ABSTRACT
Diverse insects are intimately associated with specific symbiotic bacteria, where host and symbiont are integrated into an almost inseparable biological entity. These symbiotic bacteria usually exhibit host specificity, uncultivability, reduced genome size, and other peculiar traits relevant to their symbiotic lifestyle. How host-symbiont specificity is established at the very beginning of symbiosis is of interest but poorly understood. To gain insight into the evolutionary issue, we adopted an experimental approach using the recently developed evolutionary model of symbiosis between the stinkbug Plautia stali and Escherichia coli. Based on the laboratory evolution of P. stali-E. coli mutualism, we selected ΔcyaA mutant of E. coli as an artificial symbiont of P. stali that has established mutualism by a single mutation. In addition, we selected a natural cultivable symbiont of P. stali of relatively recent evolutionary origin. These artificial and natural symbiotic bacteria of P. stali were experimentally inoculated to symbiont-deprived newborn nymphs of diverse stinkbug species. Strikingly, the mutualistic E. coli was unable to establish infection and support growth and survival of all the stinkbug species except for P. stali, uncovering that host specificity can be established at a very early stage of symbiotic evolution. Meanwhile, the natural symbiont was able to establish infection and support growth and survival of several stinkbug species in addition to P. stali, unveiling that a broader host range of the symbiont has evolved in nature. Based on these findings, we discuss what factors are relevant to the establishment of host specificity in the evolution of symbiosis.
IMPORTANCE
How does host-symbiont specificity emerge at the very beginning of symbiosis? This question is difficult to address because it is generally difficult to directly observe the onset of symbiosis. However, recent development of experimental evolutionary approaches to symbiosis has brought about a breakthrough. Here we tackled this evolutionary issue using a symbiotic Escherichia coli created in laboratory and a natural Pantoea symbiont, which are both mutualistic to the stinkbug Plautia stali. We experimentally replaced essential symbiotic bacteria of diverse stinkbugs with the artificial and natural symbionts of P. stali and evaluated whether the symbiotic bacteria, which evolved for a specific host, can establish infection and support the growth and survival of heterospecific hosts. Strikingly, the artificial symbiont showed strict host specificity to P. stali, whereas the natural symbiont was capable of symbiosis with diverse stinkbugs, which provide insight into how host-symbiont specificity can be established at early evolutionary stages of symbiosis.
KEYWORDS: Plautia stali, Escherichia coli, Pantoea, stinkbug, gut symbiosis, host specificity
INTRODUCTION
Many insects are obligatorily associated with their specific bacterial symbionts, where the host insects cannot survive without their microbial partners (1–3). At the beginning, such obligatory symbiotic bacteria must have been derived from environmental free-living bacteria. Understanding how originally unrelated host and symbiont have established symbiosis, developed interdependence, and become integrated into a coherent biological entity is an intriguing issue in the field of evolutionary biology (4, 5).
In highly mutualistic associations such as aphid-Buchnera and tsetse-Wigglesworthia symbioses (6, 7), the host phylogeny generally mirrors the symbiont phylogeny, reflecting stable vertical symbiont transmission and consequent host-symbiont co-speciation over evolutionary time (8, 9). During the course of co-evolution, the bacterial symbionts tend to develop dependence on their hosts, lose the capability of independent life, become uncultivable, suffer genome reduction, etc., thereby constituting a highly integrated symbiotic system where neither the host nor the symbiont can thrive independently (10, 11). Here, each host lineage is consistently associated with its own specific symbiont lineage, where high host-symbiont specificity is likely to evolve (12, 13). On the other hand, among facultative symbiotic associations such as Wolbachia, Serratia and Sodalis with diverse insects (14–16), or even among evolutionarily conserved mutualistic associations such as Riptortus-Caballeronia symbioses (17, 18), the bacterial symbionts are occasionally or constantly moving around different host lineages, where they tend to remain less dependent on their hosts, often retain the capability of surviving outside their hosts, remain cultivable in some cases, keep large genome sizes, etc., which will end up with such evolutionary consequences as host-symbiont phylogenetic promiscuity and compromised/context-dependent host-symbiont interdependence and specificity (13, 19). Theoretical as well as empirical studies have shed light on what factors may affect the evolutionary trajectories toward diverse symbiotic associations ranging from loose/exchangeable ones to strict/specific ones (20, 21).
How are host-symbiont interdependence and specificity established at the very beginning of the evolution of symbiosis? Needless to say, this question is difficult to address because it is difficult to observe the onset of symbiotic evolution in most cases. However, recent development of experimental evolutionary approaches to symbiosis has opened a window to look into this unexplored research area (22, 23). Here we tackle this evolutionary issue by experimentally comparing an artificial insect symbiont that was created in laboratory with a natural insect symbiont that has evolved in nature.
The brown-winged green stinkbug Plautia stali (Hemiptera: Pentatomidae) has a specialized symbiotic organ consisting of numerous crypts in a posterior region of the midgut, in which a specific bacterial symbiont of the genus Pantoea densely populates (24). The symbiont is essential for growth and survival of the host insect and vertically transmitted to newborn nymphs by maternal smearing of symbiont-containing excrement onto eggshell (24, 25). Notably, the essential symbiotic bacteria in natural populations of P. stali exhibit geographic polymorphism across the Japanese archipelago: an uncultivable symbiont Pantoea sp. A (=Sym A) is fixed in the main islands, whereas an uncultivable symbiont Pantoea sp. B coexists with cultivable symbionts Pantoea sp. C–F in the southwestern Ryukyu islands (24). Hence, both uncultivable and cultivable bacterial strains are available as essential natural symbionts of P. stali, which are useful for a variety of experimental purposes (24). In this study, we used a Pantoea sp. C (Sym C) strain as a natural cultivable symbiont of P. stali.
Recently, we established an experimental symbiotic system between P. stali as host and the model bacterium Escherichia coli as symbiont (26). We orally inoculated a hypermutating E. coli strain to symbiont-free newborn nymphs of P. stali, obtained a few adult insects that managed to survive and attained adulthood, dissected and homogenized the E. coli-populated symbiotic organ from each of them, inoculated the homogenate to symbiont-free newborn nymphs in the same way, and repeated the process for many host generations. Finally, we successfully obtained mutualistic evolutionary E. coli strains that can support growth, survival, and reproduction of P. stali and identified that single-gene mutations disrupting the carbon catabolite repression global transcriptional regulator system for bacterial metabolic switching, namely, ΔcyaA and Δcrp, make E. coli mutualistic to P. stali (26). Therefore, by introducing ΔcyaA or Δcrp mutation into the wild-type genetic background of E. coli, we can create a mutualistic E. coli strain artificially (26). In this study, we selected and used a ΔcyaA E. coli mutant as a mutualistic E. coli strain.
Besides P. stali, diverse stinkbugs are also obligatorily associated with specific symbiotic bacteria of beneficial nature, which are similarly harbored in the midgut symbiotic organ and mostly, if not always, vertically transmitted to offspring via egg surface contamination or other means (27, 28). In this study, we established stable laboratory strains of diverse stinkbugs representing seven species, three families and two superfamilies, generated newborn nymphs deprived of their original symbiont, inoculated the mutualistic E. coli strain ΔcyaA or the natural symbiont strain Sym C to them, and investigated whether these artificial and natural symbiotic bacteria of P. stali can establish symbiosis with the different host species and what phenotypic consequences are observed with the replacement of their original symbiont by the artificial and natural symbionts of P. stali.
RESULTS
Establishment of stinkbug strains, removal of original symbiotic bacteria, and inoculation of natural and artificial symbiotic bacteria from P. stali
We have established stable laboratory strains of the following diverse stinkbug species: P. stali, Glaucias subpunctatus, Nezara viridula, and Halyomorpha halys, representing the family Pentatomidae; Lampromicra miyakonus, Poecilocoris lewisi, and Eucorysses grandis, representing the family Scutelleridae; and Riptortus pedestris, representing the family Alydidae (Fig. 1A; Table S1). All the pentatomid and scutellerid species are associated with obligatory gut symbiotic bacteria, which are allied to such Enterobacteriaceae genera as Pantoea, Erwinia, and Enterobacter (Fig. 1B). Notably, the phylogenetic relationship of the symbiotic bacteria is incongruent with the systematics of the host stinkbugs, reflecting recurrent symbiont acquisitions and replacements in the evolutionary course of the pentatomid and scutellerid stinkbugs (29). Distinct from the pentatomid and scutellerid stinkbugs that vertically transmit their beneficial gut symbiont via egg surface contamination as mentioned above (27, 28), the alydid stinkbug R. pedestris does not vertically transmit but environmentally acquires its beneficial β-proteobacterial gut symbiont Caballeronia (formerly called Burkholderia) (18, 30) (Fig. 1B). In this study, for each of these stinkbug species, we generated symbiont-free newborn nymphs and orally inoculated the cultured bacteria to them as depicted in Fig. S1. We collected eggs and surface-sterilized them with ethanol and formalin, from which symbiont-free newborn nymphs hatched. The nymphs were kept without water for 1 day and then provided with bacteria-suspended water for 1 day, by which the nymphs actively ingested the bacteria-suspended water. Then, the nymphs were provided with sterilized water and food seeds in clean rearing containers and were maintained and inspected every day to monitor survival and adult emergence, as shown in Fig. S1 and Table S1. Bacterial infection was surveyed at the second instar stage and the adult stage to confirm the initial infection rate and the final establishment rate, respectively.
Fig 1.
Stinkbugs and their symbiotic bacteria. (A) Systematic relationship of the stinkbugs used in this study. The relationship of the stinkbug families is after Wu et al. (31). Stinkbug superfamilies are displayed on the right side. (B) Phylogenetic relationship of the symbiotic bacteria. A maximum-likelihood phylogeny inferred from bacterial 16S rRNA gene sequences (1,462 aligned nucleotide sites) is shown. On each node, statistical support values are indicated as posterior probability of Bayesian inference/bootstrap probability of maximum-likelihood analysis. Gut symbiotic (GS) bacteria of the stinkbugs are shown in green; E. coli is highlighted in red; and free-living proteobacterial 16S rRNA gene sequences retrieved from the DNA databases are displayed in black. Bacterial families and classes are shown on the right side. Corresponding host stinkbugs are indicated by line connections. Note that the symbiont phylogeny does not agree with the host systematics, reflecting recurrent symbiont acquisitions and replacements in the evolutionary course of the stinkbugs (29).
Symbiont replacing experiments: Plautia stali
The control insects infected with the original symbiont attained high survival rate (88.6%) and adult emergence rate (87.7%) (Fig. 2; Fig. S2). The aposymbiotic insects exhibited high survival rate (83.2%), but actually their growth was very poor: most of them remained at the second or third instar with no adult emergence (0%). These results confirmed the importance of the original symbiont Sym A for P. stali as reported previously (24). The mutualistic E. coli ΔcyaA-inoculated insects showed good survival rate (68.1%) and adult emergence rate (54.9%). The natural cultivable symbiont Sym C-inoculated insects exhibited moderate survival rate (58.0%) and adult emergence rate (28.6%). The wild-type E. coli ΔintS-inoculated insects showed moderate survival rate (37.9%) and low adult emergence rate (12.1%). Note that ΔintS is a control wild-type E. coli strain in which the phage integrase gene is disrupted without phenotypic consequences (26). While ΔintS E. coli, ΔcyaA E. coli, and Sym C symbiont exhibited moderate PCR detection rates at the second instar stage (37.5%, 54.2%, and 37.5%, respectively), they consistently showed 100% detection rates at the adult stage.
Fig 2.
Infection, survival, and growth of P. stali experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 37th day after hatching. (D) Adult emergence rate on the 37th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by a likelihood ratio test of a generalized linear model in panels C and D (a–d, P < 0.05) and by pair-wise t-test with Bonferroni correction in panels E and F (a and b, P < 0.05). NA, statistical analysis not applicable.
These results indicated that (i) both the natural cultivable symbiont Sym C and the mutualistic E. coli strain ΔcyaA can establish infection in P. stali, and (ii) both Sym C and ΔcyaA can support growth and survival of P. stali to adulthood, as reported in previous studies (24, 26).
Symbiont replacing experiments: Glaucias subpunctatus
The control insects infected with the original symbiont attained good survival rate (67.9%) and adult emergence rate (67.9%) (Fig. 3; Fig. S3). The aposymbiotic insects exhibited low survival rate (27.6%) and very low adult emergence rate (3.1%). These results indicated the importance of the original symbiont for G. subpunctatus. The mutualistic E. coli ΔcyaA-inoculated insects showed good survival rate (64.4%), but adult emergence rate was very low (8.9%). The wild-type E. coli ΔintS-inoculated insects similarly showed a good survival rate (59.7%) and a very low adult emergence rate (0.6%). The Sym C-inoculated insects exhibited relatively low survival rate (21.9%), but adult emergence rate was not so low (18.1%) in comparison with the E. coli-infected insects. When inoculated with ΔintS and ΔcyaA E. coli strains, none of second instar nymphs and adult insects retained bacterial infection. On the other hand, Sym C-inoculated insects exhibited a relatively low infection rate (16.7%) at the second instar stage and a high infection rate (84.6%) at the adult stage.
Fig 3.
Infection, survival, and growth of G. subpunctatus experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 38th day after hatching. (D) Adult emergence rate on the 38th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D (a–c, P < 0.05) and by pair-wise t-test with Bonferroni correction in panels E and F (a and b, P < 0.05. In panels C and D, for example, “n = 5 (112 eggs)” indicates “five experimental groups were prepared, which consisted of 112 eggs in total.” The same applies to panels C and D in Fig. 2 and 4–9. NA, statistical analysis not applicable; NS, no significant difference.
These results indicated that (i) the natural cultivable symbiont Sym C can partially establish infection and support growth and survival of G. subpunctatus, and by contrast, (ii) neither the mutualistic E. coli ΔcyaA nor the wild-type E. coli ΔintS can establish infection and support growth and survival of G. subpunctatus.
Symbiont replacing experiments: Nezara viridula
The control insects infected with the original symbiont exhibited a relatively low survival rate (22.6%) and adult emergence rate (21.3%) (Fig. 4; Fig. S4). The relatively low level of performance of the insects infected with the original symbiont suggested that the laboratory rearing system does not work perfectly for this stinkbug species. Notably, however, the aposymbiotic insects ended up with no survivors (0%) and no adult insects (0%), confirming the importance of the original symbiont for N. viridula as previously reported (32). The mutualistic E. coli ΔcyaA-inoculated insects suffered very low survival (6.6%) and few adult emergence (0.8%). The wild-type E. coli ΔintS-inoculated insects similarly showed very low survival (8.7%) and no adult emergence (0%). The Sym C-inoculated insects exhibited a relatively low survival rate (33.1%) and few adult emergence (0.7%). When inoculated with ΔintS and ΔcyaA E. coli strains, none of the second instar nymphs and an adult insect retained bacterial infection. On the other hand, Sym C-inoculated insects exhibited a relatively low infection rate (17.4%) at the second instar stage, and the only adult insect obtained was infected with Sym C.
Fig 4.
Infection, survival, and growth of N. viridula experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 38th day after hatching. (D) Adult emergence rate on the 38th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D. In panels E and F. NA, statistical analysis not applicable; NS, no significant difference.
These results indicated that (i) the natural cultivable symbiont Sym C can establish infection and support survival of N. viridula to some extent, and by contrast, (ii) neither the mutualistic E. coli ΔcyaA nor the wild-type E. coli ΔintS can establish infection and support growth and survival of N. viridula.
Symbiont replacing experiments: Halyomorpha halys
The control insects infected with the original symbiont attained good survival rate (57.1%) and adult emergence rate (38.1%), reflecting the importance of the original symbiont for H. halys as previously reported (Fig. 5; Fig. S5) (33). Strikingly, however, although similar survival rates were observed with the aposymbiotic insects (51.0%), the mutualistic E. coli ΔcyaA-inoculated insects (65.6%), the wild-type E. coli ΔintS-inoculated insects (64.7%), and the Sym C-inoculated insects (54.2%), none of them attained adulthood. While half of the ΔcyaA-inoculated insects retained bacterial infection at the second instar stage, few second instar insects exhibited bacterial infection when inoculated with ΔintS or Sym C.
Fig 5.
Infection, survival, and growth of H. halys experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 49th day after hatching. (D) Adult emergence rate on the 49th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D (a and b, P < 0.05). and by pair-wise t-test with Bonferroni correction in panels E and F. NA, statistical analysis not applicable; NS, no significant difference.
These results indicated that (i) neither the natural cultivable symbiont Sym C, the mutualistic E. coli strain ΔcyaA, nor the wild-type E. coli strain ΔintS can establish infection and support growth and survival of H. halys, and (ii) in the absence of the original symbiont, H. halys can manage to survive but cannot attain adulthood.
Symbiont replacing experiments: Lampromicra miyakonus
The control insects infected with the original symbiont attained good survival rate (55.6%) and adult emergence rate (55.6%) (Fig. 6; Fig. S6). The aposymbiotic insects exhibited low survival rate (1.6%) with no adult emergence (0%). These results confirmed the importance of the original symbiont for L. miyakonus as previously reported (34). The natural cultivable symbiont Sym C-inoculated insects efficiently established infection (93.3% in nymphs and 100% in adults) and exhibited moderate survival rate (37.5%) and adult emergence rate (35.9%). By contrast, when inoculated with the mutualistic E. coli strain ΔcyaA and the wild-type E. coli strain ΔintS, infection rates at the second instar were partial (20.0% and 46.7%, respectively), and few insects survived and attained adulthood.
Fig 6.
Infection, survival, and growth of L. miyakonus experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 45th day after hatching. (D) Adult emergence rate on the 45th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D (a and b, P < 0.05) and by pair-wise t-test with Bonferroni correction in panels E and F. NA, statistical analysis not applicable; NS, no significant difference.
These results indicated that (i) the natural cultivable symbiont Sym C can establish infection and support survival of L. miyakonus, and (ii) neither the mutualistic E. coli ΔcyaA nor the wild-type E. coli ΔintS can establish infection and support growth and survival of L. miyakonus.
Symbiont replacing experiments: Poecilocoris lewisi
The control insects infected with the original symbiont attained a high survival rate (77.8%) and adult emergence rate (77.8%) (Fig. 7; Fig. S7). The aposymbiotic insects exhibited low survival rate (22.2%) and no adult emergence (0%). These results indicated the importance of the original symbiont for Po. lewisi. The natural cultivable symbiont Sym C-inoculated insects efficiently established infection (91.7% in nymphs and 100% in adults) and exhibited high survival rate (90.5%) and adult emergence rate (90.5%). By contrast, when inoculated with the mutualistic E. coli strain ΔcyaA and the wild-type E. coli strain ΔintS, no infection was established and no adult insect emerged.
Fig 7.
Infection, survival, and growth of Po. lewisi experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 50th day after hatching. (D) Adult emergence rate on the 50th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D (a and b, P < 0.05) and by pair-wise t-test with Bonferroni correction in panels E and F. NA, statistical analysis not applicable; NS, no significant difference.
These results indicated that (i) the natural cultivable symbiont Sym C can establish infection and support survival of Po. lewisi, and (ii) neither the mutualistic E. coli ΔcyaA nor the wild-type E. coli ΔintS can establish infection and support growth and survival of Po. lewisi.
Symbiont replacing experiments: Eucorysses grandis
The control insects infected with the original symbiont attained high survival rate (100%) and adult emergence rate (92.3%) (Fig. 8; Fig. S8). The aposymbiotic insects exhibited moderate survival rate (31.9%) with no adult emergence (0%). These results indicated the importance of the original symbiont for E. grandis. By contrast, when inoculated with the natural cultivable symbiont Sym C, the mutualistic E. coli strain ΔcyaA, and the wild-type E. coli strain ΔintS, no infection was established and no adult insect emerged.
Fig 8.
Infection, survival, and growth of E. grandis experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 57th day after hatching. (D) Adult emergence rate on the 57th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D (a and b, P < 0.05). In panels E and F, NA indicates statistical analysis not applicable.
These results indicated that neither the natural cultivable symbiont Sym C, the mutualistic E. coli strain ΔcyaA, nor the wild-type E. coli strain ΔintS can establish infection and support growth and survival of E. grandis.
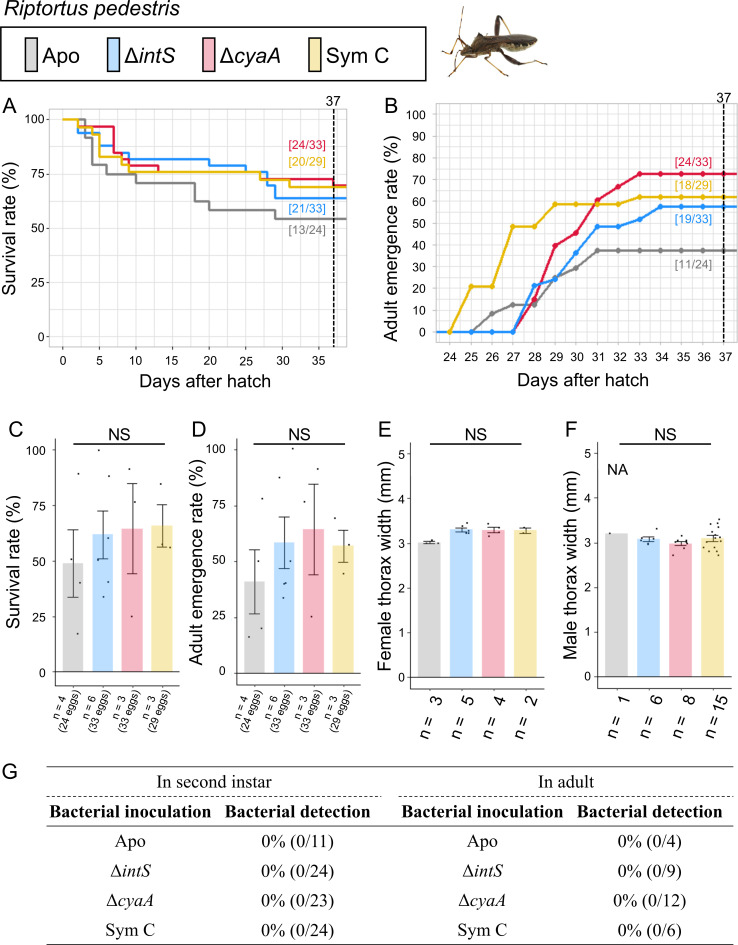
Symbiont replacing experiments: Riptortus pedestris
As for R. pedestris, the control insects that emerged from non-sterilized eggs were not examined because the insects are substantially the same as the aposymbiotic insects derived from surface-sterilized eggs (Fig. 9; Fig. S9). The aposymbiotic insects exhibited moderate survival rate (54.2%) and adult emergence rate (45.8%). Here it should be noted that aposymbiotic R. pedestris can grow to adult and reproduce, although growth rate, body size and fecundity decline in comparison with symbiotic R. pedestris (30). When inoculated with the natural cultivable symbiont Sym C, the mutualistic E. coli strain ΔcyaA, and the wild-type E. coli strain ΔintS, the insects exhibited improved survival rates (69.0%, 72.7%, and 63.6%, respectively) and adult emergence rates (62.1%, 72.7%, and 57.6%, respectively) in comparison with the aposymbiotic insects, although the differences were statistically not significant, wherein none of them established infection with the inoculated bacteria.
Fig 9.
Infection, survival, and growth of E. grandis experimentally deprived of the original mutualistic symbiont and inoculated with either a wild-type E. coli strain (ΔintS), a mutant E. coli strain mutualistic to P. stali (ΔcyaA), or a natural cultivable symbiont strain mutualistic to P. stali (Sym C). (A) Survival curve. (B) Adult emergence curve. (C) Survival rate on the 37th day after hatching. (D) Adult emergence rate on the 37th day after hatching. (E) Adult female body size. (F) Adult male body size. (G) PCR check of bacterial infection in second instar nymphs (left) and adult insects (right). Statistical tests were conducted by likelihood ratio test of a generalized linear model in panels C and D and by pair-wise t-test with Bonferroni correction in panels E and F. NA, statistical analysis not applicable; NS, no significant difference.
DISCUSSION
For understanding the evolution of symbiosis, it is of fundamental interest how host-symbiont specificity has developed during early stages of symbiotic associations. The laboratory model bacterium E. coli is originally a mammalian gut microbe and thus unrelated to stinkbugs at all. Hence, the P. stali-E. coli experimental symbiotic system we recently established (26) provides an unprecedented opportunity to investigate the early evolutionary stages of symbiosis using the experimentally tractable model insect P. stali (35) and the genetically tractable model bacterium E. coli (36). In this study, we inoculated the mutualistic E. coli strain ΔcyaA, which was artificially generated based on the outcome of the P. stali-E. coli experimental symbiotic evolution (26), to newborn nymphs of diverse stinkbug species that were experimentally deprived of their original symbiotic bacteria. In addition, we inoculated the natural cultivable symbiont strain Sym C, which is found in southwestern island populations of P. stali at low frequencies and likely at a relatively early stage of symbiotic evolution (24), to the symbiont-free newborn nymphs in the same manner. In this way, we were able to experimentally evaluate whether the artificial and natural symbiotic bacteria that have adapted to P. stali can establish symbiosis/mutualism with a diverse array of stinkbug species.
Table 1 summarizes the results of the symbiont replacing experiments conducted in this study. The remarkable finding is that E. coli can, irrespective of wild type or mutualistic genotype, establish infection and support growth and survival with P. stali only (Fig. 2). All the other stinkbug species we examined are not compatible with E. coli symbiotically (Fig. 3 to 9). This result uncovers that strict host specificity can be established at a very early stage of the evolution of mutualism. Considering our experience that the establishment of the P. stali-E. coli experimental symbiotic system was rather straightforward, we planned to launch similar E. coli experimental symbiotic systems with other stinkbug species. However, it turned out that such an optimistic idea may not work. It is currently elusive why only P. stali can establish infection and symbiosis with E. coli. Meanwhile, we now realize that we were lucky to have attempted E. coli infection to P. stali from the beginning (24), and it serendipitously worked so nicely that the P. stali-E. coli experimental symbiotic system was established successfully (26).
TABLE 1.
Summary of the results of the symbiont replacing experimentsa
| Insect species | △intS: wild-type E. coli strain | △cyaA: E. coli mutant strain mutualistic to P. stali | Sym C: natural cultivable symbiont mutualistic to P. stali |
|---|---|---|---|
| Pentatomidae | |||
| Plautia stali | △ | ○ | ○ |
| Glaucias subpunctatus | × | × | △ |
| Nezara viridula | × | × | × |
| Halyomorpha halys | × | × | × |
| Scutelleridae | |||
| Lampromicra miyakonus | × | × | ○ |
| Poecilocoris lewisi | × | × | ○ |
| Eucorysses grandis | × | × | × |
| Alydidae | |||
| Riptortus pedestris | × | × | × |
○ denotes infection establishes, and host growth and survival are supported well. △ indicates infection establishes to some extent, and host growth and survival are supported partially. × indicates infection does not establish, and host growth and survival are not supported.
Another remarkable finding is that Sym C, the natural cultivable symbiont of P. stali, can establish infection and support growth and survival not only with P. stali but also with other stinkbug species (Table 1). In L. miyakona and Po. lewisi, Sym C inoculation significantly improved survival and adult emergence (Fig. 6 and 7), whereas the improvement was only partial in G. subpunctatus (Fig. 3). Notably, these results can be explained neither by phylogenetic affinity of the bacterial side nor by phylogenetic affinity of the host side. As for the host side, L. miyakona and Po. lewisi belong to the family Scutelleridae that is distinct from the family Pentatomidae to which P. stali belongs (Fig. 1A), but P. stali-derived Sym C worked well in L. miyakona and Po. lewisi symbiotically (Fig. 6 and 7). As for the bacterial side, certainly the original symbiont of L. miyakona is closely related to Sym C, but the original symbionts of Po. lewisi and G. subpunctatus belong to a different clade in the Enterobacteriaceae (Fig. 1B).
Why is Sym C capable of symbiosis with more diverse stinkbug species than E. coli? Although speculative, we point out several candidate factors potentially relevant to the different levels of host specificity. A candidate factor is the phylogenetic one. E. coli is outside the Pantoea/Enterobacter/Erwinia clade to which the majority of pentatomid and scutellerid symbionts, including Sym C, belong (Fig. 1B) (29). This may account for the limited symbiotic capability of E. coli in comparison with Pantoea-allied Sym C. Another candidate factor is the historical/co-evolutionary one. In the southwestern Ryukyu islands, the same cultivable symbiotic bacteria, including Sym C, Sym D, and Sym E, are shared among different stinkbug species, including P. stali, L. miyakonus, Axiogastus rosmatus, and Solenosthedium chinense, and are also detected from environmental soil samples (24). This situation suggests the possibility that these cultivable symbiotic bacteria may be ecologically shared among the different stinkbug species via heterospecific horizontal transfers or soil-mediated environmental acquisitions. The broader host insect range of Sym C may make sense in this context.
In this study, to our surprise, the mutualistic E. coli strain ΔcyaA supported survival and adult emergence of P. stali significantly better than Sym C that is the natural cultivable symbiont of P. stali (Fig. 2). It seems plausible, although speculative, that this unexpected situation is partly ascribed to the excellent symbiotic capability of the mutualistic E. coli strain ΔcyaA (26) and partly due to the fact that Sym C is derived from a southwestern island population of P. stali and experimentally inoculated to the laboratory strain derived from a mainland population of P. stali, where host-symbiont local adaptations may matter (24).
It is notable that, although the effects may be relatively minor, in multiple stinkbug species such as G. subpunctatus, N. viridula, Po. lewisi, E. grandis, and R. pedestris, bacterial inoculation was neither established nor contributing to adult emergence but was supportive for survival to some extent (Fig. 3, 4, and 7 to 9). These effects may be attributable to some trace nutritional elements like vitamins derived from the bacterial inoculum, which should be experimentally verified in future studies.
In conclusion, a series of symbiont replacing experiments using diverse stinkbug species presented in this study, wherein their original symbiont was replaced by an artificial symbiont or a natural symbiont mutualistic to the specific stinkbug species P. stali, shed light on possible evolutionary trajectories toward establishment of host specificity at the onset of symbiosis. The finding that the E. coli mutant strain mutualistic to P. stali cannot establish symbiosis with other stinkbug species uncovered that host specificity can be established at a very early stage of symbiotic evolution. On the other hand, the finding that the natural symbiont Sym C can establish symbiosis with some of the diverse stinkbug species, in addition to P. stali, highlighted that a broader host range of the symbiont can evolve in nature. These observations, based on the experimental and evolutionary manipulations of symbiosis, provide invaluable insights into how host-symbiont specificity has been established in the evolutionary course toward elaborate symbiotic associations.
MATERIALS AND METHODS
Insect strains
All the insects used in this study, namely, P. stali, G. subpunctatus, N. viridula, H. halys, L. miyakonus, E. grandis, Po. lewisi, and R. pedestris, are inbred laboratory strains maintained at least for 3 years at 25°C under a long-day regimen (16-h light and 8-h dark) in climate chambers (TOMY, CLE305, or PHCBI, MLR-352) with sterilized water containing 0.05% ascorbic acid (DWA) and food seeds as described (Table S1; Fig. S1).
Bacterial strains
Considering that the mutualistic E. coli lines obtained through evolutionary experiments are under the hyper-mutating ΔmutS genetic background (26), we expected that they would accumulate many mutations and quickly change their genetic and phenotypic properties during the experiments. Therefore, we obtained and used the ΔcyaA E. coli mutant strain from the Keio single-gene knockout mutant library (37). This E. coli mutant is disruptive of the adenylate cyclase (cyaA) gene under the wild-type E. coli genetic background, which was shown to be mutualistic when inoculated to P. stali (26). The ΔintS E. coli strain was used as a wild-type control E. coli strain. The mutualistic symbiont strain Sym C, which was closely related to Pantoea dispersa, easily cultivable, and isolated from an adult insect of P. stali collected at Ishigaki Island, Okinawa, Japan in 2009 (24), was also used as a natural symbiont of P. stali. The 16S rRNA gene sequences of these bacteria were obtained from the NCBI database (https://www.ncbi.nlm.nih.gov/) with those of representative proteobacterial species. The phylogenetic relationship of these sequences was constructed using RAxML-ng v.1.2.1 (38) and MrBayes v.3.2.7a (39) for maximum-likelihood analysis and Baysian inference, respectively.
Generation of symbiont-free newborn nymphs
Egg masses were collected from rearing cases of the insects between 13:00 and 15:00 every day. The pentatomid and scutellerid stinkbugs, P. stali, G. subpunctatus, N. viridula, H. halys, L. miyakonus, E. grandis, and Po. lewisi, vertically transmit their symbiotic bacteria to offspring by smearing symbiont-containing secretion onto the egg surface upon oviposition (27). We sterilized their egg masses by soaking in 70% ethanol for 5–10 s, treating with 4% formaldehyde for 5 min, and washing in sterilized water for 5 min. After being air-dried, each egg mass was kept in a sterilized plastic petri dish, by which symbiont-free newborn nymphs were obtained. Although the alydid stinkbug R. pedestris acquires its bacterial symbiont from surrounding environment every generation (30), we treated the eggs of R. pedestris in the same way to obtain symbiont-free newborn nymphs.
Bacterial inoculation to newborn nymphs
The bacterial inoculation procedures are summarized in Fig. S1. Each bacterial strain, ΔintS, ΔcyaA, or Sym C, was cultured in liquid LB medium at 25°C overnight and diluted with sterilized water to OD600 = 0.05. In each plastic petri dish, the symbiont-free newborn nymphs, which hatched from the surface sterilized eggs within 24 h, were kept for a day without water. Then, a cotton pad was introduced to the petri dish, to which 0.75 mL of the bacterial suspension was applied, and the nymphs were allowed to orally acquire the bacterial suspension overnight. On the next day, the cotton pad for inoculation was removed and 3 mL of sterilized DWA was added to another clean cotton pad, from which the nymphs were allowed to take water. Upon molting to the second instar, some nymphs were subjected to second instar PCR infection check, while other nymphs were transferred to new clean rearing containers and aseptically maintained with sterilized food seeds and DWA as described (Table S1) (35).
Infection check of second instar nymphs
Three days after molting, second instar nymphs were individually subjected to DNA extraction and PCR detection of either ΔintS, ΔcyaA, or Sym C. Each insect was homogenized in a plastic tube with 100 μL of lysis buffer (150 mM NaCl, 10 mM Tris-HCl [pH 8.0], 1 mM EDTA, 0.1% SDS) and subjected to proteinase K digestion (100 μg/mL) at 56°C overnight. The lysate was extracted with water-saturated phenol:chloroform:isoamylalcohol (25:24:1), precipitated and washed with ethanol, air-dried, and dissolved in 200 μL of DNA suspension buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA). To detect infection with ΔintS or ΔcyaA, a 0.22-kb region of Tn5 gene inserted into the E. coli genome was amplified by PCR with the primers Tn5-1789F (5′-TGC TCG ACG TTG TCA CTG AA-3′) and Tn5-1879R (5′-GCA GGA GCA AGG TGA GAT GA-3′) (26). PCR was performed with Gflex (TaKaRa) under the temperature profile of an initial denaturation at 94°C for 1 min followed by 30 cycles at 98°C for 10 s, 60°C for 15 s, and 68°C for 30 s, and a final extension at 68°C for 5 min. To detect infection with Sym C, a 0.48-kb region of groEL gene was amplified by PCR with the primers SymC_F (5′-GAG CTG GAA GAC AAG TTC GAG-3′) and SymC_R (5′-ATG AAT GGG CTT TCC ARC TCC-3′) (24) under the same temperature profile. For quality check of the DNA samples, a 1.6-kb region of insect mitochondrial cytochrome oxidase I gene was amplified by PCR with the primers mt16SA1 (5′-AAW AAA CTA GGA TTA GAT ACC CTA-3′) and mt16SB1 (5′-TCT TAA TYC AAC ATC GAG GTC GCA A-3′) (40) under the temperature profile of an initial denaturation at 94°C for 1 min followed by 30 cycles at 98°C for 10 s, 55°C for 15 s, and 68°C for 2 min, and a final extension at 68°C for 5 min.
Rearing, morphometry, and infection check of adult insects
The nymphs inoculated with either ΔintS, ΔcyaA, or Sym C, together with the untreated control nymphs with the original symbiont and the aposymbiotic control nymphs sterilized without bacterial inoculation, were aseptically maintained with sterilized food seeds and DWA (see Table S1) as described (35). During the maintenance, we recorded the number and the developmental stage of surviving insects from 15:00 to 20:00 every day. All the adult insects were anesthetized on ice and photographed from the dorsal side using a digital scanner (EPSON, GT-X980). Using the images, we measured their thorax width using the image analyzing software Natsumushi v.1.10 (41). Some of the adult insects were subjected to dissection of their symbiotic organ in phosphate-buffered saline. The dissected symbiotic organs were photographed to record color and size and were individually subjected to DNA extraction using QIAamp DNA mini Kit (Qiagen). A 1.5-kb region of bacterial 16S rRNA gene was amplified by PCR with the primers 16SA1 (5′-AGA GTT TGA TCM TGG CTC AG-3′) and 16SB1 (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) (42). PCR was performed with Ex Taq (TaKaRa) under the temperature profile of an initial denaturation at 95°C for 2 min followed by 35 cycles of 95°C for 30 s, 52°C for 1 min, and 72°C for 2 min and a final extension at 72°C for 5 min. The PCR products were treated with exonuclease (NEB) and shrimp alkaline phosphatase (TaKaRa), and sequenced using the primers 16SA1 and 16SB1. Sanger sequencing analyses were outsourced (Eurofins Japan). To confirm infection with E. coli or Sym C, obtained 16S rRNA gene sequences were compared with the reference sequences using MEGA v.11.0 (43).
Statistical analyses and figure preparations
Statistical analyses were conducted using R v.4.2.3 (44) and RSudio v.2023.06.0+421 (45). Images of insects and isolated midgut specimens were taken on a dissection microscope S9D with FLEXCAM C1 (Leica) controlled with v.LAS-X (Leica). The obtained images were adjusted manually using Affinity Photo v.2.3.0 (Serif Ltd) and arranged using Affinity Designer v.2.3.1 (Serif Ltd).
ACKNOWLEDGMENTS
We thank Harumi Yamazaki, Tetsuhiro Hachikawa, and Sakiko Toyoda for helping in the maintenance of stinkbug strains.
This study was supported by the Japan Science and Technology Agency ERATO grant no. JPMJER1902 to T.F. and R.K.
Footnotes
This article is a direct contribution from Takema Fukatsu, a Fellow of the American Academy of Microbiology, who arranged for and secured reviews by Yuichi Hongoh, Tokyo Kogyo Daigaku, and Hiroaki Noda, National Agriculture and Food Research Organization.
Contributor Information
Minoru Moriyama, Email: m-moriyama@aist.go.jp.
Takema Fukatsu, Email: t-fukatsu@aist.go.jp.
Edward G. Ruby, University of Hawaii at Manoa, Honolulu, Hawaii, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.01342-24.
Fig. S1-S9 and Table S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York. [Google Scholar]
- 2. Bourtzis K, Miller TA. 2003. Insect symbiosis. CRC Press, Boca Raton, FL. [Google Scholar]
- 3. Douglas AE. 2022. Insects and their beneficial microbes. Princeton University Press, Princeton, NJ. [Google Scholar]
- 4. McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110:3229–3236. doi: 10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gilbert SF, Bosch TCG, Ledón-Rettig C. 2015. Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet 16:611–622. doi: 10.1038/nrg3982 [DOI] [PubMed] [Google Scholar]
- 6. Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81–86. doi: 10.1038/35024074 [DOI] [PubMed] [Google Scholar]
- 7. Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M, Aksoy S. 2002. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet 32:402–407. doi: 10.1038/ng986 [DOI] [PubMed] [Google Scholar]
- 8. Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu Rev Microbiol 59:155–189. doi: 10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- 9. Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu Rev Genet 42:165–190. doi: 10.1146/annurev.genet.41.110306.130119 [DOI] [PubMed] [Google Scholar]
- 10. McCutcheon JP, Moran NA. 2012. Extreme genome reduction in symbiotic bacteria. Nat Rev Microbiol 10:13–26. doi: 10.1038/nrmicro2670 [DOI] [PubMed] [Google Scholar]
- 11. McCutcheon JP, Boyd BM, Dale C. 2019. The life of an insect endosymbiont from the cradle to the grave. Curr Biol 29:R485–R495. doi: 10.1016/j.cub.2019.03.032 [DOI] [PubMed] [Google Scholar]
- 12. Kiers ET, West SA. 2015. Evolving new organisms via symbiosis. Science 348:392–394. doi: 10.1126/science.aaa9605 [DOI] [PubMed] [Google Scholar]
- 13. Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nat Rev Microbiol 8:218–230. doi: 10.1038/nrmicro2262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6:741–751. doi: 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 15. Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266. doi: 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- 16. Renoz F, Arai H, Pons I. 2024. The genus Sodalis as a resource for understanding the multifaceted evolution of bacterial symbiosis in insects. Symbiosis 92:187–208. doi: 10.1007/s13199-023-00966-0 [DOI] [Google Scholar]
- 17. Kikuchi Y, Hosokawa T, Fukatsu T. 2011. An ancient but promiscuous host–symbiont association between Burkholderia gut symbionts and their heteropteran hosts. ISME J 5:446–460. doi: 10.1038/ismej.2010.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takeshita K, Kikuchi Y. 2017. Riptortus pedestris and Burkholderia symbiont: an ideal model system for insect–microbe symbiotic associations. Res Microbiol 168:175–187. doi: 10.1016/j.resmic.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 19. Itoh H, Jang S, Takeshita K, Ohbayashi T, Ohnishi N, Meng X-Y, Mitani Y, Kikuchi Y. 2019. Host–symbiont specificity determined by microbe–microbe competition in an insect gut. Proc Natl Acad Sci U S A 116:22673–22682. doi: 10.1073/pnas.1912397116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher RM, Henry LM, Cornwallis CK, Kiers ET, West SA. 2016. The evolution of host-symbiont dependence. Nat Commun 8:15973. doi: 10.1038/ncomms15973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohbayashi T, Mergaert P, Kikuchi Y. 2020. Host-symbiont specificity in insects: underpinning mechanisms and evolution. Adv Insect Physiol 58:27–62. doi: 10.1016/bs.aiip.2020.03.002. [DOI] [Google Scholar]
- 22. Hoang KL, Morran LT, Gerardo NM. 2016. Experimental evolution as an underutilized tool for studying beneficial animal–microbe interactions. Front Microbiol 7:1444. doi: 10.3389/fmicb.2016.01444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Drew GC, Stevens EJ, King KC. 2021. Microbial evolution and transitions along the parasite–mutualist continuum. Nat Rev Microbiol 19:623–638. doi: 10.1038/s41579-021-00550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosokawa T, Ishii Y, Nikoh N, Fujie M, Satoh N, Fukatsu T. 2016. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat Microbiol 1:15011. doi: 10.1038/nmicrobiol.2015.11 [DOI] [PubMed] [Google Scholar]
- 25. Moriyama M, Hayashi T, Fukatsu T. 2022. A mucin protein predominantly expressed in the female-specific symbiotic organ of the stinkbug Plautia stali. Sci Rep 12:7782. doi: 10.1038/s41598-022-11895-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koga R, Moriyama M, Onodera-Tanifuji N, Ishii Y, Takai H, Mizutani M, Oguchi K, Okura R, Suzuki S, Gotoh Y, Hayashi T, Seki M, Suzuki Y, Nishide Y, Hosokawa T, Wakamoto Y, Furusawa C, Fukatsu T. 2022. Single mutation makes Escherichia coli an insect mutualist. Nat Microbiol 7:1141–1150. doi: 10.1038/s41564-022-01179-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Salem H, Florez L, Gerardo N, Kaltenpoth M. 2015. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. Proc Biol Sci 282:20142957. doi: 10.1098/rspb.2014.2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hosokawa T, Fukatsu T. 2020. Relevance of microbial symbiosis to insect behavior. Curr Opin Insect Sci 39:91–100. doi: 10.1016/j.cois.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 29. Hosokawa T, Matsuura Y, Kikuchi Y, Fukatsu T. 2016. Recurrent evolution of gut symbiotic bacteria in pentatomid stinkbugs. Zoological Lett 2:24. doi: 10.1186/s40851-016-0061-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kikuchi Y, Hosokawa T, Fukatsu T. 2007. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl Environ Microbiol 73:4308–4316. doi: 10.1128/AEM.00067-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu Y-Z, Rédei D, Eger J Jr, Wang Y-H, Wu H-Y, Carapezza A, Kment P, Cai B, Sun X-Y, Guo P-L, Luo J-Y, Xie Q. 2018. Phylogeny and the colourful history of jewel bugs (insecta: hemiptera: scutelleridae). Cladistics 34:502–516. doi: 10.1111/cla.12224 [DOI] [PubMed] [Google Scholar]
- 32. Tada A, Kikuchi Y, Hosokawa T, Musolin DL, Fujisaki K, Fukatsu T. 2011. Obligate association with gut bacterial symbiont in Japanese populations of the southern green stinkbug Nezara viridula (heteroptera: pentatomidae). Appl Entomol Zool 46:483–488. doi: 10.1007/s13355-011-0066-6 [DOI] [Google Scholar]
- 33. Taylor CM, Coffey PL, DeLay BD, Dively GP. 2014. The importance of gut symbionts in the development of the brown marmorated stink bug, Halyomorpha halys (Stål). PLoS One 9:e90312. doi: 10.1371/journal.pone.0090312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hosokawa T, Imanishi M, Koga R, Fukatsu T. 2019. Diversity and evolution of bacterial symbionts in the gut symbiotic organ of jewel stinkbugs (Hemiptera: Scutelleridae). Appl Entomol Zool 54:359–367. doi: 10.1007/s13355-019-00630-4 [DOI] [Google Scholar]
- 35. Nishide Y, Onodera NT, Tanahashi M, Moriyama M, Fukatsu T, Koga R. 2017. Aseptic rearing procedure for the stinkbug Plautia stali (Hemiptera: Pentatomidae) by sterilizing food-derived bacterial contaminants. Appl Entomol Zool 52:407–415. doi: 10.1007/s13355-017-0495-y [DOI] [Google Scholar]
- 36. Tenaillon O, Skurnik D, Picard B, Denamur E. 2010. The population genetics of commensal Escherichia coli. Nat Rev Microbiol 8:207–217. doi: 10.1038/nrmicro2298 [DOI] [PubMed] [Google Scholar]
- 37. Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008. doi: 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35:4453–4455. doi: 10.1093/bioinformatics/btz305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. Mrbayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol 4:e337. doi: 10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tanahashi M, Fukatsu T. 2018. Natsumushi: image measuring software for entomological studies. Entomol Sci 21:347–360. doi: 10.1111/ens.12315 [DOI] [Google Scholar]
- 42. Fukatsu T, Nikoh N. 1998. Two intracellular symbiotic bacteria from the mulberry psyllid Anomoneura mori (insecta, homoptera). Appl Environ Microbiol 64:3599–3606. doi: 10.1128/AEM.64.10.3599-3606.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. R Core Team . 2024. R. a language and environment for statistical computing. R foundation for statistical computing. Available from: https://www.R-project.org/
- 45. R Studio Team . 2023. RStudio: integrated development for R. RStudio. PBC, Boston, MA. Available from: http://www.rstudio.com/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1-S9 and Table S1.