Abstract
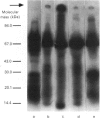
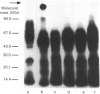
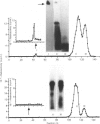
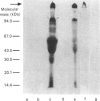
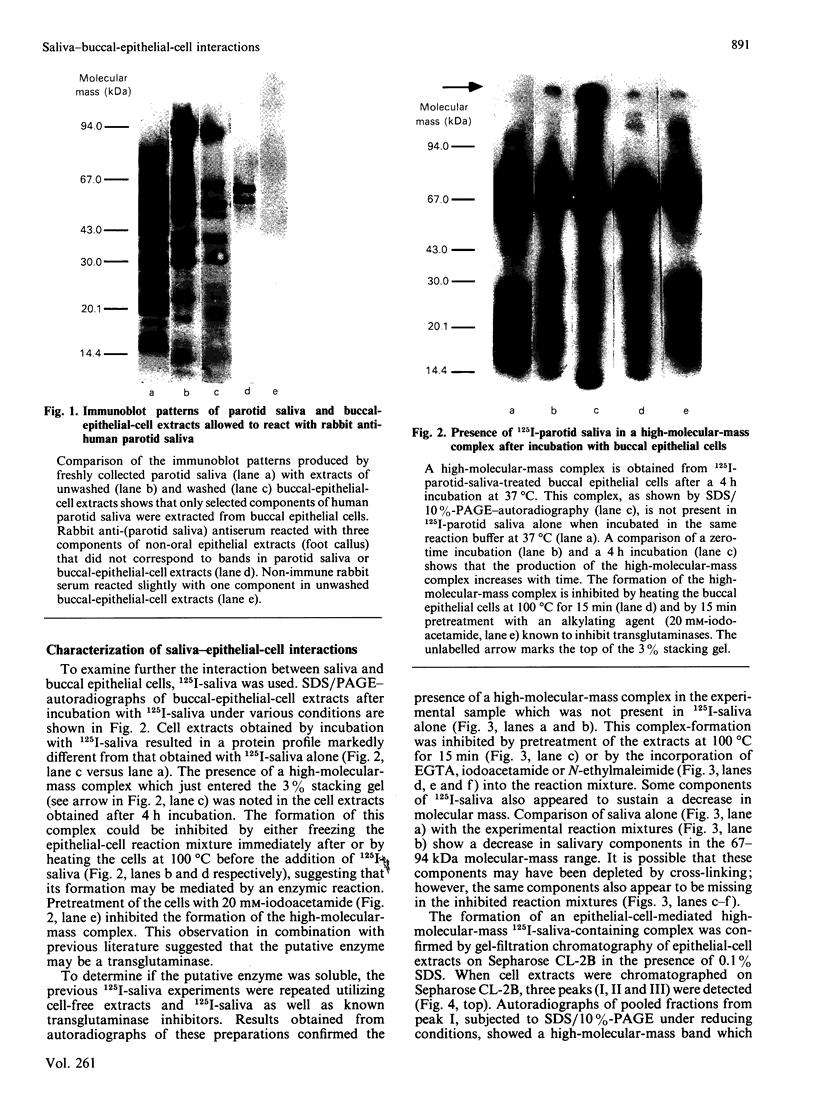
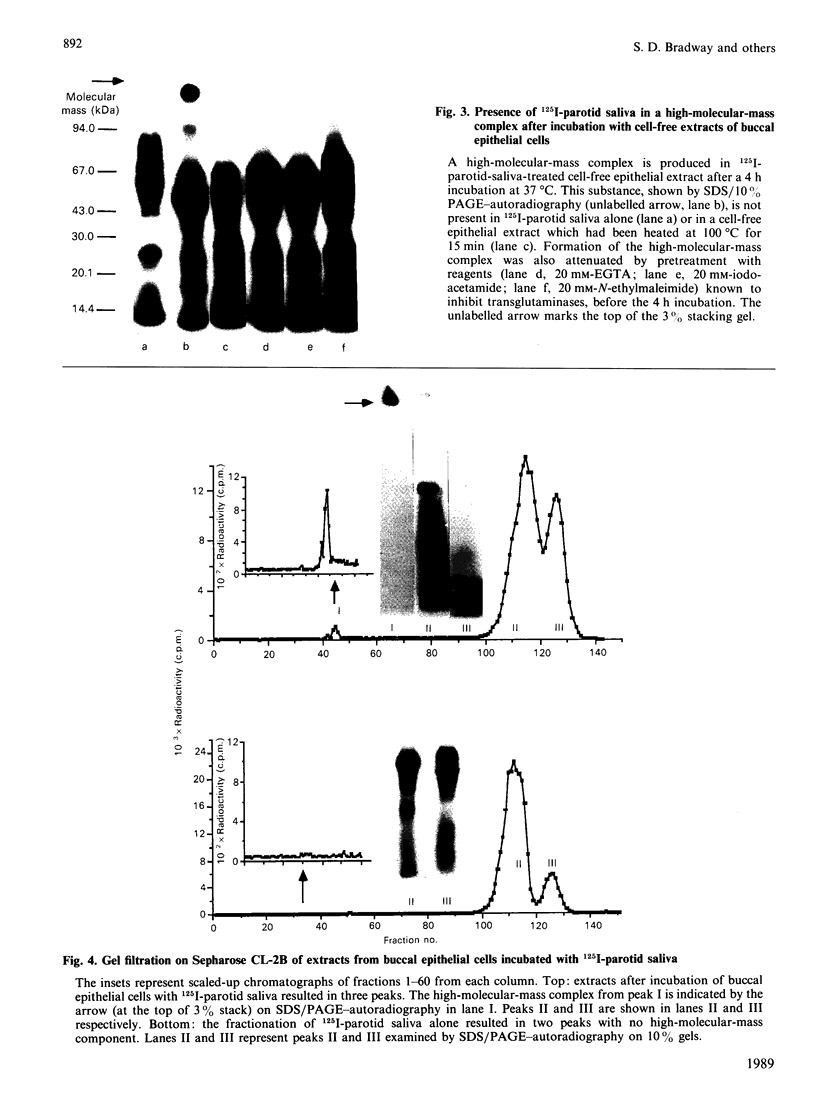
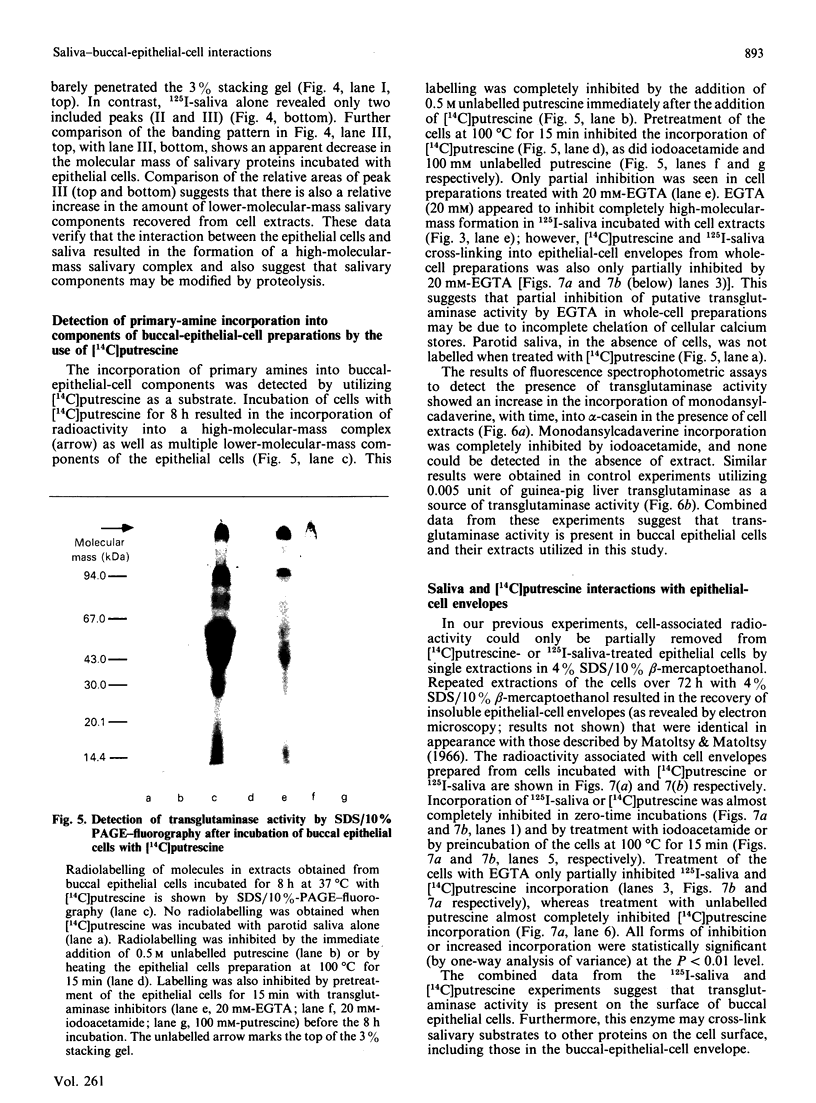
The present investigation was carried out to examine the mechanism(s) whereby salivary molecules interact with human buccal epithelial cells. By utilizing antiserum against human parotid saliva, selected salivary components were detected by electrophoretic-transfer analysis of 1.5% SDS extracts of epithelial cells. Incubation of the cells and their aqueous cell-free extracts with 125I-labelled parotid saliva resulted in the formation of an iodinated high-molecular-mass complex which was not present in 125I-labelled saline alone. Formation of this complex was time-dependent and was inhibited by treating the buccal epithelial cells or their cell-free extracts with EGTA, iodoacetamide, N-ethylmaleimide or by heating at 100 degrees C for 15 min. The epithelial cells also promoted incorporation of [14C]putrescine into high-molecular-mass complexes whose formation was inhibited by iodoacetamide, unlabelled putrescine and EGTA. Cell extracts mediated cross-linking of monodansylcadaverine into alpha-casein, and this interaction was inhibited by iodoacetamide. Significant amounts of radioactivity were recovered with the epithelial-cell envelopes after exhaustive extraction of 125I-saliva- or [14C]putrescine-treated epithelial cells with 4% (w/v) SDS/10% (v/v) beta-mercaptoethanol. The incorporation of radioactivity into epithelial-cell envelopes was inhibited by pretreatment of the cells with putrescine, EGTA, iodoacetamide, or heating at 100 degrees C for 15 min. These data suggest that: (1) oral mucosal pellicle is formed by the selective adsorption of saliva to the epithelial-cell plasma membrane and its associated cytoskeleton; and (2) the adsorbed salivary components may be cross-linked to each other or the epithelial cytoskeleton by epithelial transglutaminases.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Hashimi I., Levine M. J. Characterization of in vivo salivary-derived enamel pellicle. Arch Oral Biol. 1989;34(4):289–295. doi: 10.1016/0003-9969(89)90070-8. [DOI] [PubMed] [Google Scholar]
- Barsigian C., Fellin F. M., Jain A., Martinez J. Dissociation of fibrinogen and fibronectin binding from transglutaminase-mediated cross-linking at the hepatocyte surface. J Biol Chem. 1988 Oct 5;263(28):14015–14022. [PubMed] [Google Scholar]
- Bennick A., Cannon M. Quantitative study of the interaction of salivary acidic proline-rich proteins with hydroxyapatite. Caries Res. 1978;12(3):159–169. doi: 10.1159/000260326. [DOI] [PubMed] [Google Scholar]
- Bergey E. J., Levine M. J., Reddy M. S., Bradway S. D., Al-Hashimi I. Use of the photoaffinity cross-linking agent N-hydroxysuccinimidyl-4-azidosalicylic acid to characterize salivary-glycoprotein-bacterial interactions. Biochem J. 1986 Feb 15;234(1):43–48. doi: 10.1042/bj2340043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CURBY W. A. Device for collection of human parotid saliva. J Lab Clin Med. 1953 Mar;41(3):493–496. [PubMed] [Google Scholar]
- Edwards P. A. Is mucus a selective barrier to macromolecules? Br Med Bull. 1978 Jan;34(1):55–56. doi: 10.1093/oxfordjournals.bmb.a071459. [DOI] [PubMed] [Google Scholar]
- Ericson D. Salivary interactions with homologous and heterologous strains of oral streptococci and epithelial cells. Scand J Dent Res. 1985 Aug;93(4):320–328. doi: 10.1111/j.1600-0722.1985.tb01976.x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Etherden I., Moreno E. C. Association of neuraminidase-sensitive receptors and putative hydrophobic interactions with high-affinity binding sites for Streptococcus sanguis C5 in salivary pellicles. Infect Immun. 1983 Dec;42(3):1006–1012. doi: 10.1128/iai.42.3.1006-1012.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Qureshi J. V. Selective binding of blood group-reactive salivary mucins by Streptococcus mutans and other oral organisms. Infect Immun. 1978 Dec;22(3):665–671. doi: 10.1128/iai.22.3.665-671.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. M., King I. A., Yardley H. J. The plasma membrane of granular cells from pig epidermis: isolation and lipid and protein composition. J Invest Dermatol. 1978 Aug;71(2):131–135. doi: 10.1111/1523-1747.ep12546728. [DOI] [PubMed] [Google Scholar]
- Hatton M. N., Loomis R. E., Levine M. J., Tabak L. A. Masticatory lubrication. The role of carbohydrate in the lubricating property of a salivary glycoprotein-albumin complex. Biochem J. 1985 Sep 15;230(3):817–820. doi: 10.1042/bj2300817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juriaanse A. C., Booij M., Arends J., ten Bosch J. J. The adsorption in vitro of purified salivary proteins on bovine dental enamel. Arch Oral Biol. 1981;26(2):91–96. doi: 10.1016/0003-9969(81)90076-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Ellison S. A. Immuno-electrophoretic and chemical analyses of human parotid saliva. Arch Oral Biol. 1973 Jul;18(7):839–853. doi: 10.1016/0003-9969(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Levine M. J., Reddy M. S., Tabak L. A., Loomis R. E., Bergey E. J., Jones P. C., Cohen R. E., Stinson M. W., Al-Hashimi I. Structural aspects of salivary glycoproteins. J Dent Res. 1987 Feb;66(2):436–441. doi: 10.1177/00220345870660020901. [DOI] [PubMed] [Google Scholar]
- Lorand L., Lockridge O. M., Campbell L. K., Myhrman R., Bruner-Lorand J. Transamidating enzymes. II. A continuous fluorescent method suited for automating measurements of factor XIII in plasma. Anal Biochem. 1971 Nov;44(1):221–231. doi: 10.1016/0003-2697(71)90363-0. [DOI] [PubMed] [Google Scholar]
- Matoltsy A. G., Matoltsy M. N. The membrane protein of horny cells. J Invest Dermatol. 1966 Jan;46(1):127–129. [PubMed] [Google Scholar]
- Mayhall C. W. Concerning the composition and source of the acquired enamel pellicle of human teeth. Arch Oral Biol. 1970 Dec;15(12):1327–1341. doi: 10.1016/0003-9969(70)90021-x. [DOI] [PubMed] [Google Scholar]
- Moreno E. C., Zahradnik R. T. Demineralization and remineralization of dental enamel. J Dent Res. 1979 Mar;58(SPEC):896–903. doi: 10.1177/00220345790580024301. [DOI] [PubMed] [Google Scholar]
- Mukherjee A. B., Ulane R. E., Agrawal A. K. Role of uteroglobin and transglutaminase in masking the antigenicity of implanting rabbit embryos. Am J Reprod Immunol. 1982 Jun;2(3):135–141. doi: 10.1111/j.1600-0897.1982.tb00154.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee D. C., Agrawal A. K., Manjunath R., Mukherjee A. B. Suppression of epididymal sperm antigenicity in the rabbit by uteroglobin and transglutaminase in vitro. Science. 1983 Feb 25;219(4587):989–991. doi: 10.1126/science.6130601. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Goldsmith L. A. Human epidermal transglutaminase. Preparation and properties. J Biol Chem. 1976 Dec 10;251(23):7281–7288. [PubMed] [Google Scholar]
- Shomers J. P., Tabak L. A., Levine M. J., Mandel I. D., Hay D. I. Properties of cysteine-containing phosphoproteins from human submandibular-sublingual saliva. J Dent Res. 1982 Feb;61(2):397–399. doi: 10.1177/00220345820610020601. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Levine M. J., Cavese J. M., Prakobphol A., Murray P. A., Tabak L. A., Reddy M. S. Adherence of Streptococcus sanguis to salivary mucin bound to glass. J Dent Res. 1982 Dec;61(12):1390–1393. doi: 10.1177/00220345820610120101. [DOI] [PubMed] [Google Scholar]
- Sönju T., Rölla G. Chemical analysis of the acquired pellicle formed in two hours on cleaned human teeth in vivo. Rate of formation and amino acid analysis. Caries Res. 1973;7(1):30–38. doi: 10.1159/000259822. [DOI] [PubMed] [Google Scholar]
- Tabak L. A., Levine M. J., Mandel I. D., Ellison S. A. Role of salivary mucins in the protection of the oral cavity. J Oral Pathol. 1982 Feb;11(1):1–17. doi: 10.1111/j.1600-0714.1982.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Tolo K., Brandtzaeg P., Jonsen J. Mucosal penetration of antigen in the presence or absence of serum-derived antibody. Immunology. 1977 Nov;33(5):733–743. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Transglutaminase-mediated covalent attachment of polyamines to proteins: mechanisms and potential physiological significance. Physiol Chem Phys. 1980;12(5):457–472. [PubMed] [Google Scholar]