0.058 | −0.310 | 0.247 | 0.109 | −0.460 | −0.128 |
| Ni | −0.401 | 0.026 | −0.588 | −0.208 | −0.342 | 0.287 | −0.231 |
| Pb | −0.142 | −0.461 | 0.351 | −0.407 | 0.008 | −0.295 | −0.594 |
| V | 0.695 | −0.358 | 0.084 | 0.193 | −0.312 | 0.153 | −0.264 |
| Zn | −0.522 | −0.427 | −0.088 | 0.302 | −0.055 | 0.525 | −0.049 |
2.8. Health risk indices (non-carcinogenic effect)
2.8.1. Chronic daily intake (CDI)
Human exposure to metals primarily occurs via water consumption (oral route) and skin contact (dermal route). Chronic daily intake (CDI) was considered for both adult and child populations to ascertain the risk from exposure to metals. This assessment was uniform for oral and dermal routes among the mentioned demographics. The quantification of consumption rates and skin absorption followed the US Environmental Protection Agency (USEPA) protocols. The calculation of CDI for the oral route (CDI Ingestion) and the dermal route (CDI Dermal) was performed for each group, adhering to the formulae provided in equations (11), (12)), which are corroborated by literature [26,[68], [69], [70]].
| (11) |
| (12) |
The evaluation of human exposure risk to metals involved the assessment of two primary pathways: ingestion through water consumption and dermal exposure via skin contact. The CDI was computed for adults and children, following a consistent methodology for both ingestion and dermal exposure by the guidelines established by the Environmental Protection Agency. CDI for ingestion (CDI oral) and CDI for skin contact (CDI dermal) were determined by using a specific formula, with the element concentration (EC) measured in mg/L. For adults, the ingestion rate (IngR) was set at 2.5 L per day, while for children, it was 0.78 L per day. Exposure frequency (EF) was considered to be 365 days per year. Adults were assumed to have an exposure duration (ED) of 30 years, while children had a 6-year exposure duration. Body weight (BW) was estimated at 52 kg (adults) and 15 kg (child). The average time (AT) was 10,950 days (adult) and 2190 (child). The exposed skin area (SA) was determined for adults to be 1.8 m2 and for children to be 0.66 m2. An adherence factor (AF) of 0.07 and a dermal absorption fraction (ABSd) of 0.03 were applied. Exposure time (ET) was accounted for as 0.58 h per day. Finally, the conversion factor (CF) was set at ten⁻2 (kg/mg) (as per Table 4s), following relevant references [[71], [72], [73], [74], [75], [76], [77], [78], [79], [80]].
Table 4.
Evaluation of groundwater suitability utilizing quality metrics of water.
| Indices | Sample range |
Range | class | Samples (%) | ||
|---|---|---|---|---|---|---|
| Min value | Max.value | Mean | ||||
| DWQI | 32.54 | 101.31 | 61.88 | <50 | Excellent | 10.4 % (5 samples) |
| 50–100 | Good | 87.5 % (42 samples) | ||||
| 100–150 | Poor | 2 % (1 samples) | ||||
| 150–200 | Very poor | 0 % (0 samples) | ||||
| >200 | Unsuitable | 0 % (0 samples) | ||||
| HPI | 15.75 | 387.02 | 76.64 | <100 | Low polluted | 81.25 % (39 samples) |
| >100 | High polluted | 18.75 % (9 samples) | ||||
| MI | 1.06 | 27.60 | 9.33 | <0.3 | Very pure | 0 % (0 samples) |
| 0.3–1.0 | Pure | 0 % (0 samples) | ||||
| 1.0–2.0 | Slightly affected | 22.9 % (11 samples) | ||||
| 2.0–3.0 | Moderately affected | 8.33 % (4 samples) | ||||
| 3.0–6.0 | Strongly affected | 12.5 % (6 samples) | ||||
| >6.0 | Seriously affected | 56.25 % (27 samples) | ||||
| Cd | −8.94 | 17.60 | −0.67 | >1 | Low | 66.6 % (32 samples) |
| 1–3 | Medium | 6.25 % (3 samples) | ||||
| <3 | High | 27.08 % (13 samples) | ||||
| HI Oral (adult) | 0.10 | 3.39 | 1.04 | >1 | High risk | 35.4 % (17 samples) |
| >1 | Low risk | 64.58 % (31 samples) | ||||
| HI Oral (child) | 0.39 | 12.95 | 3.97 | >1 | High risk | 75 % (36 samples) |
| >1 | Low risk | 25 % (12 samples) | ||||
| HI dermal (adult) | 0.01 | 0.43 | 0.11 | >1 | High risk | 0 % (0 sample) |
| >1 | Low risk | 100 % (48 samples) | ||||
| HI dermal (child) | 0.02 | 1.26 | 0.31 | >1 | High risk | 6.25 % (3 samples) |
| >1 | Low risk | 93.75 % (45 samples) | ||||
The HQ was derived using the following equations: 13 and 14.
| (13) |
| (14) |
Established RfD metrics (Reference Dose) for oral consumption were delineated as 0.7 for (Fe) iron and 0.024 for (Mn) manganese. In contrast, for dermal contact, the RfD parameters were designated as 0.14 and 96 × 10⁻⁵ for Fe and Mn, respectively. The figures above were denominated in milligrams per liter per day (mg L⁻1 day⁻1).
The HI is critical in assessing non-cancer health risks attributed to metallic elements. An aggregate of HQ measures corresponding to individual metals detected at a given site is used to derive the HI for that locale. This index is computed by equation (15).
| (15) |
In the evaluation of human health, individual elements are assessed for their HQ and HI values, denoted as variable 'i.' A value below 1 indicates a low risk, while a value exceeding 1 signifies a high risk by the criteria established in reference [81].
2.8.2. RNN- based simulation model
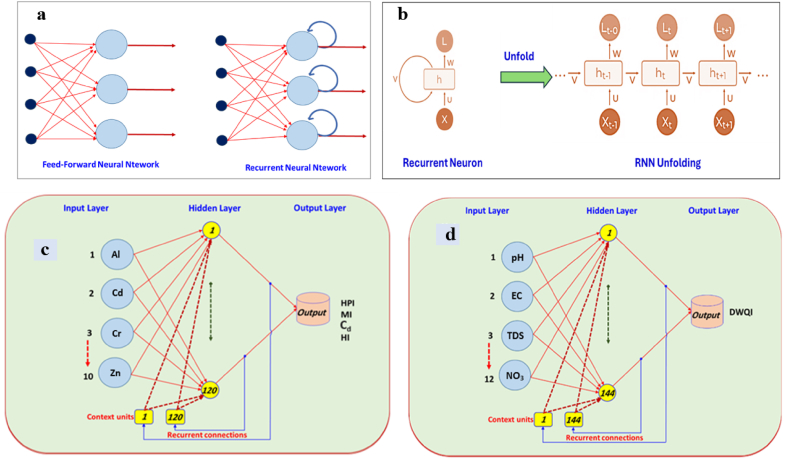
RNNs are particularly effective in tasks in which the input and output are sequences of data points, such as time-series prediction. Unlike traditional feedforward neural networks, which process inputs independently of one another, RNNs maintain a hidden state, which is the most critical component of an RNN that captures information about past inputs [82,83]. This capability enables RNNs to exhibit dynamic temporal behavior, making them particularly suitable for tasks that include sequences, such as time series prediction. At the core of RNNs is the concept of recurrence, where the output of a network at a given time step is dependent not only on the current input but also on previous inputs and the network's internal state. As shown in Fig. 2a, a feed-forward neural network operates with a singular path for information flow, moving exclusively from the input layer to the output layer while traversing through any hidden layer in between. Data progressed linearly across the network without revisiting any node more than once. However, a Recurrent Neural Network (RNN) introduces a cyclical flow of information through a loop mechanism. Before generating the output, it assesses both the current input and the knowledge accumulated from the previous inputs. This recursive nature enables an RNN to incorporate memory internally. It generates an output, replicates it, and reintroduces it into the network input for further processing.
Fig. 2.
Recurrent Neural Network versus Feed-Forward Neural Network (a), Recurrent Neural Network Unfolding (c) Topology of the RNN was used to predict (d) HPI, MI, Cd, and HI, and (b) DWQI.
An output layer, context units (components that acquire the values from the recurrent interconnections), one or more hidden layers, and an input layer comprise an RNN. Based on the sequence's input time, the hidden layers continually and repeatedly trained the network. The primary processing element within a Recurrent Neural Network (RNN) is termed a Recurrent Unit, distinct from being explicitly labeled as a "Recurrent Neuron." This component possesses a distinctive feature that enables it to retain a hidden state, facilitating the network to grasp sequential relationships by retaining past inputs during the computation. Variants such as Long Short-Term Memory (LSTM) and Gated Recurrent Unit (GRU) further enhance the RNN's capability to manage extended temporal dependencies. RNNs are classified into four types depending on the network's configuration of inputs and outputs. These include one input to one output, one input to many outputs, many inputs to one output, and many inputs to many outputs.
Unlike Deep neural networks, where we have different weight matrices for each Dense network in RNN, the weight across the network remains the same. It calculates state hidden state hi for every input Xi (Fig. 2b). By using the following equations:
| (16) |
| (17) |
Hence
| (18) |
Here, S is the State matrix, which has element si as the state of the network at timestep I The parameters in the network are W, U, V, C, and B, which are shared across timestep.
The RNN presented in this study comprises the Elman network, also known as the Simple Recurrent Network (SRN), which is one of the most widely used implicit learning neural network models. It is a type of recurrent neural network (RNN) that consisting of an input layer, hidden layer with recurrent connections, and output layer. In the case of predicting the pollution indices, the input layer contains ten neurons: Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb, V, and Zn. The output of the model (HPI, MI, Cd, or HI) is a single neuron in the output layer (Fig. 2c). In the case of predicting the DWQI, the input layer contains twelve neurons: pH, EC, TDS, Na+, Cl−, Mg2+, K+, Ca2+, SO42−, HCO3−, NO3− and CO32− (Fig. 2d). The output of the model health index is the single neuron in the output layer. Recurrent connections from the hidden layer's output to the input of the Elman network set it apart from the conventional networks. To understand the temporal patterns in successive inputs, the delay in the recurrent relationships saves information about earlier time stages. All calculations for the Recurrent Neural Network (RNN) presented in this work were conducted using MATLAB software.
2.8.3. Performance of the RNN
To measure the performance of the RNN in predicting the pollution indices and DWQI, the following four statistical indicators were utilized:
coefficient of Nash–Sutcliffe efficiency (NSE), expressed as:
| (19) |
The mean absolute error (MAD) is expressed as:
| (20) |
The absolute variance fraction, R2, is calculated as follows:
| (21) |
RMSE expressed as:
| (22) |
where PIo is the measured index, n is the number of samples or data point, PIf is the forecasted pollution index (or the forecasted DWQ index), and is the average of the corresponding measured records.
3. Results and discussion
3.1. Characterization of the groundwater chemistry
This segment will concentrate on the examination of hydrochemical characteristics. The results of the analysis are detailed in Table 1. The pH levels of the groundwater specimens oscilliated between 7.5 and 8.6, with an average of 8.10, signifying a range from slightly alkaline to alkaline conditions, as indicated in Table 1. This phenomenon is due to the intercalation of fine particles and clay within aquifer strata. The EC readings for the groundwater samples ranged from 190 to 1829 μS cm−1, with a median value of 547.5 μS cm−1, detailed in Table 1. All the EC readings were below the prescribed limit for potable water (1500 μS cm−1) as per the standards set by the organization [84], with an exception for a single sample at 1829 μS.cm-1, identified in the northeastern sector of the plain. This suggests the influence of salt dissolution processes and human activities, notably the reapplication of agriculturally contaminated water, as reported by Salem [85]. Furthermore, the salinity concentrations of the groundwater samples varied from 161 mg/L to 929.88 mg/L, averaging 356.5 mg/L, as shown in Table 1.
Within the prescribed thresholds for potable water, defined as a maximum of 1000 mg/L, every groundwater sample examined qualified as fresh water. The sodium levels in these samples varied, ranging from 8 mg/L to 150 mg/L, with an average concentration of 34 mg/L, as detailed in Table 1. Sodium exhibited a pattern similar to that of potassium but with a higher concentration. Magnesium concentration values of the groundwater samples fluctuated between 10.94 and 60.75 mg/L with a mean value of 29.16 mg/L, which falls within the standard limits for drinking water (70 mg/L) and recorded in northeastern of the plain (Table 1). The calcium concentration values of the groundwater samples fluctuated between 8 and 120 mg/L with a mean value of 58 mg/L. Approximately 30 % of the groundwater samples were found above the standard limits for drinking (Table 1), reflecting the meteoric origin of the water in the study's location and may be due to dissolution of dolomite and gypsum [86,87].
The chloride concentration values of the groundwater samples fluctuated between 24.36 and 172.38 mg/L (Table 1). The Sulfate concentration values of the groundwater samples fluctuated between 12 and 355 mg/L with a mean value of 75.80 mg/L (Table 1). One sample belonging to the El-Sadat district had a high concentration of sulfate (355 mg/L), more than the standard drinking limit (250 mg/L) [84], reflecting the seepage of sanitary drainage water. The weathering of sulfur-bearing minerals (pyrite FeS2, anhydrite CaSO4, and gypsum CaSO4.2H2O) and biological and biochemical processes are considered the main sources of sulfate [21]. Sulfate is formed during the dissolution of sulfur-bearing minerals (gypsum)
In addition to the decomposition of organic matter in the soil which leads to increased sulfate concentration in groundwater, fertilizers, industrial discharge, septic tanks, and wastewater increases the concentration of sulfate in water [88]. Concentrations of carbonate and bicarbonate in the groundwater specimens exhibited variations, ranging from a minimum of 0.00 mg/L to a maximum of 405.65 mg/L, with an average concentration determined at 141.83 mg/L, as presented in Table 1. Notably, approximately 65 % of these groundwater specimens exceeded the prescribed threshold for potable water, which was set at 120 mg/L (Organization, 2017), with the preponderance of such instances being documented in the northern segment of the area under investigation. HCO3− and CO32− ions are primarily responsible for the alkalinity of the groundwater. Two prominent sources of HCO3− ions in groundwater are the aquifer-borne dissolution of carbonate rocks like dolomite (CaMg(CO3)2) and calcite (CaCO3), in accordance with reactions [89].
Nitrate concentration values of the groundwater samples fluctuated between 4.43 mg/L to 132.84 mg/L with a mean value of 39.85 mg/L (Table 1). About 42 % of groundwater samples found above the standard drinking limit (50 mg/L) [84]. The most significant concentrations of these substances were noted in the center areas of the study, aligning with several villages such as Menouf, Quisna, El-Shohadd, and various agricultural areas. The increased concentrations of NO3− were attributed to various human activities, including septic tanks, extensive irrigation practices with the excessive application of inorganic nitrogenous fertilizers, return flow from irrigation, and discharges from households and industries [90].
Metal concentrations have previously been investigated for their toxicity and effects on human health and ecosystems [91]. However, these studies were restricted to certain geographic areas and only provided light on a small fraction of the study area. Fe, Mn, Al, and Pb concentrations, among ten elements under research, were greater in sediment samples from the study region. Still, Zn, Cr, V, Ni, Cu, and Cd concentrations were lower. The results indicated that, based on the location of the sample sites, bottom sediments collected components from comparable sources through similar mechanisms [91]. The majority of the metals under study had significant coefficient of variation values (CV > 20 %), with Zn showing the most variance (CV = 1740.67 %) and Cd showing the lowest (CV = 31.27 %). Notably, the metal contents displayed notable variability due to their high levels, which were correlated with quite high standard deviations and coefficients of variation, which may be attributable to both natural and anthropogenic loads [91].
The concentration of metals (measured in mg/L) exhibited variations among the aquifers throughout the Menoufia area (Table 1). It is also important to note the wide variety of concentrations for each metal in various places, such as Fe (64 %), Mn (53 %), Pb (25 %), and Al (9 %) of groundwater samples, which has more than standard limits for drinking. The considerable regional variation of pollution with metals in the groundwater revealed local geological differences, the impact of human activities like agriculture and industrial activities [92,93], or a combination of the two [94]. The experimental results of The Al concentration values showed that only three samples in the eastern and one in the western study area contained more than the standard drinking limit (0.2 mg/L), which was recommended by WHO [84]. The remaining samples showed no discernible concentration of Mn as specified in Table 1. Nevertheless, the elevated concentration of Al in both soil and groundwater is primarily associated with natural geological processes and may undergo modifications due to human activities, including the discharge of agricultural effluents and untreated wastewater into the study area [95,96]. The Fe concentration values of the groundwater samples showed that approximately 34 % of groundwater samples were found above the standard limits for drinking (0.3 mg/L) recommended by WHO (WHO, 2017), where the majority were recorded in the northern part of the study area. People who live close to these samples in the research region may get disorders linked to excess Fe, and the people must thus be mindful of the excess Fe [97]. Apart from that, it is considered that the Fe content in all of the samples that were obtained was safe. In general, through wastewater discharges, industries can potentially pollute the groundwater and the environment with Fe. However, by combining oxidations and microfiltration, Fe may be eliminated from groundwater [94]. The Mn concentration values revealed that about 53 % of groundwater samples found above of the standard limits for drinking (0.1 mg/L), where the majority recorded in the eastern part of study area. Additionally, through wastewater discharges, manufacturing may pollute the environment with Mn) and Fe [98]. Manganese toxicity, which damages a number of crops, including wheat, soybeans, and rice, can also result from high Mn levels in groundwater [99]. Reduced crop production, stunted development, and leaf chlorosis are signs of manganese toxicity in plants [100].
Approximately one-quarter of the groundwater specimens analyzed Pb levels surpassing the 0.01 mg/L threshold advised for potable water. Predominantly, these elevated Pb measures were detected within the northeast sector of the region under investigation. The pollution by Pb in water sources can stem from various sources, including activities like battery manufacturing and paint production. Such contamination can have serious health implications, including potential damage to the brain, difficulties with childhood development, anemia, and compromised renal function [101]. Moreover, the concentrations of potentially toxic elements (PTEs) may fluctuate because of locally concentrated sources of pollution discovered at certain sample sites, including excavated silt from these places [91]. The average metal concentrations measured were found to comply with the safe consumption thresholds delineated by the WHO [84], as outlined in Table 1, with the exception of Mn and Pb levels, which markedly surpassed the prescribed WHO benchmarks [84] (Table 1). These results point to possible dangers to the public health, if the water is ingested without being properly treated. Likewise, the pollutants may originate from a variety of places, including as industrial waste, agricultural runoff, and natural mineral deposits [94].
3.2. Groundwater evolution and water types
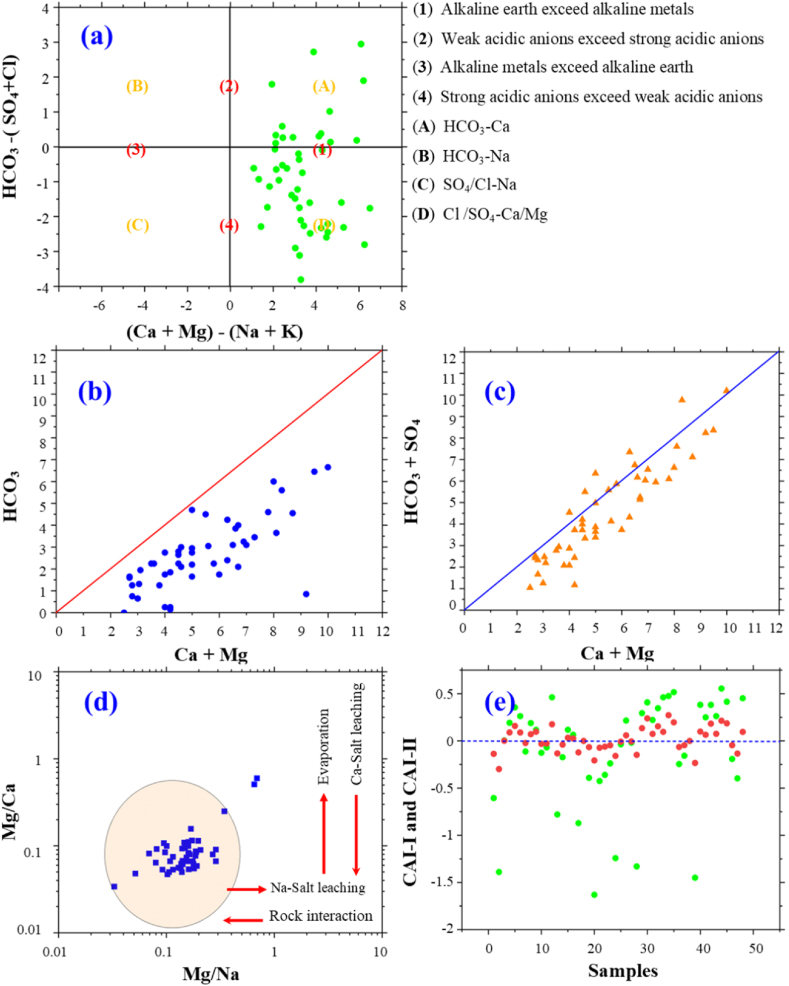
Hydrochemical processes within the aquifer may alter the composition of groundwater, as suggested in the literature [26,36]. Piper's method, as developed in 1944, was employed to elucidate the prevalent ionic species in groundwater specimens [102], as demonstrated in (Fig. 3a). The graphical representation on the Piper chart denotes the proportional ionic makeup of groundwater based on specific cation-anion conjunctions. The diagrammatic representation on the Piper chart further indicates two primary hydrochemical facies within the groundwater, characterized by the Ca–Mg–HCO3 and Ca–Mg–SO4, as depicted by the diamond field on the chart. However, the rest of the samples were under the second sub-area which is represented by the mixed water type. Zones A and B in Fig. 3 show cations, indicating that the research region's groundwater is largely of the Ca2+ and Mg2+ types. Zones F and E often include samples for anions, and these zones are characterized by a high prevalence of the bicarbonate and sulfate types of anions. In the research region, all groundwater samples are plotted in zones where the predominant ions are HCO3−, SO42−, Ca2+, and Mg2+. The cations develop in the following order: Ca2+ > Mg2+ > Na+ > K+. The anions, however, develop in the following ways: HCO3− > SO42− > Cl− > NO3− > CO32−.
Fig. 3.
Classification of geochemical facies and dominant processes as depicted by Piper (a) and Gibbs models (b, c).
According to the classification system established by Piper, the preponderance of water sources within the researched territory predominantly contains higher concentrations of alkaline earth metals, specifically Ca2+ and Mg2+, as well as HCO3−, a weak acid, in contrast to the presence of alkali metals such as Na+ and K+, along with sulfate (SO42−) and chloride (Cl−), which are strong acids. This dominance indicated Ca–Mg–HCO3 water type, followed by the Ca–Mg–SO4 type. These water facies revealed that groundwater in the region was mainly affected by processes involving groundwater rock interaction and weathering [103]. Furthermore, the high concentrations of Ca2+ and Mg2+ implied that the water is in its early stages of development, signifying the release of these ions through the weathering of carbonate minerals. The elevated HCO3− content was indicative of a meteoric water type [104].
By using the Gibbs diagram [105], which involves plotting TDS against the ratios of (Na)/(Na + Ca) and (Cl)/(Cl + HCO3), it's possible to identify the primary processes that govern the geochemistry of the groundwater. Many of the groundwater locations fall within the domains associated with weathering and rock domination, with some samples showing a trend towards evaporation processes, which leads to the plots converging into the evaporation dominance zone (Fig. 3b and c). Nevertheless, the rise in water levels resulting from more effective irrigation and rains, coupled with the shallow depth of the water table in the region, is evident. In addition, rock weathering has become the predominant factor influencing ion concentrations, followed by the rate of evaporation [106]. All of this causes salinity to rise (TDS max = 929.88 mg/L), and as water levels rise, groundwater gets worse and is more impacted by bicarbonate than by evaporation [106].
3.3. Ion exchange processes
To gain a better knowledge of groundwater chemical evolution, Chadha's diagram [107,108] was employed (Fig. 4a). All of the samples in this study fall within the first field (1), within both sub-areas (1A and 1D), where acidic solid anions surpass weak acidic anions and alkaline earths dominate alkali metals (Fig. 4a). The sub-area designated as (A) is characterized by Ca–Mg–HCO3 in its water composition, signifying the dissolution of parent rock material and the influx from overlying aquatic systems. Conversely, the Sub-area labeled (D) is associated with Ca–Mg–SO4/Cl in its hydrochemical profile, suggesting the occurrence of ion exchange in the opposite direction. These findings from Chadha's plot align with the results from the diagram of Piper (Fig. 3), pointing to the presence of water with a permanent hardness that acquires its mineralization through reverse ion exchange [106]. Mineral dissolution appears to be the primary natural process responsible for the primary solutes in natural water [109]. Illustrations presented in Fig. 4b and c delineate the correlation between (Ca2+ + Mg2+) and HCO3−, and between (Ca2+ + Mg2+) and the combined ions of HCO3− and SO42−, respectively, revealing the influence of lithological interactions, such as moderate carbonate dissolution, on the constitution of natural waters within the examined area [21]. These figures also elucidate the effect of cation exchange and its reverse process on the aquifer's chemical characteristics within the area under study. The proximity of most samples to the equiline suggests a prevalence of HCO3−a signature of cation exchange and rock weathering, the predominant processes contributing to the introduction of HCO3− into groundwater [36]. However, carbonates dissolve when water infiltrates carbonate formations in the research area.
Fig. 4.
Predominant ionic constituents and their stoichiometric ratios in the study locale: (a) [HCO3− − (SO42− + Cl−] versus [(Ca2+ + Mg2+) − (Na+ + K+)], (b) [HCO3−] versus [Mg2+ + Ca2+], (c) [Ca2+ + Mg2+] versus [SO42− + HCO3−], (d) [Mg2+/Na+] versus [Mg2+/Ca2+], and (e) CAI-I & CAI-II.
Furthermore, Fig. 4b and c illustrate a strong association between HCO3− and Mg2+/Ca2+. The graphical representation in Fig. 4c demonstrates that most of the data points coalesce around the aquiline when plotting [Ca2+ + Mg2+] against [HCO3− + SO42−], indicative of the solubilization processes of gypsum, anhydrite, and dolomite in groundwater [36,110]. However, the samples that deviate significantly from the 1:1 line indicate alternative sources of HCO3−this might involve agricultural processes, ion exchange reactions, and mineral weathering [88]. During this procedure, the clay minerals' exterior, which once held Ca2+ and Mg2+ ions, underwent ion exchange, whereby Na+ and K+ ions from groundwater took their place. This phenomenon is linked to cation exchange with clay minerals plentiful in the investigated aquifer system [85].
According to the Mg2+/cations pattern in the research region, the groundwater is one rocky zone plot (Fig. 4d). The samples showed average Mg2+/Na+ and Mg2+/Ca2+ ratios and were discovered in the rock-dominated zone (Fig. 4d). The plot illustrates that the primary ions are primarily a result of interactions between water and rock, with evaporation being a minor factor compared to soil-salt leaching, which might be a subdominant process [106]. Investigations revealed that the interaction of groundwater with the aquifer's geological components involved a mechanism identified as chloro-alkaline. In the majority of instances, the CAI indices were negative, notably CAI-I and CAI-II as illustrated in Fig. 4e, suggesting varied locations within the study area underwent distinct cation exchange reactions. Furthermore, concurrent data indicated contrasting CAI readings among some specimens, reflective of a marked inclination for the substitution of K+ and Na+ ions in the groundwater with Mg2+ and Ca2+ from the surrounding mineral strata [103,111].
3.4. Statistical analysis
3.4.1. Cluster analysis (CA)
R-mode has been employed to perform and create CA. These methods have been used for the creation and merging of consistent sets of samples into meaningful clusters, and for assessing spatial similarities and location clustering within the sampling stations [19]. Ward's linkage criterion was utilized for the clustering process (dendrogram) [37]. The CA executed for the chemicals elements on GW samples produces three clusters (Fig. 5a). A total of ten variables were split into two controlled clusters by TDS. Cluster 1 consists of HCO3−, this group demonstrated how bicarbonates separate from other chemical components found in GW, which reflected many origins of this element (carbonated for HCO3−). Cluster 2 includes Ca2+, Mg2+, K+, Na2+, SO42−, Cl−, and it represented the impact of lateral flow from the nearby aquifer and saltwater intrusion. This cluster's SO42− content denotes surface pollutants, agricultural practices such as fertilizer use, and the removal of dissolved minerals from sedimentary rocks. The Ca2+ and Mg2+, which may be characterized by natural processes such as extreme evaporation and weathering of rich minerals, as well as anthropogenic activities such as agricultural practices, sewage activities, and industrial waste water discharge [19]. However, Cluster 3 contains TDS, elucidated by salinity factor due to mineral dissolution. This cluster shows that the salinity of this study area has influenced by the all variables, in particularly by the HCO3−, SO42−, Ca2+, and Mg2+ as the elements which dominants in the study area.
Fig. 5.
Cluster dendrogram for chemicals variables and metals.
Ten variables were separated into two clusters primarily influenced by Total Dissolved Solids (TDS). Cluster 1 comprises HCO3−, revealed that bicarbonates had separated from other chemical components in GW. This dissociation reflected the various sources of this element, particularly from carbonated origins for HCO3−. Cluster 2 includes Ca2+, Mg2+, K+, Na2+, SO42−, and Cl−. It is indicative of the influence of saltwater intrusion and lateral flow from adjacent aquifers. The presence of SO42− in this cluster revealed surface contamination likely originating from agricultural activities, such as fertilizer use, and the leaching of evaporated minerals from sedimentary rocks. Ca2+ and Mg2+ in this cluster may result from natural processes like severe evaporation, mineral weathering, and anthropogenic activities like agricultural practices, sewage, and industrial wastewater discharge. Cluster 3 contains TDS, which is primarily elucidated by the salinity indicator due to mineral or salt dissolution. This cluster demonstrated that TDS (salinity), influenced by HCO3−, SO42−, Ca2+, and Mg2+, which were the dominant elements in the study area. However, there were six primary clustering groups in the CA results for the various examined metals in the research region samples (Fig. 5b). The element of Zn belonged to G1, while Pb was belonged to G2. The G3 consists of V, Cr, Cu and Ni. The fourth group (G4) formed by the Al. The fifth group (G5) was composed by Mn and the last group (G6) consists by the Fe. Despite the existence of hazardous substances and variations in their quantities being identical, the dendrogram demonstrates that there were little Euclidean distances between such groupings [44].
4. Principal Component Analysis (PCA)
Two main tests pf PCA were conducted (Bartlett's test and correlation matrix) to determine efficiency and acceptability to use PCA. The Bartlett's test value (0.000) was lower than 0.05, while the resultant KMO value (0.534) was higher than 0.5. Measures of the data's suitability for factor analysis include the KMO and Bartlett's tests, which assess sample adequacy for each individual variable in the model.
In this study, PCA was used to examine the information from the 48 samples and 10 variables (Fig. 6ab and Table 2). After the analysis statistical, three components have been selected, where it found the correlation between the factor and the parameters greater than 0.5 (>0.5). Results using PCA are more useful when there are fewer components [44]. More than 67.09 % of the overall variance was represented, and Fig. 6ab and Table 2 displays the parameter loading for the 3 PCs from the dataset's PCA. The first factor's expression of variance as a percentage (53.71 %) demonstrated that the sampling process had a reasonable amount of organization due to the interconnectedness of several elements that impact on the sample structures [19]. This was demonstrated by a strong link between Na+, Cl−, Mg2+, K+, SO42−, Ca2+, and salinity (TDS). These connection or correlations reveal the source of the GW salinity.
Fig. 6.
Plots of PCA scores for parameters chemicals (a,b) and metals (c,d,e,f,g,h): (a) F2 vs. F1, (b) F3 vs. F1, (c) F2 vs. F1, (d). F3 vs. F1, (e) F4 vs. F1, (f) F5 vs. F1, (g) F6 vs. F1, and (h). F7 vs. F1.
According to the factorial analysis, factor 1 accounts for 53.71 percent of the variation, with extremely positive loadings in TDS, Ca2+, Na+, Cl−, Mg2+, SO42−, and K+, respectively, of 0.989, 0.888, 0.876, 0.808, 0.775, 0.712, and 0.679. High TDS values revealed several hydrogeochemical processes affecting lithologic factors (mineralized water), which emphasize the ion exchange activities in the water system as well as the weathering of limestone, dolomite, halite, and gypsum as the source of the salinity. Factor 2 contributed about 11.85 % of the total variance of the sample, with significant positive loading in CO32− and negative loading in HCO3−, which were 0.869 and −0.509, respectively (Fig. 6a). This suggests that the element has no impact on the water's total mineralization, and predicts the genesis of HCO3− as a result of weathering carbonates as showing in the above reactions (Eqs. (3), (4), (5)), and might suggest the influence of acid-base equilibrium conditions on GW chemistry. F3 explains 11.53 % of the total variance of the data set and showed a strong positive coefficient for NO3− (0.893) (Fig. 6b). Therefore, F3 is associated with chemical fertilizers, and organic pollution from domestic wastewater. In addition, the effluent from household wastewater treatment facilities, which generated biodegradable organic pollutants and nutrients, in addition to industrial effluents, introducing organic and inorganic detritus, were identified as sources of NO3− pollution in the research region [88].
On the other hand, the PCA of the metals demonstrated many PCA explaining in total 85.84 % of the variance (Table 3 and Fig. 6 c, d, e, f, g, h). In total seven factors, F1, F2, F3, F4, F5, F6, F7 explains 16.28, 15.09, 14.47, 11.62, 11.13, 9.44, 7.88 % respectively. Where, the F1 represented by the Cu (−0.619) and V (0.695). The F2 represented by Fe (−0.658) and Al (−0.670), this factor showed significant loading of Fe and Al in the fundamental components, indicating that these metals are sourced from anthropogenic sources or exist naturally in sediment with a distribution pattern controlled via iron oxides and clay minerals. The F3 represented by Mn (−0.602) and Ni (−0.588). The F4 represented by Cr (0.745). F5 represented by Cd (−0.884). F6 represented by Zn (0.525). And F7 represented by Pb (−0.594). These findings can thus be linked to the interplay between soil and water, industrial operations, human behaviors, and the natural dispersion of clay minerals in sediment could result in individual metal pollution of GW networks, particularly the large loadings of Fe, Mn, Al, and Pb.
4.1. Groundwater quality assessment
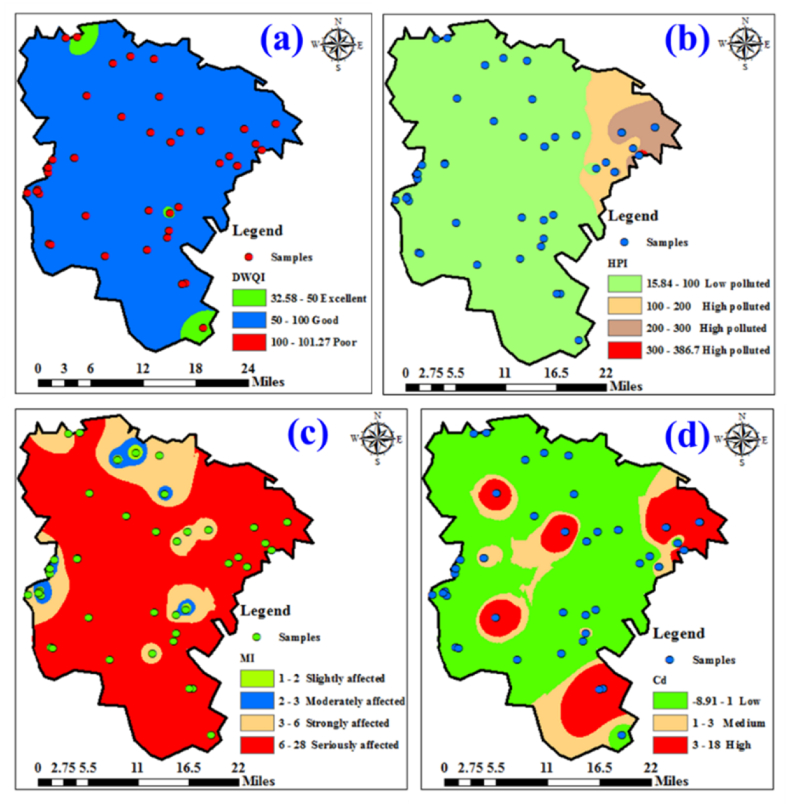
WQIs are essential instruments for evaluating water potability, with a particular emphasis on its metallic constituents. Descriptive statistical summaries for the HPI, DWQI, MI, and Cd indices are encapsulated in Table 4. The DWQI figures spanned a spectrum from 32.54 to 101.31, averaging at 61.88. The analysis delineated that a mere 10.4 % of groundwater specimens were rated as superior in quality, 87.5 % were deemed to be of satisfactory quality, and a small fraction, 2 %, were considered as substandard for consumption. Cartographical representation of the DWQI divulged that the majority of groundwater specimens, notably in the northern and southern segments of the study zone, conformed to the criteria for consumable water. Contrastingly, specific locales were pinpointed as having compromised water quality, as depicted in Fig. 7a. Spanning a spectrum, the values for HPI oscillated between a minimum of 15.75 and a maximum of 387.02, while the mean settled at 76.64. About 18.75 % of samples exceeded the permissible HPI value (HPI>100), indicating a high level of pollution in the water resources, while 81.25 % remained within the standard limit (HPI<100), suggesting low pollution levels with metals. The distribution map highlighted that the area of study's easternmost region exhibited high levels of metal pollution, requiring more attention from decision-makers to implement further treatment of water resources in that region (Fig. 7b). The values of MI observed in the groundwater specimens fluctuated between a minimum of 1.06 and a maximum of 27.60, with a mean established at 9.33. These values indicate that 22.9 % of water samples were slightly affected, 8.33 % moderately affected, 12.5 % strongly affected, and metals adversely impacted 56.25 %. Investigating the spatial representation of MI across the research zone reveals that limited sectors in the northern and western segments displayed minor to intermediate MI intensities (Fig. 7c). Conversely, a considerable expanse of the research zone is markedly contaminated by metallic substances, suggesting that the water quality in these regions is below potable standards and necessitates purification prior to use. The Cd was determined by assessing specific metal contamination factors above acceptable limits. The values of Cd have been spatially distributed on a map based on the selected sampling locations (Fig. 7d). The Cd of the groundwater samples fell between −8.94 and 17.60, with an average value of −0.67. The findings of Cd value showed that 66.6 % of the water samples are less than 1 (Cd < 1), which refers to good water quality regarding the investigated metals. The remaining samples indicated a medium contamination degree in 6.25 % of water samples and high contamination in 27.08 % of the collected samples concerning the investigated metals (Cd > 1).
Fig. 7.
Spatial representation of quality indices in groundwater Networks: (a) DWQI, (b) HPI, (c) MI, and (d) Cd.
The impact of pollution on groundwater, particularly concerning metals, was assessed using PI values. These values represent unique contribution of each metal to contamination in terms of groundwater grade and are classified into five types. The PI results showed three groups of metal effects (Table 5s). Specifically, the PI values showed that the groundwater samples were significantly affected by Fe with a PI of 4.54, severely affected by Mn with a PI of 12.32, and seriously affected by Pb with a PI of 10.42. In contrast, other metals did not exhibit a significant effect. Increased concentrations of Fe, Pb, and Mn could have resulted from the interplay between terrestrial and aquatic systems. The WQIs, including DWQI, HPI, MI, and Cd were applied to evaluate whether water is suitable for drinking, with a specific focus on metal concentrations. These indices provide a robust framework for evaluating water quality. Notably, the DWQI values exhibited a wide range, indicating varying levels of water quality. Importantly, the spatial distribution map revealed that most groundwater samples were suitable for drinking. Nevertheless, some localized degradation of water quality was noted. Furthermore, the study employed the HPI to identify areas with high metal pollution, emphasizing the need for focused attention and treatment of water resources. Additionally, the PIs were used to assess the suitability of water for drinking concerning metals. The results revealed significant variability in the degree of contamination, with some areas being seriously affected by metals, calling for immediate action to address water quality concerns. This research is distinguished by its incorporation of various indices, broad spatial analysis, and targeted examination of metallic pollutants in groundwater, all of which underpin its originality. The study provides an all-encompassing review of water purity and pollutants, presenting crucial information that can guide policymakers and those involved in the stewardship of water resources.
Table 5.
Evaluation of RNN model performance for measured parameters: DWQI, HPI, MI, and Cd, as well as HI values (dermal and oral) for both adults and children.
| Indices | Performance |
||||
|---|---|---|---|---|---|
| R2 | RMSE | MAD | E | ||
| Training Series | DWQI | 1.00 | 0.01 | 0.00 | 1.00 |
| HPI | 1.00 | 0.01 | 0.01 | 1.00 | |
| MI | 1.00 | 0.00 | 0.00 | 1.00 | |
| Cd | 1.00 | 0.00 | 0.00 | 1.00 | |
| HI oral children | 1.00 | 0.00 | 0.00 | 1.00 | |
| HI oral adults | 1.00 | 0.00 | 0.00 | 1.00 | |
| HI dermal children | 1.00 | 0.00 | 0.00 | 1.00 | |
| HI dermal children | 1.00 | 0.00 | 0.00 | 1.00 | |
| Testing Series | DWQI | 0.96 | 2.37 | 0.86 | 0.96 |
| HPI | 0.95 | 27.35 | 11.80 | 0.93 | |
| MI | 0.93 | 2.59 | 0.95 | 0.90 | |
| Cd | 0.94 | 1.77 | 0.55 | 0.93 | |
| HI oral children | 0.96 | 0.74 | 0.21 | 0.96 | |
| HI oral adults | 0.95 | 0.18 | 0.05 | 0.94 | |
| HI dermal children | 0.96 | 0.05 | 0.03 | 0.95 | |
| HI dermal children | 0.96 | 0.02 | 0.01 | 0.96 | |
4.2. Assessing health risk
Investigations were conducted on the potential health risks associated with metal exposure, focusing on individuals of varying ages, encompassing both minors and adults. The Minimum HQ oral (adult) values of Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn are 0.036, 0.003, 7.53E-05, 0.0001, 0.0014, 0.0002, 0.0063, and 6.03E-05, while the maximum HQ oral (adult) values are 0.115, 0.258, 0.017, 0.117, 3.09, 0.028, 2.6, and 0.1, respectively. The current findings demonstrated that HQ oral (adult) of Pb and Mn exceeded the permissible limit (HQ > 1) in 16.66 % of the water samples, whereas the hazard quotients of the other metals did not exceed the risk limit (HQ < 1) (Fig. 8a). The Minimum HQ oral (child) values of Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were 0.13, 0.012, 0.0003, 0.0004, 0.005, 0.0006, 0.024, and 0.0002, while the maximum HQ oral (child) values were 0.43, 0.98, 0.06, 0.44, 11.8, 0.1, 9.9, and 0.4, respectively. The results showed that HQ oral (child) of Pb and Mn exceeded the permissible limit (HQ > 1) in 20.8 % and 43.7 % of the water samples, respectively. In contrast, the hazard quotient of the other metals did not exceed the risk limit (HQ < 1) (Fig. 8b). The maximum HQ dermal (adult) values were 0.01, 0.1, 0.0003, 0.003, 0.37, 0.0007, 0.007, and 0.002, respectively. The results showed that the dermal Hazard Quotient (HQ) for adults for all of the metals did not exceed the permissible limits (HQ < 1) in all water samples, which revealed that there was risk from the metals through the dermal contact for adults (Fig. 8c). The Minimum HQ dermal (child) values of Cd, Cr, Cu, Fe, Mn, Ni, Pb, and Zn were 0.01, 0.003, 3.52E-06, 7.23E-06, 0.0005, 1.05E-05, 5.02E-05, and 2.53E-06, while the maximum HQ dermal (child) values were 0.03, 0.3, 0.0008, 0.008, 1.08, 0.002, 0.02, and 0.004, respectively (Fig. 8d). The results showed that there is no risk for a child through dermal contact with all investigated metals (HQ < 1) except two samples that showed high risk (HQ > 1) regarding Mn only. The HQ values revealed that Pb and Mn are the most common parameters in the study area and have noncarcinogenic risk effects on human health through oral and dermal contact, especially for children. These findings reflect the importance of further treatment of the water resources for Pb and Mn.
Fig. 8.
Box plot of the HQ oral (adult) (a), HQ oral (child) (b), HQ dermal (adult) (c), and HQ dermal (child) (d).
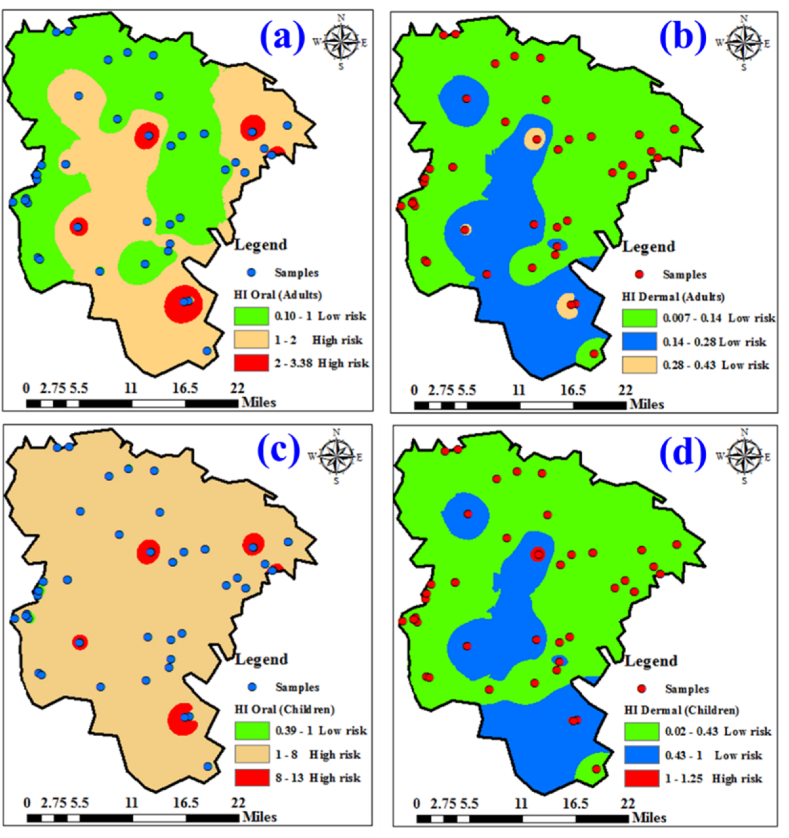
The range of HI values for adults, both minimum and maximum, for the metals under investigation encompassed 0.1 and 3.39, respectively, with a corresponding mean HI of 1.04 (Table 4). In contrast, the Hazard Index exhibited a broader range for children, fell between 0.39 and 12.95, with an average HI value of 3.97. The results showed that HI oral (adult) exceeded the permissible limit (HI > 1) in 35.4 % of the water samples, while the hazard index in 64.6 % of the samples did not exceed the risk limit (HI < 1) (Fig. 9a). In the case of a child, the HI oral values are greater than 1 (high risk) in 75 % of the water samples, and 25 % showed low risk with an HI value of less than 1 (Fig. 9b). From the distribution map of HI, the central and eastern part of the study area is more vulnerable to the noncarcinogenic health risk from the investigated metals through oral contact for children and adults. On the other hand, the HI values through dermal contact in adults fell between 0.007 and 0.42 with a mean value of 0.1, revealing no health risk effect from the dermal contact for adults (Fig. 9c). The lowest and highest HI dermal (child) values were 0.02 and 1.26, respectively, with mean value of 0.31. In case of child, the HI dermal values were greater than 1 in 6.25 % of the water samples, which indicated high health risk of metals while, 93.75 % of samples showed low risk with HI value less than 1 (Fig. 9d).
Fig. 9.
Distribution maps of Hazard index (HI) in adult (oral) (a), adult (dermal (b), child (oral) (c), and child (dermal) (d).
Based on the distribution map of HI, it is evident that the central region of the research area exhibits a higher susceptibility to noncarcinogenic health risks stemming from the analyzed metals through dermal exposure in children. The present investigation strongly advocates for additional treatment of water within central study region, particularly focusing on mitigating potential contamination from Pb and Mn. The current water quality in this zone possesses the potential to impose health hazards upon children, given their elevated water consumption relative to body weight in comparison to adults.
4.3. RNN simulation model
The most significant limitation of the present quality assessment methodology is its reliance on a profound understanding of weighting variables for determining scores associated with DWQI, HPI, MI, Cd, and HI, making the outcome somewhat ambiguous [112]. These indices are derived by combining multiple physicochemical parameters into one or single numerical value. Researchers have worked on reducing the subjectivity in these traditional methods, leading to the creation of more precise tools. One such advancement involves using entropy-based ion weights, which provides a more reliable measurement system for evaluating water quality. Nevertheless, it is essential to note that water quality research demands extensive data collection, laboratory analysis, data management, and rigorous testing procedures [113].
To simulate HPI, MI, Cd, and HI using the RNN, a data set containing ten input parameters and one output was provided (Fig. 2). Experimentation and iterative refinement are employed for neurons number determination in both the hidden layer and contextual units. Specifically, it was observed that configuring 120 neurons in the hidden layer along with 120 context units yielded the most optimal training outcomes. Nonetheless, when simulating the DWQI data set, which encompasses 12 input parameters (Fig. 10) and a single output variable, an increased configuration of 144 neurons in the hidden layer, accompanied by 144 context units, was the suitable choice. RNN was used to generate the rules in order to forecast the intended result. Thirty percent (30 %) of the available data were used for validation after the RNN was calibrated using seventy percent of the data. The relationships between the calculated DWQI, HPI, MI, Cd, and HI with RNN output for the training validation phases of the data set are displayed in Table 5, confirming the RNN models' ability to predict all indices with reasonable accuracy. To measure the performance of the developed models, the four statistical indicators used included the absolute variance fraction, R2, MAE, RMSE, and NSE. Table 5 and Fig. 10 a, b, c, d, e, f, g showed that the model performance assessment produced excellent findings for training and testing sets. Large R2 values indicate a considerable relationship between measured and simulated values.
Fig. 10.
Compares the training and testing series for (a) HPI, (b) MI, (c) Cd, (d) DWQI, (e, f) HI oral values for both adults and children, and (g, h) hazard index (HI dermal) values for both adults and children using the developed RNN models.
The RNN model exhibited remarkable proficiency in predicting WQIs, with approximately three essential parameters inferred from the HG dataset. The R2 values (training and testing datasets) stood at 1.00, anf RMSE values of 0.01 and 2.73, respectively. About the HPI, the RNN model demonstrated exceptional performance as the primary predictor. It displayed R2 values of 1.00 with an RMSE of 0.01 for the training set and an R2 value of 0.93 with an RMSE of 27.35 for the testing set. HI, oral children predictions found RNN to be the most accurate model, exhibiting R2 values of 1.00 and 0.96 and RMSE values of 0.00 and 0.74 for training and testing sets, respectively. The RNN's exceptional accuracy indicates it is a valuable tool for DWQI HPI, MI, Cd, and HI prediction.
5. Comparison with previous studies and recommendation for future work
Several studies employed different types of methodologies and indexing approaches to evaluate the risk associated with different toxic metals. Eid [26] applied Monte Carlo method for health risk assessment to predict CR and NCR (carcinogenic and non-carcinogenic risks) and reported that groundwater in Siwa Oasis (Egypt) is contaminated with several toxic metals concluding Cd, Pb, and Cr which threaten the health of children and adults. Saeed [46,67] applied ecological risk and environmental risk indices (RI and HPI) regarding PTEs in Danube River (Hungary) and reported low to medium risk of Fe, Mn, Cr, Pb, and As. Gad and Ata [40,114] used prediction models such as ANN to predict DWQI, and HI indices to predict the health risk regarding Fe and Mn in groundwater of El-Kharga Oasis, Egypt and reported that the children are vulnerable for oral exposure to PTEs. In this research,
Undoubtedly, there are some limitations in the present study, including the lack of water level data. This can be addressed in future work to determine the effects of over-extraction of water and anthropogenic activities. As an example, this could involve using contaminant transport models and stable isotopes for contaminant source detection. Further research can include Monte Carlo simulation to decrease uncertainty and increase reliability of the environmental and health risks as a perspective.
6. Conclusion
In this study, groundwater samples were taken from the Quaternary aquifer in El-Menoufia governorate (Egypt's Middle Delta). These samples were tested for physicochemical parameters and heavy metals, including Cd, Al, Cr, Fe, Cu, Pb, Ni, V, Mn, and Zn contents. Additionally, these heavy metals' regional distribution patterns, pollution levels, and health concerns were investigated. The investigation revealed the prevalence of Ca–Mg–HCO3, Ca–Mg–SO4, and mixed Ca–Mg–Cl–SO4 water facies in the Quaternary aquifer, impacted by ion exchange procedures, rock-water interactions, and silicate weathering.
Concerning metals, mainly Pb and Mn, the groundwater displayed elevated concentrations exceeding WHO drinking water standards. Most samples were deemed drinkable by the DWQI, primarily in the 'good class,' while 2 % fell into the 'poor water' category. Notably, the HPI, MI, and Cd highlighted the significant pollution of Groundwater, primarily concentrated in the central region, with Pb, Mn, and Fe being the major contributors to this pollution, falling between the 'strongly affected' and 'seriously affected' categories.
The findings of the health risk assessment showed a heightened noncarcinogenic health risk (Child) through oral and dermal contact. Similarly, the central study area reported a high risk for adults through oral contact and a lower risk through skin exposure.
Furthermore, the implementation of RNN models yielded favorable results, demonstrating the model's proficiency in predicting water quality and health indices. The RNN model excelled in predicting DWQI, with three key parameters being crucial. The R2 was 1.00 with an RMSE of 0.01 (training) and 0.96 with an RMSE of 2.73 (testing). Regarding HPI, the RNN model performed exceptionally well as the primary predictor, with R2 values of 1.00 (RMSE = 0.01) and 0.93 (RMSE = 27.35) for the training and testing sets, respectively.
The application of diverse indexing approaches, including the DWQI, HPI, MI, PI, Cd, HQ, and HI, supported by advanced techniques such as multivariate analysis, RNN models, and GIS techniques, has provided valuable insights into groundwater quality. The current findings suggest further water treatment of heavy metals in groundwater before use to protect human health in the El-Menoufia governorate and regularly monitoring the concentration. The current study and methodology could be applied in global study to investigate and predict water quality and its risk impact.
Funding
The current research work has been funded by the sustainable development and technologies national program of the Hungarian Academy of Sciences (FFT NP FTA). Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R400), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional review board statement
Not applicable.
Informed consent statement
Not applicable.
Data availability statement
All data are provided as tables and figures.
CRediT authorship contribution statement
Mohamed Gad: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Conceptualization. Aissam Gaagai: Writing – review & editing, Writing – original draft, Visualization, Software, Data curation, Conceptualization. Asmaa A. Agrama: Writing – review & editing, Writing – original draft, Validation, Resources, Investigation, Formal analysis. Walaa F.M. El-Fiqy: Writing – review & editing, Writing – original draft, Resources, Investigation, Formal analysis, Data curation. Mohamed Hamdy Eid: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology, Conceptualization. Péter Szűcs: Writing – review & editing, Writing – original draft, Visualization, Software. Salah Elsayed: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Conceptualization. Osama Elsherbiny: Writing – review & editing, Writing – original draft, Visualization, Validation, Software. Mosaad Khadr: Writing – review & editing, Visualization, Validation, Software, Data curation, Conceptualization. Mostafa R. Abukhadra: Writing – review & editing, Writing – original draft, Visualization, Software, Methodology. Haifa E. Alfassam: Writing – review & editing, Visualization, Software, Funding acquisition, Data curation. Stefano Bellucci: Writing – review & editing, Validation, Software. Hekmat Ibrahim: Writing – original draft, Software, Resources, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The current research work has been funded by the sustainable development and technologies national program of the Hungarian Academy of Sciences (FFT NP FTA). The authors acknowledge Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2024R400), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e36606.
Contributor Information
Mohamed Gad, Email: mohamed.gad@esri.usc.edu.eg.
Aissam Gaagai, Email: gaagaiaissam37@gmail.com.
Asmaa A. Agrama, Email: asmaaagrama_new2012@yahoo.com.
Walaa F.M. El-Fiqy, Email: walaa_elfiqy@yahoo.com.
Mohamed Hamdy Eid, Email: mohamed.hemida@uni-miskolc.hu.
Péter Szűcs, Email: hgszucs@uni-miskolc.hu.
Salah Elsayed, Email: salah.emam@esri.usc.edu.eg.
Osama Elsherbiny, Email: osama_algazeery@mans.edu.eg.
Mosaad Khadr, Email: mosaad.khadr@f-eng.tanta.edu.eg.
Mostafa R. Abukhadra, Email: abukhadra89@science.bsu.edu.eg.
Stefano Bellucci, Email: stefano.bellucci@lnf.infn.it.
Hekmat Ibrahim, Email: hekmat.abdulah@science.menofia.edu.eg.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Liu J., Gao M., Jin D., Wang T., Yang J. Assessment of groundwater quality and human health risk in the aeolian-sand area of yulin city, northwest China. Expo Health. 2020;12:671–680. doi: 10.1007/s12403-019-00326-8. [DOI] [Google Scholar]
- 2.Snousy M.G., Wu J., Su F., Abdelhalim A., Ismail E. Groundwater quality and its regulating geochemical processes in Assiut province, Egypt. Expo Health. 2022;14:305–323. doi: 10.1007/s12403-021-00445-1. [DOI] [Google Scholar]
- 3.Szűcs P., Dobróka M., Turai E., Szarka L., Ilyés C., Eid M.H., Szabó N.P. Combined inversion and statistical workflow for advanced temporal analysis of the Nile River's long term water level records. J. Hydrol. 2024;630 doi: 10.1016/j.jhydrol.2024.130693. [DOI] [Google Scholar]
- 4.Salam M.A., Adlii A., Eid M.H., Abukhadra M.R. Effective decontamination of Ca2+ and Mg2+ hardness from groundwater using innovative muscovite based sodalite in batch and fixed-bed column studies; dynamic and equilibrium studies. J. Contam. Hydrol. 2021;241 doi: 10.1016/j.jconhyd.2021.103817. [DOI] [PubMed] [Google Scholar]
- 5.Abukhadra M.R., Eid M.H., El-Sherbeeny A.M., Abd Elgawad A.E.E., Shim J.-J. Effective desalination of brackish groundwater using zeolitized diatomite/kaolinite geopolymer as low-cost inorganic membrane; Siwa Oasis in Egypt as a realistic case study. J. Contam. Hydrol. 2022;244 doi: 10.1016/j.jconhyd.2021.103923. [DOI] [PubMed] [Google Scholar]
- 6.Bin Jumah M.N., Eid M.H., AL-Huqail A.A., Mohammad M.A., Bin-Murdhi N.S., Abu-Taweel G.M., Altoom N., Allam A.A., AbuKhadra M.R. Enhanced remediation of as (V) and Hg (II) ions from aqueous environments using β-cyclodextrin/MCM-48 composite: batch and column studies. Journal of Water Process Engineering. 2021;42 doi: 10.1016/j.jwpe.2021.102118. [DOI] [Google Scholar]
- 7.AbuKhadra M.R., Eid M.H., Allam A.A., Ajarem J.S., Almalki A.M., Salama Y. Evaluation of different forms of Egyptian diatomite for the removal of ammonium ions from Lake Qarun: a realistic study to avoid eutrophication. Environmental Pollution. 2020;266 doi: 10.1016/j.envpol.2020.115277. [DOI] [PubMed] [Google Scholar]
- 8.Szabó N.P., Ilyés C., Turai E., Dobróka M., Kilik R., Miklós R., Eid M.H., Szücs P. 2023 14th IEEE International Conference on Cognitive Infocommunications (CogInfoCom) IEEE; Budapest, Hungary: 2023. Fourier transformation and unsupervised learning for extracting hydrogeological information from time series data. 000019–000024. [DOI] [Google Scholar]
- 9.Abukhadra M.R., Eid M.H., El-Meligy M.A., Sharaf M., Soliman A.T. Insight into chitosan/mesoporous silica nanocomposites as eco-friendly adsorbent for enhanced retention of U (VI) and Sr (II) from aqueous solutions and real water. Int. J. Biol. Macromol. 2021;173:435–444. doi: 10.1016/j.ijbiomac.2021.01.136. [DOI] [PubMed] [Google Scholar]
- 10.Bellucci S., Eid M.H., Fekete I., Péter S., Kovács A., Othman S.I., Ajarem J.S., Allam A.A., Abukhadra M.R. Synthesis of K+ and Na+ synthetic sodalite phases by low-temperature alkali fusion of kaolinite for effective remediation of phosphate ions: the impact of the alkali ions and realistic studies. Inorganics. 2022;11:14. doi: 10.3390/inorganics11010014. [DOI] [Google Scholar]
- 11.Eid M.H., Tamma A.A., Saeed O., Székács A., Abukhadra M.R., El-Sherbeeny A.M., Bence C., Mikita V., Kovács A., Szűcs P. Advanced approach combines integrated weight water quality index and potential toxic elements for environmental and health risk assessment supported by simulation technique in Oued Souf, Algeria. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-68854-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeed O., Székács A., Jordán G., Mörtl M., Abukhadra M.R., El-Sherbeeny A.M., Szűcs P., Eid M.H. Assessing surface water quality in Hungary's Danube basin using geochemical modeling, multivariate analysis, irrigation indices, and Monte Carlo simulation. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-69312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J., Han G. Distributions and source identification of the major ions in zhujiang river, southwest China: examining the relationships between human perturbations, chemical weathering, water quality and health risk. Expo Health. 2020;12:849–862. doi: 10.1007/s12403-020-00343-y. [DOI] [Google Scholar]
- 14.Li P. To make the water safer. Expo Health. 2020;12:337–342. doi: 10.1007/s12403-020-00370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salameh M.T.B., Alraggad M., Harahsheh S.T. The water crisis and the conflict in the Middle East. Sustain. Water Resour. Manag. 2021;7:69. doi: 10.1007/s40899-021-00549-1. [DOI] [Google Scholar]
- 16.Gad M., Gaagai A., Eid M.H., Szűcs P., Hussein H., Elsherbiny O., Elsayed S., Khalifa M.M., Moghanm F.S., Moustapha M.E., Tolan D.A., Ibrahim H. Groundwater quality and health risk assessment using indexing approaches, multivariate statistical analysis, artificial neural networks, and GIS techniques in El kharga Oasis, Egypt. Water. 2023;15:1216. doi: 10.3390/w15061216. [DOI] [Google Scholar]
- 17.Eid M.H., Elbagory M., Tamma A.A., Gad M., Elsayed S., Hussein H., Moghanm F.S., Omara A.E.-D., Kovács A., Péter S. Evaluation of groundwater quality for irrigation in deep aquifers using multiple graphical and indexing approaches supported with machine learning models and GIS techniques, souf valley, Algeria. Water. 2023;15:182. doi: 10.3390/w15010182. [DOI] [Google Scholar]
- 18.Keesari T., Roy A., Pant D., Sinha U.K., Kumar P.V.N., Rao L.V. Major ion, trace metal and environmental isotope characterization of groundwater in selected parts of Uddanam coastal region, Andhra Pradesh, India. J. Earth Syst. Sci. 2020;129:205. doi: 10.1007/s12040-020-01467-0. [DOI] [Google Scholar]
- 19.Katla S., Gugulothu S., Dhakate R. Spatial assessment of major ion geochemistry in the groundwater around Suryapet Region, Southern Telangana, India. Environmental Sustainability. 2021;4:107–122. doi: 10.1007/s42398-020-00148-4. [DOI] [Google Scholar]
- 20.Gad M., Dahab K., Ibrahim H. Applying of a geochemical model on the nubian sandstone aquifer in Siwa Oasis, western desert, Egypt. Environ. Earth Sci. 2018;77:401. doi: 10.1007/s12665-018-7580-6. [DOI] [Google Scholar]
- 21.Gaagai A., Aouissi H., Bencedira S., Hinge G., Athamena A., Heddam S., Gad M., Elsherbiny O., Elsayed S., Eid M., Ibrahim H. Application of water quality indices, machine learning approaches, and GIS to identify groundwater quality for irrigation purposes: a case study of sahara aquifer, doucen plain, Algeria. Water. 2023;15:289. doi: 10.3390/w15020289. [DOI] [Google Scholar]
- 22.Wu J., Sun Z. Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health. 2016;8:311–329. doi: 10.1007/s12403-015-0170-x. [DOI] [Google Scholar]
- 23.Aithani D., Jyethi D.S., Siddiqui Z., Yadav A.K., Khillare P.S. Source apportionment, pollution assessment, and ecological and human health risk assessment due to trace metals contaminated groundwater along urban river floodplain. Groundwater for Sustainable Development. 2020;11 doi: 10.1016/j.gsd.2020.100445. [DOI] [Google Scholar]
- 24.Benson N.U., Enyong P.A., Fred-Ahmadu O.H. Trace metal contamination characteristics and health risks assessment of Commelina africana L. And psammitic sandflats in the Niger delta, Nigeria. Applied and Environmental Soil Science. 2016;2016:1–14. doi: 10.1155/2016/8178901. [DOI] [Google Scholar]
- 25.Varghese J., Jaya D.S. Metal pollution of groundwater in the vicinity of valiathura sewage farm in Kerala, south India. Bull. Environ. Contam. Toxicol. 2014;93:694–698. doi: 10.1007/s00128-014-1410-7. [DOI] [PubMed] [Google Scholar]
- 26.Eid M.H., Eissa M., Mohamed E.A., Ramadan H.S., Tamás M., Kovács A., Szűcs P. New approach into human health risk assessment associated with heavy metals in surface water and groundwater using Monte Carlo Method. Sci. Rep. 2024;14:1008. doi: 10.1038/s41598-023-50000-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh R., Venkatesh A.S., Syed T.H., Reddy A.G.S., Kumar M., Kurakalva R.M. Assessment of potentially toxic trace elements contamination in groundwater resources of the coal mining area of the Korba Coalfield, Central India. Environ. Earth Sci. 2017;76:566. doi: 10.1007/s12665-017-6899-8. [DOI] [Google Scholar]
- 28.He X., Li P., Ji Y., Wang Y., Su Z., Elumalai V. Groundwater arsenic and fluoride and associated arsenicosis and fluorosis in China: occurrence, distribution and management. Expo Health. 2020;12:355–368. doi: 10.1007/s12403-020-00347-8. [DOI] [Google Scholar]
- 29.Li P., Wu J., Qian H., Lyu X., Liu H. Origin and assessment of groundwater pollution and associated health risk: a case study in an industrial park, northwest China. Environ. Geochem. Health. 2014;36:693–712. doi: 10.1007/s10653-013-9590-3. [DOI] [PubMed] [Google Scholar]
- 30.Yadav K.K., Kumar S., Pham Q.B., Gupta N., Rezania S., Kamyab H., Yadav S., Vymazal J., Kumar V., Tri D.Q., Talaiekhozani A., Prasad S., Reece L.M., Singh N., Maurya P.K., Cho J. Fluoride contamination, health problems and remediation methods in Asian groundwater: a comprehensive review. Ecotoxicol. Environ. Saf. 2019;182 doi: 10.1016/j.ecoenv.2019.06.045. [DOI] [PubMed] [Google Scholar]
- 31.Rutchik J.S., Zheng W., Jiang Y., Mo X. How does an occupational neurologist assess welders and steelworkers for a manganese-induced movement disorder? An international team's experiences in guanxi, China Part II. Journal of Occupational & Environmental Medicine. 2012;54:1562–1564. doi: 10.1097/JOM.0b013e318216d01b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snousy M.G., Wu J., Su F., Abdelhalim A., Ismail E. Groundwater quality and its regulating geochemical processes in Assiut province, Egypt. Expo Health. 2022;14:305–323. doi: 10.1007/s12403-021-00445-1. [DOI] [Google Scholar]
- 33.Chakraborty B., Roy S., Bera B., Adhikary P.P., Bhattacharjee S., Sengupta D., P.K. Shit Evaluation of groundwater quality and its impact on human health: a case study from Chotanagpur plateau fringe region in India. Appl. Water Sci. 2022;12:25. doi: 10.1007/s13201-021-01539-6. [DOI] [Google Scholar]
- 34.Chen J., Gao Y., Qian H., Ren W., Qu W. Hydrogeochemical evidence for fluoride behavior in groundwater and the associated risk to human health for a large irrigation plain in the Yellow River Basin. Sci. Total Environ. 2021;800 doi: 10.1016/j.scitotenv.2021.149428. [DOI] [PubMed] [Google Scholar]
- 35.Al-Mashreki M.H., Eid M.H., Saeed O., Székács A., Szűcs P., Gad M., Abukhadra M.R., AlHammadi A.A., Alrakhami M.S., Alshabibi M.A., Elsayed S., Khadr M., Farouk M., Ramadan H.S. Integration of geochemical modeling, multivariate analysis, and irrigation indices for assessing groundwater quality in the Al-jawf basin, Yemen. Water. 2023;15:1496. doi: 10.3390/w15081496. [DOI] [Google Scholar]
- 36.Eid M.H., Eissa M., Mohamed E.A., Ramadan H.S., Czuppon G., Kovács A., Szűcs P. Application of stable isotopes, mixing models, and K-means cluster analysis to detect recharge and salinity origins in Siwa Oasis, Egypt. Groundwater for Sustainable Development. 2024;25 doi: 10.1016/j.gsd.2024.101124. [DOI] [Google Scholar]
- 37.Eid M.H., Elbagory M., Tamma A.A., Gad M., Elsayed S., Hussein H., Moghanm F.S., Omara A.E.-D., Kovács A., Péter S. Evaluation of groundwater quality for irrigation in deep aquifers using multiple graphical and indexing approaches supported with machine learning models and GIS techniques, souf valley, Algeria. Water. 2023;15:182. doi: 10.3390/w15010182. [DOI] [Google Scholar]
- 38.Flores Y.G., Eid M.H., Szűcs P., Szőcs T., Fancsik T., Szanyi J., Kovács B., Markos G., Újlaki P., Tóth P., McIntosh R.W., Püspöki Z. Integration of geological, geochemical modelling and hydrodynamic condition for understanding the geometry and flow pattern of the aquifer system, southern nyírség–hajdúság, Hungary. Water. 2023;15:2888. doi: 10.3390/w15162888. [DOI] [Google Scholar]
- 39.Gaagai A., Aouissi H., Bencedira S., Hinge G., Athamena A., Heddam S., Gad M., Elsherbiny O., Elsayed S., Eid M., Ibrahim H. Application of water quality indices, machine learning approaches, and GIS to identify groundwater quality for irrigation purposes: a case study of sahara aquifer, doucen plain, Algeria. Water. 2023;15:289. doi: 10.3390/w15020289. [DOI] [Google Scholar]
- 40.Gad M., Gaagai A., Eid M.H., Szűcs P., Hussein H., Elsherbiny O., Elsayed S., Khalifa M.M., Moghanm F.S., Moustapha M.E., Tolan D.A., Ibrahim H. Groundwater quality and health risk assessment using indexing approaches, multivariate statistical analysis, artificial neural networks, and GIS techniques in El kharga Oasis, Egypt. Water. 2023;15:1216. doi: 10.3390/w15061216. [DOI] [Google Scholar]
- 41.Hamdy Eid M., Szűcs P., Kovács A. Problems threatening sustainability in Siwa Oasis and recommendations for understanding the sources of water quality deterioration. Geosciences and Engineering. 2022;10:138–153. doi: 10.33030/geosciences.2022.15.138. [DOI] [Google Scholar]
- 42.Li L., Wang Y., Wu Y., Li J. Major geochemical controls on fluoride enrichment in groundwater: a case study at Datong Basin, northern China. J. Earth Sci. 2013;24:976–986. doi: 10.1007/s12583-013-0385-3. [DOI] [Google Scholar]
- 43.Jing X., Yang H., Cao Y., Wang W. Identification of indicators of groundwater quality formation process using a zoning model. J. Hydrol. 2014;514:30–40. doi: 10.1016/j.jhydrol.2014.03.059. [DOI] [Google Scholar]
- 44.Zhang X., Miao J., Hu B.X., Liu H., Zhang H., Ma Z. Hydrogeochemical characterization and groundwater quality assessment in intruded coastal brine aquifers (Laizhou Bay, China) Environ. Sci. Pollut. Res. 2017;24:21073–21090. doi: 10.1007/s11356-017-9641-x. [DOI] [PubMed] [Google Scholar]
- 45.Salem S., Gaagai A., Ben Slimene I., Moussa A., Zouari K., Yadav K., Eid M., Abukhadra M., El-Sherbeeny A., Gad M., Farouk M., Elsherbiny O., Elsayed S., Bellucci S., Ibrahim H. Applying multivariate analysis and machine learning approaches to evaluating groundwater quality on the kairouan plain, Tunisia. Water. 2023;15:3495. doi: 10.3390/w15193495. [DOI] [Google Scholar]
- 46.Saeed O., Székács A., Jordán G., Mörtl M., Abukhadra M.R., Eid M.H. Investigating the impacts of heavy metal(loid)s on ecology and human health in the lower basin of Hungary's Danube River: a Python and Monte Carlo simulation-based study. Environ. Geochem. Health. 2023;45:9757–9784. doi: 10.1007/s10653-023-01769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gad M., El-Hendawy S., Al-Suhaibani N., Tahir M.U., Mubushar M., Elsayed S. Combining hydrogeochemical characterization and a hyperspectral reflectance tool for assessing quality and suitability of two groundwater resources for irrigation in Egypt. Water. 2020;12:2169. doi: 10.3390/w12082169. [DOI] [Google Scholar]
- 48.Beltrán N.H., Duarte-Mermoud M.A., Salah S.A., Bustos M.A., Peña-Neira A.I., Loyola E.A., Jalocha J.W. Feature selection algorithms using Chilean wine chromatograms as examples. J. Food Eng. 2005;67:483–490. doi: 10.1016/j.jfoodeng.2004.05.015. [DOI] [Google Scholar]
- 49.Guyon I., Elisseeff A. An introduction to variable and feature selection. J. Mach. Learn. Res. 2003;3:1157–1182. [Google Scholar]
- 50.Schulze F.H., Wolf H., Jansen H.W., van der Veer P. Applications of artificial neural networks in integrated water management: fiction or future? Water Sci. Technol. 2005;52:21–31. doi: 10.2166/wst.2005.0279. [DOI] [PubMed] [Google Scholar]
- 51.Wilson J.L., Townley L.R., Da Costa A.S. 1979. Mathematical Development and Verification of a Finite Element Aquifer Flow Model AQUIFEM-1, Massachusetts Institute of Technology, Ralph M. Parsons Laboratory for Water. [Google Scholar]
- 52.Ibrahim H., Yaseen Z.M., Scholz M., Ali M., Gad M., Elsayed S., Khadr M., Hussein H., Ibrahim H.H., Eid M.H., Kovács A., Péter S., Khalifa M.M. Evaluation and prediction of groundwater quality for irrigation using an integrated water quality indices, machine learning models and GIS approaches: a representative case study. Water. 2023;15:694. doi: 10.3390/w15040694. [DOI] [Google Scholar]
- 53.Gad M., El Osta M. Geochemical controlling mechanisms and quality of the groundwater resources in El Fayoum Depression, Egypt. Arab J Geosci. 2020;13:861. doi: 10.1007/s12517-020-05882-x. [DOI] [Google Scholar]
- 54.Gad M., El-Hendawy S., Al-Suhaibani N., Tahir M.U., Mubushar M., Elsayed S. Combining hydrogeochemical characterization and a hyperspectral reflectance tool for assessing quality and suitability of two groundwater resources for irrigation in Egypt. Water. 2020;12:2169. doi: 10.3390/w12082169. [DOI] [Google Scholar]
- 55.Ghoraba S.M., Zyedan B.A., Rashwan I.M.H. Solute transport modeling of the groundwater for quaternary aquifer quality management in Middle Delta, Egypt. Alex. Eng. J. 2013;52:197–207. doi: 10.1016/j.aej.2012.12.007. [DOI] [Google Scholar]
- 56.Rice E.W., Bridgewater L., Association A.P.H. American public health association; Washington, DC: 2012. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 57.Rice E.W., Bridgewater L., Association A.P.H. American public health association; Washington, DC: 2012. Standard Methods for the Examination of Water and Wastewater. [Google Scholar]
- 58.Yang W., Zhao Y., Wang D., Wu H., Lin A., He L. Using principal components analysis and IDW interpolation to determine spatial and temporal changes of surface water quality of xin’anjiang river in huangshan, China. IJERPH. 2020;17:2942. doi: 10.3390/ijerph17082942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prasad A., Kumar D., Singh D.V. Effect of residual sodium carbonate in irrigation water on the soil sodication and yield of palmarosa (Cymbopogon martinni) and lemongrass (Cymbopogon flexuosus) Agric. Water Manag. 2001;50:161–172. doi: 10.1016/S0378-3774(01)00103-2. [DOI] [Google Scholar]
- 60.Brown R.M., McClelland N.I., Deininger R.A., Tozer R.G. 1970. A Water Quality Index-Do We Dare, Water and Sewage Works 117. [Google Scholar]
- 61.Edet A.E., Offiong O.E. Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria) Geojournal. 2002;57:295–304. doi: 10.1023/B:GEJO.0000007250.92458.de. [DOI] [Google Scholar]
- 62.Prasad B., Bose J. Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Env Geol. 2001;41:183–188. doi: 10.1007/s002540100380. [DOI] [Google Scholar]
- 63.Caeiro S., Costa M.H., Ramos T.B., Fernandes F., Silveira N., Coimbra A., Medeiros G., Painho M. Assessing heavy metal contamination in Sado Estuary sediment: an index analysis approach. Ecol. Indicat. 2005;5:151–169. doi: 10.1016/j.ecolind.2005.02.001. [DOI] [Google Scholar]
- 64.World Health Organization . third ed. World Health Organization; Geneva: 2008. Guidelines for Drinking-Water Quality [electronic Resource]: Incorporating 1st and 2nd addenda,Vol.1, Recommendations.https://iris.who.int/handle/10665/204411 [Google Scholar]
- 65.Sobhanardakani S., Taghavi L., Shahmoradi B., Jahangard A. Groundwater quality assessment using the water quality pollution indices in Toyserkan Plain. 2017;4:21–27. [Google Scholar]
- 66.Sheykhi V., Moore F. Geochemical characterization of kor river water quality, fars province, southwest Iran. Water Qual Expo Health. 2012;4:25–38. doi: 10.1007/s12403-012-0063-1. [DOI] [Google Scholar]
- 67.Saeed O., Székács A., Jordán G., Mörtl M., Abukhadra M.R., Eid M.H. Correction: investigating the impacts of heavy metal(loid)s on ecology and human health in the lower basin of Hungary's Danube River: a Python and Monte Carlo simulation-based study. Environ. Geochem. Health. 2023;45:9785. doi: 10.1007/s10653-023-01777-4. 9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giri S., Singh A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015;187:63. doi: 10.1007/s10661-015-4265-4. [DOI] [PubMed] [Google Scholar]
- 69.Adimalla N. Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum. Ecol. Risk Assess. 2020;26:310–334. doi: 10.1080/10807039.2018.1508329. [DOI] [Google Scholar]
- 70.Saha N., Rahman M.S., Ahmed M.B., Zhou J.L., Ngo H.H., Guo W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017;185:70–78. doi: 10.1016/j.jenvman.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 71.Adimalla N. Spatial distribution, exposure, and potential health risk assessment from nitrate in drinking water from semi-arid region of South India. Hum. Ecol. Risk Assess. 2020;26:310–334. doi: 10.1080/10807039.2018.1508329. [DOI] [Google Scholar]
- 72.Alam S.M.K., Li P., Fida M. Groundwater nitrate pollution due to excessive use of N-fertilizers in rural areas of Bangladesh: pollution status, health risk, source contribution, and future impacts. Expo Health. 2024;16:159–182. doi: 10.1007/s12403-023-00545-0. [DOI] [Google Scholar]
- 73.Epa U. Supplemental guidance for developing soil screening levels for superfund sites. United States Environ. Prot. Agency. 2002;12:1–187. [Google Scholar]
- 74.Giri S., Singh A.K. Human health risk assessment via drinking water pathway due to metal contamination in the groundwater of Subarnarekha River Basin, India. Environ. Monit. Assess. 2015;187:63. doi: 10.1007/s10661-015-4265-4. [DOI] [PubMed] [Google Scholar]
- 75.Guo W., Li P., Du Q., Zhou Y., Xu D., Zhang Z. Hydrogeochemical processes regulating the groundwater geochemistry and human health risk of groundwater in the rural areas of the wei river basin, China, expo health. 2023. [DOI]
- 76.Meng Y., Wu J., Li P., Wang Y. Distribution characteristics, source identification and health risk assessment of trace metals in the coastal groundwater of Taizhou City, China. Environ. Res. 2023;238 doi: 10.1016/j.envres.2023.117085. [DOI] [PubMed] [Google Scholar]
- 77.Qian H., Ma Z.Y., Li P.Y. Geologic Publishing House; Beijing, China: 2005. Hydrogeochemistry. (in Chinese) [Google Scholar]
- 78.Saha N., Rahman M.S., Ahmed M.B., Zhou J.L., Ngo H.H., Guo W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017;185:70–78. doi: 10.1016/j.jenvman.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 79.Wu B., Zhao D.Y., Jia H.Y., Zhang Y., Zhang X.X., Cheng S.P. Preliminary risk assessment of trace metal pollution in surface water from yangtze river in nanjing section, China. Bull. Environ. Contam. Toxicol. 2009;82:405–409. doi: 10.1007/s00128-008-9497-3. [DOI] [PubMed] [Google Scholar]
- 80.Xu D., Li P., Chen X., Yang S., Zhang P., Guo F. Major ion hydrogeochemistry and health risk of groundwater nitrate in selected rural areas of the Guanzhong Basin, China. Hum. Ecol. Risk Assess. 2023;29:701–727. doi: 10.1080/10807039.2022.2164246. [DOI] [Google Scholar]
- 81.Gehlhaus M.W., Gift J.S., Hogan K.A., Kopylev L., Schlosser P.M., Kadry A.-R. Approaches to cancer assessment in EPA's integrated risk information system. Toxicol. Appl. Pharmacol. 2011;254:170–180. doi: 10.1016/j.taap.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 82.Apaydin H., Feizi H., Sattari M.T., Colak M.S., Shamshirband S., Chau K.-W. Comparative analysis of recurrent neural network architectures for reservoir inflow forecasting. Water. 2020;12:1500. doi: 10.3390/w12051500. [DOI] [Google Scholar]
- 83.Graves A., Mohamed A., Hinton G. 2013 IEEE International Conference on Acoustics, Speech and Signal Processing. IEEE; Vancouver, BC, Canada: 2013. Speech recognition with deep recurrent neural networks; pp. 6645–6649. [DOI] [Google Scholar]
- 84.Who W.H. fourth ed. 2017. Guidelines for Drinking-Water Quality: First Addendum to the. [PubMed] [Google Scholar]
- 85.Salem S.B.H., Gaagai A., Ben Slimene I., Moussa A.B., Zouari K., Yadav K.K., Eid M.H., Abukhadra M.R., El-Sherbeeny A.M., Gad M., Farouk M., Elsherbiny O., Elsayed S., Bellucci S., Ibrahim H. Applying multivariate analysis and machine learning approaches to evaluating groundwater quality on the kairouan plain, Tunisia. Water. 2023;15:3495. doi: 10.3390/w15193495. [DOI] [Google Scholar]
- 86.Jacobson A.D., Wasserburg G.J. Anhydrite and the Sr isotope evolution of groundwater in a carbonate aquifer. Chem. Geol. 2005;214:331–350. doi: 10.1016/j.chemgeo.2004.10.006. [DOI] [Google Scholar]
- 87.El Mejri H., Ben Moussa A., Zouari K. The use of hydrochemical and environmental isotopic tracers to understand the functioning of the aquifer system in the Bou Hafna and Haffouz regions, Central Tunisia. Quat. Int. 2014;338:88–98. doi: 10.1016/j.quaint.2014.04.046. [DOI] [Google Scholar]
- 88.Athamena A., Menani M.R. Nitrogen flux and hydrochemical characteristics of the calcareous aquifer of the Zana plain, north east of Algeria. Arab J Geosci. 2018;11:356. doi: 10.1007/s12517-018-3681-5. [DOI] [Google Scholar]
- 89.Gaagai A., Boudoukha A., Boumezbeur A., Benaabidate L. Hydrochemical characterization of surface water in the Babar watershed (Algeria) using environmetric techniques and time series analysis. Int. J. River Basin Manag. 2017;15:361–372. doi: 10.1080/15715124.2017.1299157. [DOI] [Google Scholar]
- 90.Rishi M.S., Kaur L., Sharma S. Groundwater quality appraisal for non-carcinogenic human health risks and irrigation purposes in a part of Yamuna sub-basin, India. Hum. Ecol. Risk Assess. 2020;26:2716–2736. doi: 10.1080/10807039.2019.1682514. [DOI] [Google Scholar]
- 91.El-Safa M.M.A., Elsayed S., Elsherbiny O., Elmetwalli A.H., Gad M., Moghanm F.S., Eid E.M., Taher M.A., El-Morsy M.H.E., Osman H.E.M., Saleh A.H. Environmental assessment of potentially toxic elements using pollution indices and data-driven modeling in surface sediment of the littoral shelf of the mediterranean sea coast and gamasa estuary, Egypt. JMSE. 2022;10:816. doi: 10.3390/jmse10060816. [DOI] [Google Scholar]
- 92.Barakat M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011;4:361–377. doi: 10.1016/j.arabjc.2010.07.019. [DOI] [Google Scholar]
- 93.Bhutiani R., Kulkarni D.B., Khanna D.R., Gautam A. Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energ. Ecol. Environ. 2017;2:155–167. doi: 10.1007/s40974-016-0019-6. [DOI] [Google Scholar]
- 94.Laonamsai J., Pawana V., Chipthamlong P., Chomcheawchan P., Kamdee K., Kimmany B., Julphunthong P. Groundwater quality variations in multiple aquifers: a comprehensive evaluation for public health and agricultural use. Geosciences. 2023;13:195. doi: 10.3390/geosciences13070195. [DOI] [Google Scholar]
- 95.Sharma N., Liang M.-C., Laskar A.H., Huang K.-F., Maurya N.S., Singh V., Ranjan R., Maurya A.S. Basin-scale geochemical assessment of water quality in the ganges river during the dry season. Water. 2023;15:2026. doi: 10.3390/w15112026. [DOI] [Google Scholar]
- 96.Chrysochoou M., Theologou E., Bompoti N., Dermatas D., Panagiotakis I. Occurrence, origin and transformation processes of geogenic chromium in soils and sediments. Curr Pollution Rep. 2016;2:224–235. doi: 10.1007/s40726-016-0044-2. [DOI] [Google Scholar]
- 97.Shaibur M.R., Ahmmed I., Sarwar S., Karim R., Hossain MdM., Islam M.S., Shah MdS., Khan A.S., Akhtar F., Uddin MdG., Rahman M.M., Salam M.A., Ambade B. Groundwater quality of some parts of coastal bhola district, Bangladesh: exceptional evidence. Urban Science. 2023;7:71. doi: 10.3390/urbansci7030071. [DOI] [Google Scholar]
- 98.Ahmad W., Alharthy R.D., Zubair M., Ahmed M., Hameed A., Rafique S. Toxic and heavy metals contamination assessment in soil and water to evaluate human health risk. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-94616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rajmohan N., Elango L. Distribution of iron, manganese, zinc and atrazine in groundwater in parts of palar and cheyyar river basins, south India. Environ. Monit. Assess. 2005;107:115–131. doi: 10.1007/s10661-005-5307-0. [DOI] [PubMed] [Google Scholar]
- 100.Podgorski J., Araya D., Berg M. Geogenic manganese and iron in groundwater of Southeast Asia and Bangladesh – machine learning spatial prediction modeling and comparison with arsenic. Sci. Total Environ. 2022;833 doi: 10.1016/j.scitotenv.2022.155131. [DOI] [PubMed] [Google Scholar]
- 101.Shalhevet J. Using water of marginal quality for crop production: major issues. Agric. Water Manag. 1994;25:233–269. doi: 10.1016/0378-3774(94)90063-9. [DOI] [Google Scholar]
- 102.Piper A.M. A graphic procedure in the geochemical interpretation of water-analyses. Trans. AGU. 1944;25:914. doi: 10.1029/TR025i006p00914. [DOI] [Google Scholar]
- 103.Gad M., El Osta M. Geochemical controlling mechanisms and quality of the groundwater resources in El Fayoum Depression, Egypt. Arab J Geosci. 2020;13:861. doi: 10.1007/s12517-020-05882-x. [DOI] [Google Scholar]
- 104.Gad M., Saleh A.H., Hussein H., Farouk M., Elsayed S. Appraisal of surface water quality of Nile River using water quality indices, spectral signature and multivariate modeling. Water. 2022;14:1131. doi: 10.3390/w14071131. [DOI] [Google Scholar]
- 105.Gibbs R.J. Mechanisms controlling world water chemistry. Science. 1970;170:1088–1090. doi: 10.1126/science.170.3962.1088. [DOI] [PubMed] [Google Scholar]
- 106.Athamena A., Gaagai A., Aouissi H.A., Burlakovs J., Bencedira S., Zekker I., Krauklis A.E. Chemometrics of the environment: hydrochemical characterization of groundwater in lioua plain (north africa) using time series and multivariate statistical analysis. Sustainability. 2022;15:20. doi: 10.3390/su15010020. [DOI] [Google Scholar]
- 107.Awasthi A., Rishi M.S., Panjgotra S. Groundwater quality assessment for drinking and industrial purposes in transboundary aquifers of Gurdaspur district, Punjab, India. Int. J. Environ. Anal. Chem. 2021:1–15. doi: 10.1080/03067319.2021.2020766. [DOI] [PubMed] [Google Scholar]
- 108.Chadha D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol. J. 1999;7:431–439. doi: 10.1007/s100400050216. [DOI] [Google Scholar]
- 109.Zhu B., Yang X., Rioual P., Qin X., Liu Z., Xiong H., Yu J. Hydrogeochemistry of three watersheds (the erlqis, zhungarer and yili) in northern xinjiang, NW China. Appl. Geochem. 2011;26:1535–1548. doi: 10.1016/j.apgeochem.2011.06.018. [DOI] [Google Scholar]
- 110.Kumari R., Datta P.S., Rao M.S., Mukherjee S., Azad C. Anthropogenic perturbations induced groundwater vulnerability to pollution in the industrial Faridabad District, Haryana, India. Environ. Earth Sci. 2018;77:187. doi: 10.1007/s12665-018-7368-8. [DOI] [Google Scholar]
- 111.Gad M., Elsayed S., Moghanm F.S., Almarshadi M.H., Alshammari A.S., Khedher K.M., Eid E.M., Hussein H. Combining water quality indices and multivariate modeling to assess surface water quality in the northern nile delta, Egypt. Water. 2020;12:2142. doi: 10.3390/w12082142. [DOI] [Google Scholar]
- 112.He S., Wu J. Relationships of groundwater quality and associated health risks with land use/land cover patterns: a case study in a loess area, Northwest China. Hum. Ecol. Risk Assess. 2019;25:354–373. doi: 10.1080/10807039.2019.1570463. [DOI] [Google Scholar]
- 113.Tiyasha T.M. Tung, Yaseen Z.M. A survey on river water quality modelling using artificial intelligence models: 2000–2020. J. Hydrol. 2020;585 doi: 10.1016/j.jhydrol.2020.124670. [DOI] [Google Scholar]
- 114.Ata A.A.E.-S.M., Aly M.H., Hussein H., Eid M.H., Abukhadra M.R., El-Sherbeeny A.M., Bellucci S., Gad M. Hydrogeochemical characteristics and air quality risks associated with gold mining operations in Egypt using geochemical modeling and risk indices. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e31086. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided as tables and figures.