Abstract
Background
Mesenchymal Stromal Cells (MSCs) are the preferred candidates for therapeutics as they possess multi-directional differentiation potential, exhibit potent immunomodulatory activity, are anti-inflammatory, and can function like antimicrobials. These capabilities have therefore encouraged scientists to undertake numerous preclinical as well as a few clinical trials to access the translational potential of MSCs in disease therapeutics. In spite of these efforts, the efficacy of MSCs has not been consistent—as is reflected in the large variation in the values of outcome measures like survival rates. Survival rate is a resultant of complex cascading interactions that not only depends upon upstream experimental factors like dosage, time of infusion, type of transplant, etc.; but is also dictated, post-infusion, by intrinsic host specific attributes like inflammatory microniche including proinflammatory cytokines and alarmins released by the damaged host cells. These complex interdependencies make a researcher’s task of designing MSC transfusion experiments challenging.
Methods
In order to identify the rules and associated attributes that influence the final outcome (survival rates) of MSC transfusion experiments, we decided to apply machine learning techniques on manually curated data collected from available literature. As sepsis is a multi-faceted condition that involves highly dysregulated immune response, inflammatory environment and microbial invasion, sepsis can be an efficient model to verify the therapeutic effects of MSCs. We therefore decided to implement rule-based classification models on data obtained from studies involving interventions of MSCs in sepsis preclinical models.
Results
The rules from the generated graph models indicated that survival rates, post-MSC-infusion, are influenced by factors like source, dosage, time of infusion, pre-Interleukin-6 (IL-6)/ Tumour Necrosis Factor- alpha (TNF-α levels, etc.
Conclusion
This approach provides important information for optimization of MSCs based treatment strategies that may help the researchers design their experiments.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13287-024-03873-3.
Keywords: Mesenchymal stromal cells, Immunomodulation, Survival rate, Sepsis, Machine learning
Background
Stem cells are considered the ‘holy grail’ for therapeutics due to their renewal and regenerative capabilities [1]. Mesenchymal Stromal/Stem Cells (MSCs) fulfill all the necessary requirements as candidates for therapeutics, namely: ability to differentiate multi-directionally, immunomodulatory activity, anti-inflammatory potential, anti-microbial capability, etc. Their translational potential is also aided by the fact that the protocols required to isolate and expand them are relatively less complicated and rapid as compared to protocols for other stem cells [2]. These biological capabilities as well as accessible experimental procedures have made treatment of diseases using MSCs treatment an attractive proposition.
As a consequence, de novo information is constantly being generated from many disease specific studies. In spite of these endeavors, the efficacy of MSCs has not been consistent in these translational studies [3]. This heterogeneity could be due to the fact that a multitude of parameters are required to be decided, by the researcher, in order to conduct a MSCs translational experiment. Some of these like animal models, dosage, time of MSCs infusion, etc. are determined by the researcher while others like physiological variability; immune environments and disease condition, etc. are intrinsic to the host and donor biology. The challenges faced by a researcher in accurately choosing these extrinsic experimental factors and accessing the intrinsic host factors, could ultimately dictate the outcome of these translational studies.
Sepsis is one such condition where the characteristics of MSCs such as their ability to differentiate multi-directionally, modulate the immune system, control inflammation, and anti-microbial properties, are entirely pertinent. Sepsis entails an abnormal host response to a microbial infection and results in a highly dysregulated immune response, hyperinflammation and microbial invasion that could lead to multiorgan failure [4]. As specific and effective therapies against sepsis are still lacking [5], it is an appropriate model for MSCs therapy. Although MSCs have been shown to reduce mortality in septic animal models [6], ambiguity exists due to differences in MSCs treatment procedures like: source of MSCs, dosage and timing of MSCs infusions, host microenvironment etc. [7].
With a goal to suggest optimization criteria for therapeutic interventions in clinical settings, we started by initially scanning the literature for terms related to the role of MSCs in preclinical models and clinical trials. We found that there was a huge variability in the extrinsic experimental factors like dose utilized, timings of infusion post sepsis induction, weight of the model, transplant type, etc. Furthermore, the intrinsic host physiology governed outcomes in terms of cytokine production, factors for organ failure, etc. and this eventually led to survival rate variability in multiple studies. These variable parameters as well as experiment outcomes make it very difficult to decide the exact methodology to achieve consistent therapeutic outcomes.
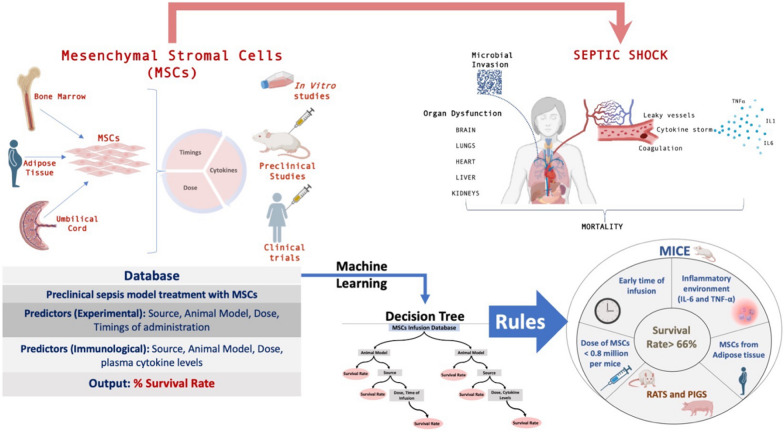
With the emergence of advanced machine learning techniques, strategies have now been developed to identify and predict the factors that govern the outcome of therapeutic interventions [8]. In order to suggest the rules and associated factors that may help the researchers overcome the challenges of using MSCs as therapeutic agents, we applied machine learning techniques to extract knowledge rules from the published data pertaining to the use of MSCs in sepsis conditions. The graphical representation of the study is depicted in Fig. 1.
Fig. 1.
Schematic representation of the study. (Created with BioRender.com)
After pre-processing and curation of these data sets, we implemented the machine learning algorithms to recognize the trajectory of outcomes post MSC infusion. We aimed to identify the extrinsic experimental factors as well as the internal host factors and their associated rules that influence the efficacy of MSCs transplantation–thereby dictating the outcome (survival rate in the studies). The generated graph models indicated the survival rates post-infusion of MSCs are influenced by factors like animal model, source, dosage and time of infusion of MSCs, basal level of inflammatory cytokines like IL-6 &TNF-α. This information is particularly useful to predict the clinical outcome of MSCs application in a specific setting and more importantly in designing targeted treatments.
Methods
All the preprocessing, filtering, and machine learning analysis was conducted using Waikato Environment for Knowledge Analysis (WEKA) 3.8.6 machine learning toolkit along with R scripts.
Data acquisition
Systematic search was carried out to collect available data on therapeutic effects of mesenchymal stem cells (MSCs) for sepsis in preclinical models. Public databases such as PubMed, Google Scholar, Litmaps were searched until May 2023. Various terms were used as key words or free text words: “sepsis” and “therapeutic” and “treatment” and “mesenchymal stem cells”. In total, 67 manuscripts were chosen (Supplementary Table 1, Additional file 1).
After extensive search, a number of common parameters were identified to analyse through machine learning techniques. These included: source of MSCs, animal model, weight, dose of MSCs, time of MSCs administration, survival rate, serum levels of different pro-inflammatory cytokines: TNF-α and IL-6 and Liver enzymes: Aspartate aminotransferase (AST) and Alanine transaminase (ALT).
Source of MSCs
The literature gathered included sources like Adipose tissue, Bone Marrow and Umbilical cord. Additionally, compatibility was either allogeneic or xenogeneic depending upon source and host of MSCs.
Animal model
Mice and rats were majorly used as model to study sepsis at preclinical levels. Two studies included pigs as well.
Dose
To treat the sepsis, MSCs were administered intravenously in most of the studies. Dose throughout the study is mentioned as number of cells (in 106) per animal.
Time of administration
Time of infusion of MSCs after establishing septic model differ in each study ranging from 0 to 48 h. Although, in majority of studies, MSCs were injected within 6 h of generating the model.
Survival rate was compared in sepsis models without MSCs infusion and septic model infused with MSCs at 48 h (Supplementary Fig. 1, Additional file 1). Levels of TNF-α, IL-6, AST and ALT, before and after MSCs infusion were noted. Cytokines levels were reported as pg/mL.
Data preprocessing and handling incomplete data
Missing data, prevalent in many clinical and experimental studies, leads to inadequacies while building predictive models [9, 10]. These inconsistencies arise due to experimental design or data acquisition problems. The possibility of missing data is higher in meta-analysis studies, such as ours, where results from multiple studies are pooled together. As a result of experimental design and methodologies followed by different studies our pooled database had missing data, ranging from 15 to 35% in 4 attributes, namely: Survival Rate, IL-6, TNF-α, and Dose.
Multiple studies have reported varied methods that deal with missing data. These methodologies range from extreme measures like removing the missing records, to imputing the missing attribute values with mean or median value. Some methods also employ “filling” in missing data by liner interpolation or extrapolation techniques. As missing data should be processed before conducting any analysis [11], we imputed the missing data for the attributes Survival_Rate by mean and of the immune attributes: IL-6 and TNF-α, by median values. Missing values in the attribute Dose were replaced by the most common dose values corresponding to the animal model used and its respective body_weight.
To maintain consistency in the nomenclature, we renamed the values of the attribute “Animal model” from “mouse” to “mice”. Additionally, the 2 values “Wharton’s jelly” and “Amniotic Fluid” were replaced by “Umbilical cord” to reduce the number of distinct nominal values in the attribute “Source_of_MSCs”.
A single outlier data record where the “Source_of_MSCs” was “Menstrual fluid” was removed.
Due to the fact that classification models require the predictive class attribute to be nominal, we discretized the class attribute “Survival_Rate” by using equal-width binning. This attribute was discretized into 3 bins: “67–100%”, “34–66%” and “0–33%”. Biologically these correspond to “high”, “median” and “low” survival rates.
Selection of input attributes
The procedure to select the most appropriate input properties, that classifies the “Survival_Rate” is challenging for our meta-analysis, as available attributes not only vary across different studies, but there exists large variation in the attribute values. To identify the researcher declared extrinsic experimental factors as well as the internal host factors and their associated rules, we decided to generate two types of models: experimental factors model and immunological factors model, by manually selecting the input properties based on the above 2 criteria. The experimental properties were: Animal_Model, Source of MSCs, Dose, Time_MSC_Infusion. Evidently, variation in the inflammatory environment in the tissues can regulate the immunoregulatory properties of MSC [12, 13].
Therefore, we tried to analyse the correlation between basal levels of the inflammatory cytokines i.e. IL-6, TNF-α before MSC infusion and the survival rate of septic animal models after MSC infusion. Two immunological models were selected: either basal/control TNF-α (Model 1) levels or basal/control IL-6 (Model 2) levels. As these also depends on the experimental properties, these two were taken along with the experimental properties for further analysis. Although AST and ALT levels were also available, we did not employ these for machine learning analysis, as these had large number of missing values (~ 50%).
We employed the “AttributeSelectedClassifier”, with tenfold cross-validation, to select the attributes that had the greatest influence in predicting the “Survival_Rate”. The score “Merit of best subset found” was used to analyze the importance of select pre and post IL-6/TNF-α attributes towards the chosen classification algorithm.
Machine learning methods
We employed a variety of classification approaches that accessed the impact of selected attributes towards predicting the efficacy of MSCs therapy. WEKA-3.8.6 machine learning toolkit along with R scripts were used for implementing and testing the classification models. We initiated the analysis by using decision tree models like j48 and Random Forest (RF) [14], which is an ensemble of decision trees. After obtaining encouraging results we tested the performance of Naïve Bayes models, Support Vector Machines (SVM using radial basis function) and Multi Layered perceptron (Neutral network). Ensemble algorithms like AdaBoostM1 (Boosting), Bagging and stacking were also tested. Logistic regression was also employed and the odds ratio of each attribute was used to access its contribution towards classifying the survival rates.
Decision trees
Although best known for their prediction capability, the true utility of machine learning algorithms on biological data is for knowledge acquisition that unravels patterns amongst various biological attributes. Decision trees are a set of classification algorithms that aid in generating novel knowledge by applying discriminating criteria on graphical representation of biological attributes. One can then use such a graph of conditions to infer possible consequences of a treatment. At the very top of a decision tree is a root node that represents the most important condition for discriminating classes. Lower internal nodes represent additional conditions for class discrimination, whereas the leaf nodes represent the final classification of a biological condition under scrutiny. By following the path from the root node to the leaf node [15], one can learn certain “rules” for the classification of records in a dataset. Therefore, in order to discover the rules (and associated attributes) that influence survival rate we gave experimental attributes and immunological attributes to the J48 decision trees algorithm, a WEKA’s implementation of the C4.5 algorithm [16].
Model performance and comparison
By using tenfold cross-validation we used percentage of correctly classified instances and F-score as measures of model accuracy. In order to compare the different algorithms, we employed “PairedCorrectedTTester” in order to find which of the algorithms performed significantly better or worse when comparing with each other. As a measure of individual classifier performance, we used 2 measures: Percent_correct (performance metric that represents the percentage of instances that are correctly classified by a machine learning algorithm) and F-measure (measure of a classifier’s accuracy that takes into account both precision and recall where a higher value indicates better performance).
Results
Curated database of mesenchymal stromal cells in septic preclinical models
Data preprocessing by the removal of outlier records, renaming of nominal values and imputation of missing values resulted in the selection of 78 records (from 67 published reports) where the septic animal models were injected with MSCs. Post attribute selection, these 78 records had 8 attributes that were chosen as inputs for further analysis. Using these attributes for the dimensionality reduction using attribute selection classifiers further revealed that three attributes: Animal_Model, Source_of_MSCs and Dose_per_Animal were common between both the experimental factors model and both the immunological factors models. For the experimental factors model, one additional attribute: Time, was selected along with the three above (Merit score ranged from 0.83 to 0.89). For the immunological model 1, the one attribute selected was TNF-α levels before infusion (Control_ TNFα) (Merit score of 0.88). The immunological model 2 had the basal levels of IL-6 (Control IL_6 with merit score of 0.92) along with the three common ones above. Thereafter, 4 attributes, for each of the respective models, were used as inputs for classification algorithms. It is noteworthy that these attributes were influencing the Survival_Rate only in the cases where the Animal_Model was mice (Figs. 2, 3, 4). In the models generated, the path from root node to outcome leaf node through the intermediate levels, suggested the following rules that influenced the survival rates (Table 1).
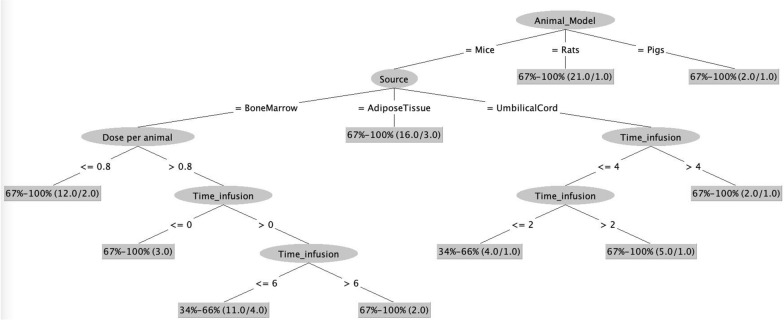
Fig. 2.
Experimental Factors Model: Decision Tree depicting the dependence of survival rate on the animal model, source, dose and time of infusion of MSCs. The numbers in the braces represent: total number of records/number of misclassified records
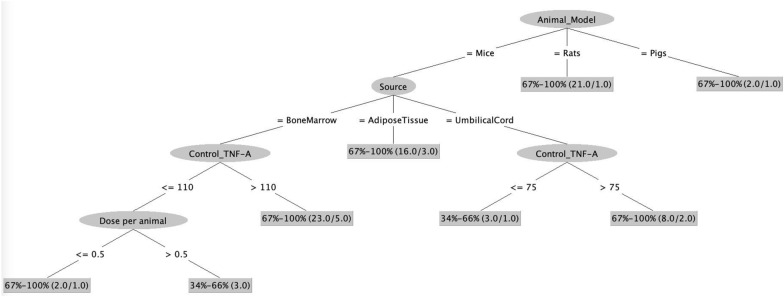
Fig. 3.
Immunological Model 1: Decision tree illustrates the dependence of survival rate on basal levels of TNF-α (Control_TNF-α) in addition to the animal model, source, dose and time of infusion of MSCs. The numbers in the braces represent: total number of records/number of misclassified records. Note: TNF-A stands for TNF- alpha
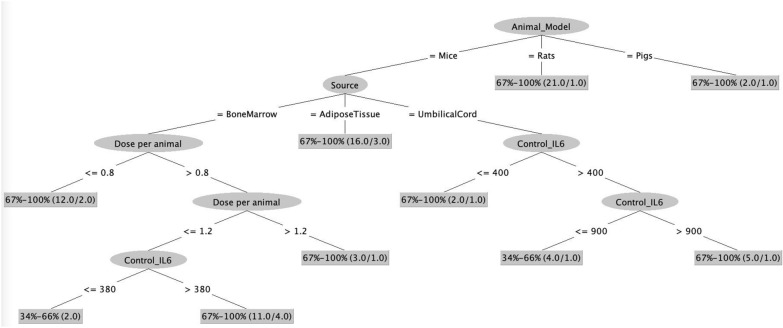
Fig. 4.
Immunological Model 2: Decision tree illustrates the dependence of survival rate on basal levels of IL-6 (Control_IL-6) in addition to the animal model, source, dose and time of infusion of MSCs. The numbers in the braces represent: total number of records/number of misclassified records
Table 1.
Set of Classification Rules contain21 rules for MSC transplantation experimental design
| 1 | If Animal Model = Rat | 67%− 100% |
|---|---|---|
| 2 | If Animal Model = Pig | 67%− 100% |
| 3 | If Animal Model = Mice and Source of MSCs = Adipose Tissue-Derived | 67%− 100% |
| 4 | If Animal Model = Mice and Source of MSCs = Bone Marrow and MSC Dose < = 0.8 × 10^6 cells | 67%− 100% |
| 5 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and MSC Dose > 0.8 × 10^6 cells and Time of MSC infusion is < = 0 | 67%− 100% |
| 6 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and MSC MSC Dose > 0.8 × 10^6 cells and Time of MSC infusion < = 6 h | 34%− 66% |
| 7 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and MSC Dose > 0.8 × 10^6 cells and Time of MSC infusion > 6 h | 67%− 100% |
| 8 | If Animal Model = Mice and Source of MSCs = Umbilical Cord derived and Time of MSC infusion < = 4 h and < = 2 h | 34%− 66% |
| 9 | If Animal Model = Mice and Source of MSCs = Umbilical Cord derived and Time of MSC infusion < = 4 h and > 2 h | 67%− 100% |
| 10 | If Animal Model = Mice and Source of MSCs = Umbilical Cord derived and Time of MSC infusion > 4 h | 67%− 100% |
| 11 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and Dose > 0.8 × 10^6 cells and > 1.2 × 10^6 cells | 67%− 100% |
| 12 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and Dose > 0.8 × 10^6 cells and < = 1.2 × 10^6 cells and Control IL-6 level < = 380 | 34%− 66% |
| 13 | If Animal Model = Mice and Source of MSCs = Bone Marrow-Derived and Dose > 0.8 × 10^6 cells and < = 1.2 × 10^6 cells and Control IL-6 level > 380 | 67%− 100% |
| 14 | If Animal Model = Mice and Source of MSCs = Umbilical Cord and Control IL-6 level < = 400 | 67%− 100% |
| 15 | If Animal Model = Mice and Source of MSCs = Umbilical Cord and Control IL-6 level > 400 and < = 900 | 34%− 66% |
| 16 | If Animal Model = Mice and Source of MSCs = Umbilical Cord and Control IL-6 level > 400 and > 900 | 67%− 100% |
| 17 | If Animal Model = Mice and Source of MSCs = Bone Marrow and Control TNF-α < = 110 and MSC Dose < = 0.5 × 10^6 cells Control TNF-α < = 110 and MSC Dose < = 0.5 × 10^6 cells | 67%− 100% |
| 18 | If Animal Model = Mice and Source of MSCs = Bone Marrow and Control TNF-α < = 110 and MSC Dose > 0.5 × 10^6 cells | 34%− 66% |
| 19 | If Animal Model = Mice and Source of MSCs = Bone Marrow and Control TNF-α > 110 | 67%− 100% |
| 20 | If Animal Model = Mice and Source of MSCs = Umbilical Cord Derived and Control TNF-α < = 75 | 34%− 66% |
| 21 | If Animal Model = Mice and Source of MSCs = Umbilical Cord Derived and Control TNF-α > 75 | 67%− 100% |
Classification: decision trees
Extrinsic experimental rules and attributes
Post tenfold cross validation, we analyzed the best representative decision tree for survival rate. As we can observe in Fig. 2, the root node of decision tree is the Animal_model, namely mice, rats, and pigs, which influences survival rate. This root node therefore predicts that only the type of animal is dictating the outcome of the MSCs transplant and that the survival rate is high (above 66%) for both rat (21 records) and pig (2 records) animal models. However, if the animal model is “mice”, there were additional rules that influenced the success of the MSCs infusion. At the second level, the attribute represents the source of MSCs used in mice model: bone marrow, adipose tissue, umbilical cord. Interestingly, adipose tissue-derived MSCs (AD-MSCs) resulted in a high survival rate (above 66%: 13 out of 16 correctly classified records). In case of umbilical cord derived MSCs (UC-MSCs) and bone marrow derived MSCs (BM-MSCs), there were additional rules that influenced the success of the MSCs infusion.
The next level of the decision tree identifies the dose of MSCs administered to the mice as the governing factor towards survival rate. Instances, where the MSCs dose was less than 0.8X106 cells reported a high survival rate (above 66%: 10 out of 12 correctly classified records). However, when the MSCs dose exceeded 0.8X106 cells, the time of infusion of the MSCs influenced the mice survival rate. When time of infusion was less than 0 h, the survival rate was above 66% (3 records) as opposed to survival rate majorly falling into 34–66% bin (7 out of 11 correctly classified records) when the time of infusion is between 0 and 6 h in the case of BM- MSCs infusion. On the other hand, in the case of UC-MSCs infusion, the time range between 2 and 4 h was resulting in a survival rate of 67–100% (4 out of 5 correctly classified records).
These findings highlight the importance of considering the type of animal model, source of MSCs, dosage and time of infusion when designing an experiment for treating animal models of sepsis. Our data driven decision tree models suggest that researchers may increase the likelihood of achieving successful outcomes in their experiments if they give importance to these attributes.
Intrinsic immunological rules and attributes
The models based upon immunological attributes showed a lot of similarity to the model based upon experimental attributes. As in the previous model, the root node of the decision tree is based on the chosen animal model: mice, rats, or pigs. Remarkably, in accordance to both the immunological models, all instances involving rat and pigs resulted in high survival rates (above 66%) for both rat (21 records) and pig (2 records) animal models. However, in the case of mice models, additional factors influenced the success of MSCs infusion. In parallel with above results, AD-MSCs resulted in a high survival rate (above 66%: 13 out of 16 correctly classified records) in mice model. The subsequent branch of the decision tree in immunological model 1 (Fig. 3) reveals the importance of basal levels of TNF-α in mice i.e. levels without MSCs infusion. Mice models with TNF-α levels more than 110 pg/mL had a survival rate more than 66% post BM-MSCs infusion as opposed to low survival rate when TNF-α levels were lower than 110 pg/mL.
Moreover, in immunological model 2 (Fig. 4), basal levels of IL-6 in mice i.e. levels without MSCs infusion, was also observed to determine the success of the MSCs infusion in terms of survival rate. In case of infusion of less than or equal to 0.8 million BM- MSCs per mice, the survival rate was invariably higher than 67% while if the dose exceeded 0.8, the survival rate was dependent upon basal IL-6 levels and when these levels in serum were more than 380, the survival rate was more than 67% (7 out of 11 correctly classified records) in comparison to lower survival rate correlating with lower basal IL-6 levels (2 correctly classified records). Likewise, in the case of UC-MSCs infusion, the basal IL-6 levels more than 900 pg/mL resulted in higher survival rate (4 out of 5 correctly classified records) while the survival rate was between 34 and 66% when basal IL-6 levels were less than 900 pg/mL.
Just like in the experimental model case, these findings emphasize the significance of the type of animal model, transplantation methods, MSCs source, dose and timing of MSCs infusion when designing an experimental study leading to MSCs therapy. Additionally, anti-inflammatory cytokines like IL-6 and TNF-α basal levels should be given careful consideration so that researchers can ensure greater probability of attaining higher survival rates from their infusion experiments.
To summarize, the path from root node to outcome leaf node through the intermediate levels, suggested the following rules that influenced the survival rates in the immunological model (ST-1), (i) Rule 1–3: Applicable to both conditions (Dose, IL-6 and TNF-α), (ii) Rules 4–11 are based on dose of MSC’s and infusion time (iii) Rules 12–16: are determined by Dose and IL-6 levels in the mice. (iv) Rules 17–21 are based on the MSC’s dose and the TNF-α levels.
Comparisons with other machine learning algorithms
We implemented classification models, using the same selected attributes as inputs, by employing a variety of classifiers. The tenfold cross validation revealed that all the classifiers, with the exception of Naïve bayes and Stacking (~ 56% accuracy for both), had high accuracy and most of these algorithms correctly classified the instances with > 70% accuracy (Table 2).
Table 2.
The Machine learning classification methods and their performance accuracy as measured by the percentage of correctly classified records and the F-Measure that combines precision and recall.
| S. No | Methods | Percent correct | F measure |
|---|---|---|---|
| 1 | J48 | 74.52 | 0.82 |
| 2 | Naïve Bayes | 55.91* | 0.71 |
| 3 | Lib SVM | 73.71 | 0.77 |
| 4 | MultiLayer Perceptron | 71.16 | 0.77 |
| 5 | Ada Boost ML | 71.29 | 0.75 |
| 6 | Bagging | 73.02 | 0.78 |
| 7 | Stacking | 56.68* | 0.72 |
| 8 | Random forest | 75.21 | 0.81 |
| 9 | Logistic | 69.34 | 0.76 |
The best performing j48 decision tree algorithm is shown in bold. The * sign depicts significantly underperforming statistic.
To find out which of the algorithms were the best performers we applied “PairedCorrectedTTester” to reveal the statistical significance of their performance. Both the Percent_correct and F-measure revealed that j48 decision tree, as well as other algorithms, had high accuracy both in terms of correctly classifies instances as well as F-measure (Table 2).
Discussion
The fact that MSCs act variably in different microenvironments compels us to standardize factors like their dosage, time of infusion and MSCs source to obtain clinically applicable outcomes. Clinical applications of MSCs can only be successful when we are aware of the parameters that influence the efficacy of the infused MSCs. Therefore, it is imperative that, during experimental design, only those factors be emphasized that enhance the efficacy of MSCs. In this study, we identify the attributes that may influence the outcome of MSCs infusion experiments, by applying machine learning techniques. Although MSCs therapy for sepsis has been widely researched over the last decade, the parameters dictating the outcome of the treatment regimen remain unknown. Analysis of the available literature revealed several factors that are dependent upon the decisions taken by the researcher during experimental design. There are also several host specific factors like the immune environment that directly influence the success rate of the MSCs treatment [17]. Our machine learning approach has not only highlighted several of these attributes, but has also generated rules informing us of the hierarchical interactions (between different attributes), as well as the values of these factors that determines the outcome. Here we discuss some of the salient features of these factors.
It is noteworthy that the decision tree algorithms, in the case of mice, highlighted the 3 way hierarchical interplay between source of MSCs, dosage and time of infusion. The rule generated by our analysis suggests that the survival rates are influenced by the source where MSCs are derived from. The analysis revealed that the studies in which septic group of mice received AD-MSCs experienced survival rate greater than 66%. Contrarily, when the tissue source was either bone marrow or umbilical cord, other factors like dose, time of infusion and basal levels of pro-inflammatory cytokines determined the survival rate.
Interestingly, an attribute, according to machine learning decision trees, that does not play a role in MSC efficacy is the transplant compatibility. According to the data, the majority of AD-MSCs were allogenically infused, as compared to BM-MSCs that were allogenic and xenogenic. Whereas, in case of UC-MSCs xenogeneic infusion was majorly reported. This attribute “Transplant type” did not have sufficient support from the decision tree algorithm and is therefore not part of the generated rules. By analysing the effect of dosage on survival rate of septic mice models Li et al. had demonstrated that those mice that were administered medium dose (~ 0.5X106 per mice) displayed highest survival rates [7], whereas low and high doses were detrimental to their survival. Adverse effects of high ADSCs, injected intravitreally, were observed by Rasiah et al. when treating mild traumatic brain injury [18]. Another study by Chen et al. on ischemic stroke rat models, came to a conclusion that a low dose of MSCs infusion displayed the most effective neurobehavioral recovery and infarction reduction [19]. Likewise, our analysis depicted an average dose of 0.8 million per mice to be appropriate and responsible for a higher survival rate post sepsis induction.
The time of administration of MSCs, to treat sepsis in animal models, varied between studies and ranged from 0 to 48 h after the induction of sepsis. These studies have shown that this time range was the most appropriate towards alleviating bacteremia by increasing the survival rate of septic models, decreasing levels of proinflammatory cytokines, and also reducing levels of liver enzymes [20–25]. There are also documented relationships between the time of MSCs administration and immune response in terms of proinflammatory, anti-inflammatory and coagulation factors. A biphasic response, in a LPS (Lipopolysaccharide) induced sepsis model, has been observed depending upon the timing of MSCs infusion. The activation or suppression of innate immune pathways was shown to be dependent on early (2 h activation) or late (4 h suppress) LPS induction [26]. Timing of MSCs infusion, in relationship to the effectiveness of the solid organ transplantation, contradicted between different studies [27]. In other diseased conditions like COVID19, early time point MSCs infusion in critically ill patients reduced their mortality [28]. Our decision tree rules also revealed that the timing of MSCs infusion dictates the outcome with MSCs infused in parallel to onset of disease (for BM-MSCs) or in a window between 2 and 6 h of sepsis induction (for UC-MSCs) leads to a relatively higher survival rate. Importantly, these rules indicate the well-established immunomodulatory activity of MSCs, as the mortality rates associated with sepsis can be mitigated by early infusion of MSCs.
Among the literature analysed for mice models in this study, three manuscripts differed wherein the time of infusion of MSCs was greater than 6 h after sepsis induction and still resulted in an improved survival rate ranging between 70 and 85% [24, 25, 29]. It is to be noted that these studies either utilised LPS preconditioning, or the MSCs were injected via retro-orbital route or sequential MSCs dosing was performed post 2 h, 24 h and 48 h of septic model generation. This clearly indicates that we can extend the window timings of MSCs infusion and improve their efficacy through different alterations in the treatment regimen.
An even more compelling observation in the current analysis was that survival rate post MSCs infusion in rats and pigs was higher (66–100%), irrespective of dose or weight. However, data analysis depicted that 98% of these studies, the infusion time was less than 3 h while majorly within 1 h of generation of model. Timing of infusion seemingly played an important role here: i.e.: if the infusion is as soon as the sepsis condition is generated, then the dose does not matter. As we increase the time window, the infusion is dependent upon dose of MSCs and weight of animals especially in case of mice. In addition to the dose/weight and time of MSCs infusion in the study, as mentioned above, internal host factors like the basal levels of pro inflammatory cytokines play a major role in response to MSCs infusion. In the sepsis model, the analysed cytokines are IL-6, TNF-α, IL-1β that indicate proinflammatory environment while IL-10 and TGF-β are indicative of anti-inflammatory environment. The cytokines that influences the survival rate were TNF-α and IL-6 in our study.
Although TNF-α is a proinflammatory cytokine and usually associated as a marker of an inflammatory microenvironment, various studies have proven that preconditioning of MSCs with TNF-α enhances the immunomodulatory capacity of MSCs. TNF-α, alone or in combination with other inflammatory mediators, influences the functional reprogramming of MSCs including higher anti-inflammatory effects [30], increasing their anti-tumour potential [31] and attenuating diseases like colitis [32].
Interestingly, IL-6 has been identified as a marker positively correlated with the severity of sepsis [33]. High levels of IL-6 are shown to be associated to mortality of septic patients and septic models in preclinical studies [34–37]. On the contrary, when Human-IL-6 was injected in mouse model, 24 h before the induction of sepsis, it promoted the survival of mice. Moreover, IL-6-/- mice had no effect on anti-inflammatory cytokines [38]. On similar lines, Remick et al.,2005 developed IL-6 knockout (KO) model of mice, and observed no difference in mortality in both IL-6 KO and control group of mice post sepsis induction [33]. In another study, IL-6 administration in neonatal mouse model of sepsis resulted in increased survival [39], whereas; total inhibition of IL-6 lowered the survival rate. These studies altogether suggest the variable roles of IL 6 being a pleiotropic cytokine and its role in regards to survival rate is uncertain because of its ubiquity and diverse functions.
It is well known that the anti-inflammatory effects of the MSCs are dependent on the host microenvironment, in vitro preconditioning of MSCs with proinflammatory cytokines is an effective strategy to boost the immunomodulatory activity of MSCs [12, 13]. On the similar note, our analysis has pointed towards a higher survival rate in an inflammatory mileu when basal TNF-α serum levels are above 110 pg/ml that primes BM-MSCs to have better functional capabilities. Basal levels of IL 6 over a threshold value of 380 pg/mL in case of BM-MSCs and 900 pg/mL in case of UC-MSCs also made them act in a better way resulting in improved survival rate. This suggests that by exposing MSCs to a simulated in vivo inflammatory milieu in advance improves their functional capabilities and improvement in survival rate in sepsis preclinical model.
The performance and accuracy of the j48 decision trees, from which these rules have been generated, were compared with 8 other machine learning algorithms. Apart from Naïve Bayes, all the other algorithms had high percentage (> 70%) of correctly classified instances (as well as high F-measure scores: > 0.7) post tenfold cross-validation. This depicts the validity of the rules generated from a machine learning perspective.
Although our study has suggested the attributes that must be considered towards planning a MSCs transplantation experiment, the dependence on data from publicly available studies has inadvertently led to undesirable inadequacies while building the decision tree models. Particularly the prevalence of missing data has forced us to replace the values of some attributes with imputed data. This “artificially generated” data could have contributed to the results being skewed. As the number of data sets were 78, removing all the instances having missing data in any of the attributes would have significantly reduced the sample size and prohibited the application of machine learning techniques. As mentioned earlier, although AST and ALT levels were also available, we did not employ these for machine learning analysis, as these had large number of missing values. This lack of ample data also prohibited the generation of a single unified tree. For example, two separate immunological models were generated i.e. for TNF-α and IL-6. When decision tree was made with both the parameters in a single dataset, the rules generated were biologically non-interpretable and obscure. The limited sample size used in the present study also means that the results were skewed towards the animal that was maximally used in the studies: mice. The other animal models, pigs and rats, were used in a lesser number of studies and detailed hierarchical decision rules could not be generated for these.
Conclusion
From the current data availability, it’s a herculean task to draw an accurate conclusion regarding the optimal conditions for administering MSCs. Our analysis depicts that a threshold level of inflammation is required for MSC driven immunomodulation which is further dependent upon source, dose and timing of MSCs infusion. It is difficult to specifically have a standard protocol as the studies involve multiple models and varied experimental approaches that makes interpretation a difficult task.
Five major points can be highlighted that guide the therapeutic methodologies involving MSCs in accordance to our analysis:
First, survival rate in a diseased model is dependent upon source of tissue from where MSCs are procured. This study suggests that AD-MSCs have better therapeutic efficacy for sepsis.
Second, an inflammatory milieu primes MSCs to perform immunoregulation. High basal cytokines levels: IL-6 and TNF-α in septic animal contributes to high survival rates, particularly in mice.
Third, an important factor is the amount of dose of MSCs. High dose can be detrimental as seen in case of mice. When MSCs were administered less than 0.8 million per mice, it remarkably improved the survival rate.
Fourth, type of animal model apparently influences the survival rate. Higher the hierarchy of the animal model better the survival rate in septic preclinical models.
Lastly, the time of infusion of MSCs markedly alters the survival rate. Through machine learning data it has been deduced that less the time of infusion of MSCs after septic induction higher is the survival rate.
The decision tree-based machine learning method has provided us with options to utilize MSCs in a more efficient manner for modifying real-time treatment options for many diseased states especially sepsis.
Supplementary Information
Abbreviations
- AD-MSCs
Adipose tissue derived mesenchymal stem cells
- ALT
Alanine transaminase
- AST
Aspartate aminotransferase
- BM-MSCs
Bone marrow derived mesenchymal stem cells
- IL-6
Interleukin 6
- KO
Knockout
- LPS
Lipopolysaccharide
- MSCs
Mesenchymal Stromal/Stem Cells
- mL
Mililitre
- pg
Picogram
- KO
Knockout
- LPS
Lipopolysaccharide
- TNFα
Tumour necrosis factor alpha
- UC-MSCs
Umbilical cord derived mesenchymal stem cells
- WEKA
Waikato Environment for Knowledge Analysis
Author contributions
AR, AA- Contributed to conception, design and drafting of manuscript, thorough evaluation of various drafts. DG & HJ have contributed to accumulation of the data, the drafting of the manuscript, applying machine learning algorithms, figures editing and its revisions post various evaluations, DM has contributed to draft revisions, data check, generation of tables. NT has contributed to manuscript by proof reading and intellectual comments due to expertise in the field of microbiology.
Funding
Nil.
Availability of data and materials
Anything additionally required would be available on request. No AI based generative text/figures/images/values were used in the study. All the preprocessing, filtering, and machine learning analysis was conducted using Waikato Environment for Knowledge Analysis (WEKA) 3.8.6 machine learning toolkit along with R scripts.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent to publication of the present manuscript.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Diksha Gakhar and Himanshu Joshi equal contribution to this work.
Contributor Information
Amit Arora, Email: arora.amit@pgimer.edu.in.
Aruna Rakha, Email: arunarakha7@gmail.com.
References
- 1.Ota K. Fuel cells: past, present and future. IEEJ Trans Fundam Mater. 2008. 10.1541/ieejfms.128.329. 10.1541/ieejfms.128.329 [DOI] [Google Scholar]
- 2.Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019. 10.3389/fimmu.2019.01191. 10.3389/fimmu.2019.01191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mastrolia I, Foppiani EM, Murgia A, Candini O, Samarelli AV, Grisendi G, et al. Challenges in clinical development of mesenchymal stromal/stem cells: concise review. Stem Cells Transl Med. 2019. 10.1002/sctm.19-0044. 10.1002/sctm.19-0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombardo E, van der Poll T, DelaRosa O, Dalemans W. Mesenchymal stem cells as a therapeutic tool to treat sepsis. World J Stem Cells. 2015. 10.4252/wjsc.v7.i2.368. 10.4252/wjsc.v7.i2.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent JL, van der Poll T, Marshall JC. The end of ”one size fits all” sepsis therapies: toward an individualized approach. Biomedicines. 2022. 10.3390/biomedicines10092260. 10.3390/biomedicines10092260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun XY, Ding XF, Liang HY, Zhang XJ, Liu SH, Duan XG, Sun TW. Efficacy of mesenchymal stem cell therapy for sepsis: a meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:1. 10.1186/s13287-020-01730-7. 10.1186/s13287-020-01730-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Wang T, Li R, Xue F, Zeng G, Zhang J, et al. Dose-specific efficacy of adipose-derived mesenchymal stem cells in septic mice. Stem Cell Res Ther. 2023. 10.1186/s13287-023-03253-3. 10.1186/s13287-023-03253-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbadawi M, Gaisford S, Basit AW. Advanced machine-learning techniques in drug discovery. Drug Discov Today. 2021. 10.1016/j.drudis.2020.12.003. 10.1016/j.drudis.2020.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu-Jones B, Finlayson SG, Chivers C, Chen I, McDermott M, Kandola J, et al. Trends and focus of machine learning applications for health research. JAMA Netw Open. 2019. 10.1001/jamanetworkopen.2019.14051. 10.1001/jamanetworkopen.2019.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ching T, Himmelstein DS, Beaulieu-Jones BK, Kalinin AA, Do BT, Way GP, et al. Opportunities and obstacles for deep learning in biology and medicine. J R Soc Interface. 2018. 10.1098/rsif.2017.0387. 10.1098/rsif.2017.0387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehrabani-Zeinabad K, Doostfatemeh M, Ayatollahi SMT. An efficient and effective model to handle missing data in classification. BioMed Res Int. 2020. 10.1155/2020/8810143. 10.1155/2020/8810143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005. 10.1182/blood-2005-04-1496. 10.1182/blood-2005-04-1496 [DOI] [PubMed] [Google Scholar]
- 13.Sudres M, Norol F, Trenado A, Grégoire S, Charlotte F, Levacher B, Lataillade JJ, Bourin P, Holy X, Vernant JP, Klatzmann D. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006. 10.4049/jimmunol.176.12.7761. 10.4049/jimmunol.176.12.7761 [DOI] [PubMed] [Google Scholar]
- 14.Cóndor JM, Rodrigues CE, de Sousa MR, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha ID, Andrade L. Treatment with human Wharton’s jelly-derived mesenchymal stem cells attenuates sepsis-induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Transl Med. 2016. 10.5966/sctm.2015-0138. 10.5966/sctm.2015-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pedrazza L, Lunardelli A, Luft C, Cruz CU, De Mesquita FC, Bitencourt S, et al. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflam Res. 2014. 10.1007/s00011-014-0745-1. 10.1007/s00011-014-0745-1 [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang L, et al. The toll-like receptor 3 Ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem Cells. 2014. 10.1002/stem.1543. 10.1002/stem.1543 [DOI] [PubMed] [Google Scholar]
- 17.Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. 2020. 10.1016/j.tips.2020.06.009. 10.1016/j.tips.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasiah PK, Jha KA, Gentry J, Del Mar NA, Townsend T, Torgbe KE, et al. A long-term safety and efficacy report on intravitreal delivery of adipose stem cells and secretome on visual deficits after traumatic brain injury. Transl Vis Sci Technol. 2022. 10.1167/tvst.11.10.1. 10.1167/tvst.11.10.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Peng D, Li J, Zhang L, Chen J, Wang L, et al. A comparative study of different doses of bone marrow-derived mesenchymal stem cells improve post-stroke neurological outcomes via intravenous transplantation. Brain Res. 2023. 10.1016/j.brainres.2022.148161. 10.1016/j.brainres.2022.148161 [DOI] [PubMed] [Google Scholar]
- 20.Pedrazza L, Lunardelli A, Luft C, Cruz CU, de Mesquita FC, Bitencourt S, et al. Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res. 2014. 10.1007/s00011-014-0745-1. 10.1007/s00011-014-0745-1 [DOI] [PubMed] [Google Scholar]
- 21.Zhao X, Liu D, Gong W, Zhao G, Liu L, Yang L, Hou Y. The toll-like receptor 3 ligand, poly (I: C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR-143. Stem cells. 2014. 10.1002/stem.1543. 10.1002/stem.1543 [DOI] [PubMed] [Google Scholar]
- 22.Ou HAO, Zhao S, Peng YUE, Xiao X, Wang Q, Liu H, et al. Comparison of bone marrow tissue- and adipose tissue-derived mesenchymal stem cells in the treatment of sepsis in a murine model of lipopolysaccharide-induced sepsis. Mol Med Rep. 2016. 10.3892/mmr.2016.5694. 10.3892/mmr.2016.5694 [DOI] [PubMed] [Google Scholar]
- 23.Liang H, Ding X, Yu Y, Zhang H, Wang L, Kan Q, et al. Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp Cell Res. 2019. 10.1016/j.yexcr.2019.06.010. 10.1016/j.yexcr.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 24.Hall SRR, Tsoyi K, Ith B, Padera RF, Lederer JA, Wang Z, et al. Mesenchymal stromal cells improve survival during sepsis in the absence of heme oxygenase-1: the importance of neutrophils. Stem Cells. 2013. 10.1002/stem.1270. 10.1002/stem.1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeedi P, Halabian R, Fooladi AAI. Antimicrobial effects of mesenchymal stem cells primed by modified LPS on bacterial clearance in sepsis. J Cell Physiol. 2019. 10.1002/jcp.27298. 10.1002/jcp.27298 [DOI] [PubMed] [Google Scholar]
- 26.Perlee D, van Vught LA, Scicluna BP, Maag A, Lutter R, Kemper EM, et al. Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single-blind, parallel group. Placebo Controlled Trial Stem Cells. 2018. 10.1002/stem.2891. 10.1002/stem.2891 [DOI] [PubMed] [Google Scholar]
- 27.Podestà MA, Remuzzi G, Casiraghi F. Mesenchymal stromal cell therapy in solid organ transplantation. Front Immunol. 2021. 10.3389/fimmu.2020.618243. 10.3389/fimmu.2020.618243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yilmaz R, Çukurova Z, Hergünsel O. The importance of timing in the application of mesenchymal stem cells in critically Ill patients with COVID-19 infection (retrospective study). Anesth Crit Care. 2022. 10.26502/acc.050. 10.26502/acc.050 [DOI] [Google Scholar]
- 29.Laroye C, Boufenzer A, Jolly L, Cunat L, Alauzet C, Merlin JL, et al. Bone marrow vs Wharton’s jelly mesenchymal stem cells in experimental sepsis: a comparative study. Stem Cell Res Ther. 2019. 10.1186/s13287-019-1295-9. 10.1186/s13287-019-1295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang M, Huang CC, Gajendrareddy P, Lu Y, Shirazi S, Ravindran S, et al. Extracellular vesicles from TNFα preconditioned MSCs: effects on immunomodulation and bone regeneration. Front Immunol. 2022. 10.3389/fimmu.2022.878194. 10.3389/fimmu.2022.878194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, et al. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018. 10.1002/stem.2730. 10.1002/stem.2730 [DOI] [PubMed] [Google Scholar]
- 32.Shin TH, Ahn JS, Oh SJ, Shin YY, Yang JW, Kang MJ, et al. Tnf-α priming elicits robust immunomodulatory potential of human tonsil-derived mesenchymal stem cells to alleviate murine colitis. Biomedicines. 2020. 10.3390/biomedicines8120561. 10.3390/biomedicines8120561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005. 10.1128/IAI.73.5.2751-2757.2005. 10.1128/IAI.73.5.2751-2757.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks J. Understanding the inflammatory cytokine response in pneumonia and sepsis. J Emerg Med. 2008. 10.1016/j.jemermed.2007.10.008.19022608 10.1016/j.jemermed.2007.10.008 [DOI] [Google Scholar]
- 35.Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007. 10.1186/cc5783. 10.1186/cc5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberholzer A, Souza SM, Tschoeke SK, Oberholzer C, Abouhamze A, Pribble JP, Moldawer LL. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock. 2005;23(6):488–93. [PubMed] [Google Scholar]
- 37.Marti L, Cervera C, Filella X, Marin JL, Almela M, Moreno A. Cytokine-release patterns in elderly patients with systemic inflammatory response syndrome. Gerontology. 2007. 10.1159/000101436. 10.1159/000101436 [DOI] [PubMed] [Google Scholar]
- 38.Yoshizawa KI, Naruto M, Ida N. Injection time of interleukin-6 determines fatal outcome in experimental endotoxin shock. J Interf Cytokine Res. 1996;16(12):995–1000. 10.1089/jir.1996.16.995. 10.1089/jir.1996.16.995 [DOI] [PubMed] [Google Scholar]
- 39.Mancuso G, Tomasello F, Migliardo M, Delfino D, Cochran J, Cook JA, et al. Beneficial effects of interleukin-6 in neonatal mouse models of group B streptococcal disease. Infect Immun. 1994. 10.1128/iai.62.11.4997-5002.1994. 10.1128/iai.62.11.4997-5002.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao S, Huang Y, Dai Z, Liao Y, Zhang J, Wang L, et al. Circular RNA mmu_circ_0001295 from hypoxia pretreated adipose-derived mesenchymal stem cells (ADSCs) exosomes improves outcomes and inhibits sepsis-induced renal injury in a mouse model of sepsis. Bioengineered. 2022. 10.1080/21655979.2022.2044720. 10.1080/21655979.2022.2044720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E 2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009. 10.1038/nm.1905. 10.1038/nm.1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato Y, Ochiai D, Abe Y, Masuda H, Fukutake M, Ikenoue S, et al. Prophylactic therapy with human amniotic fluid stem cells improved survival in a rat model of lipopolysaccharide-induced neonatal sepsis through immunomodulation via aggregates with peritoneal macrophages. Stem Cell Res Ther. 2020. 10.1186/s13287-020-01809-1. 10.1186/s13287-020-01809-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long X, Li X, Li T, Yan Q, Wen L, Yang X, et al. Umbilical cord mesenchymal stem cells enhance the therapeutic effect of imipenem by regulating myeloid-derived suppressor cells in septic mice. Ann Transl Med. 2021. 10.21037/atm-20-6371. 10.21037/atm-20-6371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu R, Pan P, Li C, Luo B, Ma H, Hao H, et al. Bone mesenchymal stromal cell-derived small extracellular vesicles inhibit inflammation and ameliorate sepsis via delivery of microRNA-21a-5p. Cytotherapy. 2023. 10.1016/j.jcyt.2023.02.002. 10.1016/j.jcyt.2023.02.002 [DOI] [PubMed] [Google Scholar]
- 45.Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG, Hong Q, et al. Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock. 2014. 10.1097/SHK.0000000000000080. 10.1097/SHK.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Liu D, Zhang XH, Yang LM, Xia Z, Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discov. 2022. 10.1038/s41420-021-00785-6. 10.1038/s41420-021-00785-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laroye C, Lemarié J, Boufenzer A, Labroca P, Cunat L, Alauzet C, et al. Clinical-grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs. Intensive Care Med Exp. 2018. 10.1186/s40635-018-0194-1. 10.1186/s40635-018-0194-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chao YH, Wu HP, Wu KH, Tsai YG, Peng CT, Lin KC, et al. An increase in CD3+CD4+CD25 + regulatory T cells after administration of umbilical cord-derived mesenchymal stem cells during sepsis. PLoS ONE. 2014. 10.1371/journal.pone.0110338. 10.1371/journal.pone.0110338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding XF, Liang HY, Sun JY, Liu SH, Kan QC, Wang LX, et al. Adipose-derived mesenchymal stem cells ameliorate the inflammatory reaction in CLP-induced septic acute lung injury rats via sTNFR1. J Cell Physiol. 2019. 10.1002/jcp.28329. 10.1002/jcp.28329 [DOI] [PubMed] [Google Scholar]
- 50.Jerkic M, Masterson C, Ormesher L, Gagnon S, Goyal S, Rabani R, et al. Overexpression of IL-10 enhances the efficacy of human umbilical-cord-derived mesenchymal stromal cells in E. coli pneumosepsis. J Clin Med. 2019. 10.3390/jcm8060847. 10.3390/jcm8060847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Darwish I, Monroy MF, Prockop DJ, Liles WC, Kain KC. Mesenchymal stromal (stem) cells suppress pro-inflammatory cytokine production but fail to improve survival in experimental staphylococcal toxic shock syndrome. BMC Immunol. 2014. 10.1186/1471-2172-15-1. 10.1186/1471-2172-15-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009. 10.1136/gut.2008.168534. 10.1136/gut.2008.168534 [DOI] [PubMed] [Google Scholar]
- 53.Varkouhi AK, He X, Teixeira Monteiro AP, Amatullah H, Tsoporis JN, Gupta S, et al. Immunophenotypic characterization and therapeutics effects of human bone marrow- and umbilical cord-derived mesenchymal stromal cells in an experimental model of sepsis. Exp Cell Res. 2021. 10.1016/j.yexcr.2021.112473. 10.1016/j.yexcr.2021.112473 [DOI] [PubMed] [Google Scholar]
- 54.Xu S, Zhou Z, Li H, Liu Z, Pan X, Wang F, et al. BMSCs ameliorate septic coagulopathy by suppressing inflammation in cecal ligation and puncture-induced sepsis. J Cell Sci. 2018. 10.1242/jcs.211151. 10.1242/jcs.211151 [DOI] [PubMed] [Google Scholar]
- 55.Liu W, Gao Y, Li H, Wang H, Ye M, Jiang G, et al. Intravenous transplantation of mesenchymal stromal cells has therapeutic effects in a sepsis mouse model through inhibition of septic natural killer cells. Int J Biochem Cell Biol. 2016. 10.1016/j.biocel.2016.08.013. 10.1016/j.biocel.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 56.Saeedi P, Halabian R, Fooladi AAI. Mesenchymal stem cells preconditioned by staphylococcal enterotoxin B enhance survival and bacterial clearance in murine sepsis model. Cytotherapy. 2019. 10.1016/j.jcyt.2018.11.002. 10.1016/j.jcyt.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 57.Deng H, Zhu L, Zhang Y, Zheng L, Hu S, Zhou W, et al. Differential lung protective capacity of exosomes derived from human adipose tissue, bone marrow, and umbilical cord mesenchymal stem cells in sepsis-induced acute lung injury. Oxid Med Cell Longev. 2022. 10.1155/2022/7837837. 10.1155/2022/7837837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lo CC, Leu S, Sung HC, Zhen YY, Cho CL, Chen A, et al. Impact of apoptotic adipose-derived mesenchymal stem cells on attenuating organ damage and reducing mortality in rat sepsis syndrome induced by cecal puncture and ligation. J Transl Med. 2012. 10.1186/1479-5876-10-244. 10.1186/1479-5876-10-244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mei SHJ, Haitsma JJ, Dos Santos CC, Deng Y, Lai PFH, Slutsky AS, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010. 10.1164/rccm.201001-0010OC. 10.1164/rccm.201001-0010OC [DOI] [PubMed] [Google Scholar]
- 60.Cóndor JM, Rodrigues CE, de Sousa MR, Canale D, Volpini RA, Shimizu MH, Camara NO, Noronha ID, Andrade L. Treatment with human Wharton’s jelly-derived mesenchymal stem cells attenuates sepsis-induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Transl Med. 2016;5(8):1048–57. 10.1152/ajpregu.00098.2018. 10.1152/ajpregu.00098.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007. 10.4049/jimmunol.179.3.1855. 10.4049/jimmunol.179.3.1855 [DOI] [PubMed] [Google Scholar]
- 62.Gonzalez H, Keane C, Masterson CH, Horie S, Elliman SJ, Higgins BD, et al. Umbilical cord-derived CD362+ mesenchymal stromal cells attenuate polymicrobial sepsis induced by caecal ligation and puncture. Int J Mol Sci. 2020. 10.3390/ijms21218270. 10.3390/ijms21218270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fazekas B, Alagesan S, Watson L, Ng O, Conroy CM, Català C, et al. Comparison of single and repeated dosing of anti-inflammatory human umbilical cord mesenchymal stromal cells in a mouse model of polymicrobial sepsis. Stem Cell Rev Rep. 2022. 10.1007/s12015-021-10323-7. 10.1007/s12015-021-10323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Y, Dou H, Li X, Zhao X, Li Y, Liu D, et al. Exosomal miR-146a contributes to the enhanced therapeutic efficacy of interleukin-1β-primed mesenchymal stem cells against sepsis. Stem Cells. 2017. 10.1002/stem.2564. 10.1002/stem.2564 [DOI] [PubMed] [Google Scholar]
- 65.Mei S, Wang S, Jin S, Zhao X, Shao Z, Zhang R, et al. Human adipose tissue-derived stromal cells attenuate the multiple organ injuries induced by sepsis and mechanical ventilation in mice. Inflammation. 2019. 10.1007/s10753-018-0905-5. 10.1007/s10753-018-0905-5 [DOI] [PubMed] [Google Scholar]
- 66.Li S, Wu H, Han D, Ma S, Fan W, Wang Y, et al. A novel mechanism of mesenchymal stromal cell-mediated protection against sepsis: restricting inflammasome activation in macrophages by increasing mitophagy and decreasing mitochondrial ROS. Oxid Med Cell Longev. 2018. 10.1155/2018/3537609. 10.1155/2018/3537609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Z, Song Y, Yuan P, Guo W, Hu X, Xing W, et al. Antibacterial fusion protein BPI21/LL-37 modification enhances the therapeutic efficacy of hUC-MSCs in sepsis. Mol Ther. 2020. 10.1016/j.ymthe.2020.05.014. 10.1016/j.ymthe.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo C, Luo F, Che L, Zhang H, Zhao L, Zhang W, et al. Mesenchymal stem cells protect against sepsis-associated acute kidney injury by inducing Gal-9/Tim-3 to remodel immune homeostasis. Ren Fail. 2023. 10.1080/0886022X.2023.2187229. 10.1080/0886022X.2023.2187229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu Y, Xu L, Collins JJP, Vadivel A, Cyr-Depauw C, Zhong S, et al. Human umbilical cord mesenchymal stromal cells improve survival and bacterial clearance in neonatal sepsis in rats. Stem Cells Dev. 2017. 10.1089/scd.2016.0329. 10.1089/scd.2016.0329 [DOI] [PubMed] [Google Scholar]
- 70.Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflam. 2012;9:1. 10.1186/1476-9255-9-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu C, Xue J, Xu B, Zhang A, Qin L, Liu J, et al. Exosomes derived from miR-146a-5p-enriched mesenchymal stem cells protect the cardiomyocytes and myocardial tissues in the polymicrobial sepsis through regulating MYBL1. Stem Cells Int. 2021. 10.1155/2021/1530445. 10.1155/2021/1530445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sung PH, Chiang HJ, Wallace CG, Yang CC, Chen YT, Chen KH, Chen CH, Shao PL, Chen YL, Chua S, Chai HT. Exendin-4-assisted adipose derived mesenchymal stem cell therapy protects renal function against co-existing acute kidney ischemia-reperfusion injury and severe sepsis syndrome in rat. Am J Transl Res. 2017;9(7):3167. [PMC free article] [PubMed] [Google Scholar]
- 73.Lee FY, Chen KH, Wallace CG, Sung PH, Sheu JJ, Chung SY, et al. Xenogeneic human umbilical cord-derived mesenchymal stem cells reduce mortality in rats with acute respiratory distress syndrome complicated by sepsis. Oncotarget. 2017. 10.18632/oncotarget.17320. 10.18632/oncotarget.17320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflam. 2014. 10.1186/1476-9255-11-1. 10.1186/1476-9255-11-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sung PH, Chiang HJ, Chen CH, Chen YL, Huang TH, Zhen YY, et al. Combined therapy with adipose-derived mesenchymal stem cells and ciprofloxacin against acute urogenital organ damage in rat sepsis syndrome induced by intrapelvic injection of cecal bacteria. Stem Cells Transl Med. 2016. 10.5966/sctm.2015-0116. 10.5966/sctm.2015-0116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng D, Zhou H, Wang H, Zhu Y, Wu Y, Li Q, et al. Mesenchymal stem cell-derived microvesicles improve intestinal barrier function by restoring mitochondrial dynamic balance in sepsis rats. Stem Cell Res Ther. 2021. 10.1186/s13287-021-02363-0. 10.1186/s13287-021-02363-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jerkic M, Gagnon S, Rabani R, Ward-Able T, Masterson C, Otulakowski G, et al. Human umbilical cord mesenchymal stromal cells attenuate systemic sepsis in part by enhancing peritoneal macrophage bacterial killing via heme Oxygenase-1 induction in rats. Anesthesiology. 2020. 10.1097/ALN.0000000000003018. 10.1097/ALN.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 78.Anderson P, Souza-Moreira L, Morell M, Caro M, O’Valle F, Gonzalez-Rey E, et al. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013. 10.1136/gutjnl-2012-302152. 10.1136/gutjnl-2012-302152 [DOI] [PubMed] [Google Scholar]
- 79.Deng H, Wu L, Liu M, Zhu L, Chen Y, Zhou H, et al. Bone marrow mesenchymal stem cell-derived exosomes attenuate LPS-induced ARDS by modulating macrophage polarization through inhibiting glycolysis in macrophages. Shock. 2020. 10.1097/SHK.0000000000001549. 10.1097/SHK.0000000000001549 [DOI] [PubMed] [Google Scholar]
- 80.Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, et al. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012. 10.1152/ajplung.00180.2011. 10.1152/ajplung.00180.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carlos Sepúlveda J, Tomé M, Eugenia Fernández M, Delgado M, Campisi J, Bernad A, et al. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cells. 2014. 10.1002/stem.1654. 10.1002/stem.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva AYO, Amorim ÉA, Barbosa-Silva MC, Lima MN, Oliveira HA, Granja MG, et al. Mesenchymal stromal cells protect the blood-brain barrier, reduce astrogliosis, and prevent cognitive and behavioral alterations in surviving septic mice. Crit Care Med. 2020. 10.1097/CCM.0000000000004219. 10.1097/CCM.0000000000004219 [DOI] [PubMed] [Google Scholar]
- 83.Park KS, Svennerholm K, Shelke GV, Bandeira E, Lässer C, Jang SC, et al. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019. 10.1186/s13287-019-1352-4. 10.1186/s13287-019-1352-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pedrazza L, Cubillos-Rojas M, De Mesquita FC, Luft C, Cunha AA, Rosa JL, et al. Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res Ther. 2017. 10.1186/s13287-017-0734-8. 10.1186/s13287-017-0734-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee W, et al. The therapeutic effect of human adult stem cells derived from adipose tissue in endotoxemic rat model. Int J Med Sci. 2013. 10.7150/ijms.5385. 10.7150/ijms.5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wu Y, Zhou J, Bi L, Huang M, Han Y, Zhang Q, et al. Effects of bone marrow mesenchymal stem cells on the cardiac function and immune system of mice with endotoxemia. Mol Med Rep. 2016. 10.3892/mmr.2016.5151. 10.3892/mmr.2016.5151 [DOI] [PubMed] [Google Scholar]
- 87.Eshghi F, Tahmasebi S, Alimohammadi M, Soudi S, Khaligh SG, Khosrojerdi A, et al. Study of immunomodulatory effects of mesenchymal stem cell-derived exosomes in a mouse model of LPS induced systemic inflammation. Life Sci. 2022. 10.1016/j.lfs.2022.120938. 10.1016/j.lfs.2022.120938 [DOI] [PubMed] [Google Scholar]
- 88.Asano K, Yoshimura S, Nakane A. Adipose tissue-derived mesenchymal stem cells attenuate staphylococcal enterotoxin A—induced toxic shock. Infect Immun. 2015. 10.1128/IAI.00730-15. 10.1128/IAI.00730-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li J, Jiang R, Hou Y, Lin A. Mesenchymal stem cells-derived exosomes prevent sepsis-induced myocardial injury by a CircRTN4/miR-497-5p/MG53 pathway. Biochem Biophys Res Commun. 2022. 10.1016/j.bbrc.2022.05.094. 10.1016/j.bbrc.2022.05.094 [DOI] [PubMed] [Google Scholar]
- 90.Bai X, Li J, Li L, Liu M, Liu Y, Cao M, et al. Extracellular vesicles from adipose tissue-derived stem cells affect Notch-miR148a-3p axis to regulate polarization of macrophages and alleviate sepsis in mice. Front Immunol. 2020. 10.3389/fimmu.2020.01391. 10.3389/fimmu.2020.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu F, Xie J, Zhang X, Wu Z, Zhang S, Xue M, et al. Overexpressing TGF-β1 in mesenchymal stem cells attenuates organ dysfunction during CLP-induced septic mice by reducing macrophage-driven inflammation. Stem Cell Res Ther. 2020. 10.1186/s13287-020-01894-2. 10.1186/s13287-020-01894-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaki OS, Safar MM, Ain-Shoka AA, Rashed LA. Bone marrow mesenchymal stem cells combat lipopolysaccharide-induced sepsis in rats via amendment of P38-MAPK signaling cascade. Inflammation. 2018. 10.1007/s10753-017-0710-6. 10.1007/s10753-017-0710-6 [DOI] [PubMed] [Google Scholar]
- 93.Dinc G, Eren E, Kontas O, Doganay M. The efficacy of mesenchymal stem cell therapy in experimental sepsis induced by carbapenem-resistant K. pneumoniae in neutropenic mice model. Eur J Clin Microbiol Infect Dis. 2020;39:1739–44. 10.1007/s10096-020-03910-y. 10.1007/s10096-020-03910-y [DOI] [PubMed] [Google Scholar]
- 94.Horak J, Nalos L, Martinkova V, Tegl V, Vistejnova L, Kuncova J, et al. Evaluation of mesenchymal stem cell therapy for sepsis: a randomized controlled porcine study. Front Immunol. 2020. 10.3389/fimmu.2020.00126. 10.3389/fimmu.2020.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Perlee D, de Vos AF, Scicluna BP, Mancheño P, de la Rosa O, Dalemans W, et al. Human adipose-derived mesenchymal stem cells modify lung immunity and improve antibacterial defense in pneumosepsis caused by Klebsiella pneumoniae. Stem Cells Transl Med. 2019. 10.1002/sctm.18-0260. 10.1002/sctm.18-0260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pan Y, Li J, Wang J, Jiang Q, Yang J, Dou H, et al. Ferroptotic MSCs protect mice against sepsis via promoting macrophage efferocytosis. Cell Death Dis. 2022. 10.1038/s41419-022-05264-z. 10.1038/s41419-022-05264-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anything additionally required would be available on request. No AI based generative text/figures/images/values were used in the study. All the preprocessing, filtering, and machine learning analysis was conducted using Waikato Environment for Knowledge Analysis (WEKA) 3.8.6 machine learning toolkit along with R scripts.