Abstract
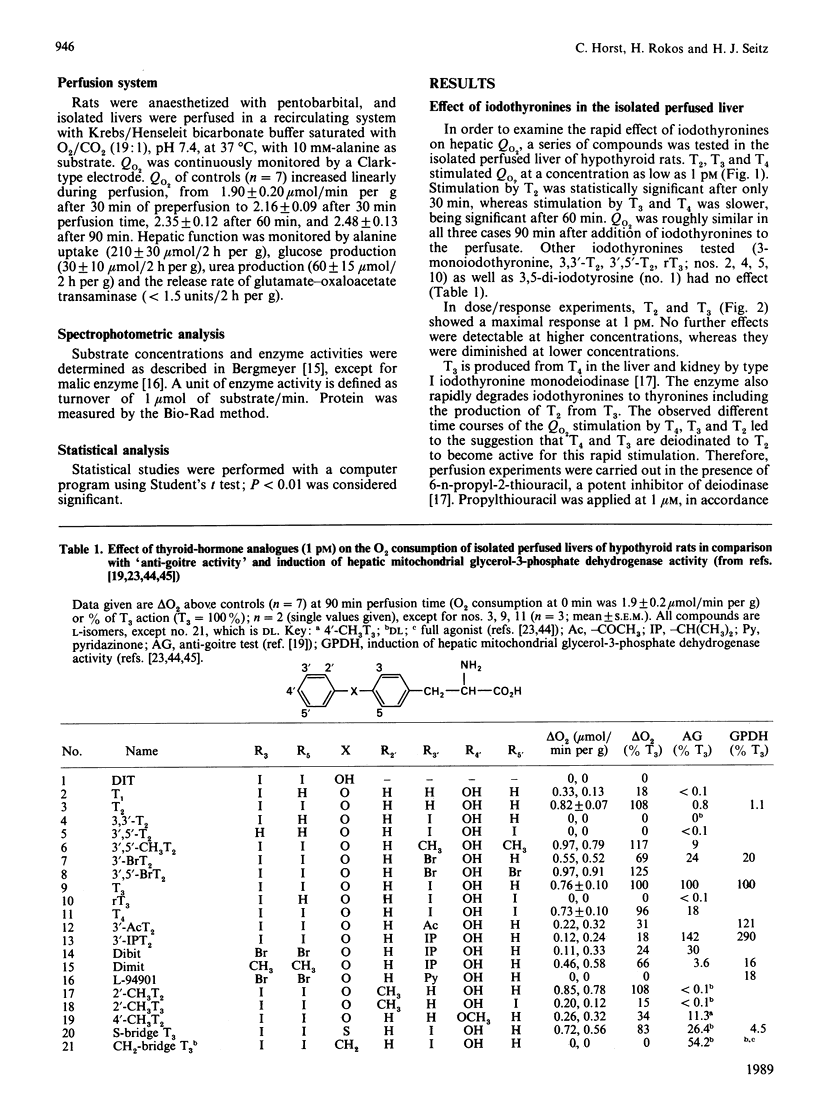
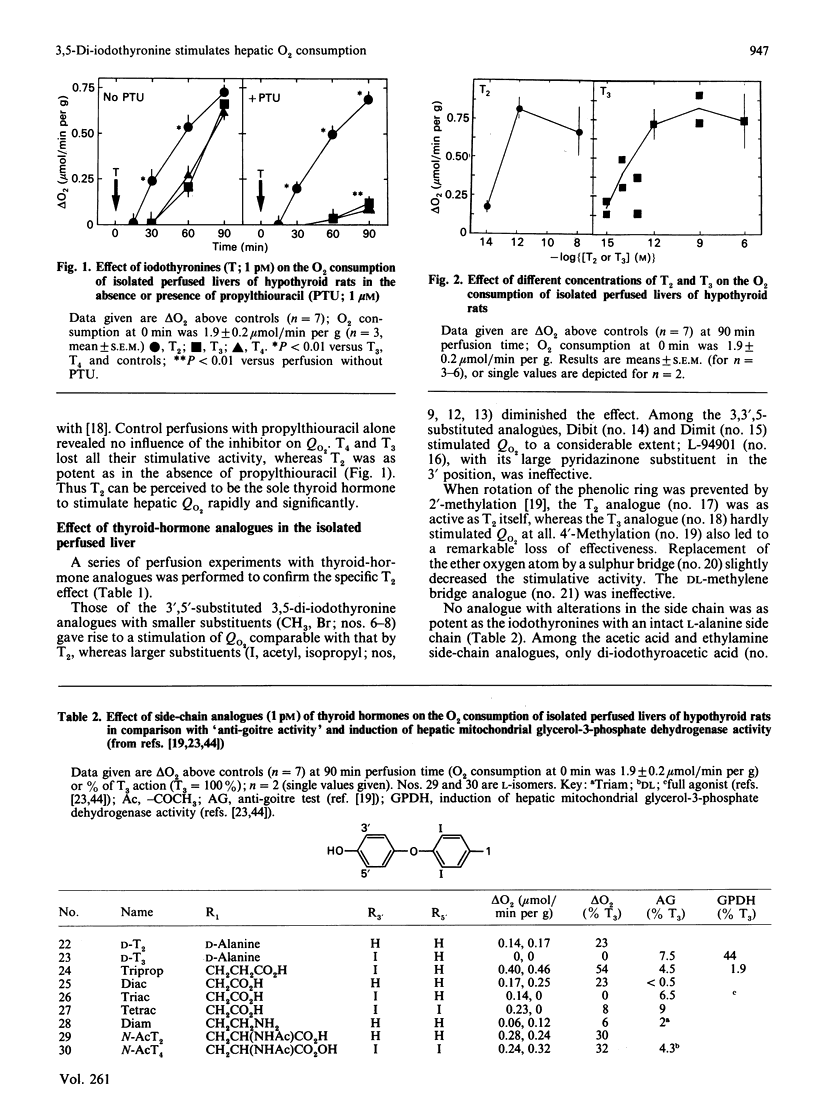
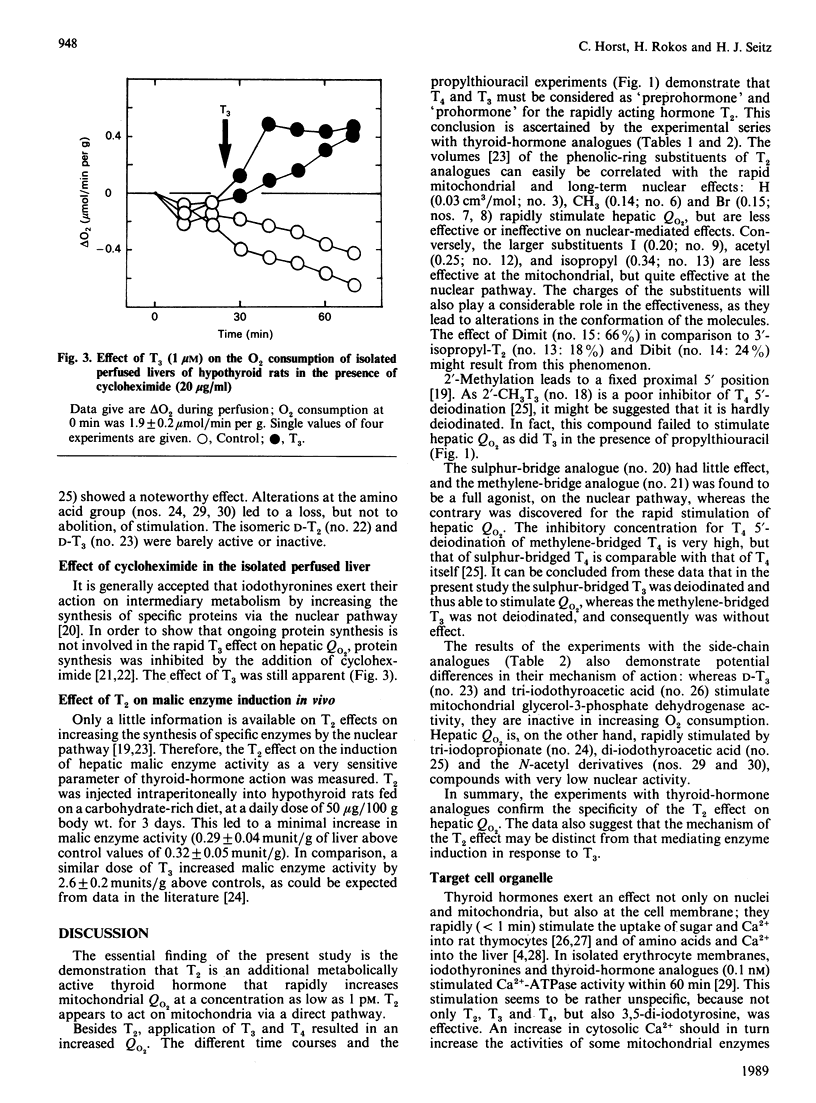
Tri-iodothyronine (T3) and thyroxine (T4) as well as 3,5-di-iodothyronine (T2) stimulated O2 consumption by isolated perfused livers from hypothyroid rats at a concentration as low as 1 pM by about 30% within 90 min. Application of T2 resulted in a faster stimulation than with application of T3 or T4. Inhibition of iodothyronine monodeiodinase by propylthiouracil, thereby blocking the degradation of T4 to T3 and of T3 to T2, demonstrated that only T2 is the active hormone for the rapid stimulation of hepatic O2 consumption: T3 and T4 lost all of their stimulative activity, whereas T2 was as potent as in the absence of propylthiouracil. Perfusion experiments with thyroid-hormone analogues confirmed the specificity of the T2 effect. The nucleus is unlikely to contribute to the rapid T2 effect, as can be deduced from perfusion experiments with cycloheximide and lack of induction of malic enzyme by T2. In conclusion, a new scheme of regulation of mitochondrial activity is proposed: T2 acts rapidly and directly via a mitochondrial pathway, whereas T3 exerts its long-term action indirectly by induction of specific enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker K. L., Lee K. L., Kenney F. T. Turnover of tyrosine transaminase in cultured hepatoma cells after inhibition of protein synthesis. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1132–1138. doi: 10.1016/0006-291x(71)90580-8. [DOI] [PubMed] [Google Scholar]
- Burger A. G., O'Connell M., Scheidegger K., Woo R., Danforth E., Jr Monodeiodination of triiodothyronine and reverse triiodothyronine during low and high calorie diets. J Clin Endocrinol Metab. 1987 Nov;65(5):829–835. doi: 10.1210/jcem-65-5-829. [DOI] [PubMed] [Google Scholar]
- Conversion of thyroxine into tri-iodothyronine by rat liver homogenate. Biochem J. 1975 Sep;150(3):489–493. doi: 10.1042/bj1500489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo-Armas A., Mowbray J. The rapid alteration by tri-iodo-L-thyronine in vivo of both the ADP/O ratio and the apparent H+/O ratio in hypothyroid-rat liver mitochondria. Biochem J. 1987 Feb 1;241(3):657–661. doi: 10.1042/bj2410657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. M., Halestrap A. P. Liver mitochondrial pyrophosphate concentration is increased by Ca2+ and regulates the intramitochondrial volume and adenine nucleotide content. Biochem J. 1987 Sep 15;246(3):715–723. doi: 10.1042/bj2460715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis F. B., Cody V., Davis P. J., Borzynski L. J., Blas S. D. Stimulation by thyroid hormone analogues of red blood cell Ca2+-ATPase activity in vitro. Correlations between hormone structure and biological activity in a human cell system. J Biol Chem. 1983 Oct 25;258(20):12373–12377. [PubMed] [Google Scholar]
- Dembri A. Effets métaboliques de la 3,5-diméthyl-3'-isopropyl-L-thyronine (DIMIT) et de la 3,5,3'-triiodo-L-thyronine (T3) dans le cerveau et le foie de rat hypothyroïdien. Analogies et différences. C R Seances Soc Biol Fil. 1987;181(4):344–354. [PubMed] [Google Scholar]
- Engler D., Burger A. G. The deiodination of the iodothyronines and of their derivatives in man. Endocr Rev. 1984 Spring;5(2):151–184. doi: 10.1210/edrv-5-2-151. [DOI] [PubMed] [Google Scholar]
- Faber J., Kirkegaard C., Lumholtz I. B., Siersbaek-Nielsen K., Friis T. Simultaneous measurement of 3,5-diiodothyronine and 3,5,3'-triiodothyronine turnover kinetics in euthyroid hyperthyroid, and hypothyroid subjects. J Clin Endocrinol Metab. 1982 Jul;55(1):8–12. doi: 10.1210/jcem-55-1-8. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Tomkins G. M. Posttranscriptional control of tyrosine aminotransferase synthesis by insulin. Proc Natl Acad Sci U S A. 1970 Jun;66(2):390–397. doi: 10.1073/pnas.66.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnoni G. V., Geelen M. J., Bijleveld C., Quagliariello E., van den Bergh S. G. Short-term stimulation of lipogenesis by triiodothyronine in maintenance cultures of rat hepatocytes. Biochem Biophys Res Commun. 1985 Apr 30;128(2):525–530. doi: 10.1016/0006-291x(85)90078-6. [DOI] [PubMed] [Google Scholar]
- Goldberg J. R., Ehrmann B., Katzeff H. L. Altered triiodothyronine metabolism in Zucker fatty rats. Endocrinology. 1988 Feb;122(2):689–693. doi: 10.1210/endo-122-2-689. [DOI] [PubMed] [Google Scholar]
- Hafner R. P., Brand M. D. Hypothyroidism in rats does not lower mitochondrial ADP/O and H+/O ratios. Biochem J. 1988 Mar 1;250(2):477–484. doi: 10.1042/bj2500477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K., Kobayashi M., Miyamoto T., Yamauchi K. Dependence of the mitochondrial uptake of triiodothyronine (T3) in rat kidney on cytosolic T3-binding protein. Endocrinology. 1986 Sep;119(3):1063–1070. doi: 10.1210/endo-119-3-1063. [DOI] [PubMed] [Google Scholar]
- Haynes R. C., Jr, Picking R. A., Zaks W. J. Control of mitochondrial content of adenine nucleotides by submicromolar calcium concentrations and its relationship to hormonal effects. J Biol Chem. 1986 Dec 5;261(34):16121–16125. [PubMed] [Google Scholar]
- Hummerich H., Soboll S. Rapid stimulation of calcium uptake into rat liver by L-tri-iodothyronine. Biochem J. 1989 Mar 1;258(2):363–367. doi: 10.1042/bj2580363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höppner W., Horst C., Rasmussen U. B., Seitz H. J. Adenine nucleotide translocase--a target of thyroid hormone action? Horm Metab Res Suppl. 1987;17:29–33. [PubMed] [Google Scholar]
- Höppner W., Süssmuth W., O'Brien C., Seitz H. J. Cooperative effect of thyroid and glucocorticoid hormones on the induction of hepatic phosphoenolpyruvate carboxykinase in vivo and in cultured hepatocytes. Eur J Biochem. 1986 Sep 1;159(2):399–405. doi: 10.1111/j.1432-1033.1986.tb09882.x. [DOI] [PubMed] [Google Scholar]
- Koehrle J., Auf'mkolk M., Rokos H., Hesch R. D., Cody V. Rat liver iodothyronine monodeiodinase. Evaluation of the iodothyronine ligand-binding site. J Biol Chem. 1986 Sep 5;261(25):11613–11622. [PubMed] [Google Scholar]
- Leeson P. D., Ellis D., Emmett J. C., Shah V. P., Showell G. A., Underwood A. H. Thyroid hormone analogues. Synthesis of 3'-substituted 3,5-diiodo-L-thyronines and quantitative structure-activity studies of in vitro and in vivo thyromimetic activities in rat liver and heart. J Med Chem. 1988 Jan;31(1):37–54. doi: 10.1021/jm00396a008. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Corrigall J. Short-term control of mitochondrial adenine nucleotide translocator by thyroid hormone. Eur J Biochem. 1984 Feb 15;139(1):95–99. doi: 10.1111/j.1432-1033.1984.tb07981.x. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Seitz H. J. Dose dependent stimulation of hepatic oxygen consumption and alanine conversion to CO2 and glucose by 3,5,3'-triiodo-L-thyronine (T3) in the isolated perfused liver of hypothyroid rats. Life Sci. 1981 May 18;28(20):2243–2249. doi: 10.1016/0024-3205(81)90576-2. [DOI] [PubMed] [Google Scholar]
- Müller M. J., Seitz H. J. Rapid and direct stimulation of hepatic gluconeogenesis by L-triiodothyronine (T3) in the isolated-perfused rat liver. Life Sci. 1980 Sep 8;27(10):827–835. doi: 10.1016/0024-3205(80)90076-4. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Mutvei A., Joste V. Regulation of biosynthesis of the rat liver inner mitochondrial membrane by thyroid hormone. Arch Biochem Biophys. 1984 Jan;228(1):41–48. doi: 10.1016/0003-9861(84)90044-4. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Inada M., Naito K., Ishii H., Tanaka K., Mashio Y., Imura H. Age-related changes of serum 3,3'-diiodothyronine, 3',5'-diiodothyronine, and 3,5-diiodothyronine concentrations in man. J Clin Endocrinol Metab. 1981 Mar;52(3):517–522. doi: 10.1210/jcem-52-3-517. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Silva E., Schwartz H. L., Surks M. I. Stimulation of hepatic mitochondrial alpha-glycerophosphate dehydrogenase and malic enzyme by L-triiodothyronine. Characteristics of the response with specific nuclear thyroid hormone binding sites fully saturated. J Clin Invest. 1977 Mar;59(3):517–527. doi: 10.1172/JCI108667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the nuclear level. Ann Intern Med. 1985 Mar;102(3):374–384. doi: 10.7326/0003-4819-102-3-374. [DOI] [PubMed] [Google Scholar]
- Palacios-Romero R., Mowbray J. Evidence for the rapid direct control both in vivo and in vitro of the efficiency of oxidative phosphorylation by 3,5,3'-tri-iodo-L-thyronine in rats. Biochem J. 1979 Dec 15;184(3):527–538. doi: 10.1042/bj1840527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal J. Biphasic stimulation of cellular calcium concentration by 3,5,3'-triiodothyronine in rat thymocytes. Biochemistry. 1988 Apr 5;27(7):2586–2590. doi: 10.1021/bi00407a047. [DOI] [PubMed] [Google Scholar]
- Segal J., Ingbar S. H. In vivo stimulation of sugar uptake in rat thymocytes. An extranuclear action of 3,5,3'-triiodothyronine. J Clin Invest. 1985 Oct;76(4):1575–1580. doi: 10.1172/JCI112139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz H. J., Müller M. J., Soboll S. Rapid thyroid-hormone effect on mitochondrial and cytosolic ATP/ADP ratios in the intact liver cell. Biochem J. 1985 Apr 1;227(1):149–153. doi: 10.1042/bj2270149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims E. A., Danforth E., Jr, Horton E. S., Bray G. A., Glennon J. A., Salans L. B. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- Soboll S., Scholz R. Control of energy metabolism by glucagon and adrenaline in perfused rat liver. FEBS Lett. 1986 Sep 1;205(1):109–112. doi: 10.1016/0014-5793(86)80875-4. [DOI] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Sakurada T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science. 1980 Oct 17;210(4467):340–342. doi: 10.1126/science.7423197. [DOI] [PubMed] [Google Scholar]
- Sterling K. Direct thyroid hormone activation of mitochondria: the role of adenine nucleotide translocase. Endocrinology. 1986 Jul;119(1):292–295. doi: 10.1210/endo-119-1-292. [DOI] [PubMed] [Google Scholar]
- Sterling K., Milch P. O., Brenner M. A., Lazarus J. H. Thyroid hormone action: the mitochondrial pathway. Science. 1977 Sep 2;197(4307):996–999. doi: 10.1126/science.196334. [DOI] [PubMed] [Google Scholar]
- Sterling K. Thyroid hormone action at the cell level (second of two parts). N Engl J Med. 1979 Jan 25;300(4):173–177. doi: 10.1056/NEJM197901253000405. [DOI] [PubMed] [Google Scholar]
- Thomas W. E., Crespo-Armas A., Mowbray J. The influence of nanomolar calcium ions and physiological levels of thyroid hormone on oxidative phosphorylation in rat liver mitochondria. A possible signal amplification control mechanism. Biochem J. 1987 Oct 15;247(2):315–320. doi: 10.1042/bj2470315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Emmett J. C., Ellis D., Flynn S. B., Leeson P. D., Benson G. M., Novelli R., Pearce N. J., Shah V. P. A thyromimetic that decreases plasma cholesterol levels without increasing cardiac activity. Nature. 1986 Dec 4;324(6096):425–429. doi: 10.1038/324425a0. [DOI] [PubMed] [Google Scholar]


