Abstract
Reaction patterns for the hydrolysis of chromophoric glycosides from cello-oligosaccharides and lactose by the cellobiohydrolases (CBH I and CBH II) purified from Trichoderma reesei and Penicillium pinophilum were determined. They coincide with those found for the parent unsubstituted sugars. CBH I enzyme from both organisms attacks these substrates in a random manner. Turnover numbers are, however, low and do not increase appreciably as a function of the degree of polymerization of the substrates. The active-site topology of the CBH I from T. reesei was further probed by equilibrium binding experiments with cellobiose, cellotriose, lactose and some of their derivatives. These point to a single interaction site (ABC), spatially restricted as deduced from the apparent independency of the thermodynamic parameters. It appears that the putative subsite A can accommodate a galactopyranosyl or glucopyranosyl group, and subsite B a glucopyranosyl group, whereas in subsite C either a glucopyranosyl or a chromophoric group can be bound, scission occurring between subsites B and C. The apparent kinetic parameters (turnover numbers) for the hydrolysis of cello-oligosaccharides (and their derivatives) by the CBH II type enzyme increase as a function of chain length, indicative of an extended binding site (A-F). Its architecture allows for specific binding of beta-(1----4)-glucopyranosyl groups in subsites A, B and C. Binding of a chromophore in subsite C produces a non-hydrolysable complex. The thermodynamic interaction parameters of some ligands common to both type of enzyme were compared: these substantiate the conclusions reached above.
Full text
PDF


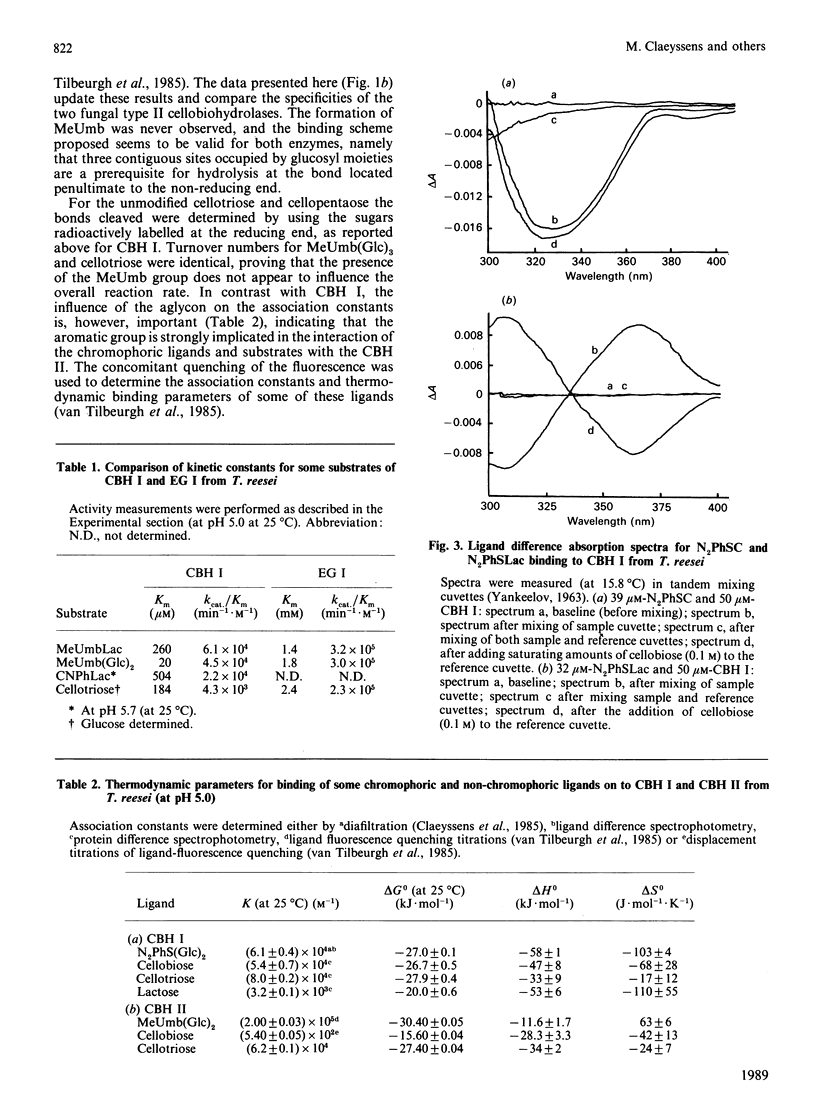
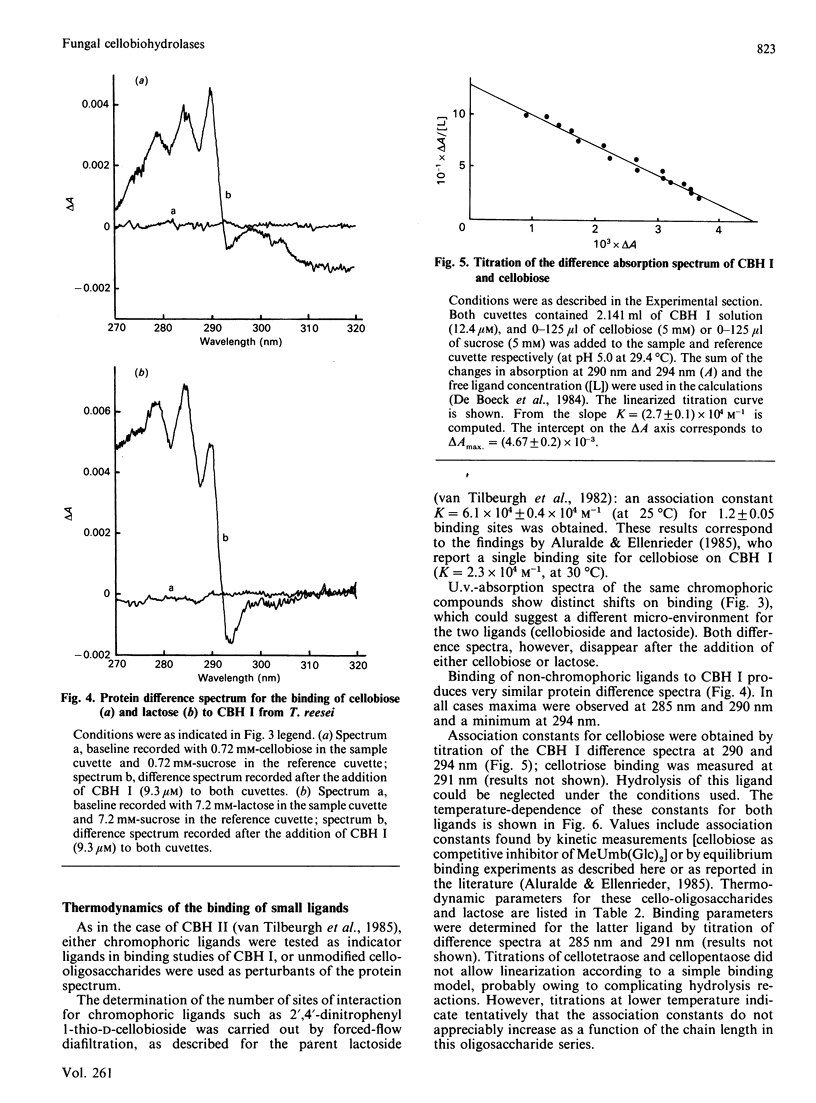
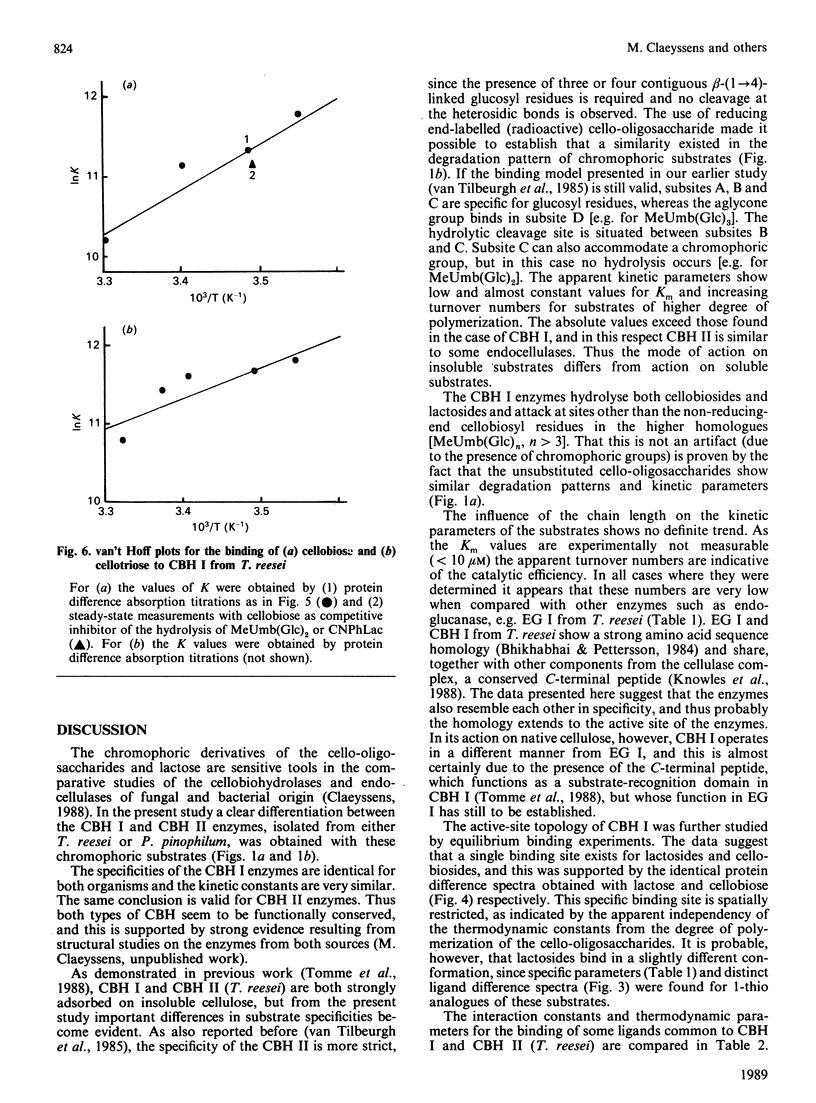

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Boeck H., Lis H., van Tilbeurgh H., Sharon N., Loontiens F. G. Binding of simple carbohydrates and some of their chromophoric derivatives to soybean agglutinin as followed by titrimetric procedures and stopped flow kinetics. J Biol Chem. 1984 Jun 10;259(11):7067–7074. [PubMed] [Google Scholar]
- Fägerstam L. G., Pettersson L. G. The cellulolytic complex of Trichoderma reesei QM 9414. An immunochemical approach. FEBS Lett. 1979 Feb 15;98(2):363–367. doi: 10.1016/0014-5793(79)80218-5. [DOI] [PubMed] [Google Scholar]
- Tomme P., Van Tilbeurgh H., Pettersson G., Van Damme J., Vandekerckhove J., Knowles J., Teeri T., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Analysis of domain function in two cellobiohydrolases by limited proteolysis. Eur J Biochem. 1988 Jan 4;170(3):575–581. doi: 10.1111/j.1432-1033.1988.tb13736.x. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Penicillium pinophilum. Synergism between enzyme components in solubilizing cellulose with special reference to the involvement of two immunologically distinct cellobiohydrolases. Biochem J. 1986 Feb 15;234(1):93–99. doi: 10.1042/bj2340093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANKEELOV J. A., Jr SPLIT-COMPARTMENT MIXING CELLS FOR DIFFERENCE SPECTROSCOPY. Anal Biochem. 1963 Sep;6:287–289. doi: 10.1016/0003-2697(63)90138-6. [DOI] [PubMed] [Google Scholar]
- van Tilbeurgh H., Pettersson G., Bhikabhai R., De Boeck H., Claeyssens M. Studies of the cellulolytic system of Trichoderma reesei QM 9414. Reaction specificity and thermodynamics of interactions of small substrates and ligands with the 1,4-beta-glucan cellobiohydrolase II. Eur J Biochem. 1985 Apr 15;148(2):329–334. doi: 10.1111/j.1432-1033.1985.tb08843.x. [DOI] [PubMed] [Google Scholar]


