Abstract
RNA signals at the ends of the genes of respiratory syncytial (RS) virus direct polyadenylation and termination of viral transcription. These gene ends contain two conserved regions, a pentanucleotide and a tract of uridylate (U) residues, separated by an A/U-rich central region that is less well conserved. The U tract is thought to be the template for polyadenylation of viral mRNAs by reiterative transcription. The cis-acting requirements for termination were investigated by mutagenesis of the matrix (M) gene end (3′-UCAAUUAUUUUUU-5′) in a dicistronic RNA replicon. Termination efficiencies were quantitated by intracellular metabolic labeling of monocistronic mRNAs and the dicistronic readthrough RNAs that result when termination fails to occur. All three regions of the gene end were necessary for termination. Mutation of each of the first 8 nucleotides of the M gene end to all other nucleotides showed that nucleotides 2 to 6 were important for termination and intolerant of change, whereas nucleotides 1 and 7 were tolerant of change. At position 8, A or U allowed termination, but G or C did not. Both the length and the position of the U tract were important for termination. U residues at positions 9 to 12 were necessary, while additional U residues at position 8, and especially position 13, enhanced termination efficiency. Altering the length of the central region abolished termination, suggesting that the position of the U tract with respect to the 3′-UCAAU-5′ sequence was critical. The termination efficiencies of each of the 10 genes of RS virus are different. Since transcription is obligatorily sequential and termination of each gene is required for transcription of the next gene downstream, these differences may contribute to gene regulation. In agreement with our data, the naturally occurring gene ends of RS virus that terminate inefficiently have short U tracts or other sequence features that correlated with decreased termination when similar mutations were analyzed in RNA replicons.
Human respiratory syncytial (RS) virus, the leading cause of bronchiolitis and pneumonia in infants, is a paramyxovirus within the order Mononegavirales, the nonsegmented negative-strand RNA viruses (9, 39). Our understanding of RS viral transcription is incomplete, and there are both similarities and important differences between transcription in RS virus and the other Mononegavirales such as the prototypic rhabdovirus, vesicular stomatitis virus (VSV) (11, 12, 16, 34). The RNA genome of RS virus, which is encapsidated with the viral nucleocapsid (N) protein, encodes 10 genes in the order NS1-NS2-N-P-M-SH-G-F-M2-L (9). Transcription of 10 capped and polyadenylated mRNAs is catalyzed by a viral RNA-dependent RNA polymerase containing the large (L) catalytic subunit, the phosphoprotein (P) cofactor, and the transcription factor M2-1 (1, 7, 15, 17, 20, 48). Monocistronic mRNAs are transcribed sequentially from a single 3′-proximal promoter (14). According to the stop-start model of transcription for the Mononegavirales (16), after a polymerase complex has transcribed a gene, it polyadenylates and releases (terminates) the mRNA and then reinitiates transcription of the next gene downstream. Termination of each gene is required to allow transcription of the downstream gene (33). After each termination event, the polymerase sometimes fails to transcribe the downstream gene (2, 13). The resulting transcriptional attenuation at each gene junction is the predominant mechanism of gene regulation in the Mononegavirales (24, 46).
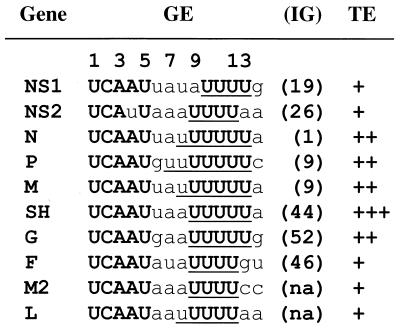
Signals that direct termination and initiation during sequential transcription are present at each gene junction of RS virus. The RNA sequences near the ends of the 10 genes of RS virus, A2 strain (6), are shown in Fig. 1 and are written 3′ to 5′ in the negative sense. Gene end (GE) sequences are 12 to 13 nucleotides (nt) long and contain two regions that are highly conserved in the different GEs of RS virus (6, 26, 49), the sequence 3′-UCAAU-5′, and a tract of four to seven uridylate (U) residues. The U tract is thought to be the template for synthesis of the poly(A) tail by reiterative transcription or slippage (25). Between these two conserved motifs is a central region, which consists of one to four nucleotides that are A/U rich but are not highly conserved. Immediately downstream of the U tract is an intergenic (IG) region, which varies in length from 1 to 52 nt with no sequence conservation, followed by a conserved 9-nt gene start (GS) signal. Previous studies of RS virus, VSV, and other Mononegavirales have shown that GE signals direct polyadenylation and termination of each mRNA, while GS signals direct initiation and capping (3, 23, 31, 33, 40, 43, 44). Replacement of the GE of RS virus with a nonviral sequence abolishes termination of the upstream gene and transcription of the downstream gene (33). The variable IG regions of RS virus are thought not to be present in monocistronic mRNAs, and their role in transcription is unclear (19, 32).
FIG. 1.
Sequences and TEs of RS virus GEs. The genomic RNA sequences near each GE of strain A2 are shown (3′ to 5′), with the genes in the same order as in the viral genome. Conserved or nonconserved nucleotides are in bold capital or lowercase letters, respectively, and the U tract is underlined. The 12 to 13 nt of the GEs are numbered, beginning with the first U of the conserved 3′-UCAAU-5′ element as position 1. The total nucleotide length of the IG region is shown in parentheses, along with the first 1 to 2 nt of the IG region. Because the M2 and L genes have no true IG region, the IG length is not applicable (na). The relative TE of each GE, as determined using a plasmid-based RNA replicon system in the presence of M2-1 protein expression (19), is indicated as follows: +, inefficient (15 to 40%); ++, efficient (65 to 80%); or +++, highly efficient (95%).
Because termination signals must be recognized to allow sequential transcription, termination plays a critical role in the life cycle of the Mononegavirales. However, unlike VSV, in which the GE sequences are identical, the IG regions are well conserved, and termination is highly efficient at all gene junctions, RS virus has both variable GEs and IG regions in its gene junctions, which direct termination with different efficiencies (19). In RS virus, termination is inefficient at the NS1/NS2, NS2/N, F/M2, and M2/L junctions; moderately efficient at the N/P, P/M, M/SH, and G/F junctions; and highly efficient at the SH/G junction (Fig. 1). Since transcription of each gene depends on termination of the upstream gene, this modulation of termination efficiency may be used to further regulate the expression of genes in RS virus, which has a more complex genome than VSV (19, 34). Also, while the variable U tract of RS virus can be as short as 4 nt (Fig. 1), the conserved U tract of VSV is 7 nt long, and shortening the U tract by even one U residue prevents termination and slippage (3). This suggests that the requirements for slippage are different in these two viruses. Furthermore, the M2-1 protein of RS virus decreases nonspecific termination within genes (7, 17) and increases the production of polycistronic transcripts, which result from failure to terminate at gene junctions (17, 19, 20). RS virus is the first member of the Mononegavirales that has been reported to encode a separate factor involved in transcriptional processivity or antitermination.
To understand termination and its possible role in regulating RS viral gene expression, we investigated the cis-acting requirements for termination. In this study, we used dicistronic RNA replicons of RS virus, which directed viral transcription when coexpressed in cells with the N, P, L, and M2-1 proteins by means of a vaccinia virus-T7 expression system (20, 48). We made alterations in a GE signal and observed the effects on termination, which were assayed by quantitating monocistronic and polycistronic mRNAs transcribed from replicons. The data reported here show that a specific sequence upstream of a U tract is required for optimal termination. In addition, the length of the U tract and its position within the GE are important for termination.
MATERIALS AND METHODS
Plasmid constructions.
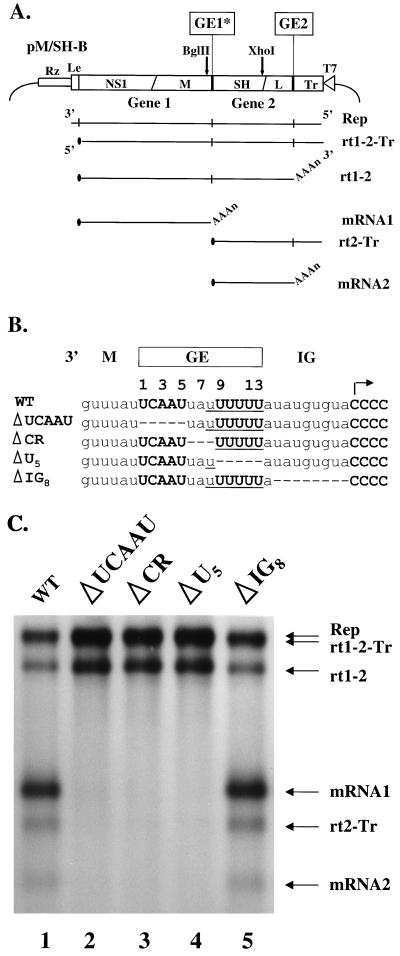
Plasmid pM/SH, which encodes a subgenomic RNA replicon, was constructed previously using cDNA clones derived from the A2 strain of RS virus (20, 48). This replicon contains the following regions (Fig. 2A), from 3′ to 5′ in the negative sense: (i) the leader region, 44 nt; (ii) gene 1, containing the first 382 nt of the NS1 gene fused to the last 318 nt of the M gene; (iii) the M/SH IG region, 9 nt; (iv) gene 2, containing the first 286 nt of the SH gene and 155 nt from two regions of the L gene; and (v) the trailer region, 154 nt. Termination of mRNA1 occurs at the M GE, which will be referred to as GE1, and termination of mRNA2 occurs at the L GE (GE2). To facilitate PCR mutagenesis of GE1, pM/SH was modified as follows to generate plasmid pM/SH-B (Fig. 2A). The BglII restriction enzyme site 260 bp upstream of GE1 was destroyed by partial digestion of pM/SH with BglII, repair of the sticky ends using the Klenow fragment of DNA polymerase I, and blunt-end ligation (42). The resulting 4-bp insertion removed the aforementioned BglII site, leaving a unique BglII site 12 bp upstream of GE1. Viral RNA replication and transcription directed by pM/SH-B were indistinguishable from the counterparts directed by pM/SH (data not shown); therefore, pM/SH-B was used as the wild-type (WT) replicon in this study.
FIG. 2.
Deletion analysis of the M/SH gene junction in a dicistronic RNA replicon. (A) Schematic of the WT replicon-expressing plasmid pM/SH-B (top), along with the RNA species synthesized by this replicon (below, horizontal lines). This subgenomic RNA replicon contains two transcriptional units: the 704-nt NS1/M gene (gene 1) and 441-nt SH/L gene (gene 2). Locations in pM/SH-B of the M GE that was mutagenized (GE1*), the hepatitis delta virus ribozyme (Rz), RS virus leader (Le), restriction enzyme sites BglII and XhoI, virus trailer (Tr), and T7 promoter (T7) are shown. RNA species synthesized from this replicon include the negative- and positive-sense replication products (Rep; only the negative strand is shown), two monocistronic tran scripts (mRNA1 and mRNA2), and three polycistronic readthrough transcripts (rt1-2-Tr, rt1-2, and rt2-Tr). The 5′ caps (filled ovals) and 3′ poly(A) tails (AAAn) of mRNAs are indicated. (B) Deletions of regions of the M/SH gene junction. The genomic RNA sequence including part of the WT M/SH gene junction is aligned with sequences of replicons from which one of the three regions of the GE, or else 8 of 9 nt of the IG region, was deleted. Dashes indicate absence of any nucleotide at those positions. Conserved or nonconserved nucleotides are in bold capital or lowercase letters, respectively. The transcription initiation site for mRNA2 at the beginning of the GS signal is indicated by an arrow. CR, central region. (C) Analysis of RNA synthesis from RS virus replicons having deletion mutations. MVA-T7 infected HEp-2 cells were transfected with support plasmids expressing the N, P, L, and M2-1 proteins of RS virus, and with a plasmid encoding the indicated WT or deletion mutant replicon, as described in Materials and Methods. RNA synthesized by RS viral polymerase was metabolically labeled with [3H]uridine in the presence of actinomycin D, analyzed on agarose-urea gels, and detected by fluorography. RNA species are identified (right) using the same nomenclature as in Fig. 2A. Rep and rt1-2-Tr comigrated on this gel.
Site-specific mutagenesis of GE1 or the M/SH intergenic region in pM/SH-B was performed by PCR (42) using an upstream mutagenic primer that annealed to the BglII site and M/SH gene junction. The downstream PCR primer annealed to a portion of the L gene sequence or to the T7 promoter downstream of the trailer region. PCR products were synthesized using Taq DNA polymerase (Life Technologies), digested with BglII and XhoI, and subcloned into these unique sites in pM/SH-B (Fig. 2A). Sequences of the relevant regions of all plasmids were determined by dideoxy sequencing (42). A similar procedure used to make the deletions shown in Fig. 2B introduced two additional nucleotide changes just upstream of the XhoI site, creating a unique AvrII site. These two nucleotide changes in the SH gene sequence did not alter RNA synthesis detectably (data not shown).
Transfections and RNA analysis.
RS viral transcription from RNA replicons was directed in cells using a vaccinia virus-T7 expression system essentially as described (20). Briefly, 5 × 105 HEp-2 cells (American Type Culture Collection) were grown overnight in 60-mm-diameter tissue culture dishes and infected with MVA-T7 (2 PFU per cell). MVA-T7, a recombinant vaccinia virus expressing T7 RNA polymerase (47), was kindly provided by B. Moss and propagated in chicken embryonic fibroblasts. We and others (37) have found that MVA-T7 allows more efficient RNA replication and transcription with lower background than another recombinant vaccinia virus, vTF7-3, which was used in some previous studies (20). Approximately 1 h after MVA-T7 infection, cells were transfected with plasmids encoding a dicistronic RNA replicon of RS virus (6 μg) and RS virus proteins (20, 48)—pN (5 μg), pP (1.3 μg), pL (1.0 μg), and pORF1 (0.3 μg)—using 20 μl of Lipofectin (Life Technologies). T7 promoters directed intracellular transcription of these plasmids. The relative amounts of these plasmids were important for optimal RNA synthesis, but in some experiments the absolute amounts of all plasmids were reduced by half with no noticeable effect on transcription or replication.
Approximately 16 h after transfection, the cells were exposed to [3H]uridine (33 μCi/ml) for 6 h in the presence of actinomycin D (10 μg/ml). Cytoplasmic extracts were prepared by scraping cells in 0.4 ml of chilled lysis buffer (1% NP-40, 0.4% sodium deoxycholate, 66 mM EDTA, 10 mM Tris-HCl [pH 7.4]), vortexing, and removal of the nuclei by brief centrifugation. RNA was purified by phenol-chloroform extraction and recovered by ethanol precipitation in the presence of 0.1 M NaCl and 10 μg of tRNA/ml. 3H-labeled RNAs were analyzed by 1.75% agarose–6 M urea–25 mM citrate gel electrophoresis (36) and detected by fluorography using presensitized X-ray film (35).
RNA quantitation.
The major RNA species synthesized by RS viral polymerase from the M/SH replicon (Fig. 2A) were identified previously by annealing with specific oligonucleotides followed by RNase H digestion (20). These include the positive- and negative-sense products of RNA replication (Rep; 1352 nt) and the following five transcription products. (i) Monocistronic mRNA1 (704 nt) is transcribed from gene 1 when the polymerase terminates at GE1. (ii) Monocistronic mRNA2 (441 nt) is transcribed from gene 2 when the polymerase terminates at GE2. (iii) The dicistronic readthrough transcript rt1-2 is synthesized when the polymerase fails to terminate at GE1. (iv) Another readthrough transcript, rt1-2-Tr, is synthesized when polymerase fails to terminate at both GE1 and GE2. (v) rt2-Tr is a readthrough from mRNA2 into the trailer region. Transcripts ending with the trailer sequence are thought to be unstable in RS virus-infected cells (31, 33). However, rt1-2-Tr and rt2-Tr are polyadenylated and stable in our replicon system (20), possibly due to the presence of vaccinia virus. The readthrough RNAs A, B, and C identified previously (20) have been renamed in this work as rt2-Tr, rt1-2, and rt1-2-Tr, respectively, in order to indicate the RNAs that each contains.
To measure the termination efficiency (TE) at GE1, the RNA species mRNA1, rt1-2, and rt1-2-Tr (Fig. 2A) were quantitated by densitometry using a Howtek Scanmaster 3 and PDI Quantity One software with in-lane background subtraction, as recommended by the manufacturer. TE was then calculated using the formula TE = mRNA1/(mRNA1 + rt1-2 + rt1-2-Tr), as explained in Results. Trace optical density (OD) readings measured by densitometry were converted to RNA molar equivalents by dividing the trace OD of each RNA species by the number of uridine residues in that RNA, and these ratios were substituted in the formula for TE. Average TE values and standard deviations were determined from three or more independent experiments for each mutant replicon. Although the replication products usually comigrated with rt1-2-Tr on the gel, replication represented a small fraction of labeled RNA in this band (data not shown). Therefore the trace OD of rt1-2-Tr was not adjusted to eliminate replication.
RESULTS
Deletion analysis of the M/SH gene junction.
Two regions of the GE sequence are highly conserved among the genes of RS virus: the sequence 3′-UCAAU-5′, and a U tract of at least 4 nt. These two motifs occur separately 35 and 192 times, respectively, in the RS viral genome (A2 strain). Only at the 10 GE, however, are both motifs found, with a short, A/U-rich central region between them (Fig. 1). This observation suggests that neither the 3′-UCAAU-5′ sequence nor the U tract can direct termination independently. To test this, we deleted each of these regions of the GE individually from a dicistronic replicon (Fig. 2A) and observed the effects on termination. We selected the M/SH gene junction as the context for these studies because the M gene end directs termination efficiently (19) and because it is the only GE sequence that is identical in two genes (the N and M genes [Fig. 1]). A dicistronic replicon was used because it provided a direct assay for termination at GE1. Discrete monocistronic mRNA1 is produced only when termination occurs at GE1 (Fig. 2A), whereas dicistronic readthrough RNAs (rt1-2 and rt1-2-Tr) are produced when termination fails to occur at GE1. The RNAs transcribed from the M/SH replicon were characterized previously (20) and are described in more detail in Materials and Methods.
The three regions of GE1 were deleted individually, as shown in Fig. 2B. We deleted either the conserved positions 1 to 5 of GE1 (replicon ΔUCAAU), positions 6 to 8 in the central region (ΔCR), or 5 nt of the U6 tract, positions 9 to 13 (ΔU5). Previous studies of RS virus showed that a GE sequence alone was sufficient to signal termination and that the downstream IG region and GS were not required (32, 33). To confirm this observation and to serve as a control, we deleted 8 nt of the 9-nt M/SH IG region to generate the replicon ΔIG8. To determine the effects of these deletions on transcription, we transfected plasmids encoding the WT or mutant replicons into MVA-T7-infected cells along with plasmids encoding the N, P, L, and M2-1 proteins, as described in Materials and Methods. This experimental system allows the formation in cells of RS virus nucleocapsid complexes that are competent for replication and transcription (20, 48). RNA synthesized from these replicons was metabolically labeled in the presence of actinomycin D and analyzed by electrophoresis.
Results of the deletion analysis are shown in Fig. 2C. The WT replicon directed transcription that terminated efficiently at GE1 to yield discrete mRNA1 (Fig. 2C, lane 1). Additionally, mRNA2 was produced, confirming that termination at GE1 allowed transcription of mRNA2. Approximately 40% of the time, termination failed to occur at the WT GE1, resulting in the generation of the readthrough RNAs rt1-2 and rt1-2-Tr. In contrast, replicons that had a region of GE1 deleted (ΔUCAAU, ΔCR, and ΔU5) produced no monocistronic mRNA1, showing that termination did not occur at GE1 (Fig. 2C, lanes 2 to 4). Correspondingly, these mutant replicons produced increased levels of readthrough RNAs compared to WT. Therefore, deletion of any of these three regions of GE1 abolished termination of mRNA1, allowing only readthrough transcription. In addition, no mRNA2 was produced from these mutant replicons. Thus, when termination did not occur at GE1, transcription of mRNA2 was prevented, consistent with the sequential mechanism of transcription (14, 33).
These data showed that all three regions of the GE (the 3′-UCAAU-5′ element, the central region, and the U tract) were required for termination. By contrast, deletion of 8 nt of the 9-nt intergenic region (ΔIG8; lane 5) had no obvious effect on termination of mRNA1 or transcription of mRNA2. Similarly, changing the first nucleotide of the IG region (the A residue at position 14 [Fig. 2B]) to any other nucleotide did not significantly affect termination (data not shown). Thus, the entire 9-nt M/SH IG region was not required for termination or reinitiation, consistent with previous studies (32, 33).
U-tract length and position are important for termination.
A U tract, which is thought to be the template for polyadenylation of viral mRNAs, is present in all 10 of the GE sequences of RS virus (Fig. 1). However, the length of the U tract varies from 4 to 7 nt at the different GEs. The position of the U tract within the GEs also varies. In GE1, for example, the U6 tract extends from position 8 to 13 (Fig. 1). The first U of the U tract varies from positions 7 to 10, while the last U is at position 12 or 13. Positions 10 to 12 are U residues in all 10 GEs. It is not known what effect these variations in the length and position of the U tract have on termination.
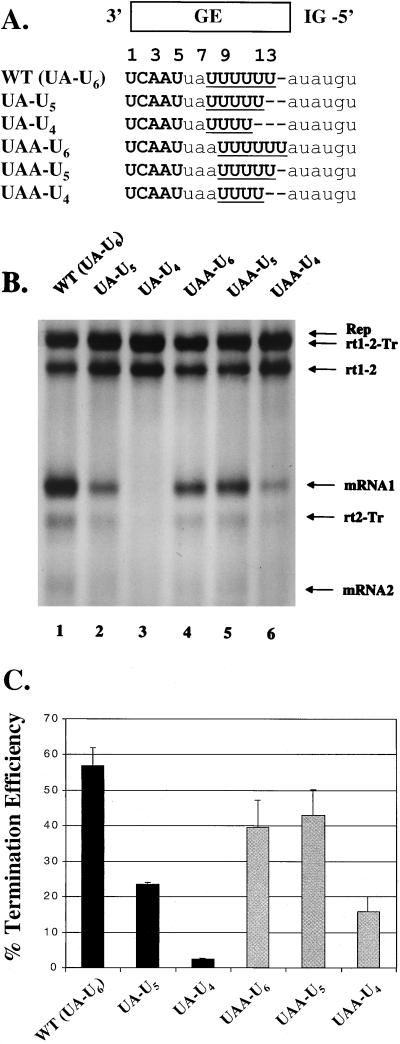
To determine the minimal length of the U tract required for termination at GE1, we deleted one or two U residues from the WT U6 tract, generating the replicons UA-U5 and UA-U4 (Fig. 3A). These replicons are referred to by the sequence of the central region and U tract, which is 3′-UA-U6-5′ in the WT replicon. Reduction of the U-tract length caused a gradual decrease in termination (Fig. 3B, lanes 1 to 3), unlike the all-or-none effect seen with the deletions described above (Fig. 2). To quantitate the effects of these mutations, we calculated the TE of each replicon.
FIG. 3.
Effects on termination of changes in the U-tract length and position. (A) Sequence of the WT M/SH gene junction aligned with sequences of mutant replicons having alterations in the U-tract length and position. Replicon names (left) give the sequences (3′ to 5′ in the negative sense) of the central region and the U tract, separated by a dash. The sequences are represented as in Fig. 2B. (B) Analysis of viral RNA synthesis directed by WT and the indicated mutant replicons. The nomenclature for RNA species (right) and the experimental procedures are the same as for Fig. 2C. (C) TE at GE1, calculated for each replicon by densitometric analysis of fluorograms as described in Materials and Methods. The average percent TE values (bars) and standard deviations (error bars) were determined for all replicons from at least three independent experiments.
TE, a standard measure of termination, is defined as the fraction of polymerase complexes that terminate at any given point on the template (45). Thus, the TE at GE1 equals the ratio of the mRNAs that are terminated at GE1 (i.e., mRNA1) to all the mRNAs that are transcribed up to GE1: TE = mRNA1 / (mRNA1 + rt1-2 + rt1-2-Tr). The relative molar amounts of mRNA1 and the readthrough RNAs were quantitated by densitometry, as described in Materials and Methods, and substituted in this formula. Since TE compares the molar amounts of three RNA species in the same lane of a gel, it is independent of experimental variability in the amount of total labeled RNA. Thus, TE values can be compared between experiments. In general, lower amounts of mRNA2 were synthesized by replicons in which termination at GE1 was inefficient, due to the sequential nature of transcription (for example, Fig. 3B, lane 2). However, the amounts of mRNA2 and rt2-Tr were not measured because these bands on the gel were too faint for accurate quantitation when termination at GE1 was substantially reduced.
The TE values for the replicons in Fig. 3B, averaged from three independent experiments, are shown in Fig. 3C. The average TE of the WT replicon was about 60%. This is lower than the TE at the M/SH gene junction in RS virus-infected cells, which was estimated at about 90% by Northern hybridization (10, 13). Part of this difference may be due to our use of metabolic labeling, which measures synthesis of RNA over a short period of time, rather than hybridization, which measures the accumulation of RNA over a longer period. It is also possible that some aspect of the vaccinia virus-T7 expression system affects termination. For example, because expression of M2-1 protein increases the production of readthrough RNAs (17, 19, 20), we examined whether transfection of excessive amounts of the M2-1 plasmid was responsible for reducing the TE. However, transfection of between 0.1 and 1 μg of the M2-1 plasmid did not greatly affect the TE of the WT replicon, which varied from 55 to 60% (data not shown). Transfection of lower amounts of M2-1 plasmid resulted in a profile of RNA synthesis similar to that seen with no M2-1 expression (20), presumably because the plasmid was not distributed to all cells. We therefore chose to examine the cis-acting requirements for termination under a constant set of conditions by transfection of 0.3 μg of the M2-1 plasmid throughout this study.
When the U tract in GE1 was shortened from 6 to 5 nt, the average TE was reduced more than twofold (Fig. 3C). Further shortening of the U tract to 4 nt reduced the TE to 4%; 4% is near the background level for TE, since mRNA1 production by UA-U4 was not detectable visually in multiple experiments (Fig. 3B, lane 3). These results show that the length of the U tract in the GE is important for termination. Four of the GEs of strain A2 contain a U4 tract (Fig. 1). These GE signals direct termination, albeit with low efficiency, 15 to 40% (19), while replicon UA-U4 failed to direct termination. However, a U4 tract is not found in conjunction with a central region of less than 3 nt in the viral GE sequences (Fig. 1), while the central region in the replicon UA-U4 was only 2 nt long (Fig. 3A). In the replicon UA-U4, position 12 of the GE was not a U residue (Fig. 3A), which is strictly conserved in the viral GE sequences (Fig. 1).
These observations suggested that when the U tract was shortened to the minimum conserved length, the position of this U4 tract became critical for termination. To test this hypothesis, we made replicons with a 3-nt-long central region (3′-UAA-5′, as in the SH gene end [Fig. 1]), followed by a U tract of variable length, 4 to 6 nt (Fig. 3A). The products of RNA synthesis from these replicons, UAA-U6, UAA-U5, and UAA-U4, are shown in Fig. 3B, lanes 4 to 6, and the TE values are shown in Fig. 3C. UAA-U4 directed termination and produced readily detectable mRNA1, unlike UA-U4 (compare Fig. 3B, lanes 3 and 6). The TE of UAA-U4 (16% [Fig. 3C]) was higher than that of UA-U4, which had a U tract of the same length but in a different position (Fig. 3A). Similarly, UAA-U5 terminated more efficiently than UA-U5 (Fig. 3C).
Taken together, these results suggest that the position of the U tract, as well as its length, is important for termination. Increasing the length of the U tract improved TE in some cases but not in others, depending on the positions of the U residues that were added. Specifically, TE was increased by extending the U tract from 4 to 5 nt to include position 13 (from UAA-U4 to UAA-U5) or position 8 (from UAA-U4 to UA-U5). TE was also increased by lengthening the U tract from 5 to 6 nt to include position 8 (from UAA-U5 to UA-U6). However, TE was not improved by further increasing the length of the U tract by 3 nt upstream of position 8 (A7U in Fig. 4) or by 1 to 2 nt downstream of position 13 (UAA-U6 [Fig. 3] and the mutant A14U [data not shown]). Thus, U residues at some positions were more important for termination than U residues at other positions. Our data suggest that U residues at positions 9 to 12 were essential for termination, while additional U residues at position 8, and especially position 13, enhanced TE.
FIG. 4.
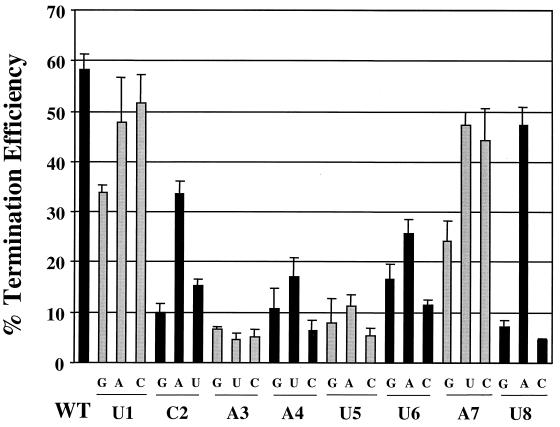
Effects on TE of nucleotide changes at positions 1 to 8 of GE1. The efficiency of termination at GE1 was calculated for RNA replicons having point mutations in nucleotide positions 1 to 8 of GE1 as described in Materials and Methods. Position numbers of the nucleotides are as shown in Fig. 1 and 2B. Average percent TE values (bars) and standard deviations (error bars) were determined for all replicons from at least three independent experiments.
In summary, efficient termination at GE1 required a U tract of at least 5 nt. The minimal conserved U tract of 4 nt allowed termination with lower efficiency, but only if the central region was 3 nt long. Thus, the length and position of the U tract were both important for termination.
An upstream sequence element required for termination.
The sequence 3′-UCAAU-5′ is conserved in nine of the GEs of strain A2 (Fig. 1) but not in that of NS2, which is an inefficient terminator (19, 31). This suggests that the sequence 3′-UCAAU-5′ is essential for termination. By contrast, the central region varies in length as well as in sequence (Fig. 1). However, the combined length of the central region and U tract is 7 to 8 nt at all GEs except that of NS1, another inefficient terminator (Fig. 1). Deletion of the central region abolished termination (Fig. 2), and the length of this region was important when the U tract was reduced to 4 nt (Fig. 3B). Thus, while the length of the central region may be important for termination, its sequence is less conserved than the 3′-UCAAU-5′ element just upstream. The role of the upstream nucleotides of the GE in termination has not been examined.
To determine which nucleotides in positions 1 to 8 of GE1 were important for termination, we made replicons in which each of these nucleotides was individually replaced with each of the three other possible nucleotides. These replicons are referred to by the WT nucleotide, its position, and the substituted nucleotide. For example, in U1G, the U residue at position 1 of GE1 was substituted with a G residue. We analyzed the TE of these replicons, and Fig. 4 shows the average TE values for the WT and mutant replicons.
Most nucleotide changes in the first eight positions of GE1 reduced the efficiency of termination, but the extent of this reduction varied significantly depending on the nucleotide position (Fig. 4). At positions 2 to 6, all nucleotide changes reduced termination substantially. Thus, positions 2 to 6 were intolerant to change, suggesting that these specific nucleotides were important for termination. At position 8, an A or U residue allowed efficient termination, but a G or C residue abolished termination. In contrast, positions 1 and 7 were tolerant to change, since termination was reduced moderately or slightly by mutations at these positions. However, changing position 1 or 7 to a G residue reduced the TE by half, indicating that G was tolerated least well at these positions. These results show that the sequence upstream of the U tract was important for termination, although some positions of the GE tolerated change more than other positions.
Importance of the length and sequence of the central region.
To further evaluate the importance of the length and sequence of the central region, we constructed replicons in which positions 6 to 8 of GE1 (3′-UAU-5′) were changed to either 3′-UAUA-5′, 3′-UAA-5′, 3′-UUA-5′, or 3′-UGG-5′ (Fig. 5A). These four mutant replicons all had the WT 3′-UCAAU-5′ element upstream of the altered central region and a U5 tract downstream. When the length of the central region was increased to 4 nt in the replicon UAUA-U5, termination was reduced to a very low level compared to WT (Fig. 5B, lanes 1 and 2). This effect was likely due to the increased length of the central region, since termination was not abolished by changing the sequence of the central region, provided this region was 2 to 3 nt long and A/U rich (Fig. 5B, lanes 4 and 5; Fig. 4). The replicon UAUA-U4, which had a U4 tract instead of a U5 tract but was otherwise identical to UAUA-U5, also terminated at a very low level (Fig. 5B, lane 3). Thus, increasing or decreasing the length of the central region by a single nucleotide abolished termination (Fig. 3B and 5B). It is unlikely that termination requires proper spacing between the upstream part of the GE and the downstream IG region or GS, since mutation of the IG region (Fig. 2C, lane 5) or the GS (33) had no effect on termination. Therefore, our data suggest that a critical function of the central region is to maintain the correct position of the U tract with respect to the upstream sequence element.
FIG. 5.
Mutational analysis of the central region in the GE. (A) Sequence of the WT M/SH gene junction aligned with sequences of mutant replicons having alterations in the length and/or sequence of the central region of the GE. Replicon names (left) give the sequences (3′ to 5′ in the negative sense) of the central region and the U tract, separated by a dash. The sequences are represented as in Fig. 2B. The replicon UAA-U5 was identical to replicon U8A (Fig. 4). (B) Fluorogram showing viral RNA synthesis directed by the WT and mutant replicons. The nomenclature for the RNA species (right) and the experimental procedures are the same as for Fig. 2C.
The replicon UGG-U5, which had G substitutions at positions 7 and 8 of GE1, failed to terminate (Fig. 5B, lane 6). This result was consistent with the failure of the U8G mutant to terminate (Fig. 4) and with the absence of G residues in positions 7 and 8 of the GE (Fig. 1). Therefore, the sequence of the central region is important for termination, suggesting that this region is more than simply a spacer between the U tract and the rest of the GE.
DISCUSSION
We investigated the cis-acting requirements for transcriptional termination of RS virus using a dicistronic RNA replicon. We found that the efficiency of termination was affected by alterations to three regions of the gene end: (i) nucleotides within the 3′-UCAAU-5′ sequence element, (ii) the length and sequence of the central region, and (iii) the length and position of the U tract.
The upstream sequence element.
The GEs of all Mononegavirales contain a conserved sequence upstream of a U tract, although the specific upstream sequence varies among different viruses (3, 8, 29, 34). In general, the results of our mutagenesis of positions 1 to 8 of the GE, using a functional assay for termination (Fig. 4), correlate with the pattern of conservation of the upstream sequence in RS virus (Fig. 1). Positions 2 to 5, which were sensitive to change in the functional assay, are also conserved in the different GEs of RS virus. Position 7, which was tolerant of changes, is less well conserved, and as found in the viral GEs, position 8 tolerated A or U but not G or C. However, the nonconserved position 6 was more sensitive to change than the conserved position 1. The reason for this result is unclear. Since we limited ourselves to individual mutations of each position of GE1, we could not determine whether different combinations of nucleotides at multiple positions might cooperate to signal termination. For example, while we found that a G residue at position 6 inhibited termination in the context of the M gene end (Fig. 4), it apparently allows termination in the P and G GEs (Fig. 1) (19).
The importance for termination of the sequence in the central region (positions 6 to 8) shows that it is more than an A/U-rich spacer within the GE, although position 7 comes closest to fitting this description. Position 8 was unusual in that it was extremely intolerant of G and C but tolerated A and U almost equally. Thus, one specific nucleotide was not required at position 8. These data suggest that weak base pairing between the template and mRNA just upstream of the U tract may be critical at some point during the process of slippage or termination. In the rhabdovirus VSV, changing the C residue just upstream of the U tract to any other nucleotide abolished termination (3). In RS virus, however, there is at least some flexibility in the sequence at positions 7 and 8, which are upstream of the conserved U tract. The different positions of the critical upstream nucleotides with respect to the U tract in RS virus and VSV, along with the different required length of the U tract, may reflect differences in the requirements for slippage or termination.
The length of the U tract.
The U tract is thought to be the template for synthesis of a poly(A) tail by slippage, which occurs prior to termination (3, 25). The length of the U tract varies among the different GEs of RS virus (Fig. 1). When we examined the effect of varying the U-tract length in an RNA replicon, we found that the minimal U tract that allowed efficient (>40%) termination was 5 nt, although a U4 tract did allow termination with lower efficiency (16% [Fig. 3]). Consistent with our results, termination is less efficient at the gene junctions of RS virus that have a U tract of 4 nt (Fig. 1), whether termination is assayed using replicons (19) or in infected cells (10, 13, 17). Thus, the inefficient termination at these junctions might be explained by the shortened U tract alone. However, other sequence differences in these gene junctions may also affect termination. Further work is required to analyze the effects of varying the length and position of the U tract in sequence contexts other than the M/SH junction.
The fact that the minimum required size of the U tract is shorter in RS virus than in VSV suggests that there may be differences in the process of slippage for different viruses. Slippage, which occurs during mRNA editing as well as polyadenylation in the Mononegavirales, requires a homopolymeric tract in the template that is proposed to form a duplex with the nascent mRNA (3, 21, 25). VSV and other rhabdoviruses have an invariant U tract of 7 nt at each GE, and shortening the U tract of VSV by a single U residue completely abolished termination and prevented slippage (3, 23). By contrast, the minimum functional length of the U tract is 4 to 5 nt for the paramyxoviruses RS virus (Fig. 3) and simian virus 5 (40, 41). One interpretation of these data is that during slippage, the template-mRNA duplex is shorter for paramyxoviruses than for the rhabdovirus VSV. In other RNA polymerases, the template-mRNA duplex has a defined length and is a critical component of the elongation complex (28, 38, 45). By analogy, if a 4- to 5-nt template-mRNA duplex were required in RS virus, a shorter U tract would not allow slippage. However, if the U tract were longer than the duplex, slippage would not necessarily be increased, because the extra U residue(s) could not base pair with the mRNA. This could explain why shortening the U tract of RS virus or VSV below a certain minimum length prevents termination, but excessive lengthening of the U tract does not increase TE (3, 23). Interestingly, the shortest U tract that is known to allow slippage during RNA replication of RS virus is 5 nt (18), which is similar to the minimum U-tract length required for termination (Fig. 3).
Position of the U tract.
Our data suggest that the distance between the upstream sequence and the U tract is also critical for transcriptional termination by RS virus. Increasing or decreasing the length of the central region by a single nucleotide abolished termination (Fig. 3 and 5). Our data suggest that efficient termination requires U residues at specific physical locations within the GE, optimally at positions 8 to 13 (Fig. 3). A model of slippage that occurs during mRNA editing in paramyxoviruses has been proposed (21). If a similar model of slippage applies to polyadenylation, then the U tract base pairs with the 3′ end of the mRNA, and the last U of the U tract remains in the active site. Our data suggest that the distance from the polymerase active site to the factor or site that recognizes the upstream nucleotides is critical for termination.
The precise function of the upstream sequence element in termination is unknown, nor is it known whether these nucleotides are recognized in the template strand, the mRNA strand, or both. However, the distance from these upstream nucleotides to the end of the U tract remains constant only in the template during slippage. The corresponding distance in the nascent mRNA increases by one nucleotide with each slippage event. Whether the critical distance between the U tract and upstream element is therefore recognized in the template strand rather than in the mRNA strand requires further study. In general, the mechanism of termination is poorly understood. Further work is needed to investigate the steps that may occur during termination (e.g., polymerase pausing, slippage, polyadenylation, and mRNA release), as well as the roles of the upstream sequence and U tract in these putative steps. For VSV, it has been demonstrated that slippage is not sufficient for termination (3).
Sequences outside the GE.
The sequence of GE1 in the replicon UAA-U5 was identical to the sequence of the GE of the SH gene (Fig. 1 and 3A). However, the TE of UAA-U5 was less than 50%, while the TE of a replicon containing the entire SH/G gene junction and portions of the SH and G genes was >90% (19). Termination is also highly efficient at the SH/G junction in RS virus-infected cells (10). Thus, our data show that the sequence of the SH GE was not sufficient to direct highly efficient termination in a heterologous context. Similarly, the sequence of GE1 in the replicon UAUA-U4 was identical to the sequence of the GE of the NS1 gene (Fig. 1 and 5A). However, termination was substantially less efficient at GE1 in UAUA-U4 than at the NS1 GE in the context of a replicon containing parts of the NS1 and NS2 genes (19).
These results suggest that sequences outside the GE may affect the efficiency of termination. The GE and GS are the only conserved sequences in the gene junctions of RS virus, and previous studies have indicated that the GE is sufficient for termination (33). It has therefore been assumed that nonconserved sequences outside the GE have little or no role in signaling termination. However, for simian virus 5, the first nucleotide of the nonconserved IG region is critical for termination when the U tract is reduced to the minimal conserved length, 4 nt (40). For RS virus, further work is required to determine whether the IG region or other sequences may be responsible for enhancing TE at the NS1 and SH GEs.
Termination and gene regulation.
In the Mononegavirales, the relative expression levels of the genes are critical, and gene expression is controlled by transcriptional termination, attenuation, and other mechanisms (24, 27, 34, 46). Our experiments showed that different point mutations in the M gene end decreased the efficiency of termination to various degrees and increased readthrough transcription into the downstream SH gene. In a natural RS virus infection, the downstream SH cistron of a dicistronic M-SH mRNA would be translated inefficiently (30), and thus SH protein expression would be downregulated. Genes further downstream would be upregulated, since there would be no transcriptional attenuation across the M/SH junction. Since RNA viruses exist as quasispecies (22), a virus with decreased termination at the M gene end could be selected, given sufficient selective pressure to downregulate SH gene expression or to upregulate downstream genes. Indeed, sequence differences in the M gene end of group B isolates of RS virus are associated with increased readthrough of the M/SH junction and decreased expression of the SH gene compared to group A isolates (D. A. Buonagurio, personal communication). For example, in the group B strain 18537, the sequence of the M gene end is 3′-UCCAUUUAUUUU-5′ (26). According to our data, both the C residue at position 3 and the U4 tract (underlined) would be expected to decrease the TE at the M gene end in this strain (Fig. 3 and 4).
Theoretically, expression of any gene of RS virus could be downregulated by transcriptional readthrough. For example, expression of the matrix protein of measles virus is sometimes downregulated by this mechanism in subacute sclerosing panencephalitis (5). Selection for mutations in the GE is thus a simple mechanism by which a nonsegmented negative-strand RNA virus can downregulate transcription of an internal gene, despite the fact that transcription is obligatorily sequential (13, 34). Most sequence analyses of RS virus have focused on the coding regions of selected genes, while few published studies have focused on the sequences of the gene junctions (6, 26, 49). Our data will facilitate the identification of viral isolates in which selection for increased transcriptional readthrough has occurred.
ACKNOWLEDGMENTS
We thank John Barr and Sean Whelan for critical reading of the manuscript, and we thank other past and present members of the Wertz and L. A. Ball laboratories for helpful suggestions on this work. We thank Bernard Moss for providing MVA-T7, and we thank Xiaoling Tang, Sarah Ballard, Claire Hankin, and Kristen Rogers for technical assistance.
This work was supported by Public Health Service grant AI20181 from the NIH to G.W.W.
REFERENCES
- 1.Barik S. The structure of the 5′ terminal cap of the respiratory syncytial virus mRNA. J Gen Virol. 1993;74:485–490. doi: 10.1099/0022-1317-74-3-485. [DOI] [PubMed] [Google Scholar]
- 2.Barik S. Transcription of human respiratory syncytial virus genome RNA in vitro: requirement of cellular factor(s) J Virol. 1992;66:6813–6818. doi: 10.1128/jvi.66.11.6813-6818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr J N, Whelan S P, Wertz G W. cis-acting signals involved in termination of vesicular stomatitis virus mRNA synthesis include the conserved AUAC and the U7 signal for polyadenylation. J Virol. 1997;71:8718–8725. doi: 10.1128/jvi.71.11.8718-8725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr J N, Whelan S P, Wertz G W. Role of the intergenic dinucleotide in vesicular stomatitis virus RNA transcription. J Virol. 1997;71:1794–1801. doi: 10.1128/jvi.71.3.1794-1801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaneo R, Schmid A, Rebmann G, Baczko K, Ter Meulen V, Bellini W J, Rozenblatt S, Billeter M A. Accumulated measles virus mutations in a case of subacute sclerosing panencephalitis: interrupted matrix protein reading frame and transcription alteration. Virology. 1986;154:97–107. doi: 10.1016/0042-6822(86)90433-2. [DOI] [PubMed] [Google Scholar]
- 6.Collins P L, Dickens L E, Buckler-White A, Olmsted R A, Spriggs M K, Camargo E, Coelingh K V. Nucleotide sequences for the gene junctions of human respiratory syncytial virus reveal distinctive features of intergenic structure and gene order. Proc Natl Acad Sci USA. 1986;83:4594–4598. doi: 10.1073/pnas.83.13.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins P L, Hill M G, Cristina J, Grosfeld H. Transcription elongation factor of respiratory syncytial virus, a nonsegmented negative-strand RNA virus. Proc Natl Acad Sci USA. 1996;93:81–85. doi: 10.1073/pnas.93.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins P L, McIntosh K, Chanock R M. Parainfluenza viruses. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1205–1241. [Google Scholar]
- 9.Collins P L, McIntosh K, Chanock R M. Respiratory syncytial virus. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1313–1351. [Google Scholar]
- 10.Collins P L, Wertz G W. cDNA cloning and transcriptional mapping of nine polyadenylated RNAs encoded by the genome of human respiratory syncytial virus. Proc Natl Acad Sci USA. 1983;80:3208–3212. doi: 10.1073/pnas.80.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conzelmann K K. Nonsegmented negative-strand RNA viruses: genetics and manipulation of viral genomes. Annu Rev Genet. 1998;32:123–162. doi: 10.1146/annurev.genet.32.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Curran J, Kolakofsky D. Replication of paramyxoviruses. Adv Virus Res. 1999;54:403–422. doi: 10.1016/s0065-3527(08)60373-5. [DOI] [PubMed] [Google Scholar]
- 13.Dickens L E. Ph.D. thesis. University of North Carolina at Chapel Hill; 1985. [Google Scholar]
- 14.Dickens L E, Collins P L, Wertz G W. Transcriptional mapping of human respiratory syncytial virus. J Virol. 1984;52:364–369. doi: 10.1128/jvi.52.2.364-369.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupuy L C, Dobson S, Bitko V, Barik S. Casein kinase 2-mediated phosphorylation of respiratory syncytial virus phosphoprotein P is essential for the transcription elongation activity of the viral polymerase; phosphorylation by casein kinase 1 occurs mainly at Ser215 and is without effect. J Virol. 1999;73:8384–8392. doi: 10.1128/jvi.73.10.8384-8392.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerson S U. Transcription of vesicular stomatitis virus. In: Wagner R R, editor. The Rhabdoviruses. New York, N.Y: Plenum Press; 1987. pp. 245–269. [Google Scholar]
- 17.Fearns R, Collins P L. Role of the M2–1 transcription antitermination protein of respiratory syncytial virus in sequential transcription. J Virol. 1999;73:5852–5864. doi: 10.1128/jvi.73.7.5852-5864.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Barreno B, Delgado T, Melero J A. Oligo(A) sequences of human respiratory syncytial virus G protein gene: assessment of their genetic stability in frameshift mutants. J Virol. 1994;68:5460–5468. doi: 10.1128/jvi.68.9.5460-5468.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy R W, Harmon S B, Wertz G W. Diverse gene junctions of respiratory syncytial virus modulate the efficiency of transcription termination and respond differently to M2-mediated antitermination. J Virol. 1999;73:170–176. doi: 10.1128/jvi.73.1.170-176.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy R W, Wertz G W. The product of the respiratory syncytial virus M2 gene ORF1 enhances readthrough of intergenic junctions during viral transcription. J Virol. 1998;72:520–526. doi: 10.1128/jvi.72.1.520-526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hausmann S, Garcin D, Delenda C, Kolakofsky D. The versatility of paramyxovirus RNA polymerase stuttering. J Virol. 1999;73:5568–5576. doi: 10.1128/jvi.73.7.5568-5576.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holland J J, De La Torre J C, Steinhauer D A. RNA virus populations as quasispecies. Curr Top Microbiol Immunol. 1992;176:1–20. doi: 10.1007/978-3-642-77011-1_1. [DOI] [PubMed] [Google Scholar]
- 23.Hwang L N, Englund N, Pattnaik A K. Polyadenylation of vesicular stomatitis virus mRNA dictates efficient transcription termination at the intercistronic gene junctions. J Virol. 1998;72:1805–1813. doi: 10.1128/jvi.72.3.1805-1813.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iverson L E, Rose J K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981;23:477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- 25.Jacques J, Kolakofsky D. Pseudo-templated transcription in prokaryotic and eukaryotic organisms. Genes Dev. 1991;5:707–713. doi: 10.1101/gad.5.5.707. [DOI] [PubMed] [Google Scholar]
- 26.Johnson P R, Collins P L. The A and B subgroups of human respiratory syncytial virus: comparison of intergenic and gene-overlap sequences. J Gen Virol. 1988;69:2901–2906. doi: 10.1099/0022-1317-69-11-2901. [DOI] [PubMed] [Google Scholar]
- 27.Kato A, Kiyotani K, Hasan M K, Shioda T, Sakai Y, Yoshida T, Nagai Y. Sendai virus gene start signals are not equivalent in reinitiation capacity: moderation at the fusion protein gene. J Virol. 1999;73:9237–9246. doi: 10.1128/jvi.73.11.9237-9246.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kireeva M L, Komissarova N, Waugh D S, Kashlev M. The 8-nucleotide-long RNA:DNA hybrid is a primary stability determinant of the RNA polymerase II elongation complex. J Biol Chem. 2000;275:6530–6536. doi: 10.1074/jbc.275.9.6530. [DOI] [PubMed] [Google Scholar]
- 29.Kolakofsky D, Pelet T, Garcin D, Hausmann S, Curran J, Roux L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: the rule of six revisited. J Virol. 1998;72:891–899. doi: 10.1128/jvi.72.2.891-899.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo L, Fearns R, Collins P L. Analysis of the gene start and gene end signals of human respiratory syncytial virus: quasi-templated initiation at position 1 of the encoded mRNA. J Virol. 1997;71:4944–4953. doi: 10.1128/jvi.71.7.4944-4953.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo L, Fearns R, Collins P L. The structurally diverse intergenic regions of respiratory syncytial virus do not modulate sequential transcription by a dicistronic minigenome. J Virol. 1996;70:6143–6150. doi: 10.1128/jvi.70.9.6143-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuo L, Grosfeld H, Cristina J, Hill M G, Collins P L. Effects of mutations in the gene-start and gene-end sequence motifs on transcription of monocistronic and dicistronic minigenomes of respiratory syncytial virus. J Virol. 1996;70:6892–6901. doi: 10.1128/jvi.70.10.6892-6901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamb R A, Kolakofsky D. Paramyxoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fields virology. 3rd ed. Vol. 1. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1177–1204. [Google Scholar]
- 35.Laskey R A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65:363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- 36.Lehrach H, Diamond D, Wozney J M, Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977;16:4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- 37.Nagai Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Nudler E, Mustaev A, Lukhtanov E, Goldfarb A. The RNA-DNA hybrid maintains the register of transcription by preventing backtracking of RNA polymerase. Cell. 1997;89:33–41. doi: 10.1016/s0092-8674(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 39.Pringle C R, Easton A J. Monopartite negative strand RNA genomes. Semin Virol. 1997;8:49–57. [Google Scholar]
- 40.Rassa J C, Parks G D. Highly diverse intergenic regions of the paramyxovirus simian virus 5 cooperate with the gene end U tract in viral transcription termination and can influence reinitiation at a downstream gene. J Virol. 1999;73:3904–3912. doi: 10.1128/jvi.73.5.3904-3912.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rassa J C, Wilson G M, Brewer G A, Parks G D. Spacing constraints on reinitiation of paramyxovirus transcription: the gene end U tract acts as a spacer to separate gene end from gene start sites. Virology. 2000;274:438–449. doi: 10.1006/viro.2000.0494. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 43.Stillman E A, Whitt M A. Mutational analyses of the intergenic dinucleotide and the transcriptional start sequence of vesicular stomatitis virus (VSV) define sequences required for efficient termination and initiation of VSV transcripts. J Virol. 1997;71:2127–2137. doi: 10.1128/jvi.71.3.2127-2137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stillman E A, Whitt M A. Transcript initiation and 5′-end modifications are separable events during vesicular stomatitis virus transcription. J Virol. 1999;73:7199–7209. doi: 10.1128/jvi.73.9.7199-7209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.von Hippel P H. An integrated model of the transcription complex in elongation, termination, and editing. Science. 1998;281:660–665. doi: 10.1126/science.281.5377.660. [DOI] [PubMed] [Google Scholar]
- 46.Wertz G W, Perepelitsa V P, Ball L A. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc Natl Acad Sci USA. 1998;95:3501–3506. doi: 10.1073/pnas.95.7.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyatt L S, Moss B, Rozenblatt S. Replication-deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology. 1995;210:202–205. doi: 10.1006/viro.1995.1332. [DOI] [PubMed] [Google Scholar]
- 48.Yu Q, Hardy R W, Wertz G W. Functional cDNA clones of the human respiratory syncytial (RS) virus N, P, and L proteins support replication of RS virus genomic RNA analogs and define minimal trans-acting requirements for RNA replication. J Virol. 1995;69:2412–2419. doi: 10.1128/jvi.69.4.2412-2419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamora M, Samal S K. Gene junction sequences of bovine respiratory syncytial virus. Virus Res. 1992;24:115–121. doi: 10.1016/0168-1702(92)90035-8. [DOI] [PubMed] [Google Scholar]