Abstract
Chain elongating bacteria are a unique guild of strictly anaerobic bacteria that have garnered interest for sustainable chemical manufacturing from carbon-rich wet and gaseous waste streams. They produce C6–C8 medium-chain fatty acids, which are valuable platform chemicals that can be used directly, or derivatized to service a wide range of chemical industries. However, the application of chain elongating bacteria for synthesizing products beyond C6–C8 medium-chain fatty acids has not been evaluated. In this study, we assess the feasibility of expanding the product spectrum of chain elongating bacteria to C9–C12 fatty acids, along with the synthesis of C6 fatty alcohols, dicarboxylic acids, diols, and methyl ketones. We propose several metabolic engineering strategies to accomplish these conversions in chain elongating bacteria and utilize constraint-based metabolic modelling to predict pathway stoichiometries, assess thermodynamic feasibility, and estimate ATP and product yields. We also evaluate how producing alternative products impacts the growth rate of chain elongating bacteria via resource allocation modelling, revealing a trade-off between product chain length and class versus cell growth rate. Together, these results highlight the potential for using chain elongating bacteria as a platform for diverse oleochemical biomanufacturing and offer a starting point for guiding future metabolic engineering efforts aimed at expanding their product range.
One-Sentence Summary
In this work, the authors use constraint-based metabolic modelling and enzyme cost minimization to assess the feasibility of using metabolic engineering to expand the product spectrum of anaerobic chain elongating bacteria.
Keywords: Chain elongation, Anaerobic bioproduction, Metabolic engineering, Metabolic modelling, Resource allocation, Circular bioeconomy
Graphical Abstract
Graphical Abstract.

Introduction
Circular economies require the recycling of societal “wastes” into new bioproducts to support sustainable human activity. Anaerobic fermentation processes can upcycle carbon from agricultural residues, food waste, and industrial off-gases into useful fuels, chemicals, and materials. One promising process is microbial chain elongation, which uses anaerobic microbiomes in open-culture systems to synthesize medium-chain fatty acids (MCFAs) from complex organic and gaseous waste streams (Angenent et al., 2016; Holtzapple et al., 2022; Scarborough et al., 2022). The process works by producing key intermediates, such as lactate and/or ethanol, through either organic waste fermentation (breaking down) or gas fermentation (building up), which subsequently undergo a secondary fermentation to produce MCFAs via chain elongation. Several studies have demonstrated stable MCFA production from organic waste (Grootscholten et al., 2014; Stamatopoulou et al., 2020) and gaseous feedstocks (Bäumler et al., 2022; Diender et al., 2016; Fernández-Blanco et al., 2022) via chain elongation at the bench scale and pilot scale, and more recently, a demonstration plant has been built in the Netherlands by the Dutch company ChainCraft (https://www.chaincraft.nl/).
At the heart of the process is a functional guild of obligate anaerobes called “chain elongating bacteria” that use a native reverse beta-oxidation (RBO) pathway (see Fig. 1B) to ferment lactate, ethanol, and other electron-rich organic substrates (e.g. sugars and glycerol) into C4–C8 carboxylates (i.e. butyrate, hexanoate, and octanoate) as part of their growth. The pathway is unique because it allows redox balancing, while also conserving energy through a novel flavin-based electron bifurcation mechanism (Buckel & Thauer, 2018; Li et al., 2008). Almost all known chain elongators belong to the phylum Bacillota (previously Firmicutes) and are a phylogenetically and physiologically diverse group. While most chain elongators remain uncultivated, over 15 strains have been isolated and sequenced to date, with most new isolates being reported in the last 10 years (Candry & Ganigué, 2021). These isolates, particularly Clostridium kluyveri, have been used to establish synthetic co-cultures to convert gaseous or sugar-based feedstocks to MCFAs and their corresponding alcohols (Bäumler et al., 2022; Diender et al., 2016; Haas et al., 2018; Lynd et al., 2022; Otten et al., 2022) which could be expanded to more complex feedstocks by selecting lactate- and/or ethanol-producing partners with improved hydrolytic capabilities.
Fig. 1.
(A) Anaerobic conversion of organic waste to oleochemicals using synthetic co-cultures inspired by natural systems. Complex polymers are degraded by lactic acid bacteria (LAB), which in turn provide lactic acid to chain elongating bacteria for medium-chain fatty acid synthesis. (B) The reverse beta-oxidation pathway in chain elongating bacteria and proposed product spectrum expansion (brown boxes). EMP = Embden–Meyerhof–Parnas glycolysis; LDH = electron-confurcating lactate dehydrogenase/electron-transferring flavoprotein; ADH = alcohol dehydrogenase; ADA = acetaldehyde dehydrogenase; PFOR = pyruvate ferredoxin oxidoreductase; PFL = pyruvate formate lyase; PTA = phosphate acetyltransferase; ACK = acetate kinase; ACACT = acetyl-CoA C acetyltransferase; HACD = 3-hydroxyacyl-CoA dehydrogenase; ECOAH = enoyl-CoA hydratase; EBACD = electron bifurcating acyl-CoA dehydrogenase; CoAT = CoA transferase; Fd = ferredoxin; RNF = proton translocating ferredoxin: NAD+ oxidoreductase complex; HYD = Fe–Fe hydrogenase. Created with BioRender.com.
The development of synthetic co-cultures, inspired by mixed culture chain elongation processes, could represent a platform for improving titres, rates, and yields of MCFA production (Fig. 1A). Moreover, there is growing interest in genetically modifying the native RBO cycle in chain elongators to expand the product spectrum beyond C4–C8 monocarboxylates, including alcohols, diols, dicarboxylates, and other bulk chemicals (Agena et al., 2023; Guss & Riley, 2021; Strik et al., 2022). This would enable the anaerobic synthesis of diverse medium-chain oleochemicals from wet and gaseous waste streams, which is expected to have improved economic feasibility compared to sugar-based aerobic fermentations (Holtzapple et al., 2022). However, the technical feasibility of making products other than C4–C8 monocarboxylates via metabolic engineering of chain elongators remains unexplored. As tools to genetically modify chain elongators emerge (Agena et al., 2023; Cheng et al., 2019; Guss & Riley, 2021), methodologies to rationally design these new biocatalysts are needed.
In this study, we use constraint-based metabolic modelling along with thermodynamic analyses to evaluate the feasibility of synthesizing diverse medium-chain oleochemicals (C6–C12 fatty acids [FAs], primary alcohols, dicarboxylates, diols, and methyl ketones [MKs]) from key intermediate substrates (lactate, ethanol, sugars, and glycerol) using anaerobic chain elongating bacteria (Fig. 1). We first analyse MCFA production scenarios beyond natural C8 production up to C12, highlighting trade-offs between ATP yield and expected growth rate using a combination of metabolic modelling and enzyme cost minimization (ECM) analyses. Subsequently, we propose several modifications to the RBO cycle to synthesize target medium-chain oleochemical products and use constraint-based metabolic modelling to determine overall pathway stoichiometry, thermodynamic feasibility, and theoretical product yields. Our results indicate that metabolic engineering of chain elongating bacteria could enable the anaerobic synthesis of diverse medium-chain oleochemicals at industrially relevant yields. Moreover, we identify challenges with engineering the RBO pathway in chain elongators and offer potential solutions to overcome them via metabolic engineering. We anticipate that these results will provide a useful starting point for engineering microbial chain elongation to serve as a platform for sustainable chemical manufacturing.
Methods
Predicting Pathway Stoichiometry with Parsimonious Flux Balance Analysis
Python3 and CobraPy were used for metabolic modelling. Previously, the iFermCell215 model was created to represent a generalized chain elongation microbiome with several functional guilds capable of utilizing different electron donors for elongation up to C8 (Scarborough et al., 2020). iFermCell215 was simplified by removing some inapplicable reactions and further modified with 26 metabolites and 30 reactions required for the production of C9–C12 carboxylates to produce iFermCell193. iFermCell193 was further constrained to capture the metabolism of typical CEB, in alignment with the proposed stoichiometric models previously described to obtain a generalized core model for chain elongating bacteria (Angenent et al., 2016; Scarborough et al., 2018). Using iFermCell193, the production of FAs from lactate (main text), ethanol, glucose, xylose, and glycerol (Supplemental Material S1) was modelled with parsimonious enzyme usage flux balance analysis (pFBA) (Lewis et al., 2010) where net ATP yield was set as the objective function, used here as a proxy for growth.
iFermCell193 was further modified with 70 metabolites and 163 reactions to produce iFermCell356 based on the proposed production pathways for expanding the chain elongating bacteria product spectrum to produce fatty alcohols, dicarboxylates, diols, MKs. The production of these bioproducts from different electron donors was modelled with pFBA where net ATP yield was set as the objective function, except MK production.
The python package equilibrator-api (https://gitlab.com/equilibrator) was used to predict the Gibbs free energy of formation for metabolites at standard conditions of T = 25°C, pH = 7, pMg = 0, ionic strength = 0 M. To calculate overall Gibbs free energy change of reaction for the production of the different products from lactate, the Gibbs free energy change of formation and the overall pathway reaction stoichiometry predicted by the model were used. The full flux solutions and redox balance tables for production from lactate are provided in Supplemental Material S3 and the model files are available on GitHub (https://github.com/e-taan/JIMB2024).
Enzyme Cost Minimization
ECM was developed by Flamholz et al. (2013) and performed here with the Python packages equilibrator-api and equilibrator-pathway (https://gitlab.com/equilibrator). A reversible Michaelis–Menten kinetic expression is substituted with a flux-force efficiency term,  , to yield an expression for the enzyme concentration [E] required to sustain a given flux J through a pathway reaction:
, to yield an expression for the enzyme concentration [E] required to sustain a given flux J through a pathway reaction:
 |
where kcat is the enzyme's rate constant, [Ci] is the concentration of reactant i, KM,i is the enzyme's saturation constant for reactant i, mi is the stoichiometric coefficient of reactant i, and ΔG′ is the Gibbs free energy change, estimated via the component contribution method at pH 6 (Noor et al., 2013). This expression can be decomposed into three factors to facilitate interpretation:  represents the stoichiometric enzyme demand;
represents the stoichiometric enzyme demand;  encapsulates the cost incurred when the enzyme is not saturated; and
encapsulates the cost incurred when the enzyme is not saturated; and  reflects the increased enzyme requirement to sustain a net flux when a reaction is near equilibrium. The total enzyme cost per unit pathway flux (gram protein/mole product/hour) is calculated by summing reaction costs multiplied by both their stoichiometry in the pathway ni and their enzyme's molar weight Mi, then dividing by the arbitrary pathway flux J in units of moles per hour. Total enzyme cost per unit flux is minimized by gradient descent given bounds on metabolite concentrations and the requirement for all reactions to be exergonic:
reflects the increased enzyme requirement to sustain a net flux when a reaction is near equilibrium. The total enzyme cost per unit pathway flux (gram protein/mole product/hour) is calculated by summing reaction costs multiplied by both their stoichiometry in the pathway ni and their enzyme's molar weight Mi, then dividing by the arbitrary pathway flux J in units of moles per hour. Total enzyme cost per unit flux is minimized by gradient descent given bounds on metabolite concentrations and the requirement for all reactions to be exergonic:
 |
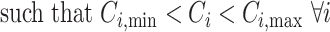 |
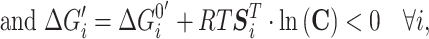 |
where 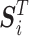 is the ith row of the stoichiometric matrix's transpose, containing the stoichiometric coefficients for each metabolite in the ith reaction, and ln(C) is the vector of logarithms of each metabolite's concentration. As a first approximation, all enzymes are assumed to share rate and saturation constants and molecular weight (kcat = 200 s−1; Km = 0.2 μM, MW = 40 kDa). Pathway specific metabolites are bounded between 1 μM and 10 mM, apart from oxoacyl-CoAs, which can drop to 0.4 μM. Universal metabolites such as ATP and nicotinamide adenine dinucleotide (NADH) are set to concentrations derived from literature (Bennet et al., 2009). The reduction potential of ferredoxin is set to −400 mV. Dissolved oxygen concentration was set to 6.6 μM (0.005 atm at equilibrium), which is expected to be sub-lethal to obligate anaerobes (Loesche, 1969). Dissolved hydrogen concentration was 1.4 mM (0.2 atm) and dissolved CO2 concentration was 10 μM. Ionic strength was set to 250 mM. A proton motive force of 200 mV was used for butyrate production, whereas 100 mV was used in all other cases, due to the reversal of RNF activity required for butyrate production without an electron acceptor. ECM solutions are available in Supplemental Material S2, and model files and functions are available on GitHub (https://github.com/e-taan/JIMB2024).
is the ith row of the stoichiometric matrix's transpose, containing the stoichiometric coefficients for each metabolite in the ith reaction, and ln(C) is the vector of logarithms of each metabolite's concentration. As a first approximation, all enzymes are assumed to share rate and saturation constants and molecular weight (kcat = 200 s−1; Km = 0.2 μM, MW = 40 kDa). Pathway specific metabolites are bounded between 1 μM and 10 mM, apart from oxoacyl-CoAs, which can drop to 0.4 μM. Universal metabolites such as ATP and nicotinamide adenine dinucleotide (NADH) are set to concentrations derived from literature (Bennet et al., 2009). The reduction potential of ferredoxin is set to −400 mV. Dissolved oxygen concentration was set to 6.6 μM (0.005 atm at equilibrium), which is expected to be sub-lethal to obligate anaerobes (Loesche, 1969). Dissolved hydrogen concentration was 1.4 mM (0.2 atm) and dissolved CO2 concentration was 10 μM. Ionic strength was set to 250 mM. A proton motive force of 200 mV was used for butyrate production, whereas 100 mV was used in all other cases, due to the reversal of RNF activity required for butyrate production without an electron acceptor. ECM solutions are available in Supplemental Material S2, and model files and functions are available on GitHub (https://github.com/e-taan/JIMB2024).
Results and Discussion
Impact of MCFA Chain Length on Growth Rate and ATP Yield
Modelling Chain Elongation Beyond Octanoic Acid
Currently, the products of chain elongation are restricted to butyric and hexanoic acids (C4–C6), with limited synthesis of octanoic acid (C8) (Nelson et al., 2017; Zhu et al., 2017). Genetically modifying chain elongating bacteria to improve selectivity and increase MCFA chain length could benefit product yields, while also expanding the process to produce MCFAs with larger markets (C8–C12).
To assess the feasibility of MCFA production beyond C8, we predicted overall pathway stoichiometry, redox balance, free energy change, and theoretical ATP and product yields of C4–C12 FAs via pFBA with ATP yield set as the objective function using a simplified metabolic model describing core chain elongation metabolism (iFermCell193, see the “Methods” section) (Table 1). We focused on lactate utilizing chain elongating bacteria (e.g. Pseudoramibacter alactolyticus, Megasphaera hexanoica, Caproicibacterium lactatifermentans) as lactate is the primary substrate in mixed culture processes converting organic wastes (Fig. 1) (Contreras-Dávila et al., 2020). Model simulations with different electron donors, including ethanol, glucose, xylose, and glycerol, were also evaluated (Supplemental Material S1, Tables S1–S5) to demonstrate process feasibility for a range of other substrates known to be consumed by chain elongating bacteria (e.g. ethanol by Clostridium kluyveri).
Table 1.
Predicted Stoichiometry for the Synthesis of C4–C12 FAs from Lactate
| Chain length | Overall equation | Product yield (mol P/mol lactate) | ATP yield (mol ATP/mol lactate) | ΔGr°′ (kJ/mol lactate) | ΔGr°′ per ATP (kJ/mol/mol ATP) | Yield (g P/g lactate) |
|---|---|---|---|---|---|---|
| C4 | Lactate + 0.5 H+ + 0.25 ADP → 0.5 butyrate + CO2 + H2 + 0.25 ATP | 0.500 | 0.250 | −14.99 | −59.96 | 0.489 |
| C6 | Lactate + 0.667 H+ + 0.333 ADP → CO2 + 0.667 H2 + 0.333 H2O + 0.333 hexanoate + 0.333 ATP | 0.333 | 0.333 | −26.75 | −80.24 | 0.431 |
| C8 | Lactate + 0.75 H+ + 0.375 ADP → CO2 + 0.5 H2 + 0.5 H2O + 0.25 octanoate + 0.375 ATP | 0.250 | 0.375 | −35.10 | −93.60 | 0.402 |
| C10 | Lactate + 0.8 H+ + 0.4 ADP → CO2 + 0.2 decanoate + 0.4 H2 + 0.6 H2O + 0.4 ATP | 0.200 | 0.400 | −40.11 | −100.29 | 0.385 |
| C12 | Lactate + 0.833 H+ + 0.417 ADP → CO2 + 0.167 dodecanoate + 0.333 H2 + 0.667 H2O + 0.417 ATP | 0.167 | 0.417 | −43.45 | −104.29 | 0.373 |
The model predicted that ATP yield and overall reaction free energy change increase with MCFA chain length from C4 to C12 (Table 1), indicating that MCFA synthesis beyond C8 could be theoretically possible. Moreover, the production of C4–C12 chain lengths from lactate released ≥50 kJ/mol per ATP, indicating that these reactions produce sufficient energy for ATP synthesis (Thauer et al., 1977). C9–C12 carboxylate production with other electron donors, including ethanol, glucose, xylose, and glycerol, was also found to be feasible (Supplemental Material S1, Tables S1–S5). This observation agrees with past modelling results that suggested hexanoic and octanoic acid production should improve ATP yields from lactate compared to butyric acid production (Scarborough et al., 2018).
The predicted increase in net ATP production is attributed to a greater flux through the proton translocating ferredoxin: NAD+ oxidoreductase complex (RNF) for each turn of the RBO cycle, which leads to greater ATP production via the ion motive force (IMF). In the RBO cycle, each successive elongation generates reduced ferredoxin from the electron-bifurcating acyl-CoA dehydrogenase (EBACD). This ferredoxin goes on to drive RNF to recover some NADH and/or is used by a soluble ferredoxin hydrogenase (HYD1) to evolve H2 as a terminal electron sink. As confirmed in the predicted flux distributions, elongation to longer chain lengths increases the total NADH demand required to reduce the acyl chains (HACD and EBACD in Fig. 1). Thus, with increasing chain length, a greater proportion of the reduced ferredoxin produced by EBACD is used to regenerate NADH via RNF, rather than driving HYD1. This ultimately leads to increased ATP production through the IMF along with decreased H2 evolution for longer chain lengths. These results also indicate that past a certain chain length (>C12), H2 evolution will cease as all electron equivalents will be required solely for chain elongation.
Intracellular Thermodynamic Landscape and Resource Allocation
Our initial modelling analysis suggested that the improved ATP yield with longer chain lengths should naturally select for MCFAs beyond C8. However, all pure and mixed culture chain elongation studies have only observed C4–C8 MCFA products. This points to a potential trade-off between growth yield (or ATP yield) and growth rate (or ATP production rate), resulting from optimal resource allocation. In a model based on resource allocation theory (Supplementary Material S1; inspired by a similar model developed by Flamholz et al., 2024), where catabolic ATP production and anabolic ATP consumption rates are linearly related to the biomass fractions allocated to either function, RBO enzyme cost per unit ATP production flux and chain elongating bacteria growth rate are inversely correlated (Fig. 2). This simplified model captures the fact that a higher growth rate is expected to necessitate both a larger pool of anabolic enzymes and ribosomes (Basan, 2018) and a higher ATP production flux, requiring that catabolic machinery produces ATP faster with less enzyme.
Fig. 2.
ECM results of RBO for production of C4–C12 carboxylates from lactate and the estimated relative effect on growth rate. Enzyme cost per unit ATP flux is reported as a multiple of butyrate production's cost. Each reaction carries a cost which accounts for the pathway's stoichiometry (bottom bar), increased cost incurred due to a reaction's proximity to equilibrium (middle bar), and the cost due to sub-saturation reactant concentrations (top bar). Bottom right panel: Enzyme cost per ATP flux relative to butyrate versus specific growth rate relative to butyrate, as derived from a simplified resource allocation model (Supplemental Material S1) (Top). Anabolic fraction of biomass corresponding to specific growth rate and catabolic fraction of biomass remaining to supply ATP at a given growth rate (Middle). ATP yield on lactate for different chain elongation simulated by pFBA (Bottom).
To evaluate the enzyme investment per ATP flux required to produce carboxylates of different chain lengths, we performed ECM analysis. ECM places a lower bound on a pathway's enzyme demand per unit flux [g/(mol/h)] by accounting for the pathway's length and stoichiometry, reaction thermodynamics, and saturation effects following from the optimal set of metabolite concentrations, given appropriate bounds on those concentrations and parametrization of enzyme kinetics (Flamholz et al., 2013). This analysis coupled with ATP yield results from our model showed that minimal enzyme cost per unit ATP flux increases with chain length (Fig. 2). This suggests that chain elongating bacteria making longer length products invest more of their proteome into high yield catabolism, at the trade-off of having less catalytic capacity for growth. These modelling predictions align with experimental results indicating that increased selectivity and production for hexanoic acid over butyrate is associated with a decrease in growth rate in C. lactatifermentans when grown on lactate as compared to glucose (Wang et al., 2022). Moreover, caproate selectivity has been shown to increase with hydraulic retention time (reduced growth rate) in continuous cultures of C. kluyveri and a chain elongation microbiome (Grootscholten et al., 2013; Kenealy & Waselefsky, 1985).
According to the optimized free energy changes predicted by ECM, thiolase reactions (ACACT) are the least exergonic in RBO (Supplemental Material S1, Fig. 1). Since these reactions operate near equilibrium, they have a smaller forward-to-reverse flux ratio (Noor et al., 2014) and therefore require a larger enzyme pool to sustain a given net flux through the pathway. The set of metabolite concentrations that minimizes pathway enzyme cost fixes the thiolase products (oxoacyl-CoAs) at low concentrations to drive these reactions (Supplemental Material S2). This heightens the demand for the proceeding enzymes in the pathway, 3-hydroxyacyl-CoA dehydrogenases (HACDs) since they are far from saturation (Fig. 2). Similarly, enoyl-CoA hydratase (ECOAH) reactions are thermodynamically constrained, meaning their reactant and product concentrations are kept high and low, respectively (Supplemental Material S2). This further increases the protein burden of HACD as well as the proceeding electron bifurcating acyl-CoA dehydrogenase (EBACD) (Fig. 2). At longer chain lengths, ECOAH reactions are predicted to be more favourable (Supplemental Material S1, Fig. 1), causing the rate at which enzyme cost increases with chain length to decrease.
Interestingly, when oxoacyl-CoA concentrations are constrained by a lower bound of 1 µM, as is the case for all other metabolites, products beyond C4 are deemed infeasible due to endergonic subsequent thiolase reactions. To reflect observations of chain elongation beyond C4, oxoacyl-CoAs can fall to sub-micromolar levels in this model. An alternative solution, as previously suggested in a similar instance with the citric acid cycle (Noor et al., 2014), is to posit enzymatic channelling. Channelling between ACACT and HACD would reduce the protein cost of both reactions by effectively merging the two into one favourable reaction. Indeed, channelling between ACACT, HACD, and ECOAH has been described with crystal structures of bacterial and human beta-oxidation complexes (Ishikawa et al., 2004; Xia et al., 2019). So long as a similar mechanism is active from C4 to C12, the trend of enzyme cost per ATP flux increasing with chain length is expected to hold. This has major implications for engineering chain elongating bacteria to produce MCFAs beyond C8, as the production of longer chain length would provide more ATP per mole of electron donor, but at the cost of a lower growth rate. As a result, compensatory efforts to bolster growth rate, for example through adaptive laboratory evolution (Sandberg et al., 2019), may be needed to achieve higher productivity.
Expanding and Controlling Chain Elongation Products with Metabolic Engineering
Metabolic engineering strategies for the expansion of RBO-derived products have been reviewed for non-chain elongating, model organisms with established genetic tools, such as Escherichia coli, Saccharomyces cerevisiae, and select acetogenic Clostridium species (Tarasava et al., 2022). The approach for these strains relies on the engineered reversal of β-oxidation accomplished through extensive strain engineering such as knockout or repression of native β-oxidation regulators and overexpression of genes from chain elongating bacteria and other oleaginous hosts (Tarasava et al., 2022). Most of these hosts do not natively rely on the RBO cycle for redox balancing or energy conservation, which is what makes the RBO cycle growth coupled in the chain elongating bacteria. Further, installing orthogonal systems for the engineered reversal of β-oxidation imposes an increased metabolic burden on non-chain elongating hosts as high expression is likely needed to ensure sufficient flux through the pathway (Liu et al., 2018). This can lead to redox limitations or inefficient substrate use for chain elongation in these non-chain elongating hosts, and approaches such as two-phase production are likely needed to maximize product yields (Lange et al., 2016). Production with non-chain elongating hosts will likely require further strain engineering to improve product yields and selectivity.
As an alternative to engineering model organisms, tools to genetically modify chain elongating bacteria are beginning to emerge (Agena et al., 2023; Cheng et al., 2019; Guss & Riley, 2021), which will open the door to expanded opportunities to produce oleochemicals via anaerobic fermentation. Chain elongating bacteria are ideal hosts for RBO-based bioproduction as the RBO cycle is innately growth coupled in these strains (no reconstruction needed) and as members of anaerobic communities can utilize solid and gaseous waste feedstocks rather than relying on refined substrates (e.g. sugars). Further, product yields in anaerobes are typically much greater as they do not synthesize as much biomass compared to aerobic systems and most electrons end up in products (Cueto-Rojas et al., 2015). However, the feasibility of pathways for the production of other oleochemicals, including, fatty alcohols, dicarboxylates, diols, and MKs, has not been explored in anaerobic chain elongating bacteria. Here, we propose potential production pathways and enzymes required to generate a wide range of oleochemicals (Fig. 3) and use pFBA and ECM to assess the feasibility and potential effects on ATP yield and growth rate.
Fig. 3.
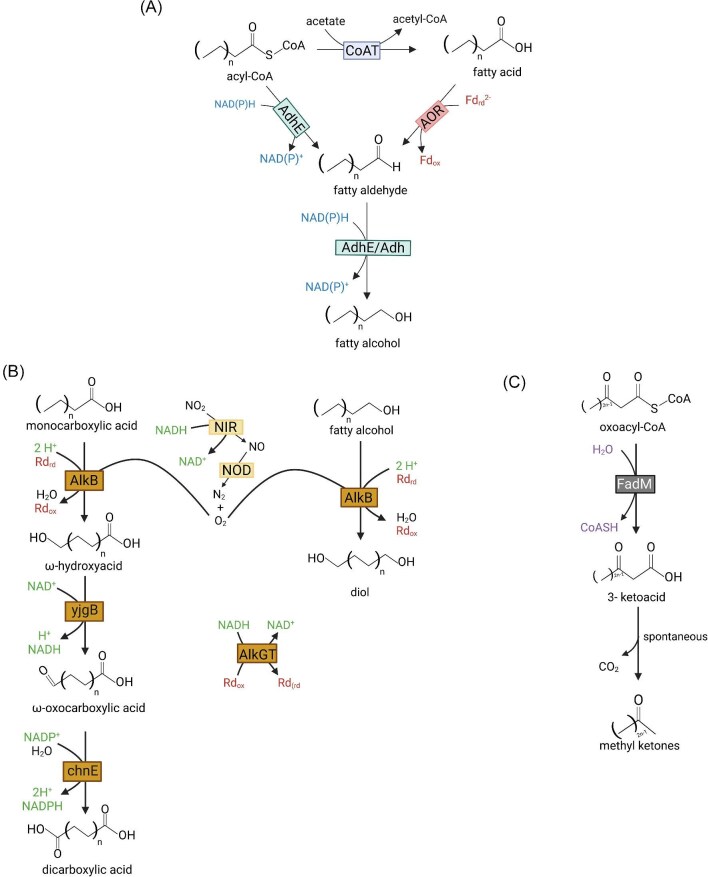
Proposed pathways for chain elongating bacteria product spectrum expansion. (A) Fatty alcohols pathway. AdhE = bifunctional aldehyde-alcohol dehydrogenase; AOR = aldehyde: ferredoxin oxidoreductase; FadM = acyl-CoA thioesterase. (B) Diol and dicarboxylic acids pathways. NIR = nitrite reductase; NOD = nitric oxide dismutase; AlkBGT = alkane 1-monooxygenase complex; yjgB = cinnamyl alcohol dehydrogenase; chnE = 6-oxohexanoate dehydrogenase; Rd = rubredoxin. (C) Methyl ketone pathway. FadM: thioesterase. Created with Biorender.com.
iFermCell193 was further modified to capture the reactions required for hexanoic acid conversion to other products (iFermCell356). Table 2 summarizes the potential pathway stoichiometries for the conversion of hexanoic acid (C6) to the corresponding fatty alcohol, dicarboxylate, and diol using lactate as an electron donor, along with the net ATP yields and overall Gibbs free energy change of reaction for each substrate–product pair as predicted by our model. All predicted pathway stoichiometries have overall favourable standard Gibbs free energies of reaction and release ≥50 kJ/mol per ATP required for ATP production. The model also predicts that net ATP yield is not negatively impacted by conversion into these other bioproducts, which indicates that their production can be growth coupled, similar to MCFAs. In the following sections, we describe potential metabolic engineering strategies to accomplish these conversions in chain elongating bacteria and discuss specific shifts in redox requirements that occur predicted by the model, along with the impact on the metabolic cost of the additional enzymes estimated by ECM (Fig. 4). The production of methyl ketones was also modelled, but it was found to negatively impact ATP yield (Fig. 5).
Table 2.
Predicted Pathway Stoichiometry for Different C6 Oleochemicals Produced from Lactate
| Product; pathway | Overall equation | Product yield (mol P/mol lactate) | ATP yield (mol ATP/mol Lactate) | ΔGr°′ (kJ/mol) | ΔGr°′ per ATP (kJ/mol/mol ATP) | Yield (g P/g lactate) |
|---|---|---|---|---|---|---|
| Carboxylate; RBO | Lactate + 0.667 H+ + 0.333 ADP → CO2 + 0.667 H2 + 0.333 H2O + 0.333 hexanoate + 0.333 ATP | 0.333 | 0.333 | −26.75 | −80.24 | 0.431 |
| Alcohol; RBO + AdhE | Lactate + H+ + 0.333 ADP → CO2 + 0.667 H2O + 0.333 hexanol + 0.333 ATP | 0.333 | 0.333 | −45.18 | −135.53 | 0.382 |
| Alcohol; RBO + AOR | Lactate + H+ + 0.5 ADP → CO2 + 0.667 H2O + 0.333 hexanol + 0.5 ATP | 0.333 | 0.500 | −45.18 | −90.35 | 0.382 |
| Dicarboxylate; RBO + AlkBGT | Lactate + H+ + 0.667 NO2− + 0.333 ADP → 0.333 adipate + CO2 + 0.667 H2 + H2O + 0.333 N2 + 0.333 ATP | 0.333 | 0.333 | −228.21 | −684.62 | 0.539 |
| Diol; RBO + AlkBGT | Lactate + 0.5 NO2− + 1.25 H+ + 0.5 ADP → 0.25 1,6-hexanediol + 0.25 acetate + CO2 + H2O + 0.25 N2 + 0.5 ATP | 0.250 | 0.500 | −191.63 | −383.26 | 0.332 |
RBO = reverse beta-oxidation, AdhE = bifunctional alcohol dehydrogenase, AOR = aldehyde:ferredoxin oxidoreductase, AlkBGT = alkane 1-monooxygenase complex
Fig. 4.
ECM results of RBO for production of novel chain elongation products from lactate. Enzyme cost per unit ATP flux is reported as a multiple of butyrate production's cost.
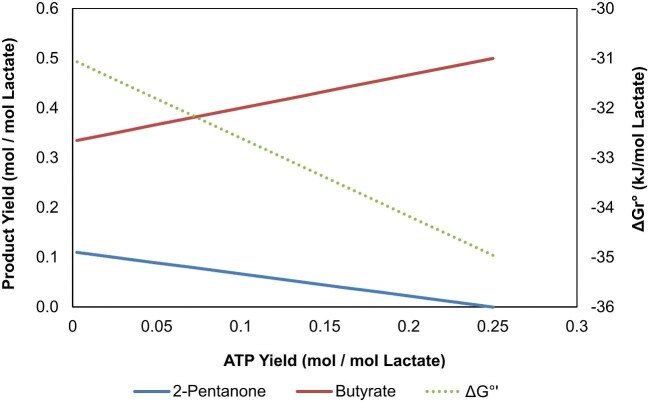
Fig. 5.
Production profiles for the co-production of 2-pentanone and butyrate from lactate and standard Gibbs free energy of reaction. Molar yields of ATP are negatively related to increasing production of 2-pentanone. Co-production of butyrate is required with increasing 2-pentanone production.
Fatty Alcohol Production
Medium-chain fatty alcohols (FAOHs) could be produced using MCFAs as building blocks. Even though FAOH production pathways (Fig. 3A) exist in anaerobic bacteria, such as acetogens and butanol-producing clostridia (Moon et al., 2016), this has yet to be observed in most chain elongating bacteria. In nature, acetogenic bacteria use aldehyde:ferredoxin oxidoreductase (AOR) to convert FAs into fatty aldehydes in monoculture or when they are co-cultured with chain elongating bacteria (Benito-Vaquerizo et al., 2020; Diender et al., 2016). In another instance, aldehyde-alcohol dehydrogenase (AdhE) can convert acyl-CoAs into aldehydes (Mehrer et al., 2018). In these two scenarios, specific electron carriers [one reduced ferredoxin and one NAD(P)H in the former and two NAD(P)H in the latter] are necessary to obtain the final product. Final conversion relies on the alcohol dehydrogenase (Adh) activity or the bifunctionality of AdhE (Liew et al., 2017). Ultimately, both AOR- and AdhE-based FAOH production should result in reduction in hydrogen evolution if implemented in the chain elongating bacteria as 4 electron equivalents are required for the additional reduction reactions.
We incorporated both AOR and AdhE FAOH synthesis pathways in our model and assessed their effects on FAOH production, redox balances, and ATP yield. In chain elongation to FAs, H2 is evolved as an electron sink to maintain redox balance. We hypothesized that instead of producing H2, these electron equivalents could be used to reduce the carboxylate to alcohol. Consistent with this, the model predicted that hexanol (C6 FAOH) production does not result in H2 production (Table 2), and instead, flux is redirected from HYD1 to RNF to produce NADH required by AOR and AdhE pathways. However, in the AOR pathway, the model predicted a net increase in ATP flux yield through the combined action of IMF and substrate-level phosphorylation as compared to hexanoic acid production (Supplementary Material S3).
ECM results show that hexanol production via AOR carries an enzyme cost per ATP flux of 2.1 times that of butyrate (Fig. 4), whereas hexanoic acid production requires 2.7 times more than butyrate (Fig. 2). This reflects the increased ATP yield from FAOH production with AOR and the prediction that the additional protein needed to reduce hexanoate to hexanol represents a small fraction of the pathway's total protein demand. In the AdhE case, ECM results show that hexanoic acid and hexanol production require approximately equal enzyme investments of 2.7 and 2.6 times the cost of butyrate production, due to bypassing the use of phosphate acetyltransferase (PTA) and acetate kinase (ACK) for alcohol production. In both AOR and AdhE cases, it can be expected that chain elongating bacteria engineered for FAOH production will not experience significant growth rate penalties compared to production of MCFA of the same chain length. In fact, growth rate could theoretically increase if the AOR pathway is implemented due to a reduction of enzyme cost per unit ATP flux (Fig. 6). A controlled metabolic engineering intervention would be required to avoid overexpression of heterologous FAOH production genes, which could be detrimental to growth rate, while successfully diverting flux from HYD1 to RNF to simultaneously balance the increased NADH demand and generate a proton motive force.
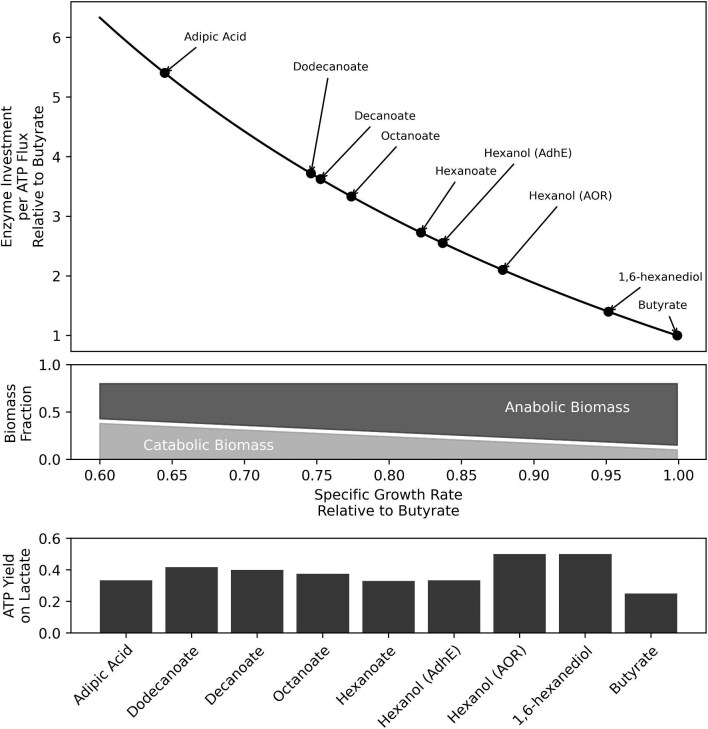
Fig. 6.
Top: Enzyme cost per ATP flux relative to butyrate versus specific growth rate relative to butyrate for bioproducts evaluated in this study, as derived from a simplified resource allocation model (Supplemental Material S1). Middle: Anabolic fraction of biomass corresponding to specific growth rate and catabolic fraction of biomass remaining to supply ATP at a given growth rate. Bottom: ATP yield on lactate for different chain elongation simulated by pFBA.
Dicarboxylate and Diol Production: Pathways Requiring Oxygen
The anaerobic oxidation of MCFAs into diols and dicarboxylic acids poses a significant metabolic engineering challenge in chain elongators, as the lack of oxygen as an electron acceptor results in a considerable decrease in reaction energetics. Methane oxidation is a clear example where the anaerobic pathway needs to be coupled to nitrite or sulphate reduction (Knittel & Boetius, 2009). Alkane-degrading microbes can anaerobically oxidize C–H bonds by fixing CO2 and converting alkanes into FAs. It is hypothesized that this mechanism is initiated by an ethylbenzene dehydrogenase and the whole pathway happens at the great expense of 6 ATP equivalents, suggesting that an unexplored energy-coupling mechanism may take place in these organisms given the low ATP yields in anaerobes (Supplemental Material S1, Fig. 2) (Heider et al., 2016; Shou et al., 2021). Alternatively, alkane monooxygenase complexes such as alkane 1-monooxygenase complex (AlkBGT) have been used in the hydroxylation of terminal ω-carbons of different compounds, including FAs (Clomburg et al., 2015). However, implementing AlkBGT in anaerobes is currently infeasible due to its requirement for O2.
To solve these problems, we envision making use of another hypothesized hydroxylation mechanism in anaerobes: the intra-aerobic pathway (Ettwig et al., 2010). Achieving such conditions requires “oxygen donors”, such as nitrite, hydrogen peroxide, or perchlorate, whose decomposition provides the required oxygen for AlkBGT. Since oxygen presence can be problematic in this scenario, it is important to maintain its intracellular concentrations low, which could be achieved by tuning nitrite reductase/nitric oxide dismutase (NIR/NOD) and AlkBGT expression or even improving AlkBGT rates through protein engineering. If oxygen levels are still high enough to disrupt cell function, a metabolic strategy that could be explored would be to confine the oxygen generation reaction within microcompartments, pseudo-organelles common in various organisms, including anaerobes, which have been used in several applications, such as protecting cells from toxic intermediates and oxygen (Heinhorst & Cannon, 2020; Kennedy et al., 2021).
In our proposed pathway, the first module comprises oxygen generation, where nitrite is reduced to nitric oxide by NIR (Wang et al., 2019), followed by the action of NOD to yield N2 and O2. The second module encompasses the oxidation reactions, where AlkB would be used to oxidize FAs and FAOHs to ω-hydroxyacids and diols, respectively, using rubredoxin as an electron carrier (Fig. 3B). The remaining portion of the AlkBGT complex is responsible for regenerating reduced rubredoxin by consuming NADH. Further oxidation of ω-hydroxyacids to dicarboxylic acids, however, would require additional enzymes, such as cinnamyl alcohol dehydrogenase (yjgB) and 6-oxohexanoate dehydrogenase (chnE) (Clomburg et al., 2015), even though it is reported that AlkBGT complex can further oxidize hydroxyls to carboxyls and even ester groups, a phenomenon called overoxidation (van Nuland et al., 2017). Protein discovery and engineering will play a key role in bringing these pathways to life in the chain elongating bacteria. For example, understanding anaerobic carboxylation mechanisms and discovering other related pathways would circumvent the need of using oxygen and microcompartments. Additionally, to the best of our knowledge, no FAOHs have been used as substrates for AlkBGT, which would require protein characterization and engineering to achieve diol production if intra-aerobic conditions are necessary.
The reactions described above were incorporated into our model and the effect on redox balance and ATP yield was assessed for the production of adipic acid and 1,6-hexanediol from hexanoic acid. NO2− was supplied in our simulations as a substrate to source O2 for the hydroxylation step. Nitrite reduction via NIR requires an investment of electron equivalents from NADH. However, the model predicted that the production of adipic acid does not require additional electron equivalents from H2 nor lactate as H2 evolution and molar yield of adipic acid from lactate was the same as in the hexanoic acid case. For adipic acid, the ω-hydroxyacid intermediate that is produced by AlkBGT is subsequently oxidized, releasing an NADH that is required for the reduction of NO2− and balancing redox. However, in the case of 1,6-hexanediol, an additional electron source is required for the initial reduction of hexanoic acid to hexanol. Thus, diol production is not as carbon efficient because additional electron equivalents from lactate are required to reduce both terminal ends into alcohols, leading to the co-production of acetate to balance carbon, yielding a net increase in ATP yield due to substrate-level phosphorylation through PTA/ACK. ECM found that 1,6-hexanediol carries an enzyme cost lower than that of hexanol produced via AOR, 1.4 times the cost of butyrate. Cost decreases since both pathways share an ATP yield of 0.5, while diol production does away with the CoA transferase (CoAT) reaction. This reaction is itself enzymatically expensive due to its low driving force, and it increases the cost of PTA and ACK reactions as CoAT requires a higher acetate concentration (Supplemental Material S2). Adipic acid production requires 5.4 times more enzyme investment per ATP flux than butyrate (Fig. 4). The heightened enzyme cost is largely due to the unfavourable YJGB reaction, in which 6-hydroxyhexanoate reduces NAD+ to form 6-oxohexanoate. Minimal enzyme cost is achieved when 6-hydroxyhexanoate concentration is high, driving the YJGB reaction but indirectly increasing the demand for termination enzymes (CoAT, PTA, and ACK) through compensatory heightening of hexanoate and acetate concentrations (Supplemental Material S2). This suggests that it may be more challenging to achieve high adipic acid productivity due to a reduction in growth rate. On the other hand, 1,6-hexanediol production could even increase growth rate vs. hexanoic acid, similarly to hexanol production via AOR (Fig. 6).
MK Production
MKs are another chemical class that can be produced using MCFAs as precursors. Currently, in the engineered RBO cycle, MKs are obtained from the hydrolysis of oxoacyl-CoA molecules by the thioesterase FadM. This reaction initially generates ketoacids, which in turn spontaneously decarboxylate to result in the final MK (Fig. 3C) (Yan et al., 2020). In addition to FadM introduction, other metabolic engineering strategies are necessary to achieve MK production in chain elongating bacteria. The first strategy would be to substitute the native CoATs by FadM, translocating termination from acyl-CoA to oxoacyl-CoA. Since the RBO cycle is essential to redox balance and ATP production in chain elongating bacteria, sole acetone and 2-butanone production would not be possible, as FadM would terminate before one complete turn of the RBO cycle. Therefore, to optimize MK production, it is necessary to allow MCFA with MK co-production.
FadM was incorporated into our model to assess the potential to produce 2-pentanone from 6-oxohexanoyl-CoA. The model predicted that 2-pentanone production is not optimal for ATP yield, which aligns with the early termination initiated by FadM at 6-oxohexanoyl-CoA, where the ATP from the complete elongation to hexanoic acid is not produced. To assess the effect of 2-pentanone production on ATP yield, we fixed 2-pentanone production at various values and determined the overall pathway stoichiometry at each point using pFBA with ATP yield set as the objective function (Table 3). It was found that while 2-pentanone production is thermodynamically feasible for the scenarios modelled, its production is negatively coupled with butyrate and ATP production (Fig. 5). CO2 production also increases with increasing 2-pentanone production due to the spontaneous decarboxylation of the ketoacid. In isolation, this loss of CO2 severely impacts the carbon efficiency of this production pathway. However, it could be alleviated if MK production was conducted in the context of a microbial community, where other functional guilds could recycle the CO2 (Baleeiro et al., 2023).
Table 3.
Selected Predicted Pathway Stoichiometries for 2-Pentanone Production
| Product; pathway | Overall equation | Product yield (mol P/mol lactate) | ATP yield (mol ATP/mol lactate) | ΔGr°′ (kJ/mol) | ΔGr°′ per ATP (kJ/mol/mol ATP) | Yield (g P/g lactate) |
|---|---|---|---|---|---|---|
| Methyl ketone; RBO + FadM | Lactate + 0.5 H+ + 0.25 ADP → 0.5 butyrate + CO2 + H2 + 0.25 ATP | 0.000 | 0.250 | −14.99 | −59.96 | 0.000 |
| Lactate + 0.545 H+ + 0.183 ADP → 0.03 2pentanone + 0.455 butyrate + 1.03 CO2 + 1.03 H2 + 0.183 ATP | 0.030 | 0.183 | −12.13 | −66.48 | 0.029 | |
| Lactate + 0.575 H+ + 0.138 ADP → 0.05 2-pentanone + 0.425 butyrate + 1.05 CO2 + 1.05 H2 + 0.138 ATP | 0.050 | 0.138 | −10.23 | −74.38 | 0.048 | |
| Lactate + 0.605 H+ + 0.093 ADP → 0.07 2-pentanone + 0.395 butyrate + 1.07 CO2 + 1.07 H2 + 0.093 ATP | 0.070 | 0.093 | −8.32 | −89.97 | 0.067 | |
| Lactate + 0.665 H+ + 0.003 ADP → 0.11 2-pentanone + 0.335 butyrate + 1.11 CO2 + 1.11 H2 + 0.003 ATP | 0.110 | 0.003 | −4.51 | −1804.79 | 0.105 |
RBO = reverse beta-oxidation, FadM = oxoacyl-CoA thioesterase
Conclusion
Developing chain elongating bacteria as a bioproduction platform for oleochemical synthesis could play a crucial role in sustainable manufacturing from carbon-rich waste streams. Our analyses demonstrate that the synthesis of C9–C12 FAs, as well as conversion to their corresponding fatty alcohols, dicarboxylic acids, diols, and MKs, is thermodynamically feasible and generates sufficient ATP production for growth coupling. In the case of C9–C12 FAs, enzyme cost analyses point towards a trade-off between growth rate and ATP yield with increasing chain length, which may explain why chain elongation in nature terminates at chain lengths of C8. While some experimental data support our results for selectivity for hexanoate over butyrate production in C. lactatifermentans and C. kluyveri at lower growth rates (Kenealy & Waselefsky, 1985; Wang et al., 2022), investigating the effect of chain length on growth rate and ATP yield considering other substrates across the diversity of chain elongating bacteria is still needed.
Other aspects that need to be investigated to understand limitations in chain length include the effect of product toxicity and enzyme–substrate affinity for longer chain FAs. This includes strategies chain elongators employ to protect against MCFA toxicity and evaluation of the substrate range that can be accommodated by native RBO cycle enzymes. To address these problems, genetic engineering tools need to be developed, including genetic cargo delivery systems, the construction of regulatory element libraries, and gene knock-in/knock-out methods to delete native chain elongating bacteria genes and to enable heterologous gene expression. These genetic modifications should allow the production of a broad range of oleochemicals through chain elongation using the pathways proposed in this work. The production of longer chain length FAs and their subsequent conversion into alcohols are ideal targets for initial demonstrations of oleochemical production using engineered chain elongating bacteria as these products are not redox limited in our analyses. However, the functionalization of the aliphatic end of the FAs required for dicarboxylate and diol production will be a significant challenge due to the anaerobic requirements of chain elongating bacteria. While we present an avenue to accomplish this through nitrite reduction and the use of microcompartments, screening of other enzymes that can functionalize FAs without oxygen will be important to chain elongating bacteria metabolic engineering efforts. Additionally, the production of MKs was found to be feasible, though not growth coupled as it requires carboxylate co-production for ATP production. Given previous demonstrations of MK production in E. coli under microaerobic conditions (Lan et al., 2013), other conditions and production pathways should be investigated to improve yields in chain elongators.
As a proof of concept, our modelling work focused on the conversion of single carbon substrates to various oleochemicals using chain elongating bacteria in isolation. The deployment of such engineered strains will likely require investigation into how mixed substrates and potential interactions with other microbes will affect product profiles. For example, the presence of more electron-rich substrates, such as glucose, may push fermentation towards less desirable, shorter chain products (e.g. acetate, butyrate) as it is advantageous for growth rather than chain elongation based on the insights from our resource allocation modelling.
Overall, our analyses indicate that chain elongating bacteria are a promising biomanufacturing chassis for accessing several oleochemical product classes. This work provides a basis for which bioproducts could be accessed through metabolic engineering of chain elongating bacteria and highlights potential barriers, including trade-offs between growth and product yield, which need to be further investigated. Developing genetic tools for chain elongating bacteria will be critical for validating our modelling predictions, understanding the fundamental physiology of this functional guild, and expanding their product spectrum, in isolation as pure cultures, and in the context of self-assembled and synthetic microbiomes.
Supplementary Material
Acknowledgement
The authors would like to thank Dr. Adam M. Guss for their helpful discussion of the results.
Contributor Information
Ethan Agena, Department of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, ON M5T 3E5, Canada.
Ian M Gois, Department of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, ON M5T 3E5, Canada.
Connor M Bowers, Department of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, ON M5T 3E5, Canada.
Radhakrishnan Mahadevan, Department of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, ON M5T 3E5, Canada; Institute of Biomedical Engineering, 164 College St., Toronto, ON M5S 3E2, Canada.
Matthew J Scarborough, Department of Civil and Environmental Engineering, University of Vermont, Burlington, VT 05405-0156, USA.
Christopher E Lawson, Department of Chemical Engineering & Applied Chemistry, University of Toronto, Toronto, ON M5T 3E5, Canada.
Funding
This work was supported by the Natural Sciences and Engineering Research Council of Canada (ALLRP 580897-22, RGPIN-2021-02684) awarded to C.E.L.
Conflict of interest
The authors declare no conflicts of interest.
References
- Agena E., Gois I., Mahadevan R., Dempster A., Nigel N., Lawson C. (2023). Investigating the genetic tractability of anaerobic chain elongating bacteria for use towards sustainable chemical production. SIMB 2023 Annual Meeting, Minneapolis, MN. [Google Scholar]
- Angenent L. T., Richter H., Buckel W., Spirito C. M., Steinbusch K. J. J., Plugge C. M., Strik D. P. B. T. B., Grootscholten T. I. M., Buisman C. J. N., Hamelers H. V. M. (2016). Chain elongation with reactor microbiomes: open-culture biotechnology to produce biochemicals. Environmental Science & Technology, 50(6), 2796–2810. 10.1021/acs.est.5b04847 [DOI] [PubMed] [Google Scholar]
- Baleeiro F. C. F., Raab J., Kleinsteuber S., Neumann A., Sträuber H. (2023). Mixotrophic chain elongation with syngas and lactate as electron donors. Microbial Biotechnology, 16(2), 322–336. 10.1111/1751-7915.14163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basan M. (2018). Resource allocation and metabolism: The search for governing principles. Current Opinion in Microbiology, 45, 77–83. 10.1016/j.mib.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Bäumler M., Schneider M., Ehrenreich A., Liebl W., Weuster-Botz D. (2022). Synthetic co-culture of autotrophic Clostridium carboxidivorans and chain elongating Clostridium kluyveri monitored by flow cytometry. Microbial Biotechnology, 15(5), 1471–1485. 10.1111/1751-7915.13941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B. D., Kimball E. H., Gao M., Osterhout R., Van Dien S. J., Rabinowitz J. D. (2009). Absolute Metabolite Concentrations and Implied Enzyme Active Site Occupancy in Escherichia Coli. Nat Chem Biol, 5(8), 593–599. 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Vaquerizo S., Diender M., Parera Olm I., Martins dos Santos V. A. P., Schaap P. J., Sousa D. Z., Suarez-Diez M. (2020). Modeling a co-culture of Clostridium autoethanogenum and Clostridium kluyveri to increase syngas conversion to medium-chain fatty-acids. Computational and Structural Biotechnology Journal, 18, 3255–3266. 10.1016/j.csbj.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckel W., Thauer R. K. (2018). Flavin-based electron bifurcation, a new mechanism of biological energy coupling. Chemical Reviews, 118(7), 3862–3886. 10.1021/acs.chemrev.7b00707 [DOI] [PubMed] [Google Scholar]
- Candry P., Ganigué R. (2021). Chain elongators, friends, and foes. Current Opinion in Biotechnology, 67, 99–110. 10.1016/j.copbio.2021.01.005 [DOI] [PubMed] [Google Scholar]
- Cheng C., Li W., Lin M., Yang S.-T. (2019). Metabolic engineering of Clostridium carboxidivorans for enhanced ethanol and butanol production from syngas and glucose. Bioresource Technology, 284, 415–423. 10.1016/j.biortech.2019.03.145 [DOI] [PubMed] [Google Scholar]
- Clomburg J. M., Blankschien M. D., Vick J. E., Chou A., Kim S., Gonzalez R. (2015). Integrated engineering of β-oxidation reversal and ω-oxidation pathways for the synthesis of medium chain ω-functionalized carboxylic acids. Metabolic Engineering, 28, 202–212. 10.1016/j.ymben.2015.01.007 [DOI] [PubMed] [Google Scholar]
- Contreras-Dávila C. A., Carrión V. J., Vonk V. R., Buisman C. N. J., Strik D. P. B. T. B. (2020). Consecutive lactate formation and chain elongation to reduce exogenous chemicals input in repeated-batch food waste fermentation. Water Research, 169, 115215. 10.1016/j.watres.2019.115215 [DOI] [PubMed] [Google Scholar]
- Cueto-Rojas H. F., van Maris A. J. A., Wahl S. A., Heijnen J. J. (2015). Thermodynamics-based design of microbial cell factories for anaerobic product formation. Trends in Biotechnology, 33(9), 534–546. 10.1016/j.tibtech.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Diender M., Stams A. J. M., Sousa D. Z. (2016). Production of medium-chain fatty acids and higher alcohols by a synthetic co-culture grown on carbon monoxide or syngas. Biotechnology for Biofuels, 9(1), 82. 10.1186/s13068-016-0495-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettwig K. F., Butler M. K., Le Paslier D., Pelletier E., Mangenot S., Kuypers M. M. M., Schreiber F., Dutilh B. E., Zedelius J., de Beer D., Gloerich J., Wessels H. J. C. T., van Alen T., Luesken F., Wu M. L., van de Pas-Schoonen K. T., Op den Camp H. J. M., Janssen-Megens E. M., Francoijs K.-J., Strous M. (2010). Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature, 464(7288), 543–548. 10.1038/nature08883 [DOI] [PubMed] [Google Scholar]
- Fernández-Blanco C., Veiga M. C., Kennes C. (2022). Efficient production of n-caproate from syngas by a co-culture of Clostridium aceticum and Clostridium kluyveri. Journal of Environmental Management, 302, 113992. 10.1016/j.jenvman.2021.113992 [DOI] [PubMed] [Google Scholar]
- Flamholz A., Noor E., Bar-Even A., Liebermeister W., Milo R. (2013). Glycolytic strategy as a tradeoff between energy yield and protein cost. Proceedings of the National Academy of Sciences, 110(24), 10039–10044. 10.1073/pnas.1215283110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamholz A. I., Goyal A., Fischer W. W., Newman D. K., Phillips R. (2024). The proteome is a terminal electron acceptor. bioRxiv, 10.1101/2024.01.31.578293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grootscholten T. I. M., Strik D. P. B. T. B., Steinbusch K. J. J., Buisman C. J. N., Hamelers B. (2014). Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Applied Energy, 116(1), 223–229. 10.1016/j.apenergy.2013.11.061 [DOI] [Google Scholar]
- Grootscholten T. I. M., Steinbusch K. J. J., Hamelers H. V. M., Buisman C. J. N. (2013). Improving Medium Chain Fatty Acid Productivity Using Chain Elongation by Reducing the Hydraulic Retention Time in an Upflow Anaerobic Filter. Bioresource Technology, 136, 735–738. 10.1016/j.biortech.2013.02.114. [DOI] [PubMed] [Google Scholar]
- Guss A. M., Riley L. A. (2021). Engineered microbes for conversion of organic compounds to butanol and methods of use (United States Patent US20210024965A1). https://patents.google.com/patent/US20210024965A1/en (1 March 2024, date last accessed).
- Haas T., Krause R., Weber R., Demler M., Schmid G. (2018). Technical photosynthesis involving CO2 electrolysis and fermentation. Nature Catalysis, 1(1), 32–39. 10.1038/s41929-017-0005-1 [DOI] [Google Scholar]
- Heider J., Szaleniec M., Sünwoldt K., Boll M. (2016). Ethylbenzene dehydrogenase and related molybdenum enzymes involved in oxygen-independent alkyl chain hydroxylation. Microbial Physiology, 26(1-3), 45–62. 10.1159/000441357 [DOI] [PubMed] [Google Scholar]
- Heinhorst S., Cannon G. C. (2020). Bacterial microcompartments. In D. Jendrossek (Ed.), Bacterial organelles and organelle-like inclusions (Vol. 34). Springer. 10.1007/978-3-030-60173-7_6 [DOI] [Google Scholar]
- Holtzapple M. T., Wu H., Weimer P. J., Dalke R., Granda C. B., Mai J., Urgun-Demirtas M. (2022). Microbial communities for valorizing biomass using the carboxylate platform to produce volatile fatty acids: A review. Bioresource Technology, 344, 126253. 10.1016/j.biortech.2021.126253 [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Tsuchiya D., Oyama T., Tsunaka Y., Morikawa K. (2004). Structural basis for channelling mechanism of a fatty acid β-oxidation multienzyme complex. The EMBO Journal, 23(14), 2745–2754. 10.1038/sj.emboj.7600298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy W. R., Waselefsky D. M. (1985) Studies on the Substrate Range of Clostridium Kluyveri; the Use of Propanol and Succinate. Arch. Microbiol, 141 (3), 187–194. 10.1007/BF00408056. [DOI] [Google Scholar]
- Kennedy N. W., Mills C. E., Nichols T. M., Abrahamson C. H., Tullman-Ercek D. (2021). Bacterial microcompartments: Tiny organelles with big potential. Current Opinion in Microbiology, 63, 36–42. 10.1016/j.mib.2021.05.010 [DOI] [PubMed] [Google Scholar]
- Knittel K., Boetius A. (2009). Anaerobic oxidation of methane: progress with an unknown process. Annual Review of Microbiology, 63(1), 311–334. 10.1146/annurev.micro.61.080706.093130 [DOI] [PubMed] [Google Scholar]
- Lan E. I., Dekishima Y., Chuang D. S., Liao J. C. (2013). Metabolic engineering of 2-pentanone synthesis in Escherichia coli. AIChE Journal, 59(9), 3167–3175. 10.1002/aic.14086 [DOI] [Google Scholar]
- Lange J., Takors R., Blombach B. (2017). Zero-growth bioprocesses: A challenge for microbial production strains and bioprocess engineering. Engineering in Life Sciences, 17(1), 27–35. 10.1002/elsc.201600108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. E., Hixson K. K., Conrad T. M., Lerman J. A., Charusanti P., Polpitiya A. D., Adkins J. N., Schramm G., Purvine S. O., Lopez-Ferrer D., Weitz K. K., Eils R., König R., Smith R. D., Palsson B. Ø. (2010). Omic data from evolved E. coli are consistent with computed optimal growth from genome-scale models. Molecular Systems Biology, 6,1, 390. 10.1038/msb.2010.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Hinderberger J., Seedorf H., Zhang J., Buckel W., Thauer R. K. (2008). Coupled ferredoxin and crotonyl coenzyme A (CoA) reduction with NADH catalyzed by the butyryl-CoA dehydrogenase/Etf complex from Clostridium kluyveri. Journal of Bacteriology, 190(3), 843–850. 10.1128/jb.01417-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F., Henstra A. M., Kӧpke M., Winzer K., Simpson S. D., Minton N. P. (2017). Metabolic engineering of Clostridium autoethanogenum for selective alcohol production. Metabolic Engineering, 40, 104–114. 10.1016/j.ymben.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Schumacher J., Wan X., Lou C., Wang B. (2018). Orthogonality and burdens of heterologous AND gate gene circuits in E. coli. ACS Synthetic Biology, 7(2), 553–564. 10.1021/acssynbio.7b00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. (1969). Oxygen sensitivity of various anaerobic bacteria. Applied Microbiology, 18(5), 723–727. 10.1128/am.18.5.723-727.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynd L. R., T. Beckham G., M. Guss A., N. Jayakody L., M. Karp E., Maranas C., L. McCormick R., Amador-Noguez D., J. Bomble Y., H. Davison B., Foster C., E. Himmel M., K. Holwerda E., S. Laser M., Yu Ng C., G. Olson D., Román-Leshkov Y., T. Trinh C., A. Tuskan G., E. Wyman C. (2022). Toward low-cost biological and hybrid biological/catalytic conversion of cellulosic biomass to fuels. Energy & Environmental Science, 15(3), 938–990. 10.1039/D1EE02540F [DOI] [Google Scholar]
- Mehrer C. R., Incha M. R., Politz M. C., Pfleger B. F. (2018). Anaerobic production of medium-chain fatty alcohols via a β-reduction pathway. Metabolic Engineering, 48, 63–71. 10.1016/j.ymben.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. G., Jang Y.-S., Cho C., Lee J., Binkley R., Lee S. Y. (2016). One hundred years of clostridial butanol fermentation. FEMS Microbiology Letters, 363(3), fnw001. 10.1093/femsle/fnw001 [DOI] [PubMed] [Google Scholar]
- Nelson R. S., Peterson D. J., Karp E. M., Beckham G. T., Salvachúa D. (2017). Mixed carboxylic acid production by Megasphaera elsdenii from glucose and lignocellulosic hydrolysate. Fermentation,10, 3(1), Article 1. 10.3390/fermentation3010010 [DOI] [Google Scholar]
- Noor E., Bar-Even A., Flamholz A., Reznik E., Liebermeister W., Milo R. (2014). Pathway thermodynamics highlights kinetic obstacles in central metabolism. PLOS Computational Biology, 10(2), e1003483. 10.1371/journal.pcbi.1003483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor E., Haraldsdóttir H. S., Milo R., Fleming R. M. T. (2013). Consistent estimation of Gibbs energy using component contributions. PLOS Computational Biology, 9(7), e1003098. 10.1371/journal.pcbi.1003098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten J. K., Zou Y., Papoutsakis E. T. (2022). The potential of caproate (hexanoate) production using Clostridium kluyveri syntrophic cocultures with Clostridium acetobutylicum or Clostridium saccharolyticum. Frontiers in Bioengineering and Biotechnology, 10, 965614. 10.3389/fbioe.2022.965614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg T. E., Salazar M. J., Weng L. L., Palsson B. O., Feist A. M. (2019). The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metabolic Engineering, 56, 1–16. 10.1016/j.ymben.2019.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough M. J., Hamilton J. J., Erb E. A., Donohue T. J., Noguera D. R. (2020). Diagnosing and predicting mixed-culture fermentations with unicellular and guild-based metabolic models. mSystems, 5(5), e00755–20 10.1128/msystems.00755-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarborough M. J., Lawson C. E., DeCola A. C., Gois I. M. (2022). Microbiomes for sustainable biomanufacturing. Current Opinion in Microbiology, 65, 8–14. 10.1016/j.mib.2021.09.015 [DOI] [PubMed] [Google Scholar]
- Scarborough M. J., Lawson C. E., Hamilton J. J., Donohue T. J., Noguera D. R. (2018). Metatranscriptomic and thermodynamic insights into medium-chain fatty acid production using an anaerobic microbiome. mSystems, 3(6), e00221–18. 10.1128/mSystems.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou L.-B., Liu Y.-F., Zhou J., Liu Z.-L., Zhou L., Liu J.-F., Yang S.-Z., Gu J.-D., Mu B.-Z. (2021). New evidence for a hydroxylation pathway for anaerobic alkane degradation supported by analyses of functional genes and signature metabolites in oil reservoirs. AMB Express, 11(1), 18. 10.1186/s13568-020-01174-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatopoulou P., Malkowski J., Conrado L., Brown K., Scarborough M. (2020). Fermentation of organic residues to beneficial chemicals: A review of medium-chain fatty acid production. Processes,1571, 8(12), Article 12. 10.3390/pr8121571 [DOI] [Google Scholar]
- Strik D. P. B. T. B., Ganigué R., Angenent L. T. (2022). Editorial: Microbial chain elongation- close the carbon loop by connecting-communities. Frontiers in Bioengineering and Biotechnology, 10, 894490. https://www.frontiersin.org/articles/10.3389/fbioe.2022.894490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasava K., Lee S. H., Chen J., Köpke M., Jewett M. C., Gonzalez R. (2022). Reverse β-oxidation pathways for efficient chemical production. Journal of Industrial Microbiology and Biotechnology,49, 2, kuac003. 10.1093/jimb/kuac003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. (1977). Energy Conservation in Chemotrophic Anaerobic Bacteria. Bacteriological Reviews, 41 (1), 100–180. 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nuland Y. M., de Vogel F. A., Scott E. L., Eggink G., Weusthuis R. A. (2017). Biocatalytic, one-pot diterminal oxidation and esterification of n-alkanes for production of α,ω-diol and α,ω-dicarboxylic acid esters. Metabolic Engineering, 44, 134–142. 10.1016/j.ymben.2017.10.005 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhou W., Gao J., Ren C., Xu Y. (2022). Revealing the Characteristics of Glucose- and Lactate-Based Chain Elongation for Caproate Production by Caproicibacterium lactatifermentans through Transcriptomic, Bioenergetic, and Regulatory Analyses. mSystems, 7(5), e00534–22. 10.1128/msystems.00534-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tamiev D., Alagurajan J., DiSpirito A. A., Phillips G. J., Hargrove M. S. (2019). The role of the NADH-dependent nitrite reductase, Nir, from Escherichia coli in fermentative ammonification. Archives of Microbiology, 201(4), 519–530. 10.1007/s00203-018-1590-3 [DOI] [PubMed] [Google Scholar]
- Xia C., Fu Z., Battaile K. P., Kim J.-J. P. (2019). Crystal structure of human mitochondrial trifunctional protein, a fatty acid β-oxidation metabolon. Proceedings of the National Academy of Sciences, 116(13), 6069–6074. 10.1073/pnas.1816317116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Simmons T. R., Cordell W. T., Hernández Lozada N. J., Breckner C. J., Chen X., Jindra M. A., Pfleger B. F. (2020). Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone and 2-undecanone. Metabolic Engineering, 61, 335–343. 10.1016/j.ymben.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhou Y., Wang Y., Wu T., Li X., Li D., Tao Y. (2017). Production of high-concentration n-caproic acid from lactate through fermentation using a newly isolated Ruminococcaceae bacterium CPB6. Biotechnology for Biofuels, 10(1), 102. 10.1186/s13068-017-0788-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.