Abstract
Background
Gestational age (GEAA) estimated by newborn DNA methylation (GAmAge) is associated with maternal prenatal exposures and immediate birth outcomes. However, the association of GAmAge with long-term overweight or obesity (OWO) trajectories is yet to be determined.
Methods
GAmAge was calculated for 831 children from a US predominantly urban, low-income, multi-ethnic birth cohort based on cord blood DNA methylation profile using Illumina EPIC array. Repeated anthropometric measurements aligned with pediatric primary care schedule allowed us to calculate body-mass-index percentiles (BMIPCT) at specific age and to define long-term weight trajectories from birth to 18 years.
Results
GAmAge was associated with BMIPCT trajectories, defined by 4 groups: stable (consistent OWO: “early OWO”; constant normal weight: “NW”) or non-stable (OWO by year 1 of follow-up: “late OWO”; OWO by year 6 of follow-up: “NW to very late OWO”). GAmAge differentiated between the group with consistently normal BMIPCT pattern and the non-stable groups with late and very late OWO development. Such differentiation was observed in the age periods of birth to 1year, 3years, 6years, 10years, and 14years (p < 0.05 for all). The findings persisted after adjusting for GEAA, maternal smoking, delivery method, and child’s sex in multivariate models. Birth weight was a mediator for the GAmAge effect on OWO status for specific groups at multiple age periods.
Conclusions
GAmAge is associated with BMIPCT trajectories from birth to age 18 years, independent of GEAA and birth weight. If further confirmed, GAmAge may serve as an early biomarker for predicting BMI trajectory to inform early risk assessment and prevention of OWO.
Trial registration
ClinicalTrials.gov (NCT03228875).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-024-03568-9.
Keywords: Epigenetic clock, Pediatrics, Overweight or obesity, BMI percentiles
Background
Childhood obesity has profound implications for immediate and long-term health outcomes. These include elevated blood pressure, type 2 diabetes, cardiovascular diseases, and asthma [1]. Previous studies indicate that early onset of obesity, as early as the age of 2 years (y), can significantly predict obesity 30 years later, particularly among children with severe obesity [2]. Among perinatal risk factors associated with later life obesity and cardiometabolic complications are method of delivery (cesarean section) [3], maternal weight gain during pregnancy [4], maternal gestational diabetes [5], maternal smoking during pregnancy [6], low birth weight for gestational age [7], and premature birth [8]. These findings underscore the importance of early intervention and continuous monitoring of children’s weight status to mitigate the long-term risks of obesity-related morbidity in adulthood. An early marker that can predict the trajectory of overweight or obesity (OWO) is central to initiating early intervention. Moreover, a marker based on a delivery week or fetal development may have a good predictive ability for later-life complications, including OWO.
In adults, age prediction by DNA methylation (DNAm), known as epigenetic clock or methylation age (mAge), was found to be associated with body mass index (BMI) [9], abdominal adipose tissues [10], and liver fat [10]. The residuals of mAge regressed on age (age acceleration), or the differences between methylation and chronological ages (“age diff” or Δage), is considered a strong predictor of all-cause mortality [11, 12], cardiovascular mortality [13], and the incidence of cardiovascular disease [14]. In newborns and children, epigenetic clocks can estimate the gestational and chronological age using DNA extracted from different tissues (saliva, peripheral, and cord blood) [15]. Cord-blood age acceleration (the residuals of gestational age (GEAA) methylation age (GAmAge) regressed on GEAA) was associated with maternal exposures such as vitamin D supplementation during pregnancy in a sample of White, African American, and Hispanic mothers [16], pre-pregnancy BMI and smoking in a sample of British mothers [17, 18], and gestational diabetes in Chinese mothers [19]. In European decedents’ newborns, GAmAge and cord-blood age acceleration were associated with higher cord-blood vitamin B12 levels [20], delivery method (c-section) [15, 17], and immediate birth outcomes of weight, length, and head circumference [17, 21]. Data on the long-term associations of GAmAge and age acceleration measured in cord blood and child’s phenotypes are sparse; Cord blood age acceleration was directly associated with a child’s weight and height up to 6 months and inversely associated with a child’s weight at 10y of age [21].
In this analysis, we used data from a multi-ethnic mostly Black and Hispanic population birth cohort with extended time points for anthropometric measurements from birth to 21y. We examined GAmAge as a predictor of childhood obesity and longitudinal trajectories from birth up to age 18y, as reflected by distinct overweight or obesity (OWO) groups. We also examined whether birth weight can mediate these long-term associations. All of the above associations were examined beyond the impact of GEAA to elucidate further the role of DNAm-based biological age as an indicator of health status.
Methods
This study included 831 mother–newborns pairs from the Boston Birth Cohort (BBC; registered in ClinialTrial.gov NCT03228875), a US predominantly urban, low-income, Black and Hispanic population. The BBC was initiated in 1998 with rolling enrollment at the Boston Medical Center in Boston, MA, as detailed elsewhere [22, 23]. In brief, mothers who delivered a singleton live birth at the Boston Medical Center were invited to participate 24–72 h after a vaginal delivery. The BBC is enriched by preterm (< 37 weeks of gestation) and low birth weight (< 2500 g) births by design of over-sampling preterm birth at enrollment. Pregnancies resulting from in vitro fertilization, multiple gestations (e.g., twins, triplets), fetal chromosomal abnormalities, major birth defects, or preterm birth due to maternal trauma were excluded. After mothers provided written informed consent, research assistants (RAs) administered a standardized questionnaire interview on maternal sociodemographic characteristics, lifestyle, including smoking and alcohol consumption, diet, and reproductive and medical history. Maternal and newborn clinical information, including birth outcomes, was abstracted from the medical records. The study protocol has received initial and annual approval from the Institutional Review Boards (IRBs) of Boston Medical Center and the Johns Hopkins Bloomberg School of Public Health.
Main covariates
Mother–child characteristics
For background characteristics and adjustment of the statistical models, we used the following data: maternal age at delivery, parity (nulliparous or multiparous), maternal education (below college or college and higher), maternal self-reported race (Black/African American, White, and Hispanic), maternal pre-pregnancy BMI, maternal weight gain during pregnancy, maternal diabetes (non, gestational diabetes or pre-existing diabetes mellitus), delivery method, child’s sex (female or male), maternal smoking, birth weight (in kg or binary with below 2500g defined as a low birth weight (LBW) [24]), and GEAA (days or weeks). We further characterized groups according to delivery week: extremely preterm (< 28 weeks) and very preterm (28 to 32 weeks) grouped into extremely to very preterm group, moderate to late preterm (week 32 to 37; World Health Organization (WHO) definitions), term (37 to 41 weeks), and late to post-term based on late-term (41 to 42 weeks) and post-term (> 42 weeks; American College of Obstetricians and Gynecologists definitions; [25]). The estimation of GEAA was detailed before [22] and was performed using an established algorithm based on both the last menstrual period and the result of early ultrasound (< 20 weeks’ gestation). Fetal growth groups– small for gestational age (SGA), appropriate for gestational age (AGA), and large for gestational age (LGA) were determined by the birth weight and gestational age as described before [26].
Long-term obesity groups and BMIPCT
Out of the data available for the BBC, we selected 3029 children with height and weight measurements with sufficient follow-up data. We calculated BMIPCT using WHO (birth to 2y; [27]) and Centers for Disease Control (CDC) and Prevention growth charts (age 2 y and up [28];) for these children. As child well-care visits had different frequencies, the BMIPCT data was divided into the following 32 time windows: monthly measurements in the first year, quarterly measurements in the second, and yearly measurements from month 36 (3rd year) to the 216th month. Out of the 3029 children with BMIPCT, 940 had available DNAm measured.
Obesity-related age periods
In accordance with our previous work [29], we primarily focused on seven age periods representing different developmental phases: birth to 1y old (Additional file 1: Figure S1a), birth to 2y, birth to 3y, birth to 6y (Additional file 1:Fig. S1b), birth to 10y, birth to 14y and birth to 18y. Those age periods were previously identified as obesity-related critical periods, with ages 1y and 10y associated with changes in total body fat content [30], OWO at the age of 3y is a risk factor for becoming OWO adolescents (ages 15-18y), and BMI increase at ages 2-6y may be an early marker for OWO at 14y [31].
Based on the first age period in this analysis (birth to 1) and the availability of children with DNAm analysis, we selected children with at least one BMIPCT measurement at each age period. We refined the similar sample size at each period, thus resulting in a sample size of 831 for each age period allowing us to follow the same sample of children across multiple age periods for their observed or discovered BMIPCT trajectory.
BMIPCT missing data and OWO groups
Imputation of BMIPCT missing data was detailed before [32] and in Additional file 1: Methods S1 [32, 33]. The OWO groups were constructed separately for each period, as follows: first, we applied k-means clustering with k = 2. Next, we used Principal Component Analysis (PCA) to find the 1st and 2nd principal components. Since the first principal component primarily determines the k-means clustering, we divided the two groups above into four using the 2nd principal component, as previously demonstrated [34]. The groups resulting from this procedure represent four distinctive OWO trajectories, with two consistent-weight groups and two non-consistent weight-increase groups named retrospectively after examining the trajectories, as we published before in an Epigenome-Wide Association Study of long-term obesity trajectories [29]: 1. Early OWO: children with early onset OWO who demonstrated a consistent high BMIPCT from birth to the end of each age period; 2. Late OWO: late onset OWO children that were NW at birth but experienced a rapid weight increase in the first months of life to become OWO by year one; 3. NW to very late OWO: children distinguished from the late OWO by maintaining NW at early ages but becoming OWO by year six; 4. NW children consistently kept NW from birth to the end of each age period.
DNA methylation profiling and calculation of GAmAge
The blood draw procedure and quality control (QC) steps were detailed before [29, 35] for the same population in the current study. In summary, the labor and delivery service’s trained nursing staff obtained cord blood after delivery. Genome-wide DNA profiling from 963 samples (plus 21 replicates) was performed using the MethylationEPIC BeadChip (850K) [36]. Sample-level QC: We excluded 23 samples: 7 sex mixed-up samples, 2 samples with call rate < 98% methylation sites, 12 samples with mean log2 intensity < 10, and 2 samples with logistic error. Probe-level QC: We performed the single-sample Noob (ssNoob) methods for background and dye bias correction [37]. For > 865,000 CpG sites, we extracted beta values. For the current study, we primarily employed a GAmAge published by Haftorn et al. [38]. This GAmAge includes 176 CpGs (no CpGs missing in our data). No probes were removed to calculate GAmAge, per the above publication by Haftorn et al., which presented a GAmAge prediction model based on the EPIC array. GAmAge, measured in days, was calculated for 831 children with available DNAm data from cord blood samples using the “methylclock” R package [39]. We also included two other methylation clocks, based on 27K and 450K methylation arrays: Knight (GEAA prediction based on 148 CpGs, six missing in our data) [40] and Bohlin (96 CpGs, eight missing) [41]. These clocks evaluate GEAA in weeks. In our current analysis, we primarily used the Haftorn methylation clock as the main GAmAge clock and referred to it as “GAmAge”. When results for other methylation clocks are presented, we refer to them as “Knight” or “Bohlin” clocks. As the three clocks estimate GEAA in different type of measurement (i.e., days or weeks), we transformed GEAA originally recorded in weeks to a measurement of days for direct comparison and comparable effect size when analyzing the Haftorn GAmAge models. A plot showing overlapping CpGs between the three clocks is presented in Additional file 1: Figure S2. The CpGs, coefficients, gene names, position, and chromosome data for each clock are presented in Additional file 2: Table S1-Table S3. Further methodological explanation of the employed methylation clocks is presented in Additional file 1: Methods S2 [38, 40, 41]
Statistical analysis
The primary aim of this study is to examine the association between GAmAge and BMIPCT trajectories across several age periods during childhood. Summary statistics were performed to compare newborns’ demographic and clinical characteristics across OWO groups using the chi-square test or the Fisher's exact test for categorical variables and ANOVA for continuous variables. ANOVA post hoc correction for multiple comparisons was performed using Bonferroni correction. Pearson correlation was used to examine the correlation between continuous variables and 95% confidence interval (CI) presented to compare differences between the different correlation coefficients. Multinomial regression was used to associate OWO groups with GAmAge, with adjustment for covariates that may affect birth weight and week and were associated with OWO groups, including gestational age, child’s sex, maternal smoking, and delivery method based on the existing literature and findings in the BBC [42, 43]. Linear regression models were used to examine the association with continuous dependent outcomes. GAmAge acceleration was calculated by regressing out GEAA from GAmAge in a linear regression. We stratified the association between GAmAge or GEAA and birth weight by four groups of birth week: extremely preterm to very preterm (extremely preterm and very preterm grouped due to a small sample size), moderate to late preterm, term, late to post-term (grouped late-term and post-term). Mediation analysis using the “mediation” R package [44] was performed to examine the mediatory role of birth weight in the association between GAmAge and OWO groups. Since OWO trajectory is a four-factor variable, we used logistic regression to perform the mediation analysis with the NW as the reference group and performed 3 comparisons for the mediation (reference group vs. early OWO, late OWO or NW to very late) per age period. All statistical analyses were performed using R (version 4.1; R Foundation for Statistical Computing).
Results
Population characteristics
Maternal and child characteristics across OWO birth to 1y groups were presented in Table 1. BMIPCT at the end of each age period is presented in Additional file 1: Table S4). Significant differences were observed in the children’s sex (p = 0.031), with the smallest relative number of girls in the NW to very late OWO group and maternal smoking (p = 0.003), with most mothers reporting ever smoking in the NW to very late OWO group. The early OWO group had the highest birth weight (p = 1.2e-10 vs. late OWO, p = 1.5e-4 vs. NW). The NW to very late OWO group had the lowest birth weight compared with the early OWO (p = 1.4e-38) and NW (p = 4.03e-19) and were born in an earlier week compared with the other three groups (p = 1.6e-14 vs. early OWO, p = 8.1e-07 vs. late OWO, p = 9.1e-12 vs. NW). The NW to very late OWO had the highest percentage of children born preterm and SGA.
Table 1.
Prenatal and perinatal characteristics across subgroups of child BMI longitudinal trajectories from birth to 1ya
|
Total (N = 831) |
Early OWO (N = 229) |
Late OWO (N = 216) |
NW to very late OWO (N = 187) |
NW (N = 199) |
P-value2 | |
|---|---|---|---|---|---|---|
| GAmAge (days) | ||||||
| Mean (SD) | 275 (12.5) | 279 (8.08) | 275 (12.2) | 269 (15.8) | 278 (11.3) | < 0.001 |
| Maternal age at delivery (y) | ||||||
| Mean (SD) | 28.4 (6.54) | 28.4 (6.71) | 29.3 (6.26) | 27.8 (6.56) | 28.0 (6.56) | 0.086 |
| Maternal pre-pregnancy BMI (kg/m2) | ||||||
| Mean (SD) | 26.9 (6.41) | 27.4 (6.61) | 27.2 (6.89) | 26.6 (5.9) | 26.2 (6.07) | 0.208 |
| Gestational age at delivery (weeks) | ||||||
| Mean (SD) | 38.6 (2.5) | 39.2 (1.67) | 38.6 (2.46) | 37.3 (3.14) | 39.1 (2.21) | < 0.001 |
| Term groups (n (%) | ||||||
| Extremely to very preterm | 24 (2.9%) | 0 (0%) | 5 (2.3%) | 17 (9.1%) | 2 (1.0%) | < 0.001 |
| Moderate to late preterm | 123 (14.8%) | 24 (10.5%) | 34 (15.7%) | 46 (24.6%) | 19 (9.5%) | |
| Term | 612 (73.6%) | 178 (77.7%) | 158 (73.1%) | 119 (63.6%) | 157 (78.9%) | |
| Late to post-term | 72 (8.7%) | 27 (11.8%) | 19 (8.8%) | 5 (2.7%) | 21 (10.6%) | |
| Fetal growth groups (n (%)) | ||||||
| SGA | 87 (10.5%) | 12 (5.2%) | 20 (9.3%) | 36 (19.3%) | 19 (9.5%) | < 0.001 |
| AGA | 661 (79.5%) | 170 (74.2%) | 180 (83.3%) | 150 (80.2%) | 161 (80.9%) | |
| LGA | 82 (10.0%) | 47 (20.5%) | 16 (7.4%) | 1 (0.5%) | 19 (9.5%) | |
| Parity (n (%)) | ||||||
| Nulliparous | 374 (45.0%) | 89 (38.9%) | 100 (46.3%) | 97 (51.9%) | 88(44.2%) | 0.064 |
| Multiparous | 457 (55.0%) | 140 (61.1%) | 116 (53.7%) | 90 (48.1%) | 111 (55.8%) | |
| Maternal race (n (%)) | ||||||
| Black/African American | 602 (72.4%) | 164 (71.6%) | 158 (73.2%) | 137 (73.3%) | 143 (71.9%) | 0.812 |
| White | 44 (5.3%) | 11 (4.8%) | 15 (6.9%) | 10 (5.3%) | 8 (4.0%) | |
| Hispanic | 185 (22.3%) | 54 (23.6%) | 43 (19.9%) | 40 (21.4%) | 48 (24.1%) | |
| Maternal diabetes (n (%))b | ||||||
| No | 763 (92.5%) | 214 (94.3%) | 198 (92.6%) | 174 (93.6%) | 177 (89.4%) | 0.194 |
| Gestational diabetes | 35 (4.2%) | 11 (4.8%) | 8 (3.7%) | 6 (3.2%) | 10 (5.1%) | |
| Pregestational diabetes | 27 (3.3%) | 2 (0.9%) | 8 (3.7%) | 6 (3.2%) | 11 (5.5%) | |
| Maternal education (n (%)) | ||||||
| Below college | 551 (66.3%) | 156 (68.1%) | 144 (66.7%) | 124 (66.3%) | 127 (63.8%) | 0.825 |
| College and higher | 280 (33.7) | 73 (31.9%) | 72 (33.3%) | 63 (33.7%) | 72 (36.2%) | |
| Maternal smoking (n (%)) | 0.003 | |||||
| Never smoked | 619 (74.5%) | 178 (77.7%) | 155 (71.8%) | 124 (66.3%) | 162 (81.4%) | |
| Ever smoked | 212 (25.5%) | 51 (22.3%) | 61 (28.2%) | 63 (33.7%) | 37 (18.6%) | |
| Baby’s sex (n (%)) | ||||||
| Female | 396 (47.7%) | 112 (48.9%) | 114 (52.8%) | 72 (38.5%) | 98 (49.2%) | 0.031 |
| Male | 435 (52.3%) | 117 (51.1%) | 102 (47.2%) | 115 (61.5%) | 101 (50.8%) | |
| Child’s birth weight (g) | ||||||
| Mean (SD) | 3120 (667) | 3460 (552) | 3080 (601) | 2650 (671) | 3220 (580) | < 0.001 |
aBMI trajectory is defined using longitudinal BMI percentile data from birth to 12 months of age. Early OWO: children with consistently high BMIPCT; Late OWO: children with BMIPCT increased to OWO by the end of the first year; NW to very late OWO: children with NW in early life that was increased to OWO by the 6th year; NW: children with consistently normal BMIPCT
2Tested using ANOVA or chi-square or the Fisher's exact tests. AGA Appropriate for gestational age, BMI Body mass index, GAmAge Gestational methylation age, LGA Large for gestational age, NW Normal weight, OWO Overweight or obesity, SGA Small for gestational age, y Years
bData available for N = 825
GAmAge associations with long-term obesity
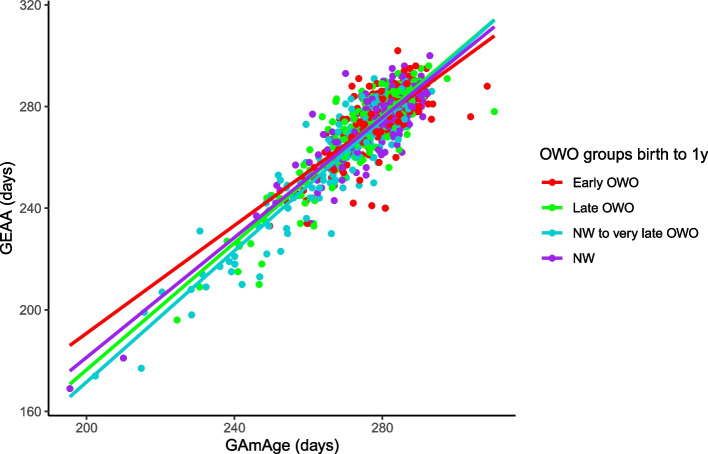
GAmAge (275.5 ± 12.5 days) and GEAA (270.0 ± 17.5 days) were strongly correlated (r = 0.89, 95% CI [0.87, 0.90], p = 2.8e-279). Stratifying by the OWO groups at each age period examined, we observed the strongest correlation of GAmAge and GEAA among NW to very late OWO children, compared with the other groups, across multiple age periods (Fig. 1; Additional file 1: Figure S3). For example, in the age period of birth to 1y, the following correlations were observed: early OWO vs. late OWO vs. NW to very late OWO vs. NW: 0.734 (95% CI [0.67, 0.79]) vs. 0.886 (95% CI [0.85, 0.91]) vs. 0.926 (95% CI [0.90, 0.94]) vs. 0.858 (95% CI [0.82, 0.89]), respectively. Knight (38.8 ± 2.3weeks) and Bohlin (39.8 ± 1.6 weeks) clocks were also strongly correlated with GEAA (38.6 ± 2.5) (r = 0.78 95% CI [0.75, 0.80] and r = 0.86, 95% CI [0.84, 0.88], Knight and Bohlin, respectively; Additional file 1: Figure S4 presents these correlations across OWO groups for the age period of birth to 1y).
Fig. 1.
GAmAge and GEAA. The correlation between GAmAge and GEAA across OWO group at age period birth to 1y
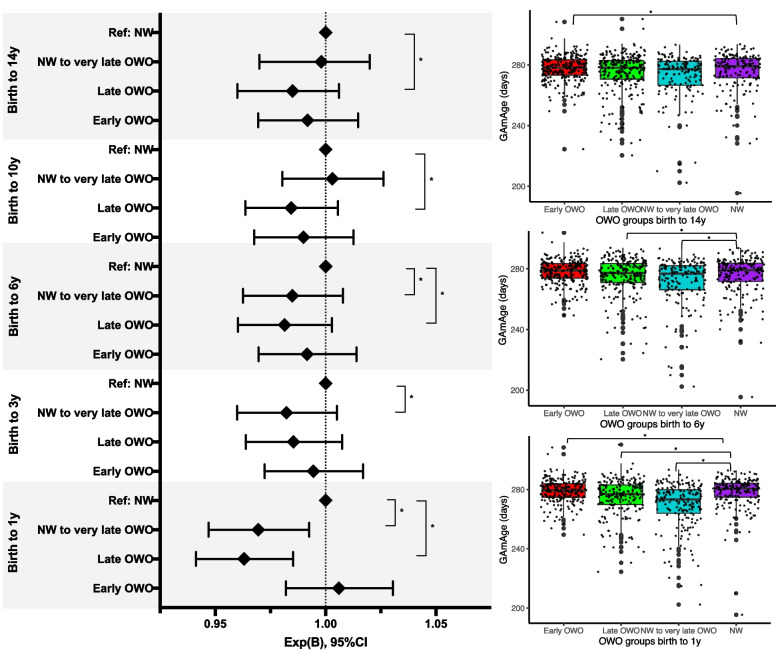
Next, we examined whether GAmAge could predict long-term BMIPCT patterns represented by OWO groups. The OWO groups differed in GAmAge in the following age periods (birth to 1y: p = 5.9e-16; birth to 2y: 0.028; birth to 3y: p = 0.019; birth to 6y: p = 8.6e-03; birth to 10y: p = 6.2e-03; birth to 14y: p = 0.022; birth to 18y: p = 0.048). Post-hoc correction for multiple comparisons showed that significant differences between early age periods (birth to 1y, 2y, 3y, and 6y) were mostly observed between the early OWO and the late OWO and NW to very late OWO (Additional file 1: Table S5). For later age periods (birth to 10y and birth to 14y), the differences were mostly between the early OWO and NW to very late OWO (p = 1.8e-04 and p = 0.001, respectively). After accounting for GEAA, child’s sex, delivery method, and maternal smoking, GAmAge was associated with OWO groups in multiple age periods: significantly higher GAmAge was observed in both consistent BMIPCT groups, early OWO and NW, compared with the non-consistent late and NW to very late OWO groups (Fig. 2;Table 2). Setting NW as the reference group, the relative odds ratio of 0.98 for a one-unit increase in GAmAge in the NW vs. the very late OWO group was consistent for the age periods birth to 1y, 3y, and 6y (p < 0.05 for all). A similar observation for the relative odds ratio of 0.96–0.98 for a one-unit increase in GAmAge in the NW vs. the late OWO was found for age periods birth to 1y, birth to 6y, birth to 10y, and birth to 14y (p < 0.05 for all).
Fig. 2.
Child’s GAmAge across OWO groups in selected age periods. Left: results of the multinomial regression with NW as the reference group. Models adjusted for GEAA in days, maternal smoking, delivery method, and child sex. Results presented for the GAmAge and gestational age predictors. N = 831. Right: box plots for GAmAge across OWO groups for age periods birth to 1y, birth to 6y, and birth to 14y. GAmAge, gestational methylation age; GEAA, gestational age; NW, normal weight; OWO, overweight or obese; y, years. *Denotes significant difference at p < 0.05 level
Table 2.
The association between GAmAge, GEAA, and OWO groups across different age periods
| Birth to 1y | Effect | GAmAge | GEAA | ||||
|---|---|---|---|---|---|---|---|
| z-score | p-value | OR [95%CI] | z-score | p-value | OR [95% CI] | ||
| Ref: NW | Early OWO | 0.53 | 0.60 | 1.00 [0.98,1.03] | 0.29 | 0.77 | 1.00 [0.98,1.03] |
| Late OWO | -2.63 | 0.008 | 0.96 [0.94,0.99] | 0.36 | 0.72 | 1.00 [0.98,1.03] | |
| NW to very late OWO | -2.03 | 0.04 | 0.98 [0.95,0.99] | -1.79 | 0.07 | 0.98 [0.95, 1.00] | |
| Ref: NW to very late OWO | Early OWO | 2.58 | 0.009 | 1.03 [1.01,1.06] | 2.10 | 0.035 | 1.03 [1.00,1.05] |
| Late OWO | -0.60 | 0.54 | 0.99 [0.97,1.01] | 2.33 | 0.019 | 1.03 [1.00,1.05] | |
| Ref: Late OWO | Early OWO | 3.23 | 0.001 | 1.04 [1.01,1.06] | -0.06 | 0.95 | 0.99 [0.97,1.02] |
| Birth to 2y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.002 | 0.99 | 0.99 [0.97,1.02] | 0.73 | 0.36 | 1.01 [0.98,1.03] |
| Late OWO | -1.25 | 0.21 | 0.98 [0.96,1.01] | 0.39 | 0.69 | 1.00 [0.98,1.03] | |
| NW to very late OWO | -1.35 | 0.17 | 0.98 [0.96,1.01] | -1.61 | 0.11 | 0.98 [0.96,1.00] | |
| Ref: NW to very late OWO | Early OWO | 1.35 | 0.18 | 1.02 [0.99,1.04] | 2.33 | 0.02 | 1.03 [1.00,1.05] |
| Late OWO | 0.14 | 0.89 | 1.00 [0.98,1.02] | 2.08 | 0.04 | 1.02 [1.00,1.05] | |
| Ref: Late OWO | Early OWO | 1.27 | 0.21 | 1.01 [0.99,1.04] | 0.36 | 0.72 | 1.00 [0.98,1.03] |
| Birth to 3y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.49 | 0.63 | 0.99 [0.97,1.02] | 1.48 | 0.14 | 1.02 [0.99.1.04] |
| Late OWO | -1.78 | 0.07 | 0.98 [0.96,1.00] | 0.84 | 0.40 | 1.01 [0.99,1.03] | |
| NW to very late OWO | -2.03 | 0.04 | 0.98 [0.95,0.99] | -0.62 | 0.53 | 0.99 [0.97,1.02] | |
| Ref: NW to very late OWO | Early OWO | 1.52 | 0.13 | 1.02 [0.99,1.04] | 2.06 | 0.04 | 1.02 [1.00,1.05] |
| Late OWO | 0.28 | 0.78 | 1.00 [0.98,1.03] | 1.48 | 0.14 | 1.02 [0.99,1.04] | |
| Ref: Late OWO | Early OWO | 1.30 | 0.19 | 1.01 [0.99,1.04] | 0.66 | 0.51 | 1.01 [0.99,1.03] |
| Birth to 6y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.74 | 0.46 | 0.99 [0.97,1.01] | 1.83 | 0.07 | 1.02 [0.99,1.04] |
| Late OWO | -2.43 | 0.02 | 0.97 [0.95,0.99] | 1.85 | 0.06 | 1.02 [0.99,1.04] | |
| NW to very late OWO | -2.04 | 0.04 | 0.98 [0.95,0.99] | 0.04 | 0.97 | 1.00 [0.98,1.02] | |
| Ref: NW to very late OWO | Early OWO | 1.29 | 0.20 | 1.01 [0.99,1.04] | 0.47 | 0.08 | 1.02 [0.99,1.05] |
| Late OWO | -0.31 | 0.75 | 0.9 [0.97,1.02] | 0.43 | 0.07 | 1.02 [0.99,1.04] | |
| Ref: Late OWO | Early OWO | 1.69 | 0.09 | 1.02 [0.99,1.04] | 0.006 | 0.99 | 1.00 [0.98,1.02] |
| Birth to 10y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.87 | 0.38 | 0.99 [0.97,1.01] | 2.07 | 0.04 | 1.02 [1.00,1.05] |
| Late OWO | -2.28 | 0.02 | 0.97 [0.95,0.99] | 1.87 | 0.06 | 1.02 [0.99,1.05] | |
| NW to very late OWO | -0.60 | 0.55 | 0.99 [0.97,1.02] | -0.52 | 0.60 | 0.99 [0.97,1.02] | |
| Ref: NW to very late OWO | Early OWO | -0.26 | 0.79 | 0.99 [0.97,1.02] | 2.58 | 0.009 | 1.03[1.01,1.06] |
| Late OWO | -1.67 | 0.09 | 0.98 [0.96,1.00] | 2.43 | 0.01 | 1.03 [1.01,1.05] | |
| Ref: Late OWO | Early OWO | 1.45 | 0.15 | 1.02 [0.99,1.04] | 0.26 | 0.79 | 1.00 [0.98,1.02] |
| Birth to 14y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.70 | 0.48 | 0.99 [0.97,1.01] | 1.72 | 0.08 | 1.02 [0.99,1.04] |
| Late OWO | -2.05 | 0.04 | 0.98 [0.96,0.99] | 1.35 | 0.17 | 1.01 [0.99,1.04] | |
| NW to very late OWO | -0.79 | 0.43 | 0.99 [0.97,1.01] | -0.32 | 0.75 | 0.99 [0.97,1.02] | |
| Ref: NW to very late OWO | Early OWO | 0.13 | 0.89 | 1.00 [0.98,1.02] | 2.05 | 0.04 | 1.02 [1.00,1.05] |
| Late OWO | -1.21 | 0.23 | 0.99 [0.96,1.01] | 1.71 | 0.09 | 1.02 [0.99,1.04] | |
| Ref: Late OWO | Early OWO | 1.43 | 0.15 | 1.02 [0.99,1.04] | 0.46 | 0.65 | 1.00 [0.98,1.03] |
| Birth to 18y | Effect | z-score | p-value | OR [95% CI] | z-score | p-value | OR [95% CI] |
| Ref: NW | Early OWO | -0.20 | 0.84 | 0.99 [0.97,1.02] | 1.37 | 0.17 | 1.02 [0.99,1.04] |
| Late OWO | -1.36 | 0.17 | 0.98 [0.96,1.01] | 0.64 | 0.52 | 1.01 [0.98,1.03] | |
| NW to very late OWO | 0.56 | 0.58 | 1.01 [0.98,1.03] | -0.79 | 0.43 | 0.99 [0.96,1.02] | |
| Ref: NW to very late OWO | Early OWO | -0.79 | 0.42 | 0.99 [0.97,1.01] | 2.21 | 0.03 | 1.03 [1.00,1.05] |
| Late OWO | -1.99 | 0.046 | 0.98 [0.96,0.99] | 1.52 | 0.13 | 1.01 [0.99,1.04] | |
| Ref: Late OWO | Early OWO | 1.28 | 0.20 | 1.01 [0.99,1.03] | 0.86 | 0.39 | 1.01 [0.99,1.03] |
The model includes mutual adjustment for GAmAge and GEAA and the following covariates: maternal smoking, delivery method, and child sex. Results presented for the GAmAge and GEAA (both in days) predictors. N = 831
GAmAge Gestational methylation age, GEAA Gestational age
We also examined whether GAmAge provides additional information over GEAA to predict long-term OWO. We compared the GAmAge and GEAA coefficients in the multivariate models that included mutual adjustment for both predictors across several age periods (Table 2). In the age periods of birth to 1, 3, 6, 10, 14, and 18y, GAmAge explained more than GEAA the differences between some OWO groups, as reflected by significant z-score (e.g., birth to 1y, late OWO vs. early OWO: 3.23 vs. -0.06 z-scores for GAmAge vs. GEAA, respectively; birth to 3y, NW vs. NW to very late OWO: -2.03 vs. -0.62; birth to 6y, NW vs. late OWO: -2.43 vs. 1.85). On the other hand, at some age periods (birth to 1, 2, 3, 10, 14, and 18y), GEAA explained more of the association with some OWO groups (e.g., birth to 1y, late OWO vs. NW to very late OWO: -0.60 vs. 2.33 z-scores for GAmAge vs. GEAA, respectively; birth to 2y, early OWO vs. NW to very late OWO: 1.35 vs. 2.33).
As a sensitivity analysis, we added maternal weight gain during pregnancy to our model, predicting OWO at different age periods by GAmAge, GEAA, maternal smoking, delivery method, and child sex (Additional file 1: Table S6). Adding maternal weight gain did not affect most of the associations observed between GAmAge and OWO groups at different age periods.
We repeated the analysis for the associations with long-term obesity to examine the association with GAmAge acceleration (the residuals from linear regression using GEAA as a predictor for GAmAge). Results from this analysis are presented in Additional file 1: Table S7. This analysis yielded similar results for the age period of birth to 1y. While we did not reach statistical significance for the other age periods, the direction of the effect size was similar to the main analysis.
We have also repeated the analysis for the association with long-term obesity, replacing GAmAge with the Knight (Additional file 1: Table S8) or Bohlin (Additional file 1: Table S9) clocks. While the direction of the associations of the Knight and Bohlin clocks were in accordance with the GAmAge across the different age periods, the associations did not reach statistical significance. Most of the GEAA associations remained similar to the main model with the mutual adjustment with GAmAge.
GAmAge and GEAA contribution to birth weight variation
Birth weight was associated with GAmAge after adjusting for GEAA, child’s sex, delivery method, and maternal smoking (beta = 7.66, p = 0.0064). Examining the R2 of this model, i.e., how much variation in birth weight was explained by the model, starting with the association of birth weight with the child’s sex, delivery method, and maternal smoking (R2 = 0.031), adding GAmAge (R2 = 0.449) or GEAA (R2 = 0.516), or both, showed the highest R2 with both GAmAge and GEAA in the model (R2 = 0.521), suggesting GAmAge explained additional 0.5% variation in birth weight on top of GEAA, sex, delivery method, and maternal smoking.
Since GEAA showed a similar high proportion of the explained variance in birth weight for the above model, we further stratified the birthweight model by subgroups of the delivery week. We found that the association of birth weight and GAmAge was the strongest in the extremely to very preterm strata (extremely to very preterm: beta = 21.60, p = 0.024, R2 = 0.582 when both GAmAge and GEAA in the model; moderate to late preterm: beta = 12.63, p = 0.029, R2 = 0.305; term: beta = 6.39, p = 0.08, R2 = 0.198; late to post-term: beta = -11.13, p = 0.264, R2 = 0.053). In the extremely to very preterm strata, for a model adjusted just for child’s sex, delivery methods, and maternal smoking (R2 = 0.059), adding GAmAge (R2 = 0.575) contributed more to the model than adding GEAA (R2 = 0.442). This was not observed within the moderate to late preterm strata, where adding GEAA to the model contributed more than adding GAmAge (R2 of a model without GAmAge or GEAA = 0.048; R2 for adding GAmAge = 0.221; R2 for adding GEAA instead of GAmAge = 0.276). This was also observed within the term strata (R2 = 0.045, R2 = 0.1234, R2 = 0.194; for models without GAmAge or GEAA, a model with GAmAge added, a model with GEAA added, respectively).
Mediation of the GAmAge association with OWO groups by birth weight
In the subsequent analysis, we examined whether birth weight mediated the association between GAmAge and long-term OWO groups. We performed a mediation analysis using the NW group as a reference in a two-group comparison logistic regression model. We found that birth weight mediated the association between GAmAge and the OWO groups, consistently between the NW and early OWO groups in the age periods of birth to 2, 3, 6, 14, and 18y. A summary of the casual mediation analysis is presented in Additional file 1: Table S10.
Discussion
In our study of 831 children, the GAmAge was associated with long-term obesity and was lower in late and very late OWO trajectories compared to early OWO. These associations were mediated by birth weight in multiple age periods, specifically for the associations between the consistent BMIPCT groups: early OWO and NW.
We found that among children assigned to the group NW to very late OWO, the strongest correlation between GAmAge and GEAA was observed compared to the other OWO group at multiple age periods. The NW to very late OWO group was characterized by having the lowest birth week and the highest percentage of preterm and SGA children. When later stratified the association of GAmAge with birth weight by delivery week, we found that the association of birth weight and GAmAge was the strongest in the extremely to very preterm strata. Moreover, GAmAge contributed more to the multivariate model when examining associations with birth weight than adding GEAA. This was not observed for later birth weeks of term and moderate to late preterm. DNAm, and more specifically, GAmAge, can also be a marker for fetal development and differentiate preterm and term newborns, as previous studies showed that preterm delivery was associated with decreased GAmAge acceleration [45, 46]. This may be due to differences in the preterm immune system compared to term newborns in cell composition and function [47]. These observations highlight the need to investigate further DNAm patterns and regulatory mechanisms among delivery week groups.
The data on the associations between GAmAge and long-term weight trajectories are limited. In a prospective study that followed 785 children from birth to 10y [21], the association of regressed GAmAge of GEAA was directly associated with the increase in age-specific time windows weight measurements up to 6 months. However, these associations reversed from the age of 5 years onwards, and the regressed GAmAge of age was inversely associated with the child’s weight: a non-significant trend in the ages of 5y to 9y and a significant association at the age of 10 were observed. In our analysis, we demonstrated the associations of GAmAge with OWO patterns in several age periods from birth to 18y. The use of epigenetic markers as an early indicator for later life obesity was also demonstrated in our previous epigenome-wide association study, where specific DNAm sites were associated with OWO trajectory patterns, differentiating between the OWO groups [29]. Here, we found that consistent BMIPCT trajectories groups early OWO (children with elevated BMIPCT from birth) and NW (children with NW pattern from birth) significantly differ in GAmAge from the non-consistent BMIPCT trajectories groups late (OWO by the end of year 1) and NW to very late OWO (NW until 6y old, and OWO onwards).
For the NW to very late OWO, GEAA and not GAmAge was a differentiating factor for the association with early and late OWO groups, but not with NW, across different age periods. This may be explained by the significantly lower GEAA for this group, compared with other groups, thus potentially having continuous effect on weight in later life. Of note, this OWO group also had higher SGA rates than other groups. A previous meta-analysis of 28 studies showed that LBW and SGA were associated with glycemic-related complications in childhood and adolescence [7]. Thus, our current findings highlight the need to monitor non-consistent BMIPCT patterns and provide early predictive markers to detect children at risk of cardiometabolic morbidity. The accumulating evidence for the predictive ability of early-life epigenetic signatures on later-life obesity should be further examined. Moreover, the associations of early epigenetic signatures with later-life morbidity should also be studied.
Previous studies in adults concerning the methylation clocks have highlighted its utility in disease and all-cause mortality prediction [9, 11–13]. Pediatrics clocks, using DNAm to predict GEAA using cord-blood samples, may have different utility. Previous findings concerning the GAmAge described associations with developmental features, such as birth weight, length, and head circumference [17, 21], and in older age—weight and height up to 6 and at 10 y [21]. In our study, we employed three methylation clocks; our primary methylation clock was based on the EPIC array, as opposed to the other two older clocks, based on the 27K and 450K arrays. The commonality among the three clocks is the population the models were trained and tested on, mainly the White/European population. While all three clocks showed a good correlation with GEAA, we could not validate the results of the association study. The CpG overlap between the different clocks is low, with two shared CpGs between Knight and Bohlin clocks and 11 overlaps between the GAmAge and Bohlin. Those results suggest that while different CpGs may provide a good prediction of GEAA, the association with longitudinal outcomes depends on the distribution of the employed CpGs.
Birth weight was a mediator for the GAmAge effect on OWO status for specific groups at multiple age periods. The two non-consistent BMIPCT trajectory groups started with a median BMIPCT below the 50th percentile, but by the end of year 1 and year 6, respectively, children in these groups become OWO. Birth weight has been studied for the associations with short- and long-term obesity and other health outcomes; a U-shaped association between birth weight and childhood obesity was observed in a cohort of 5141 children between the ages of 9 to 11 [48]. In that study, beyond factors such as highest parental education, maternal history of gestational diabetes, child age, infant feeding mode, gestational age, unhealthy diet pattern scores, and sleep quality, the odds ratio of being > 4000g at birth was 1.77 for boys and 2.48 for girls. Also, children from high-income countries had a higher risk of childhood obesity with birth weight > 4000g, whereas children from low- or middle-income countries had an increased risk starting at 3500g of birth weight. On the other hand, low birth weight was associated with cardiometabolic diseases in adulthood [49] and with childhood and adulthood obesity [50, 51]. Yet, it has to be noted that not all findings indicate that low birth weight might lead to childhood obesity [50]. Therefore, utilizing GAmAge as an independent marker at birth may assist in identifying late-onset obesity in children that are NW and with a lower birth week in their early life without other indication for the long-term OWO trajectory.
There are some limitations to this study. First, in the casual mediation analysis – birth weight and GAmAge were measured at the same time. Second, the findings’ reproducibility depends on available birth cohorts with dense repeated BMIPCT measurements and DNAm, as the BBC has. Our main association model included GAmAge and GEAA, which were highly correlated. Yet, including both age estimations in the model was valuable for the direct comparison of effect size and for identifying OWO groups/age periods where the GAmAge had a larger effect size than the GEAA. Additionally, in our linear models for the association with birth weight, we compared the R2 of the models, with and without GAmAge, to demonstrate how much variation in birth weight was explained by the model that includes GAmAge. Finally, to address co-linearity issues, we also included an age acceleration model as a sensitivity analysis. The strengths of this study, beyond its large sample size and extended time points for BMIPCT measurements from birth to 18y, are the novel associations described of GAmAge with long-term OWO trajectories.
Conclusions
Biological signatures based on DNAm are independent of GEAA (clinically defined) in long-term association with OWO. The findings of this study, along with previous studies showing an association between methylation clocks and anthropometric measurements in the pediatric population, support the notion that GAmAge may be a marker of developmental features for BMI trajectories. GAmAge may help in the early detection of the onset of late and very late OWO. Utilizing a robust marker based on DNAm instead of searching for specific CpGs may be a useful tool to identify individuals at risk for future OWO and initiate early intervention.
Supplementary Information
Additional file 1: Methods S1: BMIPCT imputation; Methods S2: Array, sample size, population, and methods for the employed clocks; Table S4: BMIPCT across OWO groups at the end of each age period; Table S5: Differential level of GAmAge across OWO groups in different age periods; Table 6: The association between GAmAge, GEAA, and OWO groups across different age periods; Table S7: The association between age acceleration and OWO groups across different age periods; Table S8: The association between Knight clock, GEAA, and OWO groups across different age periods; Table S9: The association between Bohlin clock, GEAA, and OWO groups across different age periods; Table S10: Mediation analysis; Figure S1(a-b): OWO trajectories; Figure S2: Overplaying CpGs between three gestational methylation age clocks; Figure S3: GAmAge and GEAA correlations across OWO groups in different age periods; Figure S4: GAmAge clocks and GEAA correlations across OWO groups in the age period of birth to 1y.
Additional file 2: Table S1: Knight gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome; Table S2: Bohlin gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome; Table S3: Haftorn (GAmAge) gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome.
Acknowledgements
Not applicable.
Abbreviations
- AGA
Appropriate for gestational age
- BBC
Boston Birth Cohort
- BMI
Body mass index
- BMIPCT
Body mass index percentile
- CDC
Centers for Disease Control
- DNAm
DNA methylation
- GAmAge
Gestational methylation age
- GEAA
Gestational age
- LBW
Low birth weight
- LGA
Large for gestational age
- NW
Normal weight
- OWO
Overweight or obesity
- SGA
Small for gestational age
- QC
Quality control
- y
Year/s
Authors’ contributions
AYM, XH, FBH, XW, and LL were responsible for study conception. XW supervised collection of phenotypic data and biospecimens. AYM and LL verified the underlying data. AYM was responsible for drafting of the manuscript. XH, GW, LL, and XW supervised DNA methylation data generation. AYM and XH performed DNA methylation quality control and data cleaning. AYM performed most of the statistical analyses under the guidance and technical support of LL. XW was responsible for overseeing acquisition of the epidemiological and clinical data as well as biospecimens. FBH, XW, and LL obtained funding. All the authors were responsible for critical review and revision of the manuscript and contributed to data interpretations. All authors read and approved the final version of the manuscript.
Funding
The Boston Birth Cohort (the parent study) was supported in part by the National Institutes of Health (NIH) grants (2R01HD041702, R01HD098232, R01ES031272, R21AI154233, R01ES031521, and U01 ES034983); and the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) (UT7MC45949). Dr. Yaskolka Meir is supported by the Council for Higher Education- Zuckerman support program for outstanding postdoctoral female researchers. Dr Hong is partly supported by NIAID (R21AI171059) and March of Dimes Grant (No. 6-FY23-0011). This information or content and conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by any funding agencies.
Availability of data and materials
The data, data dictionary, and analytical programs for this manuscript are not currently available to the public. However, they can be made available upon reasonable request and after the review and approval of the institutional review board.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all the study mothers. Institutional Review Boards of the Boston Medical Center and the Johns Hopkins Bloomberg School of Public Health approved the study. The study is also registered on ClinicalTrials.gov (NCT03228875).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xiaobin Wang, Email: xwang82@jhu.edu.
Liming Liang, Email: lliang@hsph.harvard.edu.
References
- 1.Kinlen D, Cody D, O’Shea D. Complications of obesity. QJM. 2018;111:437–43. 10.1093/QJMED/HCX152. [DOI] [PubMed] [Google Scholar]
- 2.Ward ZJ, Long MW, Resch SC, Giles CM, Cradock AL, Gortmaker SL. Simulation of Growth Trajectories of Childhood Obesity into Adulthood. N Engl J Med. 2017;377:2145–53. 10.1056/NEJMOA1703860/SUPPL_FILE/NEJMOA1703860_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baran J, Weres A, Baran R, Czenczek-Lewandowska E, Leszczak J, Wyszyńska J. Preterm birth and the type of birth and their impact on the incidence of overweight and obesity in children. Int J Environ Res Public Health. 2022;19:12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin J, Kwon Y, Kim JH, Jeong SJ. Association between maternal weight gain during pregnancy and child’s body mass index at preschool age. Clin Exp Pediatr. 2023;66:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Wang L, Liu H, Zhang S, Li W, Leng J, et al. Maternal gestational diabetes and childhood adiposity risk from 6 to 8 years of age. Int J Obes. 2024;48:414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnurr TM, Ängquist L, Nøhr EA, Hansen T, Sørensen TIA, Morgen CS. Smoking during pregnancy is associated with child overweight independent of maternal pre-pregnancy BMI and genetic predisposition to adiposity. Sci Rep. 2022;12:3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martín-Calvo N, Goni L, Tur JA, Martínez JA. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: Systematic review and meta-analysis. Obes Rev. 2022;23: e13380. [DOI] [PubMed] [Google Scholar]
- 8.Ou-Yang M-C, Sun Y, Liebowitz M, Chen C-C, Fang M-L, Dai W, et al. Accelerated weight gain, prematurity, and the risk of childhood obesity: A meta-analysis and systematic review. PLoS ONE. 2020;15:e0232238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dugué P-A, Bassett JK, Joo JE, Baglietto L, Jung C-H, Wong EM, et al. Association of DNA methylation-based biological age with health risk factors and overall and cause-specific mortality. Am J Epidemiol. 2018;187:529–38. [DOI] [PubMed] [Google Scholar]
- 10.Yaskolka Meir A, Keller M, Bernhart SH, Rinott E, Tsaban G, Zelicha H, et al. Lifestyle weight-loss intervention may attenuate methylation aging: the CENTRAL MRI randomized controlled trial. Clin Epigenetics. 2021;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, McGue M, et al. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell. 2016;15:149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perna L, Zhang Y, Mons U, Holleczek B, Saum K-U, Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin Epigenetics. 2016;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lind L, Ingelsson E, Sundström J, Siegbahn A, Lampa E. Methylation-based estimated biological age and cardiovascular disease. Eur J Clin Invest. 2018;48:e12872. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhou W-H. Epigenetic clocks in the pediatric population: when and why they tick? Chin Med J (Engl). 2021;134:2901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Wagner CL, Dong Y, Wang X, Shary JR, Huang Y, et al. Effects of maternal vitamin D3 supplementation on offspring epigenetic clock of gestational age at birth: a post-hoc analysis of a randomized controlled trial. Epigenetics. 2020;15:830–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khouja JN, Simpkin AJ, O’Keeffe LM, Wade KH, Houtepen LC, Relton CL, et al. Epigenetic gestational age acceleration: a prospective cohort study investigating associations with familial, sociodemographic and birth characteristics. Clin Epigenetics. 2018;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpkin AJ, Hemani G, Suderman M, Gaunt TR, Lyttleton O, Mcardle WL, et al. Prenatal and early life influences on epigenetic age in children: a study of mother–offspring pairs from two cohort studies. Hum Mol Genet. 2016;25:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiau S, Wang L, Liu H, Zheng Y, Drong A, Joyce BT, et al. Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics. 2021;16:186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monasso GS, Küpers LK, Jaddoe VWV, Heil SG, Felix JF. Associations of circulating folate, vitamin B12 and homocysteine concentrations in early pregnancy and cord blood with epigenetic gestational age: the Generation R Study. Clin Epigenetics. 2021;13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bright HD, Howe LD, Khouja JN, Simpkin AJ, Suderman M, O’Keeffe LM. Epigenetic gestational age and trajectories of weight and height during childhood: a prospective cohort study. Clin Epigenetics. 2019;11:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. [DOI] [PubMed] [Google Scholar]
- 23.Pearson C, Bartell T, Wang G, Hong X, Rusk SA, Fu L, et al. Boston Birth Cohort profile: rationale and study design. Precision Nutrition. 2022;1:e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. 2018 Global reference list of 100 core health indicators (plus health-related SDGs). No. WHO/HIS/IER/GPM/2018.1. World Health Organization, 2018.
- 25.Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup. JAMA. 2013;309:2445–6. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Mueller N, Wang G, Hong X, Chen T, Ji Y, et al. The joint association of small for gestational age and nighttime sleep with blood pressure in childhood. Sci Rep. 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Body mass index-for-age (BMI-for-age). OMS 2021:1–6. https://www.who.int/toolkits/child-growth-standards/standards/body-mass-index-for-age-bmi-for-age (accessed 10 Feb 2022).
- 28.Growth Charts - Individual Growth Charts n.d. https://www.cdc.gov/growthcharts/charts.htm (accessed 10 Feb 2022).
- 29.Meir AY, Huang W, Cao T, Hong X, Wang G, Pearson C, et al. Umbilical cord DNA methylation is associated with body mass index trajectories from birth to adolescence. EBioMedicine. 2023;91:104550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Körner A, Kiess W, Landgraf K. White Adipose Tissue Accumulation and Dysfunction in Children with Obesity. Pediatr Obes: Springer; 2018. p. 95–115. [Google Scholar]
- 31.Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379:1303–12. 10.1056/NEJMoa1803527. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Meir AY, Olapeju B, Wang G, Hong X, Venkataramani M, et al. Defining longitudinal trajectory of body mass index percentile and predicting childhood obesity: methodologies and findings in the Boston Birth Cohort. Precision Nutrition. 2023;2: e00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 34.Cao T, Zhao J, Hong X, Wang G, Hu FB, Wang X, et al. Cord Blood Metabolome and BMI Trajectory from Birth to Adolescence: A Prospective Birth Cohort Study on Early Life Biomarkers of Persistent Obesity. Metabolites. 2021;11:739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu R, Hong X, Zhang B, Huang W, Hou W, Wang G, et al. DNA methylation mediates the effect of maternal smoking on offspring birthweight: a birth cohort study of multi-ethnic US mother–newborn pairs. Clin Epigenetics. 2021;13:1–13. 10.1186/s13148-021-01032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong X, Hao K, Ji H, Peng S, Sherwood B, Di Narzo A, et al. Genome-wide approach identifies a novel gene-maternal pre-pregnancy BMI interaction on preterm birth. Nat Commun. 2017;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fortin J-P Jr, TJT, Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33:558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haftorn KL, Lee Y, Denault WRP, Page CM, Nustad HE, Lyle R, et al. An EPIC predictor of gestational age and its application to newborns conceived by assisted reproductive technologies. Clin Epigenetics. 2021;13:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelegí-Sisó D, de Prado P, Ronkainen J, Bustamante M, González JR. methylclock: a Bioconductor package to estimate DNA methylation age. Bioinformatics. 2021;37:1759–60. [DOI] [PubMed] [Google Scholar]
- 40.Knight AK, Craig JM, Theda C, Baekvad-Hansen M, Bybjerg-Grauholm J, Hansen CS, et al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol. 2016;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohlin J, Håberg SE, Magnus P, Reese SE, Gjessing HK, Magnus MC, et al. Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol. 2016;17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou W, Zhang M, Ji Y, Hong X, Wang G, Xu R, et al. A prospective birth cohort study of maternal prenatal cigarette smoking assessed by self-report and biomarkers on childhood risk of overweight or obesity. Precis Nutr. 2022;1:e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makker K, Wang X. Early life origins of cardiometabolic outcomes in Boston Birth Cohort: review of findings and future directions. Precision Nutrition. 2023;2: e00050. [PMC free article] [PubMed] [Google Scholar]
- 44.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for Causal Mediation Analysis. J Stat Softw. 2014;59:1–38. 10.18637/JSS.V059.I05.26917999 [Google Scholar]
- 45.Daredia S, Huen K, Van Der Laan L, Collender PA, Nwanaji-Enwerem JC, Harley K, et al. Prenatal and birth associations of epigenetic gestational age acceleration in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort. Epigenetics. 2022;17:2006–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bozack AK, Rifas-Shiman SL, Gold DR, Laubach ZM, Perng W, Hivert M-F, et al. DNA methylation age at birth and childhood: performance of epigenetic clocks and characteristics associated with epigenetic age acceleration in the Project Viva cohort. Clin Epigenetics. 2023;15:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Goede OM, Lavoie PM, Robinson WP. Cord blood hematopoietic cells from preterm infants display altered DNA methylation patterns n.d. 10.1186/s13148-017-0339-1. [DOI] [PMC free article] [PubMed]
- 48.Qiao Y, Ma J, Wang Y, Li W, Katzmarzyk PT, Chaput JP, et al. Birth weight and childhood obesity: a 12-country study. Int J Obes Suppl. 2015;5:S74–9. 10.1038/ijosup.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Mendonça ELSS, de Lima MM, Bueno NB, de Oliveira ACM, Mello CS. Premature birth, low birth weight, small for gestational age and chronic non-communicable diseases in adult life: A systematic review with meta-analysis. Early Hum Dev. 2020;149: 105154. 10.1016/J.EARLHUMDEV.2020.105154. [DOI] [PubMed] [Google Scholar]
- 50.Chen C, Jin Z, Yang Y, Jiang F, Huang H, Liu S, et al. Association of low birth weight with thinness and severe obesity in children aged 3–12 years: a large-scale population-based cross-sectional study in Shanghai. China BMJ Open. 2019;9: e028738. 10.1136/BMJOPEN-2018-028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jornayvaz FR, Vollenweider P, Bochud M, Mooser V, Waeber G, Marques-Vidal P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc Diabetol. 2016;15:1–10. 10.1186/S12933-016-0389-2/TABLES/5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Methods S1: BMIPCT imputation; Methods S2: Array, sample size, population, and methods for the employed clocks; Table S4: BMIPCT across OWO groups at the end of each age period; Table S5: Differential level of GAmAge across OWO groups in different age periods; Table 6: The association between GAmAge, GEAA, and OWO groups across different age periods; Table S7: The association between age acceleration and OWO groups across different age periods; Table S8: The association between Knight clock, GEAA, and OWO groups across different age periods; Table S9: The association between Bohlin clock, GEAA, and OWO groups across different age periods; Table S10: Mediation analysis; Figure S1(a-b): OWO trajectories; Figure S2: Overplaying CpGs between three gestational methylation age clocks; Figure S3: GAmAge and GEAA correlations across OWO groups in different age periods; Figure S4: GAmAge clocks and GEAA correlations across OWO groups in the age period of birth to 1y.
Additional file 2: Table S1: Knight gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome; Table S2: Bohlin gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome; Table S3: Haftorn (GAmAge) gestational methylation age clock. CpGs, coefficients, annotated gene, position, and chromosome.
Data Availability Statement
The data, data dictionary, and analytical programs for this manuscript are not currently available to the public. However, they can be made available upon reasonable request and after the review and approval of the institutional review board.