Abstract
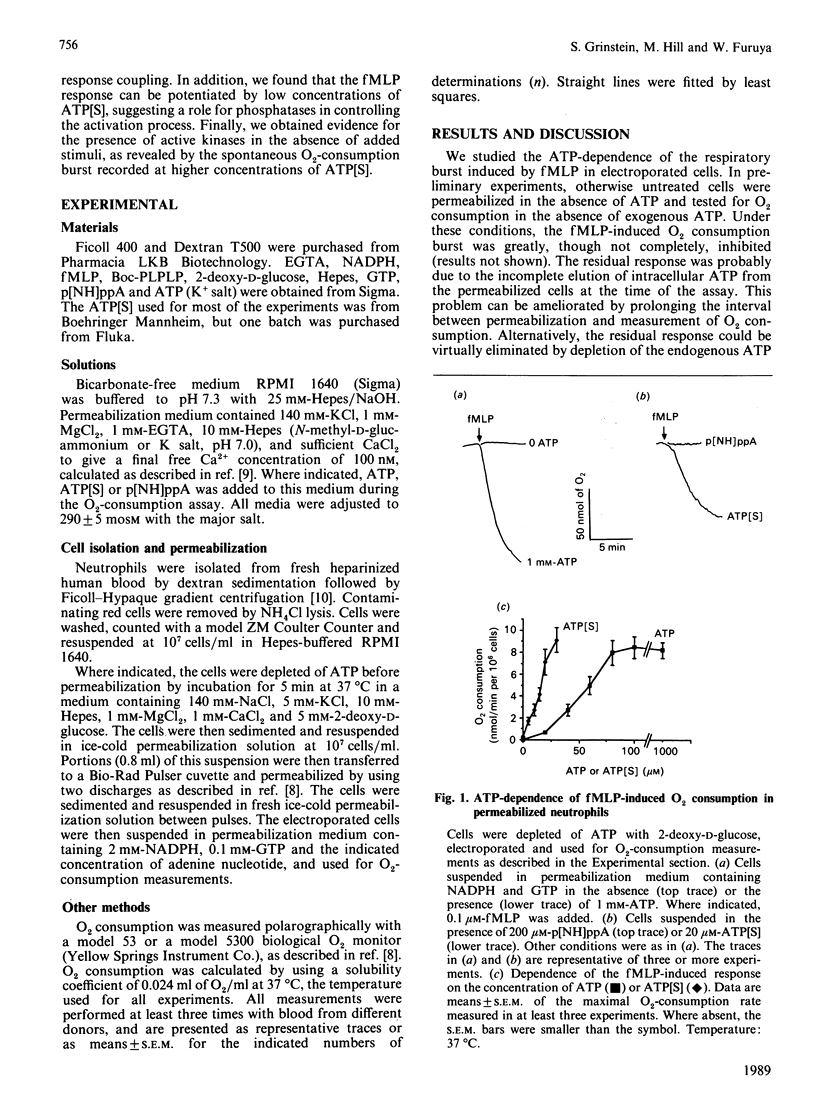
Electrically permeabilized human neutrophils were used to study the mechanism of activation of the NADPH oxidase by chemotactic factors. The respiratory burst elicited by formyl-methionyl-leucyl-phenylalanine (fMLP) was strictly dependent on the addition of ATP. The response was also supported by adenosine 5'-[gamma-thio]triphosphate (ATP[S]), but not by the non-hydrolysable analogue (p[NH]ppA). In the presence of ATP, displacement of fMLP from its receptor by antagonist peptides resulted in the abrupt termination of the O2-consumption burst. In contrast, the response persisted after displacement of fMLP when ATP[S] was present. This finding is consistent with the formation of biologically active thiophosphoproteins which are resistant to cleavage by cellular phosphatases. Accordingly, lower concentrations of ATP[S], as compared with ATP, were required to support the fMLP response. The data indicate that protein phosphatases control the extent and duration of the response in cells stimulated with chemoattractants. Unlike ATP, sub-millimolar concentrations of ATP[S] elicited a spontaneous respiratory burst in the absence of fMLP or other stimuli. This effect was inhibited by p[NH]ppA and was not observed in intact (non-permeabilized) cells, indicating interaction of ATP[S] with an intracellular adenine-nucleotide-binding site, possibly a protein kinase. These results suggest that protein kinases are active in neutrophils in the absence of exogenous stimuli, but that accumulation of the essential phosphoprotein(s) is normally prevented by the ongoing vigorous phosphatase activity. It is conceivable that control of the respiratory burst is exerted by inhibition of phosphatase activity, instead of or in addition to the more commonly postulated activation of protein kinases.
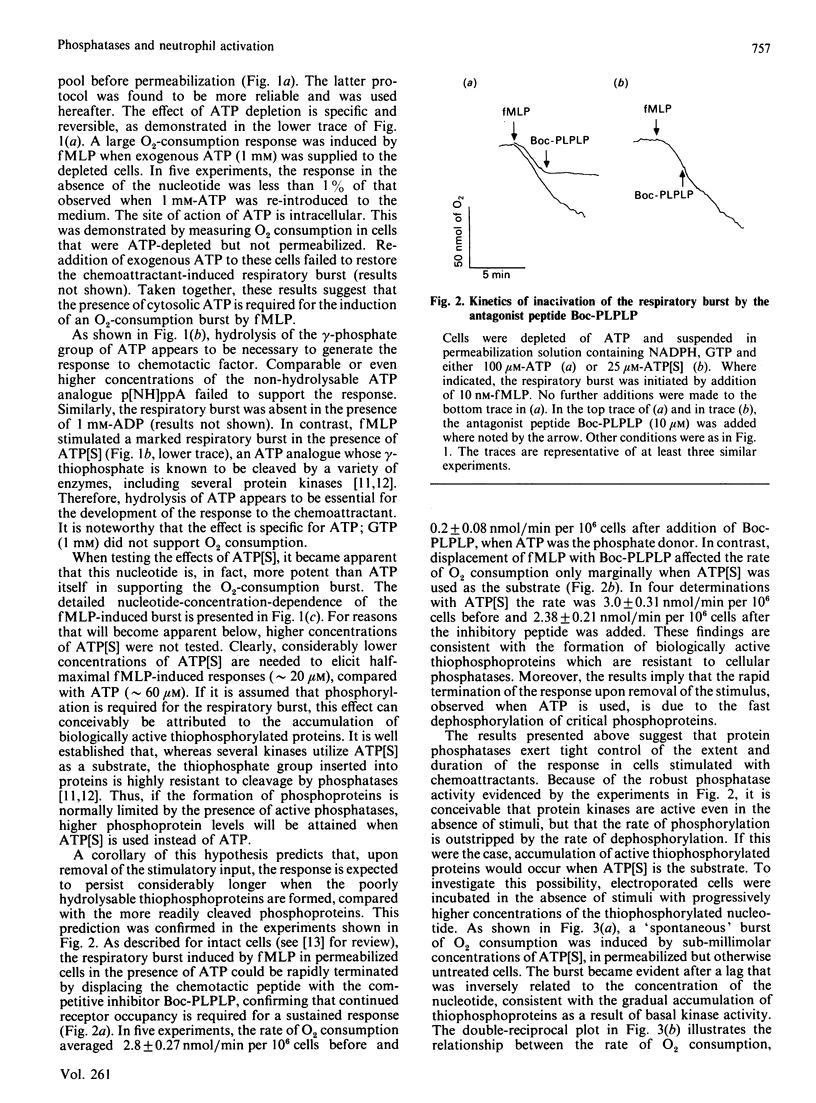
Full text
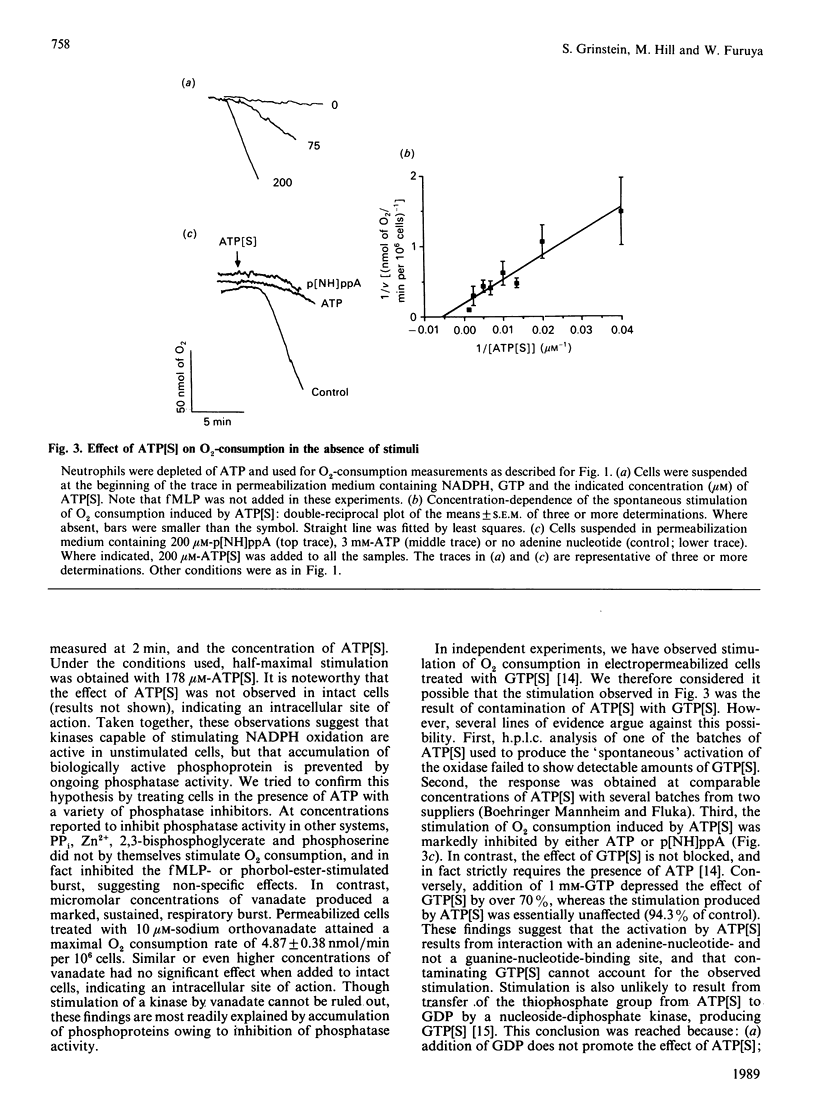
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy P., Hoar P. E., Kerrick W. G. Irreversible thiophosphorylation and activation of tension in functionally skinned rabbit ileum strips by [35S]ATP gamma S. J Biol Chem. 1979 Nov 10;254(21):11148–11153. [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide production by digitonin-stimulated guinea pig granulocytes. The effects of N-ethyl maleimide, divalent cations; and glycolytic and mitochondrial inhibitors on the activation of the superoxide generating system. J Clin Invest. 1978 Apr;61(4):1088–1096. doi: 10.1172/JCI109008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne M. D., Luszczynska H. M., Kunzi M. Evidence for protein kinase C in bovine adrenocortical membrane preparations using [35S] gamma-thio-ATP as a phosphate donor. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(6):433–444. [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Effects of anaerobiosis and inhibitors on O2-production by human granulocytes. Blood. 1975 Jun;45(6):851–861. [PubMed] [Google Scholar]
- Curnutte J. T., Kuver R., Scott P. J. Activation of neutrophil NADPH oxidase in a cell-free system. Partial purification of components and characterization of the activation process. J Biol Chem. 1987 Apr 25;262(12):5563–5569. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Receptor-mediated activation of electropermeabilized neutrophils. Evidence for a Ca2+- and protein kinase C-independent signaling pathway. J Biol Chem. 1988 Feb 5;263(4):1779–1783. [PubMed] [Google Scholar]
- Kraft A. S., Berkow R. L. Tyrosine kinase and phosphotyrosine phosphatase activity in human promyelocytic leukemia cells and human polymorphonuclear leukocytes. Blood. 1987 Aug;70(2):356–362. [PubMed] [Google Scholar]
- Ligeti E., Doussiere J., Vignais P. V. Activation of the O2(.-)-generating oxidase in plasma membrane from bovine polymorphonuclear neutrophils by arachidonic acid, a cytosolic factor of protein nature, and nonhydrolyzable analogues of GTP. Biochemistry. 1988 Jan 12;27(1):193–200. doi: 10.1021/bi00401a029. [DOI] [PubMed] [Google Scholar]
- Nasmith P. E., Mills G. B., Grinstein S. Guanine nucleotides induce tyrosine phosphorylation and activation of the respiratory burst in neutrophils. Biochem J. 1989 Feb 1;257(3):893–897. doi: 10.1042/bj2570893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura N., Curnutte J. T., Roberts R. L., Babior B. M. Relationship of protein phosphorylation to the activation of the respiratory burst in human neutrophils. Defects in the phosphorylation of a group of closely related 48-kDa proteins in two forms of chronic granulomatous disease. J Biol Chem. 1988 May 15;263(14):6777–6782. [PubMed] [Google Scholar]
- Seifert R., Rosenthal W., Schultz G., Wieland T., Gierschick P., Jakobs K. H. The role of nucleoside-diphosphate kinase reactions in G protein activation of NADPH oxidase by guanine and adenine nucleotides. Eur J Biochem. 1988 Jul 15;175(1):51–55. doi: 10.1111/j.1432-1033.1988.tb14165.x. [DOI] [PubMed] [Google Scholar]
- Sklar L. A. Ligand-receptor dynamics and signal amplification in the neutrophil. Adv Immunol. 1986;39:95–143. doi: 10.1016/s0065-2776(08)60349-1. [DOI] [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]


