Summary
Cold storage of potato tubers is commonly used to reduce sprouting and extend postharvest shelf life. However, cold temperature stimulates the accumulation of reducing sugars in potato tubers. Upon high‐temperature processing, these reducing sugars react with free amino acids, resulting in brown, bitter‐tasting products and elevated levels of acrylamide—a potential carcinogen. To minimize the accumulation of reducing sugars, RNA interference (RNAi) technology was used to silence the vacuolar invertase gene (VInv), which encodes a protein that breaks down sucrose to glucose and fructose. Because RNAi often results in incomplete gene silencing and requires the plant to be transgenic, here we used transcription activator‐like effector nucleases (TALENs) to knockout VInv within the commercial potato variety, Ranger Russet. We isolated 18 plants containing mutations in at least one VInv allele, and five of these plants had mutations in all VInv alleles. Tubers from full VInv‐knockout plants had undetectable levels of reducing sugars, and processed chips contained reduced levels of acrylamide and were lightly coloured. Furthermore, seven of the 18 modified plant lines appeared to contain no TALEN DNA insertions in the potato genome. These results provide a framework for using TALENs to quickly improve traits in commercially relevant autotetraploid potato lines.
Keywords: transcription activator‐like effector nucleases, gene editing, vacuolar invertase, cold‐induced sweetening, acrylamide reduction, potato
Introduction
Potato (Solanum tuberosum) is the third most important food crop, with worldwide production estimated at 376 million metric tons in 2013 (http://faostat3.fao.org/browse/rankings/commodities_by_regions/E). The majority of potatoes (61% of the 2010 crop in the United States) are used by processors for potato chips, French fries and other processed products. Because potatoes are harvested only once a year, it is necessary to cold store the tubers to ensure a year‐round supply of high‐quality potatoes for processing. Without cold storage, potatoes have a shelf life of about 6 months, after which they rapidly deteriorate in quality (Bianchi et al., 2014). In addition to prolonged storage, cold temperatures also reduce sprouting, losses due to shrinkage and the spread of disease. One undesirable consequence of cold storage is cold‐induced sweetening (CIS), in which reducing sugars accumulate in the tubers. When processed at high temperatures, reducing sugars form dark‐pigmented products that are bitter and unacceptable to consumers (Dale and Bradshaw, 2003; Kumar et al., 2004; Mottram et al., 2002; Sowokinos, 2001; Stadler et al., 2002). In the United States, CIS causes up to 15% of potatoes being rejected at processing plants every year (Bhaskar et al., 2010).
In addition to producing bitter‐tasting products, heat processing causes reducing sugars (e.g. glucose and fructose) in potato tubers to react with free amino acids (e.g. asparagine) to form the potential cancer‐causing agent, acrylamide, via the nonenzymatic Maillard reaction (Tareke et al., 2002). Acrylamide is particularly prevalent in heat‐processed potatoes that have undergone CIS, due to their high levels of reducing sugars. Acrylamide in potato chips and French fries has caused global food safety concerns, and legal measures have been sought to limit acrylamide levels in fried potato products (Grob, 2007; Medeiros Vinci et al., 2012). Thus, methods that reduce acrylamide are being sought by the potato processing industry, and one effective way is to decrease reducing sugars in cold‐stored tubers (Bhaskar et al., 2010; Matsuura‐Endo et al., 2006).
Accumulation of reducing sugars during CIS is influenced by several metabolic processes, including starch synthesis, starch degradation, glycolysis, hexogenesis and mitochondrial respiration (Sowokinos, 2001). A portion of the sucrose is cleaved to produce the reducing sugars, fructose and glucose, by a family of ubiquitous enzymes termed invertases (Roitsch and Gonzalez, 2004). There are three types of invertase isoenzymes classified by their solubility, subcellular localization, pH‐optima and isoelectric point: cell wall‐bound invertases, neutral invertases and vacuolar invertases (VInv) (Roitsch and Gonzalez, 2004). VInv is localized in the vacuole and plays a particularly important role in the production of reducing sugars in cold‐stored tubers (Kumar et al., 2004; Matsuura‐Endo et al., 2006; Sowokinos, 2001). Transgenic RNA interference (RNAi) approaches have confirmed that knocking down VInv expression lowers reducing sugars and dark‐pigmented nonenzymatic browning in cold‐stored potatoes (Bhaskar et al., 2010; Wu et al., 2011; Ye et al., 2010).
Here we will report the generation of potato varieties with knockout mutations in all alleles of the VInv gene through precise genome engineering (Voytas, 2013). This was accomplished by transiently expressing transcription activator‐like effector nucleases (TALENs) designed to bind and cleave specific DNA sequences in the VInv locus. The double‐stranded breaks (DSBs) created by the TALENs were repaired by nonhomologous end joining (NHEJ), an error prone mechanism that introduces indel (insertion/deletion) mutations that compromised VInv gene function. Due to the high levels of heterozygosity in the potato genome, the task of simultaneously targeting multiple alleles required careful TALEN design and optimization (Draffehn et al., 2010). In contrast to previous RNAi work, TALENs achieved complete knockout lines without the need to incorporate foreign DNA. As a result, the new potato lines have significantly lower levels of reducing sugars and acrylamide in heat‐processed products.
Results
TALEN design and activity assessment at the VInv target site
To design TALENs targeting the VInv gene in cultivated potato, exon 1 of VInv was sequenced from Solanum tuberosum cv Ranger Russet. Ranger Russet was chosen because this variety is widely used for frying, and processing is hindered due to cold‐induced sweetening. As most cultivated potato varieties are tetraploid with high levels of heterozygosity, 454 deep sequencing was used to assess allele diversity. Sequencing primers were designed based on conserved regions identified through a multiple DNA sequence alignment of the VInv genes described in previous studies (Slugina et al., 2014). A region near the start codon (600 bp) was amplified from Ranger Russet and subjected to 454 pyro‐sequencing. From a total of 8268 reads, three distinct allele types were identified (designated as A1, A2 and A3). The ratio of read numbers for each allele approximated 1 : 2 : 1 (A1, 1814 reads; A2, 4265 reads; A3, 2189 reads) suggesting that there is one copy of A1, two copies of A2 (designated as A2(1) and A2(2)) and one copy of the A3 in Ranger Russet. Three TALEN pairs were designed to target within the first 200 bp of coding sequence using TALEN™ hit software (Figure 1b) (Haun et al., 2014). The most conserved TALEN‐binding sites are identical in sequence between the alleles A2(1), A2(2) and A3, but contain one, two, or three single nucleotide polymorphisms (SNPs) in A1 (Figure 1c). TALENs were synthesized based on the consensus target sequences among the three VInv alleles.
Figure 1.
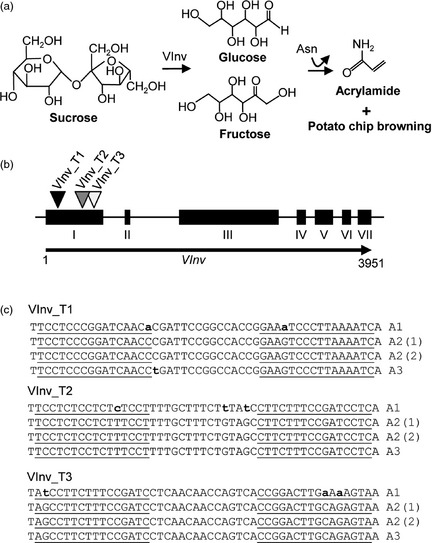
Targeting the Solanum tuberosum cv Ranger Russet VInv gene with transcription activator‐like effector nucleases (TALENs). (a) During cold storage, potato tubers accumulate acrylamide through a nonenzymatic Maillard reaction, which uses reducing sugars (primarily glucose and fructose) and free amino acids (asparagine [Asn]). Reducing sugars accumulate through hydrolysis of sucrose by vacuolar acid invertase (VInv). In addition to accumulating acrylamide, cold‐stored potatoes also produce brown‐ to black‐pigmented products. (b) Schematic of the VInv gene. TALEN target sites are indicated with black, grey and white triangles (VInv_T1, VInv_T2, VInv_T3, respectively). (c) TALEN target sites within exon 1. Single nucleotide polymorphisms are indicated by lowercase bold letters. Underlined letters indicate TALEN‐binding sites. A1, allele 1; A2(1), copy 1 of allele 2; A2(2) copy 2 of allele 2; A(3), allele 3.
TALEN activity was tested at endogenous target sites by expressing the TALENs in protoplasts and surveying the target sites for mutations introduced by NHEJ (Figure 2a). TALEN‐encoding plasmids (VInv_T1, VInv_T2 and VInv_T3) were individually introduced into Ranger Russet protoplasts by polyethylene glycol (PEG)‐mediated transformation (Figure S1). Protoplasts were sacrificed 48 h post‐transformation and genomic DNA was isolated. The genomic DNA was subjected to PCR amplification, producing a 272‐bp fragment encompassing the TALEN recognition sites. The PCR product was then subjected to 454 pyro‐sequencing, and the resulting sequencing reads were evaluated for NHEJ‐induced mutations. The frequency of mutated sequences in each sample was used to estimate the activity of the corresponding TALENs.
Figure 2.
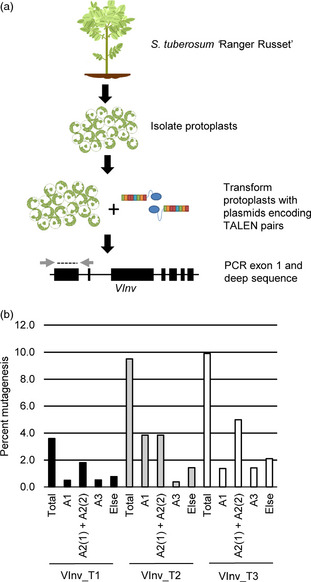
Assessment of transcription activator‐like effector nuclease (TALEN) activity in Solanum tuberosum cv Ranger Russet protoplasts. (a) Protoplasts from leaves on 3‐week‐old potato plants (Ranger Russet) were isolated and transformed with plasmids encoding TALEN pairs. Following transformation, exon 1 was amplified by PCR and mutations were assessed by 454 pyro‐sequencing. (b) Percentage of vacuolar invertase sequences containing TALEN‐induced mutations. Total refers to the combined percentage of mutations in all four alleles. Else refers to sequences that could not be assigned an allele type due to TALEN‐induced mutations that removed the allele‐defining single nucleotide polymorphisms.
The VInv TALEN pairs, encoded by plasmids VInv_T1, VInv_T2 and VInv_T3, induced NHEJ mutations at frequencies of 3.6%, 9.5% and 9.9%, respectively (Figure 2b). All three TALEN pairs induced mutations in all VInv alleles, despite the DNA sequence polymorphisms. TALEN pairs were also tested in protoplasts isolated from three other commercial varieties, namely Russet Burbank, Atlantic and Shepody. The observed mutation frequency for each TALEN pair in each variety is summarized in Figure S2. Of the three TALEN pairs, VInv_2 TALENs performed consistently well across all four varieties, with mutation frequencies ranging from 2.1% to 15.9%.
Generating potato lines with knockout mutations in VInv
To create potato lines harbouring mutations in the VInv alleles, Ranger Russet protoplasts were transformed with TALEN‐encoding plasmids and regenerated into whole plants (Figure 3a). Given the previous pyro‐sequencing results, we focused our efforts on VInv_T2, as this TALEN pair performed consistently well across all four potato varieties and contained the fewest SNPs in the TALE‐binding regions among the three different alleles. For each experiment, 100 000–200 000 protoplasts were transformed with a plasmid VInv_T2 using PEG. Transformed protoplasts were transferred to nonselective regeneration media and allowed to regenerate. Shoots that appeared from calli (approximately 12 weeks post‐transformation) were excised and transferred to rooting medium. To reduce the likelihood of recovering a chimeric potato plant (containing a mixture of WT and modified cells), several meristematic subcultures were propagated. At the time that plantlets were transferred to rooting medium, a small leaf sample was removed and DNA was isolated for genotyping of the VInv locus.
Figure 3.
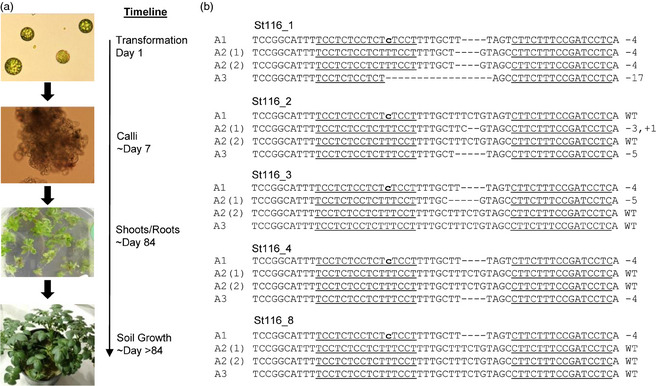
Recovery of potato lines carrying mutations within vacuolar invertase (VInv). (a) Approach and timeline to regenerate plants with mutations in VInv. Protoplasts were transformed with plasmids encoding the VInv_T2 transcription activator‐like effector nucleases pair and were cultured in nonselective regeneration medium. Following shoot and root formation, potato plantlets were transferred to soil. (b) Examples of plant lines carrying mutations in one or more of the VInv alleles. WT, wild type.
Plants were initially screened for mutations by directly sequencing PCR amplicons obtained using primers that recognize all three allele types, A1, A2 and A3. Sequencing chromatograms with two or more peaks near the TALEN target sites suggested that there were mutations in one or more VInv alleles (Figure S3). From a total of approximately 600 regenerated shoots, 18 plants were identified that likely harbour mutations in at least one VInv allele. To further characterize mutations within the VInv alleles, PCR amplicons encompassing the VInv TALEN‐binding sites were cloned, and 24–48 clones were sequenced. Of the 18 candidate plants, six contained mutations in one VInv allele, five contained mutations in two VInv alleles, two contained mutations in three VInv alleles, and five contained mutations in all four VInv alleles (Table 1).
Table 1.
Characterization of vacuolar invertase mutant plants
| Number of mutant alleles | Number of plants | Plants without transgene |
|---|---|---|
| 4 | 5 (28%) | 2 |
| 3 | 2 (11%) | 1 |
| 2 | 5 (28%) | 2 |
| 1 | 6 (33%) | 2 |
| Total | 18 | 7 (39%) |
From transformation group St116, nine mutants were isolated, seven of which had 1 or 2 mutations within the VInv alleles. Two plants were recovered with mutations in all VInv alleles (St116_1 and St116_9). Notably, plant St116_1 contained a four‐base‐pair deletion in A1, an identical four‐base‐pair deletion in both A2(1) and A2(2), and a 17‐bp deletion in A3. Because all mutations within plant St116_1 resulted in frame shifts, VInv gene function was predicted to be completely knocked out. For this reason, plant St116_1 was advanced to phenotypic characterization.
It is possible that transformation of protoplasts with plasmid DNA results in stable integration of DNA into the plant genome. To determine whether VInv_T2 plasmid DNA was present in the mutants, PCR was performed using primer pairs designed to amplify three regions of the TALEN‐expression cassette (Figure S4A). Of 18 mutant plants, 7 (39%) had no detectable TALEN vector sequence. A representative example of a PCR to detect VInv_T2 plasmid sequence is shown in Figure S4B. Taken together, our results demonstrate the usefulness of TALENs and protoplast transformation/regeneration to create modified, nontransgenic potato lines in a single generation.
Assessment of sugar levels from cold‐stored potato tubers from VInv‐knockout lines
To assess levels of reducing sugars in cold‐stored tubers, potatoes from wild‐type Ranger Russet, St116_8 (containing a mutation in one VInv allele) and St116_1 (containing mutations in all VInv alleles), were cold‐stored at 4 °C for 14 days. Following cold storage, tubers were homogenized, and the levels of sucrose, glucose and fructose were quantified using high‐pressure liquid chromatography. As shown in Figure 4a, the levels of fructose, glucose and sucrose in wild‐type tubers were 1.4, 1.5 and 1.1 mg/g (% of fresh weight), respectively. In tubers from St116‐8, the sugar levels were roughly comparable to wild type with 1.2, 1.2 and 2 mg/g of fructose, glucose and sucrose, respectively. Tubers from the full knockout mutant, St116‐1, exhibited significantly decreased levels of fructose and glucose that were below the limit of detection for this assay (<0.1 mg/g), and increased levels of sucrose (3.3 mg/g) (Figure 4a). To further assess sugar levels in our mutant potato plants, three additional lines, St116_5, St116_3 and St123_1, containing three wild‐type VInv alleles, two wild‐type VInv alleles and two wild‐type VInv alleles, respectively, were assessed for levels of fructose, glucose and sucrose (Figure S5). Compared to cold‐stored tubers from wild‐type plants and St116_1, all additional lines had intermediate sugar levels.
Figure 4.
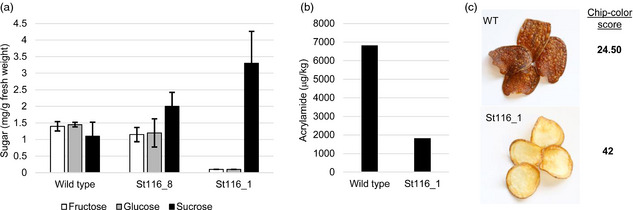
Quality assessment of mutant potato lines. (a) Analysis of sugar content within potato tubers stored at 4 °C for 14 days. Error bars represent standard deviation. (b) Analysis of acrylamide content in potato chips that were processed from tubers that were stored at 4 °C for 14 days. (c) Images of potato chips after being processed from tubers stored at 4 °C for 14 days. The colorimetric score is listed to the right of the image.
Acrylamide levels and quality assessment of potato chips produced from cold‐stored tubers from VInv‐knockout lines
Acrylamide levels were assessed in potato chips from wild‐type Ranger Russet and the St116_1 mutant. Chips were prepared from tubers stored at 4 °C for 14 days. Acrylamide levels were quantified using high‐pressure liquid chromatography. Acrylamide content in chips from St116_1 tubers was 1820 μg/kg, 73.3% less than that of chips from wild‐type tubers (6820 μg/kg) (Figure 4b). Potato chips from the wild‐type and the mutant plants were also assessed for colour. Chips processed from cold‐stored wild‐type and St116_1 mutant tubers are shown in Figure 4c. As predicted, chips from St116_1 were lighter‐coloured than those from wild type. The chip colour, as determined by the Hunter score, was substantially better for line St116_1 compared to wild type (Figure 4c).
Discussion
Potatoes are an integral part of the world's food supply; however, trait‐improvement efforts are met with challenges due to the complexity of the autotetraploid genome and the high degree of heterozygosity (Barrell et al., 2013). As a result, classic improvement schemes require long breeding cycles and screening of exceptionally large populations to accumulate desired alleles (Bradshaw et al., 2006). In recent years, new technologies, such as transgenesis/cisgenesis, RNAi and TILLING, have been developed to overcome these challenges (Barrell et al., 2013; Elias et al., 2009). The major shortcoming of these technologies is the lack of precision to produce desired traits. For example, transgenesis/cisgenesis and RNAi technologies deliver and integrate transgenes in a random fashion into the potato genomes by either direct DNA uptake or Agrobacterium‐mediated transformation. As a result, the expression of transgenes is not predictable and largely depends on the location, copy number and configuration of the transgenes. Thus, the characterization of a large population of independently transformed lines is often required to identify those with the desired expression of the transgene (Barrell and Conner, 2009; Barrell et al., 2013; Jacobs et al., 2009). TILLING is also time‐consuming and laborious, and numerous mutations occur outside the target gene that can only be removed by extensive backcrossing regimes (Elias et al., 2009; Muth et al., 2008). Recent advances in technologies and methods that enable precise gene modification hold promise to overcome these challenges and accelerate trait improvement in potato (Voytas, 2013).
To circumvent the difficulties inherent in working with a highly heterozygous autopolyploid species, the method of targeted gene modification needs to be highly efficient, such that mutations can be introduced simultaneously in multiple loci and multiple alleles. In this study, we demonstrated that TALEN‐mediated gene editing creates a valuable trait in a commercial potato cultivar with high efficiency. Sequence‐specific TALENs were designed to cleave multiple alleles of a target gene and were delivered to potato protoplasts with over 80% efficiency. Potato plants were then regenerated from single‐cell protoplasts, and about 3% of the plants were found to contain mutations in one to four VInv alleles. The ability to generate multiple mutant lines is particularly valuable for potato trait development, as somaclonal variation commonly occurs in plants derived from tissue culture leading to abnormal phenotypes (Barrell et al., 2013). In addition to the cultivar Ranger Russet, TALENs were found to be highly efficient mutagens in protoplasts derived from other commercial varieties. As plants can be readily regenerated from these transformed protoplasts, the method described in this study can be used as a framework for efficiently introducing targeted mutations into a gene‐of‐interest in a single generation in elite potato varieties.
Previous studies have indicated that off‐target mutagenesis can occur when using TALENs for genome modification (Frock et al., 2015; Mali et al., 2013). Furthermore, we have shown that TALEN pairs could tolerate up to 3–4 mismatches in the recognition sites (Juillerat et al., 2014). Therefore, it may be possible that the TALEN reagents used in this study bind and cleave unintended target sites. However, in an effort to minimize the chance of off‐target mutations, during TALEN design and synthesis, we searched publicly available potato genome sequences for potential TALEN‐binding sites—no potential target sequences were found with four or less mismatches. To completely rule out the possibility of off‐target mutations in the VInv mutant lines, it is possible to perform whole‐genome sequencing.
Previous research demonstrated that silencing of the VInv gene by RNAi decreases the reducing sugar and acrylamide levels in cold‐stored, processed potato tubers and potato chips (Bhaskar et al., 2010; Wu et al., 2011). No accumulation of the reducing sugars, glucose and fructose, was detected in the RNAi lines that suppressed VInv gene expression by more than 97%, whereas partial suppression did not control CIS effectively. This observation suggested that the VInv gene has to be almost completely silenced to confer the CIS‐resistant trait. In this study, we tested this hypothesis through targeted mutagenesis. By generating a series of mutants with 1–4 VInv alleles knocked out, our results showed a positive correlation between the number of wild‐type alleles and the levels of reducing sugars in cold‐stored potato tubers. When all four VInv alleles were mutated, reducing sugars were undetectable (<0.1 μg/kg), which is consistent with previous RNAi research. The low level of acrylamide generated within the tubers of our complete knockout line suggests the presence of reducing sugars, albeit at undetectable levels. These reducing sugars are most likely derived from invertase activity in the cytoplasm and cell wall (Bhaskar et al., 2010; Roitsch and Gonzalez, 2004). Therefore, it may be possible to further reduce acrylamide content by targeting additional, nonessential invertases. The agronomic performance of the full VInv‐knockout lines remains to be assessed in field trails in the coming years.
In the past two decades, transgenic or cisgenic approaches have been used to create potato varieties with new traits (Barrell et al., 2013; Holme et al., 2013; Rommens, 2007). Although a few new varieties have been deregulated by the USDA, transgenic potatoes have not been well accepted by the market because of their GM status (Fernandez‐Cornejo et al., 2014). In this study, several VInv‐knockout lines were created that lack foreign DNA (e.g. the TALEN‐encoding constructs) in their genome.
Regulatory authorities around the world are currently considering how to handle plants that lack transgenes and carry targeted mutations such as those created by NHEJ (Kuzma and Kokotovich, 2011). It is interesting to consider that the potato lines created in this study have no foreign DNA and thus are not different from naturally occurring varieties.
Materials and methods
Identification of TALEN target sites in the VInv gene
To completely inactivate or knockout the VInv gene in S. tuberosum cv Ranger Russet, sequence‐specific nucleases were designed that target the protein coding region in the vicinity of the start codon. Three TALEN target sites were identified (VInv_T1, VInv_T2 and VInv_T3) within the first 200 bp of the coding sequence using TALEN™ Hit software. The locations of the TALENs and their corresponding sequences are shown in Figure 1c. TALENs were synthesized using methods similar to those described elsewhere (Beurdeley et al., 2013; Cermak et al., 2011).
Protoplast isolation and transformation
Protoplast isolation and transformation were carried out as previously described with slight modifications (Craig et al., 2005). Shoots derived from tubers (grown in vermiculite at 22 °C under 120 μmol/m2/s for 3 weeks) were surface‐sterilized and cultured on StProp medium (MS basal salts plus vitamins, 2% sucrose, 0.7% agar, pH 5.8). Subcultures were made by single node propagation at biweekly intervals, and leaves from these subcultures served as the source material for protoplast isolation as previously described (Yoo et al., 2007; Zhang et al., 2013). Protoplasts were suspended in transfection buffer at a cell density of 100 000 protoplasts/mL in a final volume of 200 μL and placed in a 2‐mL Eppendorf tube containing 15 μg of the plasmid of interest. Immediately following suspension, 200 μL of 40% PEG4000 was added to the protoplast suspension. The tube was gently vortexed and incubated in the dark at room temperature for 30 min. After incubation, 1 mL of wash buffer was added to each tube. Tubes were gently vortexed and cells were pelleted at 500 g for 5 min followed by resuspension in plating medium. Transformation efficiencies were monitored by transforming protoplasts with a plasmid encoding YFP.
Validation of TALENs in potato protoplasts
To assess TALEN activity, protoplasts from varieties Ranger Russet, Atlantic, Russet Burbank and Shepody were transformed with plasmid DNA encoding VInv_T1, VInv_T2 or VInv_T3. Two days after transformation, protoplasts were sacrificed and subjected to DNA extraction using the hexadecyltrimethylammonium bromide (CTAB)‐based method (Zhang et al., 2013). Purified DNA was used as a template for PCR using primers designed to amplify VInv exon 1 (5′‐CACCCAGTACCATTCCAGTTATG‐3′ and 5′‐TTTTTGAGGTTGAAAATGGTAAGCA‐3′). The resulting amplicons were subjected to 454 pyro‐sequencing (Roche GS Junior), and TALEN‐induced mutations were quantified.
Regeneration of mutant plants
Immediately after transformation of protoplasts with a plasmid encoding VInv_T2, cells were cultured using methods and media previously described by Gamborg et al. with slight modifications (Gamborg and Shyluk, 1981). Protoplasts were suspended in P‐medium and stored at 25 °C in the dark. Two weeks after transformation, after the majority of the protoplasts had divided at least once, the protoplast culture was diluted twofold in a suspension of P‐medium. Two weeks after dilution, cultures were plated on a solid reservoir of CUL medium in a 10‐cm petri plate (Haberlach et al., 1985). Six weeks after plating, protoplast‐derived calli (p‐calli) were transferred to a solid reservoir of DIF medium (Haberlach et al., 1985). P‐calli were transferred to fresh DIF medium at biweekly intervals. As shoots formed from the calli, they were excised, sampled for DNA isolation and placed into a solid reservoir of R‐medium (Gamborg and Shyluk, 1981). As the shoots elongated, they were subcloned at individual nodes and separated into two populations: one for propagation and the other for tuber production. The propagation population was maintained and expanded on StProp, whereas the tuber population was transferred to a soilless potting substrate (SunGro, Agawam, MA, USA, All Purpose Planting Mix) and grown to maturity in a growth chamber.
Genotyping VInv mutant plantlets
DNA was isolated from fresh in vitro leaf material using methods described by Aljanabi and Martinez (Aljanabi and Martinez, 1997). Mutants were identified using a two‐step screening process. For the first step, DNA was isolated and subjected to PCR to amplify the locus of interest using primers designed for VInv (forward primer, 5′‐CACCCAGTACCATTCCAGTTATG‐3′; reverse primer, 5′‐CATTGGACCACGCATAAGAA‐3′). The resulting pool of amplicons were directly sequenced using Sanger sequencing (Eurofins MWG Operon, Huntsville, AL, USA). We predicted that if a plant contained insertions or deletions within one or more VInv alleles, the resulting sequencing reads would contain unordered traces near the predicted TALEN cut site. Sequence results were scored by hand, to check for a disordered trace. Candidate plants were subjected to further analysis: the PCR product was cloned using the CloneJet PCR Cloning Kit (Thermo Scientific, Waltham, MA, USA), and clones were subjected to Sanger sequencing. Given the highly heterozygous nature of the potato genome, a large number of clones (n ≥ 10) were sequenced to recover all allele types. Sequencing files associated with the clones were aligned with the S. tuberosum VInv gene (NCBI Accession JN661860) using the discontinuous megablast algorithm with default parameters with a standalone version of NCBI‐BLAST+ for Windows.
Sugar profiling, acrylamide analysis and colorimetry of fried potato chips
Sugar profile analysis was carried out by Rtech Laboratories (Land O'Lakes, MN) using AOAC official methods (Method 982.14; Glucose, Fructose, Sucrose, and Maltose in Presweetened Cereals; http://www.eoma.aoac.org/). The analysis was performed on 30 g (mass was determined after 14 days of storage at 4 ± 1 °C) of homogenized tuber tissue comprised of the medial one centimetre of two to three independent mature tubers from a single plant. Potato chips were processed using the method previously described (Bhaskar et al., 2010). Acrylamide analysis was performed on chips prepared from tubers that were stored at 4 ± 1 °C for 14 days. Analysis was performed by Covance laboratories (United States) using a modified method described by the United States Food and Drug Administration (Detection and Quantitation of Acrylamide in Foods; http://www.fda.gov/). The colour intensity of potato chips was measured using Hunter scores. Chip colour measurements were determined on a subset of transversely sliced chips (15 g) from the medial one centimetre using a D25LT colorimeter (HunterLab) (Bhaskar et al., 2010).
Conflict of interest
All authors are employees of Cellectis Plant Sciences. Cellectis Plant Sciences is the subsidiary of Cellectis SA.
Supporting information
Figure S1 Illustration of a representative TALEN‐expression plasmid.
Figure S2 Assessment of TALEN activity within protoplasts from four different potato varieties – Atlantic, Ranger Russet, Russet Burbank, and Shepody.
Figure S3 Examples of chromatograms for VInv alleles containing either wild type sequences (top) or mutated sequences (bottom).
Figure S4 A, Graphical representation of the TALEN expression cassette that was introduced into potato protoplasts.
Figure S5 Analysis of sugar content within potato tubers stored at 4 °C for 14 days.
References
- Aljanabi, S.M. and Martinez, I. (1997) Universal and rapid salt‐extraction of high quality genomic DNA for PCR‐based techniques. Nucleic Acids Res. 25, 4692–4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell, P.J. and Conner, A.J. (2009) Expression of a chimeric magainin gene in potato confers improved resistance to the phytopathogen Erwinia carotovora. Open Plant Sci. J. 3, 14–21. [Google Scholar]
- Barrell, P.J. , Meiyalaghan, S. , Jacobs, J.M. and Conner, A.J. (2013) Applications of biotechnology and genomics in potato improvement. Plant Biotechnol. J. 11, 907–920. [DOI] [PubMed] [Google Scholar]
- Beurdeley, M. , Bietz, F. , Li, J. , Thomas, S. , Stoddard, T. , Juillerat, A. , Zhang, F. , Voytas, D.F. , Duchateau, P. and Silva, G.H. (2013) Compact designer TALENs for efficient genome engineering. Nat. Commun. 4, 1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar, P.B. , Wu, L. , Busse, J.S. , Whitty, B.R. , Hamernik, A.J. , Jansky, S.H. , Buell, C.R. , Bethke, P.C. and Jiang, J. (2010) Suppression of the vacuolar invertase gene prevents cold‐induced sweetening in potato. Plant Physiol. 154, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, G. , Scalzo, R.L. , Testoni, A. and Maestrelli, A. (2014) Nondestructive analysis to monitor potato quality during cold storage. J. Food Qual. 37, 9–17. [Google Scholar]
- Bradshaw, J.E. , Bryan, G.J. and Ramsay, G. (2006) Genetic resources (including wild and cultivated Solanum species) and progress in their utilisation in potato breeding. Potato Res. 49, 49–65. [Google Scholar]
- Cermak, T. , Doyle, E.L. , Christian, M. , Wang, L. , Zhang, Y. , Schmidt, C. , Baller, J.A. , Somia, N.V. , Bogdanove, A.J. and Voytas, D.F. (2011) Efficient design and assembly of custom TALEN and other TAL effector‐based constructs for DNA targeting. Nucleic Acids Res. 39, e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, W. , Gargano, D. , Scotti, N. , Nguyen, T. , Lao, N. , Kavanagh, T. , Dix, P. and Cardi, T. (2005) Direct gene transfer in potato: a comparison of particle bombardment of leaf explants and PEG‐mediated transformation of protoplasts. Plant Cell Rep. 24, 603–611. [DOI] [PubMed] [Google Scholar]
- Dale, M.F. and Bradshaw, J.E. (2003) Progress in improving processing attributes in potato. Trends Plant Sci. 8, 310–312. [DOI] [PubMed] [Google Scholar]
- Draffehn, A. , Meller, S. , Li, L. and Gebhardt, C. (2010) Natural diversity of potato (Solanum tuberosum) invertases. BMC Plant Biol. 10, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias, R. , Till, B. , Mba, C. and Al‐Safadi, B. (2009) Optimizing TILLING and Ecotilling techniques for potato (Solanum tuberosum L). BMC Res. Notes, 2, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Cornejo, J. , Wechsler, S. , Livingston, M. and Mitchell, L. (2014) Genetically Engineered Crops in the United States. Washington D.C: US Department of Agriculture Economic Research Service. [Google Scholar]
- Frock, R.L. , Hu, J. , Meyers, R.M. , Ho, Y.J. , Kii, E. and Alt, F.W. (2015) Genome‐wide detection of DNA double‐stranded breaks induced by engineered nucleases. Nat. Biotechnol. 33, 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamborg, O.L. and Shyluk, J.P. (1981) Nutrition, Media and Characteristics of Plant Cell and Tissue Cultures. In: Plant Tissue Culture ( Thorpe, T.A. ed) pp. 21–44. San Diego, CA: Academic Press. [Google Scholar]
- Grob, K. (2007) Options for legal measures to reduce acrylamide contents in the most relevant foods. Food Addit. Contam. 24, 71–81. [DOI] [PubMed] [Google Scholar]
- Haberlach, G. , Cohen, B. , Reichert, N. , Baer, M. , Towill, L. and Helgeson, J.P. (1985) Isolation, culture and regeneration of protoplasts from potato and several related Solanum species. Plant Sci. 39, 67–74. [Google Scholar]
- Haun, W. , Coffman, A. , Clasen, B.M. , Demorest, Z.L. , Lowy, A. , Ray, E. , Retterath, A. , Stoddard, T. , Juillerat, A. , Cedrone, F. , Mathis, L. , Voytas, D.F. and Zhang, F. (2014) Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol. J. 12, 934–940. [DOI] [PubMed] [Google Scholar]
- Holme, I.B. , Wendt, T. and Holm, P.B. (2013) Intragenesis and cisgenesis as alternatives to transgenic crop development. Plant Biotechnol. J. 11, 395–407. [DOI] [PubMed] [Google Scholar]
- Jacobs, J.M. , Takla, M.F. , Docherty, L.C. , Frater, C.M. , Markwick, N.P. , Meiyalaghan, S. and Conner, A.J. (2009) Potato transformation with modified nucleotide sequences of the cry9Aa2 gene improves resistance to potato tuber moth. Potato Res. 52, 367–378. [Google Scholar]
- Juillerat, A. , Dubois, G. , Valton, J. , Thomas, S. , Stella, S. , Marechal, A. , Langevin, S. , Benomari, N. , Bertonati, C. , Silva, G.H. , Daboussi, F. , Epinat, J.C. , Montoya, G. , Duclert, A. and Duchateau, P. (2014) Comprehensive analysis of the specificity of transcription activator‐like effector nucleases. Nucleic Acids Res. 42, 5390–5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, D. , Singh, B.P. and Kumar, P. (2004) An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 145, 247–256. [Google Scholar]
- Kuzma, J. and Kokotovich, A. (2011) Renegotiating GM crop regulation. Targeted gene‐modification technology raises new issues for the oversight of genetically modified crops. EMBO reports 12, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali, P. , Aach, J. , Stranges, P.B. , Esvelt, K.M. , Moosburner, M. , Kosuri, S. , Yang, L. and Church, G.M. (2013) CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura‐Endo, C. , Ohara‐Takada, A. , Chuda, Y. , Ono, H. , Yada, H. , Yoshida, M. , Kobayashi, A. , Tsuda, S. , Takigawa, S. , Noda, T. , Yamauchi, H. and Mori, M. (2006) Effects of storage temperature on the contents of sugars and free amino acids in tubers from different potato cultivars and acrylamide in chips. Biosci. Biotechnol. Biochem. 70, 1173–1180. [DOI] [PubMed] [Google Scholar]
- Medeiros Vinci, R. , Mestdagh, F. and De Meulenaer, B. (2012) Acrylamide formation in fried potato products – present and future, a critical review on mitigation strategies. Food Chem. 133, 1138–1154. [Google Scholar]
- Mottram, D.S. , Wedzicha, B.L. and Dodson, A.T. (2002) Acrylamide is formed in the Maillard reaction. Nature, 419, 448–449. [DOI] [PubMed] [Google Scholar]
- Muth, J. , Hartje, S. , Twyman, R.M. , Hofferbert, H.R. , Tacke, E. and Prüfer, D. (2008) Precision breeding for novel starch variants in potato. Plant Biotechnol. J. 6, 576–584. [DOI] [PubMed] [Google Scholar]
- Roitsch, T. and Gonzalez, M.C. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci. 9, 606–613. [DOI] [PubMed] [Google Scholar]
- Rommens, C.M. (2007) Intragenic crop improvement: combining the benefits of traditional breeding and genetic engineering. J. Agric. Food Chem. 55, 4281–4288. [DOI] [PubMed] [Google Scholar]
- Slugina, M.A. , Khrapalova, I.A. , Ryzhova, N.N. , Kochieva, E.Z. and Skryabin, K.G. (2014) Polymorphism of Pain‐1 invertase gene in Solanum species. Dokl. Biochem. Biophys. 454, 1–3. [DOI] [PubMed] [Google Scholar]
- Sowokinos, J. (2001) Biochemical and molecular control of cold‐induced sweetening in potatoes. Am. J. Potato Res. 78, 221–236. [Google Scholar]
- Stadler, R.H. , Blank, I. , Varga, N. , Robert, F. , Hau, J. , Guy, P.A. , Robert, M.C. and Riediker, S. (2002) Acrylamide from Maillard reaction products. Nature, 419, 449–450. [DOI] [PubMed] [Google Scholar]
- Tareke, E. , Rydberg, P. , Karlsson, P. , Eriksson, S. and Tornqvist, M. (2002) Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J. Agric. Food Chem. 50, 4998–5006. [DOI] [PubMed] [Google Scholar]
- Voytas, D.F. (2013) Plant genome engineering with sequence‐specific nucleases. Annu. Rev. Plant Biol. 64, 327–350. [DOI] [PubMed] [Google Scholar]
- Wu, L. , Bhaskar, P.B. , Busse, J.S. , Zhang, R. , Bethke, P.C. and Jiang, J. (2011) Developing Cold‐Chipping Potato Varieties by Silencing the Vacuolar Invertase Gene All rights reserved. No part of this periodical may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Permission for printing and for reprinting the material contained herein has been obtained by the publisher. Crop Sci. 51, 981–990. [Google Scholar]
- Ye, J. , Shakya, R. , Shrestha, P. and Rommens, C.M. (2010) Tuber‐specific silencing of the acid invertase gene substantially lowers the acrylamide‐forming potential of potato. J. Agric. Food Chem. 58, 12162–12167. [DOI] [PubMed] [Google Scholar]
- Yoo, S.D. , Cho, Y.H. and Sheen, J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Zhang, F. , Li, X. , Baller, J.A. , Qi, Y. , Starker, C.G. , Bogdanove, A.J. and Voytas, D.F. (2013) Transcription activator‐like effector nucleases enable efficient plant genome engineering. Plant Physiol. 161, 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Illustration of a representative TALEN‐expression plasmid.
Figure S2 Assessment of TALEN activity within protoplasts from four different potato varieties – Atlantic, Ranger Russet, Russet Burbank, and Shepody.
Figure S3 Examples of chromatograms for VInv alleles containing either wild type sequences (top) or mutated sequences (bottom).
Figure S4 A, Graphical representation of the TALEN expression cassette that was introduced into potato protoplasts.
Figure S5 Analysis of sugar content within potato tubers stored at 4 °C for 14 days.


