Abstract
The vaccinia virus (VV) B8R gene encodes a secreted protein with homology to the gamma interferon (IFN-γ) receptor. In vitro, the B8R protein binds to and neutralizes the antiviral activity of several species of IFN-γ, including human and rat IFN-γ; it does not, however, bind significantly to murine IFN-γ. Here we report on the construction and characterization of recombinant VVs (rVVs) lacking the B8R gene. While the deletion of this gene had no effect on virus replication in vitro, rVVs lacking the B8R gene were attenuated for mice. There was a significant decrease in weight loss and mortality in normal mice, and nude mice survived significantly longer than did controls inoculated with parental virus. This is a surprising result considering the minimal binding of the B8R protein to murine IFN-γ and its failure to block the antiviral activity of this cytokine in vitro. Such reduction in virulence could not be determined in rats, since they are considerably more resistant to VV infection than are mice. Finally, deletion of the B8R gene had no detectable effects on humoral immune responses. Mice and rats vaccinated with the rVVs showed identical humoral responses to both homologous and heterologous genes expressed by VV. This study demonstrates that the deletion of the VV B8R gene leads to enhanced safety without a concomitant reduction in immunogenicity.
Vaccinia virus (VV) is the prototype member of the genus Orthopoxvirus, family Poxviridae, and it has been used extensively as a vector for the development of recombinant live vaccines (34). There are currently two effective recombinant VV (rVV) vaccines, one for rabies and the other for rinderpest (1, 18, 26, 52, 56). The rabies rVV vaccine has been used successfully to control fox rabies in Europe and, more recently, raccoon rabies in the United States (38). The rinderpest rVV vaccine protected cattle from a challenge with more than 1,000 times the lethal dose of the virus (18, 56). Not all rVVs, however, have been as effective as those for rabies and rinderpest. For example, we showed that rVVs expressing the vesicular stomatitis virus (VSV) G protein (VSV-G) protected against only a minimal experimental challenge dose (33, 57). Additionally, rhesus macaques immunized with rVVs expressing genes of the simian immunodeficiency virus (SIV) were not protected from virus infection, although there was a reduction in virus load and increased survival time (2, 20, 21, 37). It is clear that there is a definite need to enhance the efficacy of rVV vaccines against diseases for which protection is not optimal. However, it is even more critical that the safety of rVVs be validated before their approval for use as vaccines. Although VV has not been associated with any disease, it can cause severe complications in immunodeficient or immunosuppressed individuals (6, 7, 15, 43). Thus, increasing the safety of rVV vaccines without compromising their efficacy is an important consideration in vaccine development.
VV is one of many viruses with genes that code for specific proteins to suppress host immunity. These immunomodulating genes are usually not essential for virus growth in vitro. Their products include homologs of interleukin-1β (3, 47) and alpha/beta interferon (IFN-α/β) receptors (14, 49), complement control proteins (23, 28), and serine protease inhibitors (8, 25, 29, 46). In particular, VV contains a gene (B8R) that codes for a secreted protein with sequence similarity to the extracellular domain of the IFN-γ receptor (50). This protein neutralizes the antiviral activities of human, rat, rabbit, bovine, and chicken IFN-γ, but it binds with significantly lower affinity to murine IFN-γ (4, 36, 41, 51). Cytokines such as IFN-γ play essential roles in the regulation of the immune system and in defense against pathogens. Additionally, IFN-γ exhibits adjuvant activities when used with a subunit antigen (5), and it has attenuating activities (up to 106-fold for immunodeficient mice) when expressed by VV (19, 27). Thus, we hypothesized that the efficacy as well as the safety of rVVs might be increased by deleting the B8R gene. This hypothesis was tested in mice and rats inoculated with rVVs with a deletion of the B8R gene. We found that rVVs with an inactivated B8R gene are attenuated for normal and nude mice without having a concomitant reduction in immunogenicity.
MATERIALS AND METHODS
Cells and viruses.
Human HeLa S3 and A549 cells, African green monkey kidney BS-C-1 and BS-C-40 cells, hamster BHK-21 cells, and murine L929 cells were grown at 37°C under 5% CO2 in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The Western Reserve strain of VV (WR) (9), obtained from B. Moss (National Institute of Allergy and Infectious Diseases, Bethesda, Md.), a WR-derived rVV expressing VSV-G at the thymidine kinase (TK) site (v50) (33), and their derivatives were propagated in HeLa S3 cells and subjected to titer determination in BS-C-1 cells. Encephalomyocarditis virus (EMCV) was propagated in BHK-21 cells and subjected to titer determination in L929 cells. The New Jersey serotype of VSV (55) was propagated and subjected to titer determination in BHK-21 cells.
Animals.
Male athymic nude BALB/cBy (nu/nu) mice were purchased from Jackson Laboratory (Bar Harbor, Maine). Female (BALB/c × C57BL/6) F1 hybrid mice (CB6F1), male athymic nude Rowett (rnu/rnu) rats, and female Fischer 344 (F344) rats were purchased from Harlan Sprague-Dawley (Indianapolis, Ind.). All animals were maintained in accordance with National Institutes of Health guidelines and animal care protocols approved by the Animal Care Committee at the University of California, Davis.
Construction of p2B8R.
The strategy used to construct the VV transfer vector p2B8R is shown in Fig. 1. Each PCR fragment contained engineered restriction endonuclease sites to facilitate vector construction (Table 1). Briefly, a pUC18 PCR fragment was generated by amplification of plasmid pUC18 (54) with primers pUC18F and pUC18R, which contained the β-lactamase gene conferring ampicillin resistance (Ampr) and the origin of replication (Ori). Next, a B8R left PCR fragment was generated by amplification of VV WR DNA (22, 45) with primers B8RLF and B8RLR, which comprised the region to the left of the B8R gene start codon. This ATG was mutated to an ATA (engineered in the reverse B8RLR primer [shown in lowercase in Table 1]) to prevent the expression of a truncated B8R gene. Sequencing of this fragment (comprising most of the B7R gene) with primers B8RLS1, B8RLS2, B8RLS3, and B8RLS4 showed that it contains the wild-type sequence. The pUC18 and B8R left PCR fragments (digested with BglII and KpnI) were ligated to form plasmid pUCB8RLeft. Next, a B8R right PCR fragment, generated with primers B8RRF and B8RRR and containing most of the 819-bp B8R protein coding sequence (nucleotides 157 to 791), was cleaved with BamHI and BglII and ligated to BglII-cleaved pUCB8RLeft, producing pUCB8R. Then a pSC11 (12) PCR fragment (generated with primers pSC11F and pSC11R) was inserted into the BglII site of pUCB8R, resulting in the transfer vector pB8R, which directs the insertion of a cassette containing the VV P7.5 promoter and the lacZ gene (for β-galactosidase expression under the VV P11 promoter) into the B8R genomic region. Finally, the 273-bp SmaI-XbaI fragment of pB8R (containing the VV P7.5 promoter) was replaced with the 161-bp SmaI-NheI fragment of pJS5 (13), generating p2B8R, which contains two back-to-back strong synthetic VV early/late promoters (dsP).
FIG. 1.
Construction of VV transfer vector p2B8R. p2B8R was generated by joining pUC18, B8R left (B8RL), B8R right (B8RR), and pSC11 PCR fragments, as well as a DNA fragment from plasmid pJS5. The transfer vector p2B8R directs the insertional inactivation and deletion of the B8R gene of VV by homologous recombination. It contains the lacZ gene for β-galactosidase expression under the control of the VV P11 late promoter for screening of rVVs, and two back-to-back strong synthetic VV promoters (dsP) that are active in both early and late stages of infection. There are multiple cloning sites adjacent to each side of the dsP to facilitate the cloning of heterologous genes (only unique sites are shown).
TABLE 1.
Oligonucleotide primers used in this study
| Name | Sequence (5′-3′)a |
|---|---|
| pUC18F | TTTCTTAGATCTCAGGTGGCACTT |
| pUC18R | AATACGGGTACCCACAGAATCAGG |
| B8RLF | GTAATAGGTACCTTAGCATCCTAT |
| B8RLR | TATAATAGATCTtatGGTGTTGTTTG |
| B8RLS1 | CATGTTCTTTCCTGCGTTATCC |
| B8RLS2 | AAAAATAAACAAATAGGGGTTCC |
| B8RLS3 | CGTCGGATTTGATTCCATAGAT |
| B8RLS4 | CCTTTATAGCATTTTCCCTCTG |
| B8RRF | GCATCAAGATCTGGCAAACAATGT |
| B8RRR | AGTTAAGGATCCTAAGAAAAGTGGTA |
| pSC11F | GCTCACAGATCTCCCGGGATCCGT |
| pSC11R | CTGCCCAGATCTTATTATTTTTGACACC |
Engineered restriction endonuclease sites are underlined.
Generation of rVVs and analysis of their genomic DNA, stability, and purity.
rVVs were generated by standard homologous recombination using cationic liposome-mediated transfection of BS-C-1 cells infected with the VVs at 0.05 PFU/cell. The rVVs were plaque purified from transfection supernatants on BS-C-1 cell monolayers by using 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) to detect the lacZ marker gene (16).
The expression of the lacZ gene by rVVs was tested by cytochemical staining of infected cell monolayers as previously described (32), with minor modifications. Briefly, plaque assays were performed on BS-C-40 cell monolayers. After 2 days, the cells were rinsed twice with phosphate-buffered saline (pH 7.3) (PBS), fixed with a 2% paraformaldehyde–0.2% glutaraldehyde solution in 0.1 M sodium phosphate (pH 7.3) for 5 min at 4°C, rinsed again with PBS twice, and stained overnight at 37°C with X-Gal stain (0.1 M sodium phosphate [pH 7.3], 1.3 mM MgCl2, 3 mM potassium ferricyanide, 3 mM potassium ferrocyanide, 0.1% X-Gal). Next, blue plaques were counted and marked, and finally dishes were stained with crystal violet staining solution (0.5% crystal violet, 10% ethanol, 20% formaldehyde) to reveal any colorless (parental) plaques not marked previously. Restriction analysis of rVV DNA samples was performed with DNA purified by a small-scale method employing micrococcal nuclease (30).
B8R protein bioassay.
B8R protein activity was determined by its ability to prevent the antiviral activity of human IFN-γ. HeLa S3 cell suspensions were infected with rVVs at 20 PFU/cell or mock infected with DMEM for 1 h. The cells were washed twice, resuspended in DMEM, and incubated for 36 h. Supernatants were then harvested, and VV particles were removed by centrifugation at 80,000 × g (24,000 rpm in an SW28 rotor) for 75 min at 4°C on a 25% (wt/wt) sucrose cushion. The clarified supernatant was then concentrated (about 40-fold) with Centriprep-10 concentrators (10,000 molecular weight cutoff) (Amicon, Beverly, Mass.) and filtered through 0.2-μm-pore-size filters. Each supernatant was serially diluted in DMEM–5% FBS. Subsequently, 5 μl (600 U/ml) of recombinant human IFN-γ (Genzyme, Cambridge, Mass.) in DMEM–5% FBS was added to 45 μl of each dilution and incubated at 37°C for 1 h. Mixtures were then transferred to 96-well plates, seeded 4 to 6 h previously with 2 × 104 A549 cells/well in 100 μl of DMEM–5% FBS (final IFN-γ concentration, 20 U/ml). After 24 h of incubation, cells were challenged with the minimum dose of EMCV (104 PFU in 50 μl) that gave 100% cytopathic effects and stained with crystal violet staining solution 1 day later.
Virus growth curves.
Virus replication in vitro was determined by generating one-step growth curves (40). Briefly, duplicate monolayers of L929 and A549 cells were infected at 0.01 PFU/cell for 1 h in 12-well plates. The cells were then washed and resuspended in 1 ml of DMEM–2.5% FBS. At each time point, supernatants were collected, centrifuged (to pellet detached cells), and transferred to a new tube (the extracellular virus fraction). Cells in the wells were resuspended in 1 ml of DMEM, scraped, and added to the pellet of detached cells (the intracellular virus fraction). The intracellular fraction was freeze-thawed three times, trypsinized, and sonicated. Duplicates of both virus fractions were subjected to titer determination on BS-C-1 cell monolayers.
Virulence studies in mice and rats.
Groups of CB6F1 normal mice and F344 normal rats (8- to 9-week-old females) were challenged intranasally with 10-fold dilutions (in sterile PBS) of each rVV strain in a final volume of 10 μl while under light anesthesia. The animals were examined and weighed daily.
Groups of BALB/cBy nude mice (7- to 8-week-old males) and Rowett nude rats (7-week-old males) were challenged intraperitoneally with 107 PFU of each rVV strain in a final volume of 250 μl of sterile PBS. The animals were examined daily, and the mortality rate for each group was determined.
rVV immunogenicity studies.
Groups of animals (6- to 7-week-old female CB6F1 mice and F344 rats) were immunized intramuscularly with 106 PFU of each VV strain in a final volume of 50 μl of sterile PBS while under light anesthesia. Serum was collected weekly from each animal, and serum-neutralizing antibody titers to VV and VSV were determined by plaque reduction on BS-C-40 cell monolayers (24) and serum neutralization assays on BHK-21 cell monolayers (33), respectively.
Data analysis.
Statistical analyses were performed using the statistical software program SAS, release 6.11 (SAS Institute, Cary, N.C.).
RESULTS
Deletion of the B8R gene has no effect on VV replication in vitro.
The strategy used for generation of the transfer vectors employed in this study is presented in Fig. 1. rVVs derived from the pB8R transfer vector were unstable, probably due to the presence of P7.5 in the inverted terminal repeat regions of the VV genome as well as in the transfer vector (25). For this reason, p2B8R was developed for generating the two rVVs used in this study. The p2B8R transfer vector directs homologous recombination with the B8R region of the VV genome, leading to inactivation of the gene as a result of partial deletion (154 bp) and insertion of the lacZ gene. In addition, its strong synthetic promoters (dsP) allow increased levels of expression (13; P. H. Verardi, F. H. Aziz, S. Ahmad, and T. D. Yilma, unpublished data) of two heterologous genes.
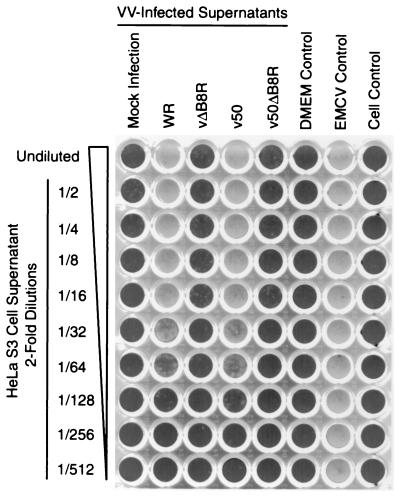
Two rVVs were generated for the study: (i) vΔB8R was derived from the WR strain of VV, selected because of its high virulence to mice, and (ii) v50ΔB8R was derived from v50, a WR-derived rVV expressing the VSV-G gene within the TK locus (33), selected to facilitate the analysis of the immune response to both homologous (VV) and heterologous (VSV-G) antigens. The purity and stability of the final preparations of viruses were confirmed by X-Gal cytochemical staining of plaques after a minimum of four consecutive plaque purifications in cell culture. Restriction analysis (HindIII digestion) of DNA purified from rVVs confirmed the insertional inactivation of the B8R and TK regions by the lacZ and VSV-G genes, respectively (data not shown). To confirm that the rVVs with a deletion of the B8R gene did not express any residual IFN-γ binding activities, HeLa S3 cells were infected with the viruses at a high multiplicity of infection (MOI) and their supernatants were harvested, clarified from VV particles, concentrated, and assayed for human IFN-γ-neutralizing activities. It is clear from the data that only the supernatants derived from cells infected with VVs with an intact B8R gene (WR and v50) expressed detectable human IFN-γ-neutralizing activities (Fig. 2). These concentrated supernatants at dilutions of 1/32 to 1/64 were able to neutralize human IFN-γ at concentrations of about 20 U/ml; IFN-γ at 1 U/ml provides 50% protection from cytopathic effects in this assay. However, there were no detectable activities from concentrated supernatants of rVVs lacking the B8R gene, even when undiluted (Fig. 2).
FIG. 2.
Human IFN-γ-neutralizing activities of VV-infected cell supernatants. Supernatants of HeLa S3 cells infected with the rVVs at 20 PFU/cell or mock infected with DMEM were collected 36 h postinfection, clarified, concentrated, and assayed for their ability to inactivate the antiviral activity of human IFN-γ. Recombinant human IFN-γ (5 μl, 600 U/ml) was added to 45 μl of serial dilutions of each supernatant, and the mixtures were incubated at 37°C for 1 h and then transferred to A549 cell monolayers in 96-well plates (final IFN-γ concentration, 20 U/ml). After 24 h, the cells were challenged with EMCV; they were stained with crystal violet 1 day later. Control wells received equivalent volumes of DMEM in lieu of cell supernatants (all controls), human IFN-γ (EMCV and cell controls), and EMCV challenge (cell control). Assays were performed in duplicate with identical results (only one plate is shown).
The plaque phenotypes of rVVs and their parental strains were indistinguishable in cell culture. Similarly, the growth of all viruses in vitro (at low MOI) was essentially identical (Fig. 3). This was true for viruses grown in human (A549) or murine (L929) cells and for both intracellular and extracellular virus fractions (Fig. 3). Infection at higher MOIs also resulted in virtually identical virus yields (data not shown). Thus, the deletion of the B8R gene has no effect on virus replication in vitro.
FIG. 3.
Growth curves for WR, vΔB8R, v50, and v50ΔB8R. Monolayers of human A549 (A and B) or murine L929 (C and D) cells were infected at 0.01 PFU/cell. At the indicated time points, both intracellular (A and C) and extracellular (B and D) virus fractions were collected and subjected to titer determination on BS-C-1 cell monolayers. The data shown represent the mean values from duplicate samples assayed in duplicate; error bars indicate the standard error of the mean.
Deletion of the B8R gene decreases VV virulence for normal mice.
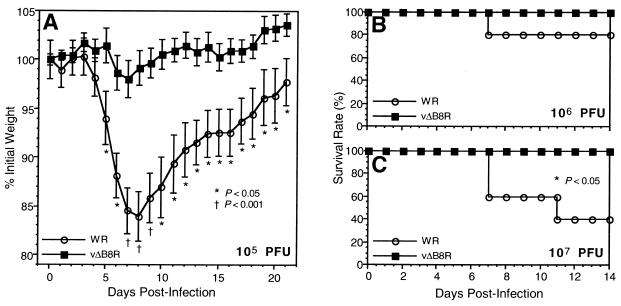
Immunocompetent mice inoculated intranasally with 103 and 104 PFU of either WR or vΔB8R exhibited no significant weight loss (data not shown). However, mice inoculated with 105 PFU of WR had marked weight loss, which was not observed in vΔB8R-infected mice (Fig. 4A). This difference was statistically significant (by analysis of variance [ANOVA]) from days 5 through 21 postinfection (P < 0.05) and highly significant on days 7, 8, and 9 postinfection (P < 0.001). When vΔB8R was used at a dose of 106 PFU, no significant weight loss was observed, while mice inoculated with 106 PFU of WR showed marked weight loss (data not shown) and one of them died (20% mortality [Fig. 4B]). Finally, when vΔB8R was used at a dose of 107 PFU, the mice had some weight loss (data not shown) but survived, while three of the mice inoculated with 107 PFU of WR died (60% mortality). This difference in survival was significant (P < 0.05, log-rank test). Taken together, these results indicate that inactivation of the B8R gene decreases VV virulence for mice. On the other hand, rats inoculated intranasally with doses as high as 107 PFU of either WR or vΔB8R did not have significant weight loss or any other signs of disease and maintained normal growth curves (data not shown).
FIG. 4.
Virulence studies in VV-infected immunocompetent mice. Normal mice (CB6F1, 11 per group in the experiment in panel A and 5 per group in the experiments in panels B and C) were inoculated intranasally with 105 (A), 106 (B) or 107 (C) PFU of either WR or vΔB8R. Animals were individually weighed and monitored for signs of disease for 21 days. (A) Mean group weight on each day, expressed relative to the mean weight for that group at the day of infection, as well as the P values obtained by statistical analysis (ANOVA). Error bars indicate the standard error of the mean. (B and C) Survival data presented as Kaplan-Meier plots (the log-rank test was applied for statistical survival analysis).
Deletion of the B8R gene attenuates VV virulence for nude mice.
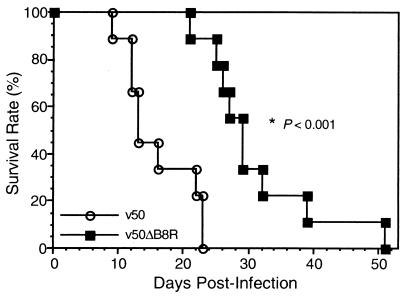
Immunodeficient, athymic nude mice inoculated intraperitoneally with 107 PFU of v50ΔB8R displayed a significantly greater (P < 0.001, Mann-Whitney-Wilcoxon test) survival rate than did nude mice inoculated with v50 (Fig. 5). While all nude mice injected with v50 died by 23 days postinfection, mice infected with v50ΔB8R had a survival rate of 89% on this day (Fig. 5). The median survival time of nude mice inoculated with v50 and v50ΔB8R was 13 and 29 days, respectively. All nude mice inoculated with v50 and v50ΔB8R developed typical pox lesions. A group of four control nude mice mock inoculated with sterile PBS remained healthy throughout the observation period of 120 days. Nude rats inoculated intraperitoneally with 107 PFU of VV resisted infection, showing no signs of disease (including pox lesions) even when injected with the highly virulent WR strain (data not shown).
FIG. 5.
Virulence studies in VV-infected immunodeficient mice. Nude mice (nine per group) were inoculated intraperitoneally with 107 PFU of each rVV, and survival rates were recorded daily. All nude mice injected with v50 died by 23 days postinfection, while mice infected with v50ΔB8R exhibited a much higher survival rate (89% on day 23). The median survival times of nude mice inoculated with v50 and v50ΔB8R were 13 and 29 days, respectively. Nonparametric ANOVA statistical analysis of survival time (Mann-Whitney-Wilcoxon test) yielded P < 0.001.
Deletion of the B8R gene does not alter the humoral response to VV.
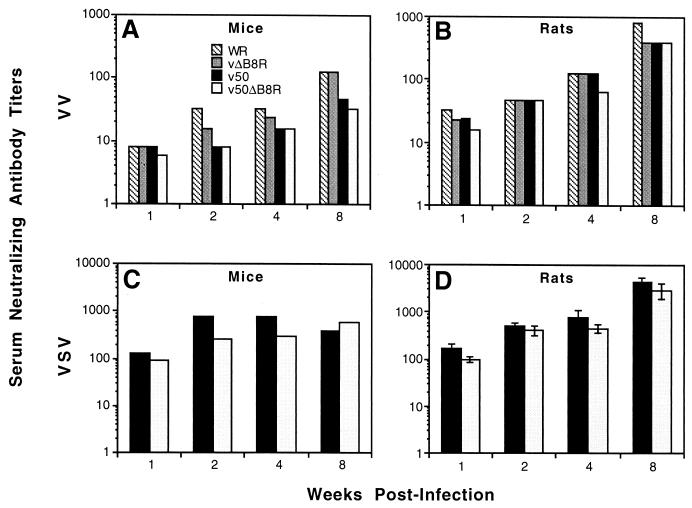
Groups of normal mice and rats (five animals/group) were inoculated intramuscularly with 106 PFU of VV. Serum was collected weekly from each animal, and neutralizing-antibody titers to VV and VSV were determined by a plaque reduction assay or serum neutralization, respectively. Although there was a significant increase in attenuation, no statistical differences were observed in the humoral immune response to either homologous (VV) or heterologous (VSV-G) antigens in mice or rats inoculated with rVVs (Fig. 6).
FIG. 6.
Immunogenicity studies of VV-infected immunocompetent mice and rats. Groups of five normal mice (CB6F1) (A and C) or rats (F344) (B and D) were inoculated intramuscularly with 106 PFU of WR, vΔB8R, v50, or v50ΔB8R. Each histogram represents the mean serum neutralizing-antibody titer to VV (A and B) or VSV (C and D). Serum samples were pooled and assayed in duplicate, except for the experiment in panel D, where samples were assayed individually (error bars represent the standard error of the mean). Undetectable levels of VV and VSV serum neutralizing antibodies (<8) were found at the time of inoculation for all groups (week 0), for all time points in a group of mice mock inoculated with sterile PBS, and for the VSV titers of WR- and vΔB8R-infected animals (data not shown). One v50-inoculated mouse died of undetermined causes 36 days postinfection.
DISCUSSION
VV is one of many viruses that code for immunomodulating proteins, including B8R. This secreted protein has sequence similarity to the extracellular domain of the IFN-γ receptor, binding and neutralizing the antiviral activity of most species of IFN-γ, including rat IFN-γ. However, even at high concentrations, the B8R protein fails to block the antiviral activity of murine IFN-γ in vitro (4, 36, 41, 51). IFN-γ is a pleiotropic cytokine that plays essential roles in the regulation of the immune system and in the host defense against pathogens. We and others showed that rVVs expressing IFN-γ were attenuated by more than 106-fold in immunodeficient mice (19, 27) and that IFN-γ has adjuvant activities when used with a subunit antigen (e.g., VSV-G) (5). This led us to the hypothesis that deleting immunomodulating genes (e.g., the B8R gene) in rVVs would allow the development of safer and more efficacious vaccines.
To test our hypothesis, we constructed two rVVs with deleted B8R genes to test the role of this gene in virulence and on the humoral immune response of animals to both homologous (VV) and heterologous (VSV-G) antigens. Although the B8R protein does not inhibit the antiviral activity of murine IFN-γ (4, 36, 41, 51) and has no effect on VV replication in vitro (Fig. 3), inactivation of the B8R gene reduced the virulence of VV in a murine intranasal model (as measured by weight loss and survival rates of normal mice) and in a nude mouse model (as measured by survival rates of nude mice). Intranasal infection of normal mice with rVVs has been reported to be an ideal route for studies of pathogenesis and virulence (53), and CB6F1 mice have been used extensively to assess the adjuvant activity of IFN-γ (39). It has also been reported that rVVs with an insertional inactivation of the TK gene (i.e., v50 and v50ΔB8R) are greatly attenuated for normal mice (10); doses as high as 5 × 106 PFU of v50 failed to cause weight loss in CB6F1 mice (P. H. Verardi, F. H. Aziz, and T. D. Yilma, unpublished data). For this reason, only WR and vΔB8R (with a higher starting virulence) were used in the virulence studies in immunocompetent mice. Normal mice inoculated intranasally with WR had significantly higher weight loss and mortality than did vΔB8R-infected mice (Fig. 4). These results indicate that the inactivation of the B8R gene attenuates VV virulence for mice. On the other hand, such reduction in virulence could not be determined in normal rats, since they are considerably more resistant to VV infection than mice (F344 rats infected with either WR or vΔB8R did not lose weight or exhibit disease symptoms even when the virus was given at a dose of 107 PFU). Intraperitoneal inoculation of nude mice is an established model for disseminated VV infection (17, 19, 42). Since VVs with an intact TK gene are highly virulent for nude mice, only v50 and v50ΔB8R (with a lower starting virulence) were used in the attenuation studies with immunodeficient mice. We demonstrated that nude mice inoculated with v50ΔB8R had significantly higher survival rates than did those inoculated with v50 (Fig. 5). The effects of the B8R protein could not be determined in nude rats due to their greater resistance to VV infection (44).
The direct inactivation of the antiviral activity of murine IFN-γ by the B8R protein cannot explain the higher virulence of rVVs with an intact B8R gene, since the B8R protein does not bind significantly to murine IFN-γ in vitro. Knockout mice with an inactivated IFN-γ receptor gene were found to be more susceptible to infection with VV or herpesvirus than were mice with an inactivated IFN-γ gene (11), although one would assume that the two groups of mice would be equally susceptible. This suggests that there may be more than one ligand for the IFN-γ receptor and its poxvirus homologs. Indeed, the myxoma virus homolog of the B8R protein (M-T7), which was shown to be a virulence factor in rabbits (35), has a number of ligands including rabbit IFN-γ and human interleukin-8; it also inhibits the biological activity of the chemokine RANTES (31). In addition, VVs expressing human IFN-γ are attenuated for nude mice (19). This is surprising since human IFN-γ has no detectable antiviral activity in mouse cells. Thus, it is reasonable to deduce that the B8R protein may bind to other immunoregulatory proteins or may have immunomodulating properties in vivo that affect VV virulence by a mechanism other than direct interference with the antiviral activity of IFN-γ.
B8R-deleted rVVs elicited humoral immune responses to both homologous (VV) and heterologous (VSV-G) antigens that did not differ significantly from those of the respective parental strains (Fig. 6). One could speculate that the deletion of the B8R gene would augment the immune response by eliminating immunomodulating functions of the B8R protein (e.g., binding IFN-γ to neutralize its antiviral activity), just as the deletion of serine protease inhibitor homolog genes of VV increases antibody response to a heterologous antigen (58). However, we have shown here that the B8R gene also acts as a virulence factor; its deletion attenuates the vector, which should result in a decreased immune response. The two competing actions could effectively cancel each other, accounting for the experimental observation that humoral immune responses elicited by both B8R+ and B8R− viruses are essentially the same. In future studies, it will be of great interest to determine the effect of the B8R gene in cell-mediated immune responses, which are essential to vaccine efficacy.
Two highly effective rVV vaccines have been developed to date: one for protection against rinderpest and the other for the eradication of rabies in wildlife (1). However, other rVVs have not been as efficacious. Also, the safety of rVVs is a major concern, especially for immunocompromised individuals. Strategies to increase safety include the use of modified VV Ankara (MVA), a highly attenuated and host cell-restricted vector (37, 48). Here we show that the B8R protein is a VV virulence factor in mice despite the lack of detectable inhibition of the antiviral activity of murine IFN-γ by the B8R protein in vitro. In addition, deletion of the B8R gene did not alter the immune responses to homologous (VV) or heterologous (VSV-G) antigens. Consequently, the B8R locus emerges as an excellent site for heterologous gene expression in rVV vaccine development; inactivation of the B8R gene attenuates VV (increasing the safety of the vector) without compromising the effectiveness of the immune response elicited by the recombinant vaccine. All of these features are highly desirable for the development of new, effective rVV vaccines.
ACKNOWLEDGMENTS
This work was supported by an NIH grant awarded to T.D.Y. (AI37182) and a grant awarded to L.A.J. by The Harold Wetterberg Foundation. Other support included NIH grants AI29207 and AI36197 and USA-DAMD contract 17-95-C-5054N to T.D.Y. P.H.V. received support from Conselho Nacional de Desenvolvimento Científico Tecnológico (CNPq), Brazil.
We are grateful to Lauretta Turin, Ian Crossley, and Kartik Nettar for critical advice and assistance. We thank Sally Owens for helpful discussions and review of the manuscript.
REFERENCES
- Ada G. Vaccine development. Real and imagined dangers. Nature. 1991;349:369. doi: 10.1038/349369a0. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Lohman B, Marthas M, Giavedoni L, El-Amad Z, Haigwood N L, Scandella C J, Gardner M B, Luciw P A, Yilma T. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res Hum Retroviruses. 1994;10:195–204. doi: 10.1089/aid.1994.10.195. [DOI] [PubMed] [Google Scholar]
- Alcamí A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- Alcamí A, Smith G L. Vaccinia, cowpox, and camelpox viruses encode soluble gamma interferon receptors with novel broad species specificity. J Virol. 1995;69:4633–4639. doi: 10.1128/jvi.69.8.4633-4639.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K P, Fennie E H, Yilma T. Enhancement of a secondary antibody response to vesicular stomatitis virus “G” protein by IFN-γ treatment at primary immunization. J Immunol. 1988;140:3599–3604. [PubMed] [Google Scholar]
- Arita I, Fenner F. Complications of smallpox vaccination. In: Quinnan G V Jr, editor. Vaccinia viruses as vectors for vaccine antigens. 1985. pp. 49–60. Proceedings of the Workshop on Vaccinia Viruses as Vectors for Vaccine Antigens. [Google Scholar]
- Baxby D. Safety of recombinant vaccinia vaccines. Lancet. 1991;337:913. doi: 10.1016/0140-6736(91)90241-g. [DOI] [PubMed] [Google Scholar]
- Boursnell M E, Foulds I J, Campbell J I, Binns M M. Non-essential genes in the vaccinia virus HindIII K fragment: a gene related to serine protease inhibitors and a gene related to the 37K vaccinia virus major envelope antigen. J Gen Virol. 1988;69:2995–3003. doi: 10.1099/0022-1317-69-12-2995. [DOI] [PubMed] [Google Scholar]
- Bronson L H, Parker R F. The neutralization of vaccine virus by immune serum: titration by the intracerebral inoculation of mice. J Bacteriol. 1941;41:56–57. [Google Scholar]
- Buller R M, Smith G L, Cremer K, Notkins A L, Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature. 1985;317:813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Cantin E, Tanamachi B, Openshaw H, Mann J, Clarke K. Gamma interferon (IFN-γ) receptor null-mutant mice are more susceptible to herpes simplex virus type 1 infection than IFN-γ ligand null-mutant mice. J Virol. 1999;73:5196–200. doi: 10.1128/jvi.73.6.5196-5200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Brechling K, Moss B. Vaccinia virus expression vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler J R, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques. 1997;23:1094–1097. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- Colamonici O R, Domanski P, Sweitzer S M, Larner A, Buller R M. Vaccinia virus B18R gene encodes a type I interferon-binding protein that blocks interferon α transmembrane signaling. J Biol Chem. 1995;270:15974–15978. doi: 10.1074/jbc.270.27.15974. [DOI] [PubMed] [Google Scholar]
- Cooney E L, Collier A C, Greenberg P D, Coombs R W, Zarling J, Arditti D E, Hoffman M C, Hu S L, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Earl P L, Moss B. Generation of recombinant vaccinia viruses. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1994. pp. 16.17.1–16.17.16. [Google Scholar]
- Flexner C, Hügin A, Moss B. Prevention of vaccinia virus infection in immunodeficient mice by vector-directed IL-2 expression. Nature. 1987;330:259–262. doi: 10.1038/330259a0. [DOI] [PubMed] [Google Scholar]
- Giavedoni L, Jones L, Mebus C, Yilma T. A vaccinia virus double recombinant expressing the F and H genes of rinderpest virus protects cattle against rinderpest and causes no pock lesions. Proc Natl Acad Sci USA. 1991;88:8011–8015. doi: 10.1073/pnas.88.18.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni L D, Jones L, Gardner M B, Gibson H L, Ng C T, Barr P J, Yilma T. Vaccinia virus recombinants expressing chimeric proteins of human immunodeficiency virus and γ interferon are attenuated for nude mice. Proc Natl Acad Sci USA. 1992;89:3409–3413. doi: 10.1073/pnas.89.8.3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavedoni L D, Planelles V, Haigwood N L, Ahmad S, Kluge J D, Marthas M L, Gardner M B, Luciw P A, Yilma T D. Immune response of rhesus macaques to recombinant simian immunodeficiency virus gp130 does not protect from challenge infection. J Virol. 1993;67:577–583. doi: 10.1128/jvi.67.1.577-583.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Jr, Elkins W R, Alvord W G, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S T, Chan Y S, Smith G L. Vaccinia virus homologues of the Shope fibroma virus inverted terminal repeat proteins and a discontinuous ORF related to the tumor necrosis factor receptor family. Virology. 1991;180:633–647. doi: 10.1016/0042-6822(91)90077-o. [DOI] [PubMed] [Google Scholar]
- Isaacs S N, Kotwal G J, Moss B. Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci USA. 1992;89:628–632. doi: 10.1073/pnas.89.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ahmad S, Chan K, Verardi P, Morton W R, Grant R, Yilma T. Enhanced safety and efficacy of live attenuated SIV vaccines by prevaccination with recombinant vaccines. J Med Primatol. 2000;29:231–239. doi: 10.1034/j.1600-0684.2000.290316.x. [DOI] [PubMed] [Google Scholar]
- Kettle S, Blake N W, Law K M, Smith G L. Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode Mr 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology. 1995;206:136–147. doi: 10.1016/s0042-6822(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Kieny M P, Lathe R, Drillien R, Spehner D, Skory S, Schmitt D, Wiktor T, Koprowski H, Lecocq J P. Expression of rabies virus glycoprotein from a recombinant vaccinia virus. Nature. 1984;312:163–166. doi: 10.1038/312163a0. [DOI] [PubMed] [Google Scholar]
- Kohonen-Corish M R, King N J, Woodhams C E, Ramshaw I A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-γ. Eur J Immunol. 1990;20:157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- Kotwal G J, Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988;335:176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- Kotwal G J, Moss B. Vaccinia virus encodes two proteins that are structurally related to members of the plasma serine protease inhibitor superfamily. J Virol. 1989;63:600–606. doi: 10.1128/jvi.63.2.600-606.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A C, Chu Y. A rapid method for screening vaccinia virus recombinants. BioTechniques. 1991;10:564–565. [PubMed] [Google Scholar]
- Lalani A S, Graham K, Mossman K, Rajarathnam K, Clark-Lewis I, Kelvin D, McFadden G. The purified myxoma virus gamma interferon receptor homolog M-T7 interacts with the heparin-binding domains of chemokines. J Virol. 1997;71:4356–4363. doi: 10.1128/jvi.71.6.4356-4363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor G R, Nolan G P, Fiering S, Roederer M, Herzenberg L A. Use of E. coli lacZ (β-galactosidase) as a reporter gene. In: Murray E J, editor. Gene transfer and expression protocols. Vol. 7. Clifton, N.J: Humana Press; 1991. pp. 217–235. [DOI] [PubMed] [Google Scholar]
- Mackett M, Yilma T, Rose J K, Moss B. Vaccinia virus recombinants: expression of VSV genes and protective immunization of mice and cattle. Science. 1985;227:433–435. doi: 10.1126/science.2981435. [DOI] [PubMed] [Google Scholar]
- Moss B. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc Natl Acad Sci USA. 1996;93:11341–11348. doi: 10.1073/pnas.93.21.11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman K, Nation P, Macen J, Garbutt M, Lucas A, McFadden G. Myxoma virus M-T7, a secreted homolog of the interferon-γ receptor, is a critical virulence factor for the development of myxomatosis in European rabbits. Virology. 1996;215:17–30. doi: 10.1006/viro.1996.0003. [DOI] [PubMed] [Google Scholar]
- Mossman K, Upton C, Buller R M, McFadden G. Species specificity of ectromelia virus and vaccinia virus interferon-γ binding proteins. Virology. 1995;208:762–769. doi: 10.1006/viro.1995.1208. [DOI] [PubMed] [Google Scholar]
- Ourmanov I, Brown C R, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch V M. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J Virol. 2000;74:2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoret P P, Brochier B. The development and use of a vaccinia-rabies recombinant oral vaccine for the control of wildlife rabies: a link between Jenner and Pasteur. Epidemiol Infect. 1996;116:235–240. doi: 10.1017/s0950268800052535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playfair J H, De Souza J B. Recombinant gamma interferon is a potent adjuvant for a malaria vaccine in mice. Clin Exp Immunol. 1987;67:5–10. [PMC free article] [PubMed] [Google Scholar]
- Price N, Tscharke D C, Hollinshead M, Smith G L. Vaccinia virus gene B7R encodes an 18-kDa protein that is resident in the endoplasmic reticulum and affects virus virulence. Virology. 2000;267:65–79. doi: 10.1006/viro.1999.0116. [DOI] [PubMed] [Google Scholar]
- Puehler F, Weining K C, Symons J A, Smith G L, Staeheli P. Vaccinia virus-encoded cytokine receptor binds and neutralizes chicken interferon-γ. Virology. 1998;248:231–240. doi: 10.1006/viro.1998.9278. [DOI] [PubMed] [Google Scholar]
- Ramshaw I A, Andrew M E, Phillips S M, Boyle D B, Coupar B E. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329:545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]
- Schuurman H J. The nude rat. Hum Exp Toxicol. 1995;14:122–125. doi: 10.1177/096032719501400130. [DOI] [PubMed] [Google Scholar]
- Smith G L, Chan Y S, Howard S T. Nucleotide sequence of 42 kbp of vaccinia virus strain WR from near the right inverted terminal repeat. J Gen Virol. 1991;72:1349–1376. doi: 10.1099/0022-1317-72-6-1349. [DOI] [PubMed] [Google Scholar]
- Smith G L, Howard S T, Chan Y S. Vaccinia virus encodes a family of genes with homology to serine proteinase inhibitors. J Gen Virol. 1989;70:2333–2343. doi: 10.1099/0022-1317-70-9-2333. [DOI] [PubMed] [Google Scholar]
- Spriggs M K, Hruby D E, Maliszewski C R, Pickup D J, Sims J E, Buller R M, VanSlyke J. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell. 1992;71:145–152. doi: 10.1016/0092-8674(92)90273-f. [DOI] [PubMed] [Google Scholar]
- Sutter G, Wyatt L S, Foley P L, Bennink J R, Moss B. A recombinant vector derived from the host range-restricted and highly attenuated MVA strain of vaccinia virus stimulates protective immunity in mice to influenza virus. Vaccine. 1994;12:1032–1040. doi: 10.1016/0264-410x(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Symons J A, Alcamí A, Smith G L. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Upton C, Mossman K, McFadden G. Encoding of a homolog of the IFN-γ receptor by myxoma virus. Science. 1992;258:1369–1372. doi: 10.1126/science.1455233. [DOI] [PubMed] [Google Scholar]
- Verardi P H. Ph.D. thesis. University of California, Davis; 1997. [Google Scholar]
- Wiktor T J, Macfarlan R I, Reagan K J, Dietzschold B, Curtis P J, Wunner W H, Kieny M P, Lathe R, Lecocq J P, Mackett M, Moss B, Koprowski H. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc Natl Acad Sci USA. 1984;81:7194–7198. doi: 10.1073/pnas.81.22.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J D, Reith R W, Jeffrey L J, Arrand J R, Mackett M. Biological characterization of recombinant vaccinia viruses in mice infected by the respiratory route. J Gen Virol. 1990;71:2761–2767. doi: 10.1099/0022-1317-71-11-2761. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yilma T, Breeze R G, Ristow S, Gorham J R, Leib S R. Immune responses of cattle and mice to the G glycoprotein of vesicular stomatitis virus. Adv Exp Med Biol. 1985;185:101–115. doi: 10.1007/978-1-4684-7974-4_6. [DOI] [PubMed] [Google Scholar]
- Yilma T, Hsu D, Jones L, Owens S, Grubman M, Mebus C, Yamanaka M, Dale B. Protection of cattle against rinderpest with vaccinia virus recombinants expressing the HA or F gene. Science. 1988;242:1058–1061. doi: 10.1126/science.3194758. [DOI] [PubMed] [Google Scholar]
- Yilma T, Mackett M, Rose J K, Moss B. Vaccinia virus recombinants expressing vesicular stomatitis genes immunize mice and cattle. In: Quinnan G V, editor. Vaccinia viruses as vectors for vaccine antigens. 1985. pp. 187–200. Proceedings of the Workshop on Vaccinia Viruses as Vectors for Vaccine Antigens. [Google Scholar]
- Zhou J, Crawford L, McLean L, Sun X Y, Stanley M, Almond N, Smith G L. Increased antibody responses to human papillomavirus type 16 L1 protein expressed by recombinant vaccinia virus lacking serine protease inhibitor genes. J Gen Virol. 1990;71:2185–2190. doi: 10.1099/0022-1317-71-9-2185. [DOI] [PubMed] [Google Scholar]