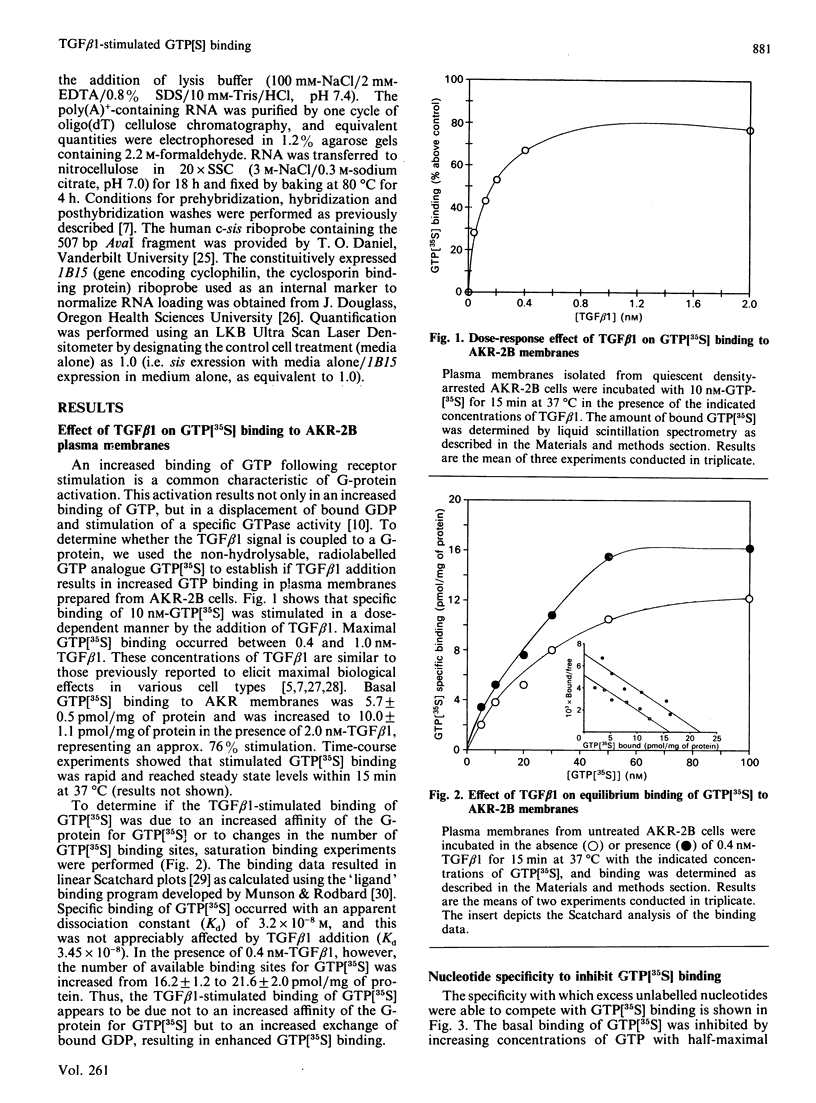
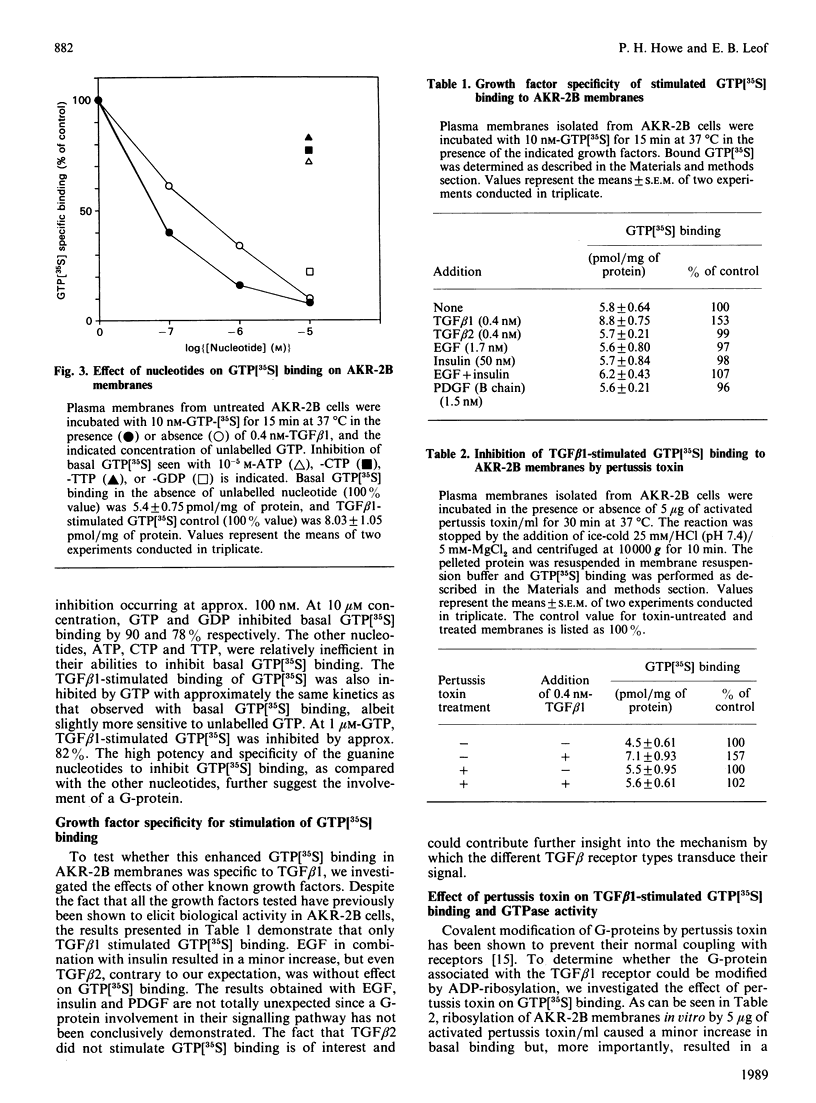
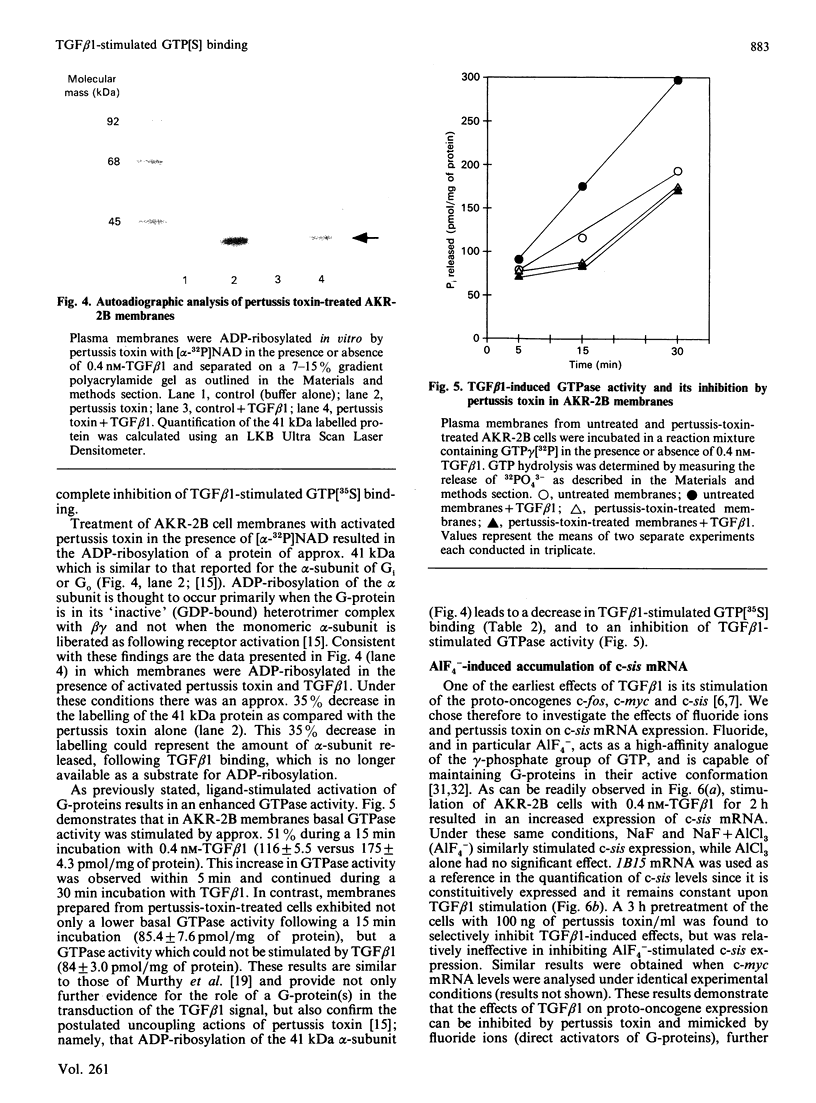
Abstract
Transforming growth factor beta (TGF beta 1) is a potent regulator of DNA synthesis and cellular proliferation. In this study, we investigated whether the growth stimulatory signal of TGF beta 1 is transduced intracellularly by guanine nucleotide regulatory proteins (G-proteins). In plasma membranes from AKR-2B cells, TGF beta 1 increased binding of the radiolabelled, non-hydrolysable GTP analogue, guanosine 5'-[gamma-[35S]thio]triphosphate (GTP[35S]), in a dose-dependent manner. Maximal effects occurred between 0.4 and 1.0 nM-TGF beta 1. Specific binding of GTP[35S] occurred with a Kd of 3.2 x 10(-8) M which was not affected by addition of TGF beta 1. Instead, TGF beta 1 increased the number of available binding sites for GTP[35S] from 16.2 +/- 1.2 to 21.6 +/- 2.1 pmol/mg of protein. GTP[35S] binding was both nucleotide- and growth-factor-specific. Only guanine nucleotides were able to compete for binding, and of the growth factors tested (epidermal growth factor, platelet-derived growth factor, insulin, TGF beta 1 and TGF beta 2) only TGF beta 1 affected GTP[35S] binding. TGF beta 1 increased GTPase activity, as determined by the release of 32PO4(3-) from GTP gamma[32P], from 116 +/- 5.5 to 175 +/- 4.3 pmol/mg of protein following a 15 min incubation. Pretreatment of the membranes with pertussis toxin inhibited both TGF beta 1-stimulated binding of GTP[35S] as well as TGF beta 1-stimulated GTPase activity. These inhibitory actions of pertussis toxin were associated with toxin-induced ADP-ribosylation of a 41 kDa protein. Furthermore, the stimulatory effects of TGF beta 1 on c-sis mRNA expression were shown to be pertussis-toxin sensitive and could be mimicked by direct activation of G-proteins with AIF4-. These results demonstrate that in AKR-2B cells a pertussis-toxin-sensitive guanine nucleotide regulatory protein(s) is coupled to TGF beta 1 receptor binding.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Ashkenazi A., Peralta E. G., Winslow J. W., Ramachandran J., Capon D. J. Functionally distinct G proteins selectively couple different receptors to PI hydrolysis in the same cell. Cell. 1989 Feb 10;56(3):487–493. doi: 10.1016/0092-8674(89)90251-1. [DOI] [PubMed] [Google Scholar]
- Avissar S., Schreiber G., Danon A., Belmaker R. H. Lithium inhibits adrenergic and cholinergic increases in GTP binding in rat cortex. Nature. 1988 Feb 4;331(6155):440–442. doi: 10.1038/331440a0. [DOI] [PubMed] [Google Scholar]
- Bigay J., Deterre P., Pfister C., Chabre M. Fluoride complexes of aluminium or beryllium act on G-proteins as reversibly bound analogues of the gamma phosphate of GTP. EMBO J. 1987 Oct;6(10):2907–2913. doi: 10.1002/j.1460-2075.1987.tb02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Burns D. L., Hewlett E. L., Moss J., Vaughan M. Pertussis toxin inhibits enkephalin stimulation of GTPase of NG108-15 cells. J Biol Chem. 1983 Feb 10;258(3):1435–1438. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambard J. C., Paris S., L'Allemain G., Pouysségur J. Two growth factor signalling pathways in fibroblasts distinguished by pertussis toxin. Nature. 1987 Apr 23;326(6115):800–803. doi: 10.1038/326800a0. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Daniel T. O., Fen Z. Distinct pathways mediate transcriptional regulation of platelet-derived growth factor B/c-sis expression. J Biol Chem. 1988 Dec 25;263(36):19815–19820. [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Williams L. T. Agents that increase cAMP accumulation block endothelial c-sis induction by thrombin and transforming growth factor-beta. J Biol Chem. 1987 Sep 5;262(25):11893–11896. [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Evans S. W., Beckner S. K., Farrar W. L. Stimulation of specific GTP binding and hydrolysis activities in lymphocyte membrane by interleukin-2. Nature. 1987 Jan 8;325(7000):166–168. doi: 10.1038/325166a0. [DOI] [PubMed] [Google Scholar]
- Fischer J. B., Schonbrunn A. The bombesin receptor is coupled to a guanine nucleotide-binding protein which is insensitive to pertussis and cholera toxins. J Biol Chem. 1988 Feb 25;263(6):2808–2816. [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Halenda S. P., Volpi M., Zavoico G. B., Sha'afi R. I., Feinstein M. B. Effects of thrombin, phorbol myristate acetate and prostaglandin D2 on 40-41 kDa protein that is ADP ribosylated by pertussis toxin in platelets. FEBS Lett. 1986 Aug 18;204(2):341–346. doi: 10.1016/0014-5793(86)80840-7. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Endo T., Massagué J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-beta. J Biol Chem. 1987 May 15;262(14):6443–6446. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Imamura K., Kufe D. Colony-stimulating factor 1-induced Na+ influx into human monocytes involves activation of a pertussis toxin-sensitive GTP-binding protein. J Biol Chem. 1988 Oct 5;263(28):14093–14098. [PubMed] [Google Scholar]
- Imamura K., Sherman M. L., Spriggs D., Kufe D. Effect of tumor necrosis factor on GTP binding and GTPase activity in HL-60 and L929 cells. J Biol Chem. 1988 Jul 25;263(21):10247–10253. [PubMed] [Google Scholar]
- Katada T., Tamura M., Ui M. The A protomer of islet-activating protein, pertussis toxin, as an active peptide catalyzing ADP-ribosylation of a membrane protein. Arch Biochem Biophys. 1983 Jul 1;224(1):290–298. doi: 10.1016/0003-9861(83)90212-6. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Srivastava S. K., Anderson P. S., Aaronson S. A. Ras p21 proteins with high or low GTPase activity can efficiently transform NIH/3T3 cells. Cell. 1986 Feb 28;44(4):609–617. doi: 10.1016/0092-8674(86)90270-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Reep B., Chang K. J. Treatment of human platelets with trypsin, thrombin, or collagen inhibits the pertussis toxin-induced ADP-ribosylation of a 41-kDa protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5880–5883. doi: 10.1073/pnas.83.16.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Getz M. J., Moses H. L. Transforming growth factor type beta regulation of actin mRNA. J Cell Physiol. 1986 Apr;127(1):83–88. doi: 10.1002/jcp.1041270111. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Moses H. L. Modulation of transforming growth factor type beta action by activated ras and c-myc. Mol Cell Biol. 1987 Jul;7(7):2649–2652. doi: 10.1128/mcb.7.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litosch I. Guanine nucleotide and NaF stimulation of phospholipase C activity in rat cerebral-cortical membranes. Studies on substrate specificity. Biochem J. 1987 May 15;244(1):35–40. doi: 10.1042/bj2440035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Possible involvement of a GTP-binding protein, the substrate of islet-activating protein, in receptor-mediated signaling responsible for cell proliferation. J Biol Chem. 1987 Sep 15;262(26):12463–12467. [PubMed] [Google Scholar]
- Murthy U. S., Anzano M. A., Stadel J. M., Greig R. Coupling of TGF-beta-induced mitogenesis to G-protein activation in AKR-2B cells. Biochem Biophys Res Commun. 1988 May 16;152(3):1228–1235. doi: 10.1016/s0006-291x(88)80416-9. [DOI] [PubMed] [Google Scholar]
- Nishimoto I., Hata Y., Ogata E., Kojima I. Insulin-like growth factor II stimulates calcium influx in competent BALB/c 3T3 cells primed with epidermal growth factor. Characteristics of calcium influx and involvement of GTP-binding protein. J Biol Chem. 1987 Sep 5;262(25):12120–12126. [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Sternweis P. C., Gilman A. G. The subunits of the stimulatory regulatory component of adenylate cyclase. Resolution of the activated 45,000-dalton (alpha) subunit. J Biol Chem. 1983 Sep 25;258(18):11369–11376. [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Olashaw N. E., Pledger W. J. Cellular mechanisms regulating proliferation. Adv Second Messenger Phosphoprotein Res. 1988;22:139–173. [PubMed] [Google Scholar]
- Paris S., Chambard J. C., Pouysségur J. Coupling between phosphoinositide breakdown and early mitogenic events in fibroblasts. Studies with fluoroaluminate, vanadate, and pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1977–1983. [PubMed] [Google Scholar]
- Pike L. J., Lefkowitz R. J. Activation and desensitization of beta-adrenergic receptor-coupled GTPase and adenylate cyclase of frog and turkey erythrocyte membranes. J Biol Chem. 1980 Jul 25;255(14):6860–6867. [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]
- Yatani A., Mattera R., Codina J., Graf R., Okabe K., Padrell E., Iyengar R., Brown A. M., Birnbaumer L. The G protein-gated atrial K+ channel is stimulated by three distinct Gi alpha-subunits. Nature. 1988 Dec 15;336(6200):680–682. doi: 10.1038/336680a0. [DOI] [PubMed] [Google Scholar]
- ten Dijke P., Hansen P., Iwata K. K., Pieler C., Foulkes J. G. Identification of another member of the transforming growth factor type beta gene family. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4715–4719. doi: 10.1073/pnas.85.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]