Abstract
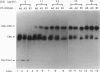
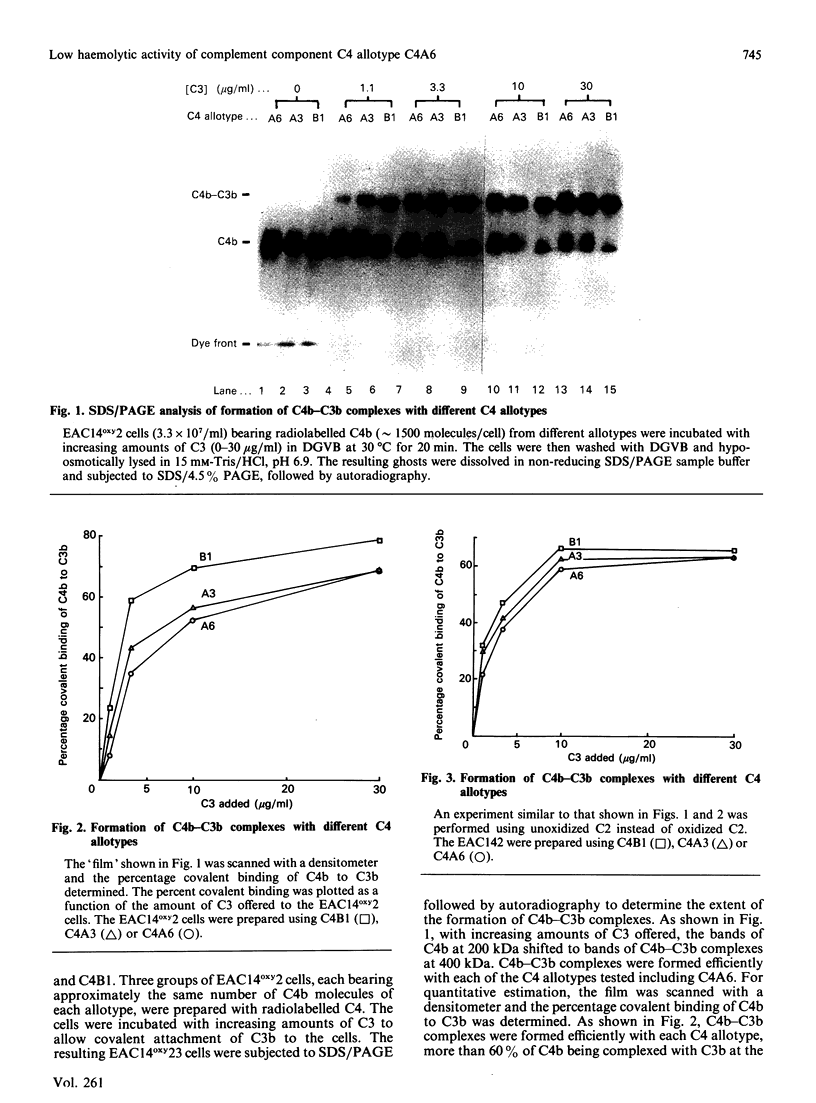
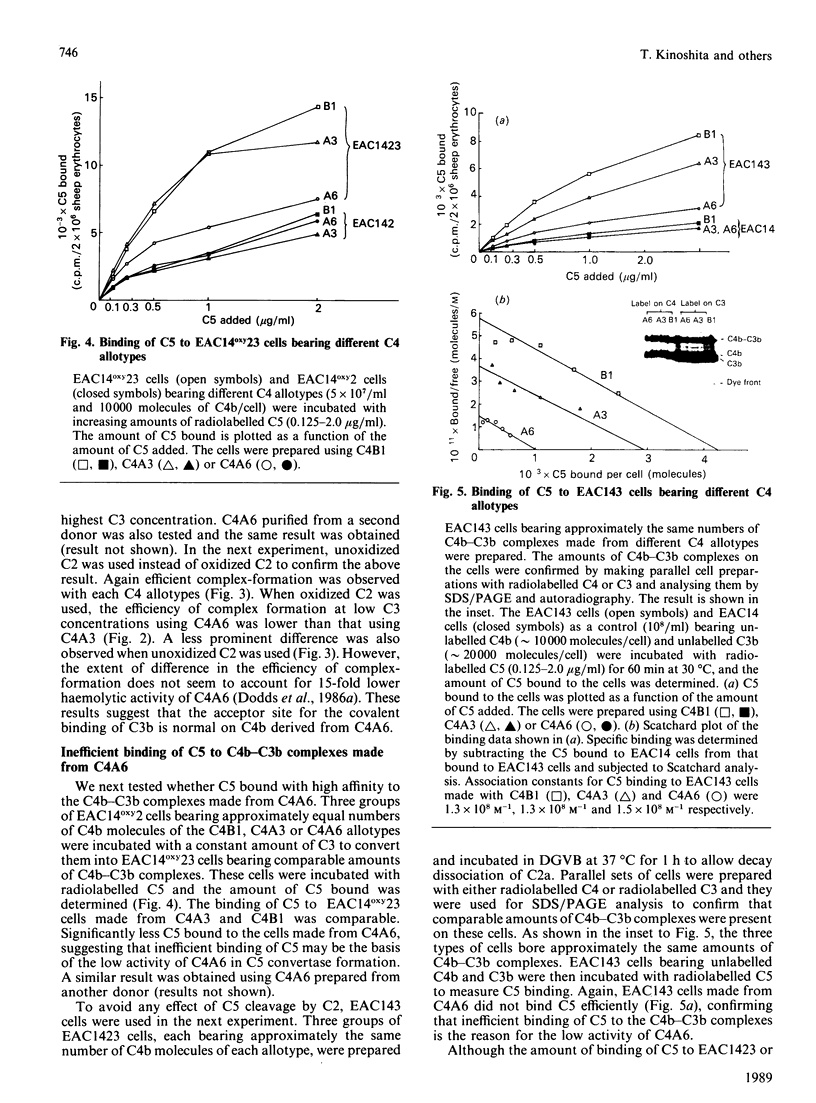
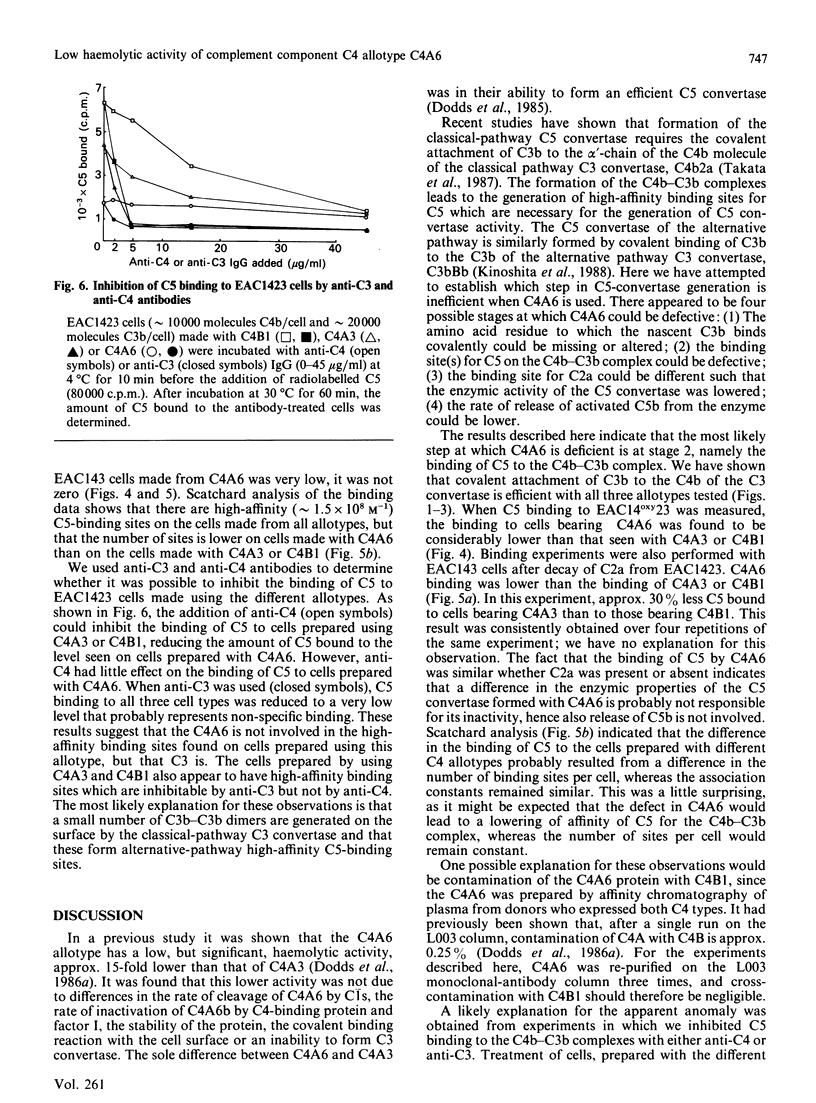
We have compared the C5-convertase-forming ability of different C4 allotypes, including the C4A6 allotype, which has low haemolytic activity and which has previously been shown to be defective in C5-convertase formation. Recent studies suggest that C4 plays two roles in the formation of the C5 convertase from the C3 convertase. Firstly, C4b acts as the binding site for C3 which, upon cleavage by C2, forms a covalent linkage with the C4b. Secondly, C4b with covalently attached C3b serves to form a high-affinity binding site for C5. Purified allotypes C4A3, C4B1 and C4A6 were used to compare these two activities of C4. Covalently linked C4b-C3b complexes were formed on sheep erythrocytes with similar efficiency by using C4A3 and C4B1, indicating that the two isotypes behave similarly as acceptors for covalent attachment of C3b. C4A6 showed normal efficiency in this function. However, cells bearing C4b-C3b complexes made from C4A6 contained only a small number of high-affinity binding sites for C5. Therefore a lack of binding of C5 to the C4b C3b complexes is the reason for the inefficient formation of C5 convertase by C4A6. The small number of high-affinity binding sites created, when C4A6 was used, were tested for inhibition by anti-C3 and anti-C4. Anti-C4 did not inhibit C5 binding, whereas anti-C3 did. This suggests that the sites created when C4A6 is used to make C3 convertase may be C3b-C3b dimers, and hence the low haemolytic activity of C4A6 results from the creation of low numbers of alternative-pathway C5-convertase sites.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belt K. T., Yu C. Y., Carroll M. C., Porter R. R. Polymorphism of human complement component C4. Immunogenetics. 1985;21(2):173–180. doi: 10.1007/BF00364869. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Gagnon J., Porter R. R. Amino acid sequence around the proposed thiolester bond of human complement component C4 and comparison with the corresponding sequences from C3 and alpha 2-macroglobulin. Biosci Rep. 1981 May;1(5):423–429. doi: 10.1007/BF01116192. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Law S. K., Reid K. B., Sim R. B. Structure, organization, and regulation of the complement genes. Annu Rev Immunol. 1988;6:161–195. doi: 10.1146/annurev.iy.06.040188.001113. [DOI] [PubMed] [Google Scholar]
- DiScipio R. G. The conversion of human complement component C5 into fragment C5b by the alternative-pathway C5 convertase. Biochem J. 1981 Dec 1;199(3):497–504. doi: 10.1042/bj1990497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds A. W., Law S. K., Porter R. R. The origin of the very variable haemolytic activities of the common human complement component C4 allotypes including C4-A6. EMBO J. 1985 Sep;4(9):2239–2244. doi: 10.1002/j.1460-2075.1985.tb03920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds A. W., Law S. K., Porter R. R. The purification and properties of some less common allotypes of the fourth component of human complement. Immunogenetics. 1986;24(5):279–285. doi: 10.1007/BF00395532. [DOI] [PubMed] [Google Scholar]
- Dodds A. W., Law S. K. Structural basis of the binding specificity of the thioester-containing proteins, C4, C3 and alpha-2-macroglobulin. Complement. 1988;5(2):89–97. doi: 10.1159/000463039. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Podack E. R., Cooper N. R. The interaction of C5 with C3b in free solution: a sufficient condition for cleavage by a fluid phase C3/C5 convertase. J Immunol. 1980 Jan;124(1):326–331. [PubMed] [Google Scholar]
- Isenman D. E., Young J. R. The molecular basis for the difference in immune hemolysis activity of the Chido and Rodgers isotypes of human complement component C4. J Immunol. 1984 Jun;132(6):3019–3027. [PubMed] [Google Scholar]
- Kinoshita T., Takata Y., Kozono H., Takeda J., Hong K. S., Inoue K. C5 convertase of the alternative complement pathway: covalent linkage between two C3b molecules within the trimolecular complex enzyme. J Immunol. 1988 Dec 1;141(11):3895–3901. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Dodds A. W., Porter R. R. A comparison of the properties of two classes, C4A and C4B, of the human complement component C4. EMBO J. 1984 Aug;3(8):1819–1823. doi: 10.1002/j.1460-2075.1984.tb02052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauff G., Alper C. A., Awdeh Z., Batchelor J. R., Bertrams J., Bruun-Petersen G., Dawkins R. L., Démant P., Edwards J., Grosse-Wilde H. Statement on the nomenclature of human C4 allotypes. Immunobiology. 1983 Mar;164(2):184–191. doi: 10.1016/s0171-2985(83)80009-6. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Molecular organization and function of the complement system. Annu Rev Biochem. 1988;57:321–347. doi: 10.1146/annurev.bi.57.070188.001541. [DOI] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- O'Neill G. J., Yang S. Y., Dupont B. Two HLA-linked loci controlling the fourth component of human complement. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5165–5169. doi: 10.1073/pnas.75.10.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. Enharncement of the hemolytic activity of the second component of human complement by oxidation. J Exp Med. 1967 Dec 1;126(6):1013–1025. doi: 10.1084/jem.126.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B., Porter R. R. The proteolytic activation systems of complement. Annu Rev Biochem. 1981;50:433–464. doi: 10.1146/annurev.bi.50.070181.002245. [DOI] [PubMed] [Google Scholar]
- Roos M. H., Mollenhauer E., Démant P., Rittner C. A molecular basis for the two locus model of human complement component C4. Nature. 1982 Aug 26;298(5877):854–856. doi: 10.1038/298854a0. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Tack B. F. The beta-Cys-gamma-Glu thiolester bond in human C3, C4, and alpha 2-macroglobulin. Springer Semin Immunopathol. 1983;6(4):259–282. doi: 10.1007/BF02116276. [DOI] [PubMed] [Google Scholar]
- Takata Y., Kinoshita T., Kozono H., Takeda J., Tanaka E., Hong K., Inoue K. Covalent association of C3b with C4b within C5 convertase of the classical complement pathway. J Exp Med. 1987 Jun 1;165(6):1494–1507. doi: 10.1084/jem.165.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura N. An incompatibility in the reaction of the second component of human complement with the fourth component of guinea-pig complement. Immunology. 1970 Feb;18(2):203–212. [PMC free article] [PubMed] [Google Scholar]
- Yu C. Y., Belt K. T., Giles C. M., Campbell R. D., Porter R. R. Structural basis of the polymorphism of human complement components C4A and C4B: gene size, reactivity and antigenicity. EMBO J. 1986 Nov;5(11):2873–2881. doi: 10.1002/j.1460-2075.1986.tb04582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]