Abstract
Sprouty and Spred {Sprouty-related EVH1 [Ena/VASP (vasodilator-stimulated phosphoprotein) homology 1] domain} proteins have been identified as antagonists of growth factor signalling pathways. We show here that Spred-1 and Spred-2 appear to have distinct mechanisms whereby they induce their effects, as the Sprouty domain of Spred-1 is not required to block MAPK (mitogen-activated protein kinase) activation, while that of Spred-2 is required. Similarly, deletion of the C-terminal Sprouty domain of Spred-1 does not affect cell-cycle progression of G0-synchronized cells through to S-phase following growth factor stimulation, while the Sprouty domain is required for Spred-2 function. We also demonstrate that the inhibitory function of Spred proteins is restricted to the Ras/MAPK pathway, that tyrosine phosphorylation is not required for this function, and that the Sprouty domain mediates heterodimer formation of Spred proteins. Growth-factor-mediated activation of the small GTPases, Ras and Rap1, was able to be regulated by Spred-1 and Spred-2, without affecting receptor activation. Taken together, these results highlight the potential for different functional roles of the Sprouty domain within the Spred family of proteins, suggesting that Spred proteins may use different mechanisms to induce inhibition of the MAPK pathway.
Keywords: cell signalling, mitogen-activated protein kinase (MAPK), Ras, Spred, Sprouty
Abbreviations: BrdU, bromodeoxyuridine; CAT, chloramphenicol acetyltransferase; DMEM, Dulbecco's modified Eagle's medium; DTT, dithiothreitol; EGF, epidermal growth factor; EGFR, EGF receptor; ERK, extracellular-signal-regulated kinase; EST, expressed sequence tag; EVH1, Ena/VASP (vasodilator-stimulated phosphoprotein) homology 1; FCS, foetal calf serum; FGF, fibroblast growth factor; FKHR, Forkhead homologue in rhabdomyosarcoma; GFP, green fluorescent protein; GST, glutathione S-transferase; HA, haemagglutinin; HEK, human embryonic kidney; JNK, c-Jun N-terminal kinase; KBD, c-kit-binding domain; MAPK, mitogen-activated protein kinase; NGF, nerve growth factor; RBD, Ras/Rap1-binding domain; RTK, receptor tyrosine kinase; Spred, Sprouty-related EVH1 domain; VEGF, vascular endothelial growth factor
INTRODUCTION
The process of normal development in an organism requires a highly co-ordinated and regulated control of cellular growth and differentiation. Positive signals for growth and differentiation are relayed by growth factors and their cognate receptors, most commonly RTKs (receptor tyrosine kinases), on cell membranes and amplified intracellularly by protein signal transduction networks. Negative control of signalling can be achieved by withdrawal of the initiator growth factor itself and/or by signal inhibitory proteins that serve to antagonize intracellular propagation of the initial stimulus. An oft-repeated paradigm of developmental control is that positive signals induce negative regulatory proteins that eventually inhibit these same signals, in a classical autoregulatory feedback loop [1].
One class of negative regulatory proteins that inhibit mitogenic signalling downstream of some RTKs is the Sprouty family. Sprouty (spry) was initially identified by genetic screens in Drosophila as an antagonist of Breathless FGF (fibroblast growth factor) receptor signalling during tracheal branching [2]. Spry mutations induced excessive branching of the tracheal network, while forced expression of spry blocked tracheal branching [2]. Subsequently, it was shown in the Drosophila system that spry action was not limited to FGF activity, but acted downstream of a range of RTKs, including Torso and Sevenless [3–6]. Unlike Drosophila, where there is but a single spry gene [7], four homologous mammalian spry genes have been identified [2,8]. Mammalian spry genes exhibit a dynamic expression pattern throughout embryonic development, and their expression can be up-regulated rapidly by certain growth factors [9–12]. Like Drosophila Sprouty, vertebrate Sprouty proteins appear to act as key regulators of developmental processes, such as limb formation [11], lung branching morphogenesis [13] and angiogenesis [14]. A distinctive feature of vertebrate Sprouty proteins is their selective antagonism of only a subset of growth factors, with Sprouty1 and Sprouty2 inhibiting FGF- and VEGF (vascular endothelial growth factor)-induced signalling, but not EGF (epidermal growth factor) nor chemical (PMA) activation of signalling [7,14].
More recently, another family of apparent RTK-negative regulatory proteins have been described [15]. Termed Spred proteins, for Sprouty-related EVH1 [Ena/VASP (vasodilator-stimulated phosphoprotein) homology 1] domain, the two mouse variants described, Spred-1 and Spred-2, contain an N-terminal EVH1 domain, a central KBD (c-kit-binding domain) and a conserved cysteine-rich (Sprouty) domain at the C-terminus, analogous to that found in Sprouty proteins. Spred proteins were able to block MAPK (mitogen-activated protein kinase) activation induced by NGF (nerve growth factor) and EGF by a mechanism requiring both the EVH1 and Sprouty domains, and appeared to function downstream of Ras [15].
In the present paper, we show that there appears to be distinct mechanisms whereby Spred-1 and Spred-2 regulate MAPK signalling. The Sprouty domain of Spred-1 is not absolutely required to block MAPK activation, in contrast with Spred-2, where it is essential for inhibitory activity. Targeting the Sprouty-domain-truncated Spred proteins to the membrane restores the inhibitory activity of the mutant Spred proteins. This indicates the inhibitory function of the Spred proteins does not reside in the Sprouty domain. The distinct functional requirements for the Sprouty domain between Spred-1 and Spred-2 is also observed in assays of neuronal differentiation and cell-cycle progression of G0-synchronized cells to S-phase following growth factor stimulation. Both Spred-1 and Spred-2 are able to reduce the levels of the active forms of the small GTPase proteins Ras and Rap1, but have no apparent effect on receptor activation following growth factor stimulation. This suggests that the target of Spred inhibitory activity lies between these two points in RTK signalling pathways. We have explored the role of the Sprouty domain, and demonstrate that this domain mediates interaction between Spred proteins, inducing Spred heterodimers, suggesting that considerable scope for fine-tuning of Spred inhibitory responses may exist.
EXPERIMENTAL
Antibodies
Anti-FLAG, anti-HA (haemagglutinin), mouse monoclonal anti-(phospho-p44/42 MAPK) [ERK1/2 (extracellular-signal-regulated kinase 1/2)] antibodies were from Sigma–Aldrich, anti-GFP (green fluorescent protein) antibody was from Clontech, anti-phospho-tyrosine monoclonal antibody P-Tyr-100 was from Cell Signaling Technology, and monoclonal antibody 4G10 was from Upstate Biotechnology, as was the sheep anti-EGFR (EGF receptor) polyclonal antibody. Other proteins were detected using the following antibodies: mouse monoclonal anti-(βIII tubulin) clone G712A (Promega), anti-(phospho-p38 kinase) rabbit polyclonal antibody, and anti-phospho-JNK (c-Jun N-terminal kinase) rabbit polyclonal antibody (Promega). Anti-phospho and pan-p44/42 MAPK rabbit polyclonal antibodies, anti-phospho-Akt (Thr 308), and anti-phospho-FKHR (Ser 256) (where FKHR is Forkhead homologue in rhabdomyosarcoma) (Cell Signaling Technology). Anti-BrdU (bromodeoxyuridine), anti-Ras and anti-Rap1 mouse monoclonal antibodies were from BD Biosciences. Rabbit polyclonal sera against hSpred-1 (where h stands for human) (amino acids 1–256) and hSpred-2 (amino acids 222–294) GST (glutathione S-transferase) fusion proteins were obtained as described below.
Plasmids
hSpred-1 cDNA was cloned by PCR amplification from a human glioblastoma cDNA library (a gift from Dr U. Novak, Department of Surgery, University of Melbourne, Royal Melbourne Hospital, Parkville, Australia) using 5′ and 3′ primers to the human spred-1 gene sequence information obtained from GenBank® human EST (expressed sequence tag) clones BF700928 and BF697562 respectively. hSpred-2 was cloned from GenBank® human EST clone BE897828 using 5′ and 3′ PCR oligonucleotides. Sequences of hSpred-1 (residues 1–255) and hSpred-2 (residues 222–294) were amplified by PCR, and cloned in-frame to GST in the vectors pGEX5X1 and pGEX5X2 respectively. hSpred-1 and hSpred-2 were cloned into pEF-BOS [16] with an N-terminal FLAG-tag. pEF-BOSFlag Spred-1ΔC comprised residues 1–285 of hSpred-1 with an N-terminal FLAG-tag. pEF-BOSFlag Spred-1ΔN contained residues 223–444 of hSpred-1. pEF-BOSFlag Spred-2ΔC comprised residues 1–257 of hSpred-2 and pEF-BOSFlag Spred-2ΔN contained residues 201–418 of hSpred-2. Automated DNA sequencing verified the sequences of all constructs.
Preparation of GST–Spred fusion proteins and antibodies
GST–Spred fusion protein antigen vectors were transformed into BL-21 Escherichia coli (Stratagene) and cultured overnight in 100 ml of Luria–Bertani broth containing 100 μg/ml ampicillin and chloramphenicol. Of the overnight culture, 50 ml was added to 1 litre of Luria–Bertani broth with ampicillin and grown to a D600 of 0.8. Induction was achieved by the addition of 25 mg of IPTG (isopropyl β-D-thiogalactoside). Cultures were incubated for a further 2 h before being harvested. GST-fusion proteins were purified using glutathione–agarose beads (Sigma), and eluted by the addition of ice-cold 5 mM glutathione (Sigma) in 50 mM Tris/HCl, pH 8.0. Assessment of quality and quantification of the GST-fusion proteins was performed by SDS/PAGE analysis (10% gel). Antigen (100 μg per animal) was injected into rabbits and subsequently boosted twice before the final sera was obtained. Antibody specificity was verified by Western blot analysis of transfected HEK-293T (human embryonic kidney) cell lysates.
Determination of Ras-GTP and Rap1-GTP levels
The GST–RBD (Ras/Rap1-binding domain) fusion proteins GST–Raf-A85K RBD [17] and GST–RalGDS RBD [18] were prepared essentially as described in [18]. GST–Raf-A85K RBD encodes the Ras-binding domain of the A85K mutant Raf1 (residues 51–131) which exhibits 5-fold higher binding affinity for Ras-GTP than that of wild-type Raf [17] and GST–RalGDS RBD (residues 1–97) binds to Rap1-GTP. Affinity precipitation and identification of Ras-GTP and Rap1-GTP levels were performed as described in [18,19].
Cell culture
HEK-293, NIH-3T3, HEK-293T and Flp-In™ T-REx 293™ (Invitrogen) cells were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FCS (foetal calf serum) and penicillin/streptomycin, and grown at 37 °C under 10% CO2. Tetracycline-inducible Spred-1 and Spred-2 cell lines were constructed as follows. Briefly, full-length cDNAs encoding human HA-tagged Spred-1 and Spred-2 were cloned into the pcDNA5/FRT/TO plasmid and transfected into Flp-In™ T-REx™ 293 cells. Stable clones were selected with hygromycin and blasticidin, according to the manufacturer's protocol.
For immuno-labelling experiments, cells were grown on glass coverslips in 12- or six-well cell-culture dishes. PC12 rat pheochromocytoma cells were plated on to laminin- (20 μg/ml) (Sigma) or poly(L-lysine)- (Sigma) pre-treated coverslips in 24-well plates in DMEM supplemented with 10% (v/v) horse serum and 5% (v/v) FCS.
Cell transfection
At 1 day before transfection, HEK-293T or NIH-3T3 cells were seeded in 12-well, six-well or 10-cm-diameter cell-culture dishes, and transiently transfected using the Effectene transfection reagent (Qiagen) or calcium phosphate. PC12 cells were seeded in either six- or 24-well cell-culture dishes, and transfected using either GenePORTER 2 transfection reagent (Gene Therapy Systems) or DMRIE-C reagent (Invitrogen).
Growth factors and inhibitors
Growth factors were used at the following concentrations: NGF-β, 100 ng/ml (Sigma, N2513); EGF, 200 ng/ml (Sigma, E9644). Mouse VEGF was used at 100 ng/ml, and was kindly provided by Dr Steven Stacker (Ludwig Institute for Cancer Research).
PCR
PCRs were performed using the high-fidelity Pfx polymerase (Invitrogen) with Reaction Enhancer, according to the manufacturer's specifications.
Immunoblots
Cells (6×105) were lysed for 1 h by rotating at 4 °C in 250 μl RIPA buffer [0.5% (v/v) Nonidet P40, 20 mM Tris/HCl, pH 7.5, 0.1% (w/v) SDS, 1% (v/v) Triton X-100, 0.15 M NaCl, 2 mM EDTA, 10% (v/v) glycerol, 1× protease inhibitor cocktail (Calbiochem) and 1× phosphatase inhibitor cocktail (Calbiochem)]. Lysates were centrifuged at 10000 g for 15 min at 4 °C. Protein concentration was determined using the BCA (bicinchoninic acid) system (Sigma–Aldrich), and equal amounts of protein were loaded into each lane of an SDS/10% polyacrylamide gel. The proteins were transferred on to PVDF (Millipore) or nitrocellulose (Osmonics) membranes and detected with Protein A, anti-mouse, -sheep or -rabbit secondary antibodies coupled to HRP (horseradish peroxidase), and chemiluminescence was detected using the SuperSignal West Dura (Pierce) or ECL® (enhanced chemiluminescence) (Amersham Biosciences) Western blotting detection reagents. Membranes were stripped using the Restore Western Blot Stripping Buffer (Pierce).
Immunoprecipitation
Cells were lysed in lysis buffer [1% (v/v) Triton X-100, 20 mM Tris/HCl, pH 7.5, 150 mM NaCl, 2 mM EDTA and 10% (v/v) glycerol] with protease and phosphatase inhibitors (Calbiochem), and lysates were precipitated with 5 μg of antibody for between 1 and 24 h at 4 °C on a wheel. Protein LA beads (Sigma, 20 μl, 1:1) were then added for a further 1 h at 4 °C. Immunocomplexes were pelleted at 10000 g in a microcentrifuge and washed five times in lysis buffer. A 50 μl volume of 1× Laemmli loading buffer with 250 mM DTT (dithiothreitol) was added to the beads and incubated at 100 °C for 5 min. The samples (20 μl amounts) were resolved on SDS/10% polyacrylamide gels for analysis.
Metabolic labelling of cells and phosphoamino acid analysis
HEK-293T cells (6×106 cells/10-cm-diameter dish) transfected with Spred-1 or Spred-2 plasmids were serum-starved for 16 h, washed twice in phosphate-free DMEM and incubated in 4 ml of phosphate-free DMEM containing 32Pi (Amersham Biosciences) (0.4 mCi/ml) for 2 h at 37 °C. Cells were stimulated (or not stimulated) with 20% (v/v) FCS for 15 min, washed once with ice-cold TBS (Tris-buffered saline) containing 1 mM sodium orthovanadate and 2 mM DTT. Cells were lysed in lysis buffer (see the previous section) with the following phosphatase inhibitors: 1 mM sodium orthovanadate, 10 mM NaF, 5 mM β-glycerophosphate, 1 mM sodium molybdate and 2 mM sodium pyrophosphate, and subjected to immunoprecipitation using anti-FLAG beads. Immunoprecipitates were washed four times with cell lysis buffer, two times with RIPA buffer, resolved by SDS/PAGE and transferred on to a PVDF membrane. Spred proteins detected by immunoblotting with anti-FLAG antibody were excised and subjected to phospho-amino acid analysis as described [20].
Immunofluorescence
Cells were plated in 24-well plates on coverslips. The cells were washed with PBS then fixed with 3% (w/v) paraformaldehyde/3% (w/v) sucrose/0.2% (v/v) Triton X-100 for 10 min. Subsequently, the cells were washed in PBS three times, then the primary antibody was added at various dilutions in 1% (w/v) BSA in PBS for 1 h. A further three washes were performed in PBS, before the addition of the secondary antibody (Alexa Fluor® 488 anti-rabbit and Alexa Fluor® 594 anti-mouse; Molecular Probes) in 1% (w/v) BSA in PBS for 1 h. A further three washes in PBS were followed by the addition of DAPI (4,6-diamidino-2-phenylindole) added at 1:10000 in PBS for 2 min. The cells were finally washed three times in PBS, then mounted using Vectashield (Vector Laboratories) mounting medium on to microscope slides and sealed with nail polish. Cells were viewed under the fluorescence microscope using oil-immersion lenses (Leica) from ×40 to ×100 magnification.
CAT (chloramphenicol acetyltransferase) reporter assays
Phospho-Elk-1 transactivation assays (Stratagene) were performed using the CAT ELISA system (Roche Diagnostics), essentially as recommended. Cells in six-well plates were transfected with a variety of mammalian expression constructs, Gal4-CAT plasmid (100 ng), pFA2-Elk-1 fusion transactivator plasmid (50 ng) and pCMV-lacZ (50 ng) for the assessment of transfection efficiency. At 24 h after transfection, cells were serum-starved for 24 h, and then stimulated with 10% (v/v) serum for 6 h. Cells were then lysed, and the lysates were loaded in duplicate on to anti-CAT antibody-coated wells.
LacZ expression was determined by absorbance spectroscopy (405 nm) on lysate samples (100 μl) added to 900 μl of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4·H2O, 10 mM KCl, 1 mM MgSO4·7H2O and 40 mM 2-mercaptoethanol), with the addition of 200 μl ONPG (o-nitrophenyl β-D-galactopyranoside) (4 mg/ml in distilled water). CAT ELISA absorbance recordings were standardized according to Lac Z expression and protein concentration (Protein Assay Dye Reagent Concentrate; Bio-Rad).
BrdU-incorporation assays and immunofluorescence
The S-phase assays were performed using NIH-3T3 cells transfected with a variety of mammalian expression constructs of Spred-1 or -2 as indicated, and, after a 12-h period, were placed in serum-free medium for 24 h. Cells were then re-stimulated with 10% (v/v) serum for 16 h. At the time of the addition of serum to the cells, BrdU (Roche) at a concentration of 10 μM was also added to the medium. Cells were processed for immunofluorescence using 3% (w/v) paraformaldehyde/0.2% (v/v) Triton X-100 fixation for 10 min. Mouse monoclonal anti-BrdU and rabbit polyclonal anti-FLAG antibodies were used as the primary antibodies, and Alexa Fluor® 488 anti-mouse and Alexa Fluor® 594 anti-rabbit as the secondary antibodies. Cells were examined under the microscope to ascertain the percentage of FLAG-positive cells that were also positive for BrdU incorporation.
RESULTS
Tyrosine phosphorylation is not required for functional activity of hSpred proteins
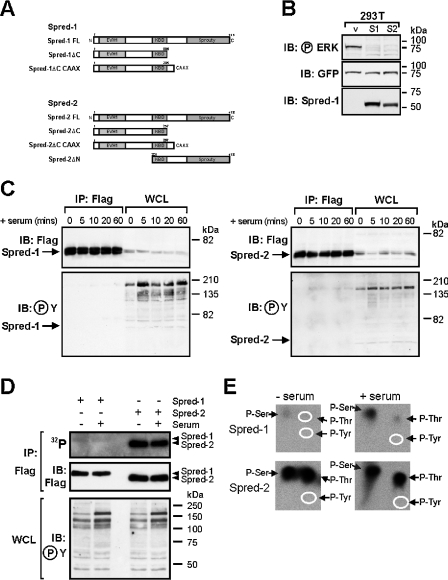
Phosphorylation of Sprouty proteins at a conserved N-terminal tyrosine residue has been reported to control the inhibitory activity of the mSprouty1 (where m stands for mouse) and mSprouty2 proteins [21], and both mSpred-1 and Spred-2 are phosphorylated on tyrosine by the c-kit receptor [15,22]. We therefore wished to assess the tyrosine phosphorylation status of Spred proteins under conditions in which they were repressing ERK activation. We transfected HEK-293T cells with full-length constructs of hSpred-1 or hSpred-2 (Figure 1A) and confirmed that they were able to clearly inhibit induction of GFP–ERK2 phosphorylation in these cells in response to serum (Figure 1B). However, immunoprecipitation of the Spred proteins throughout a time course of serum induction, followed by Western blotting with an anti-phosphotyrosine antibody, indicated no detectable phosphorylation of the abundant Spred proteins, despite readily detectable increases in tyrosine phosphorylation of several cellular proteins in response to serum stimulation (Figure 1C). Conceivably, very low levels of Spred-1 or Spred-2 tyrosine phosphorylation may be undetectable by anti-phosphotyrosine immunoblotting. We therefore used phospho-amino acid analysis to determine if Spred-1 and Spred-2 are phosphorylated on tyrosine residues before or after serum stimulation. Serum-starved HEK-293T cells expressing Spred-1 or Spred-2 were metabolically labelled with 32Pi and stimulated with serum for 15 min. Immunoprecipitated Spred proteins were then run on an SDS/polyacrylamide gel, transferred on to a PVDF membrane and subjected to autoradiography. Figure 1(D) shows that both Spred-1 and Spred-2 were 32P-labelled in serum-starved and serum-stimulated cells, indicating that both proteins are phosphorylated. However, Spred-2 was phosphorylated much more strongly than Spred-1 (Figure 1D, compare top panel with middle panel showing total Spred protein levels). Despite no change in the levels of 32P-incorporated by Spred-1 and Spred-2 after serum stimulation, we detected a significant increase in tyrosine phosphorylation of several cellular proteins in response to serum treatment (Figure 1D, bottom panel). Phospho-amino acid analysis of the 32P-radiolabelled Spred proteins revealed that Spred-1 and -2 contained phosphoserine and/or phosphothreonine, but lacked detectable phosphotyrosine, irrespective of whether cells were serum treated or not (Figure 1E). As well as containing much higher levels of both phosphoserine and phosphothreonine than Spred-1 (note the 3-fold difference in autoradiography times; see the legend to Figure 1), Spred-2 showed a significantly higher ratio of phosphothreonine to phosphoserine than Spred-1. It is noteworthy that, although Spred-1 phosphoserine and phosphothreonine levels appear to increase after serum stimulation (Figure 1E), we did not observe a corresponding serum-dependent increase in 32P-labelling of Spred-1 (Figure 1D), suggesting that some loss of the 32P-labelled Spred-1 sample from unstimulated cells may have occurred during the phospho-amino acid analysis. Together our results suggest that, while Spred-1 and Spred-2 are capable of inhibiting serum-induced ERK activation, this does not coincide with any detectable Spred-1 or Spred-2 tyrosine phosphorylation.
Figure 1. Tyrosine phosphorylation of Spred proteins is not required for suppression of ERK activation.
(A) Schematic diagram of domain structure of hSpred and mutant proteins used in the present study. Spred-1 FL, full-length Spred-1, amino acids 1–444; Spred-1ΔC, amino acids 1–285; Spred-1ΔC CAAX, amino acids 1–285 with CAAX motif of Ras; Spred-2 FL, full-length Spred-2 amino acids 1–418; Spred-2ΔC, amino acids 1–257; Spred-2ΔC CAAX, amino acids 1–257 with CAAX motif of Ras; Spred-2ΔN amino acids 210–418. (B) HEK-293T cells transfected with vector alone (v: pEFBOS; 2 μg) or with vectors encoding FLAG–Spred-1 (S1; 2 μg) or FLAG–Spred-2 (S2; 2 μg) and GFP–ERK2 (GFP; 0.2 μg) were serum-starved for 16 h, then stimulated with 10% serum for 15 min. Whole-cell lysates were probed sequentially by Western blotting with the indicated antibodies; the anti-GFP antibody detects GFP–ERK2 protein. Note that the anti-Spred-1 antibody cross-reacts with Spred-2. Molecular-mass sizes are indicated in kDa. (C) HEK-293T cells were transfected with vectors encoding FLAG–Spred-1 (5 μg) or FLAG–Spred-2 (5 μg) and serum-starved for 16 h, then stimulated with 20% serum (+ serum) for the indicated times. Whole-cell lysates were either immunoprecipitated with anti-FLAG antibody (IP: Flag) or loaded as is (WCL) and the blots probed with either anti-FLAG or anti-phosphotyrosine (℗Y) antibodies as indicated. Protein A–HRP (horseradish peroxidase) rather than anti-mouse HRP was used for detection of the mouse anti-phosphotyrosine antibody to avoid recognition of the IgH chain present in the immunoprecipitates. Molecular-mass sizes are indicated in kDa. (D) HEK-293T cells expressing FLAG–Spred-1 or -2 were serum-starved for 16 h, metabolically labelled with 32Pi for 2 h, then stimulated (+) or not stimulated (−) with 20% serum for 15 min. Spred proteins were immunoprecipitated (IP: Flag), resolved by SDS/PAGE, transferred on to PVDF, subjected to autoradiography for 80 h (32P) then probed with an anti-FLAG antibody (IB: Flag). Samples of whole-cell lysate (WCL) (3% of that used in IP) were analysed by immunoblotting with an anti-phosphotyrosine antibody (IB: ℗Y). Molecular-mass sizes are indicated in kDa. (E) Bands corresponding to FLAG–Spred-1 and -2 from unstimulated (− serum) and serum-stimulated cells (+ serum) were excised from the membrane shown in (D) and subjected to phospho-amino acid analysis [20] and autoradiography for 170 or 60 h (Spred-1 and -2 samples respectively). The positions of phosphoserine (P-Ser), phosphothreonine (P-Thr) and phosphotyrosine (P-Tyr), determined by ninhydrin staining of unlabelled phospho-amino acid standards added to the radiolabelled samples, are indicated by arrows and/or white ovals.
Spred proteins have distinct requirements for the Sprouty domain to suppress Ras/MAPK pathway activation
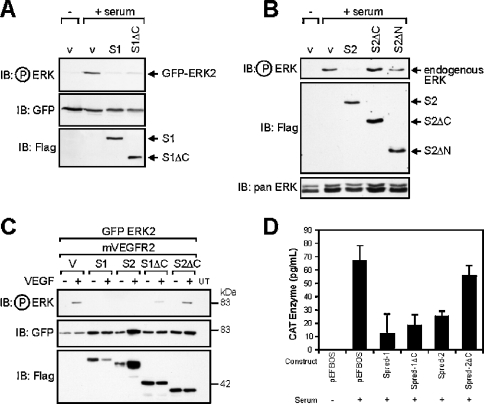
mSpred-1 and mSpred-2 have been reported to block ERK activation induced by a range of stimuli [15,22]. We wished to determine the role of the EVH1 and Sprouty domains of the Spred proteins in this activity. We compared ERK activation in the presence of full length and N- and C-terminal truncation mutants of Spred-1 and Spred-2 (Figures 2A and 2B) in response to serum. Both full-length Spred-1 and Spred-2 were similarly effective in blocking ERK2 activation induced by serum (Figures 2A and 2B). However, a very different result was obtained when we analysed the properties of C-terminal truncated mutants of Spred-1 (S1ΔC) and Spred-2 (S2ΔC), and an N-terminal truncation mutant of Spred-2 (S2ΔN). Spred-2ΔC, where the Sprouty domain is removed, was unable to suppress ERK activation downstream of serum or VEGF (Spred-2ΔC, Figures 2B and 2C). Likewise, Spred-2ΔN, which contains the KBD and Sprouty domains, was also unable to inhibit ERK activation (S2ΔN; Figure 2B). In contrast, a construct lacking the Sprouty domain of Spred-1, S1ΔC, was effective in interfering with ERK2 activation induced by any of the stimuli we used (Figures 2A and 2C). The same trend was observed in an Elk-1 transactivation assay, an in vivo readout of endogenous ERK activation, with Spred1-ΔC effective in inhibiting Elk-1 phosphorylation, but with Spred-2ΔC unable to do so (Figure 2D).
Figure 2. Differential effects of Sprouty domain deletion on Spred-1 and Spred-2 activity.
(A)–(C) HEK-293 cells were transfected with 0.2 μg of GFP–ERK2 construct and 2 μg of empty vector (v: pEFBOS), FLAG-tagged hSpred-1 or -2 (S1, S2), the mVEGF2 receptor cDNA (1 μg), FLAG-tagged C-terminal Sprouty domain deletion mutants of hSpred-1 or -2, (S1ΔC, S2ΔC; 2 μg), or an N-terminal deletion mutant (S2ΔN; 2 μg). At 24 h post-transfection, the cells were serum-starved for 12 h, then re-stimulated with either 10% serum, or stimulated (+) or not (−) with mVEGF (100 ng/ml) for 20 min, then lysed in RIPA buffer. Equal total protein amounts for each lysate were loaded on a SDS/PAGE gel, and the immunoblot (IB) was probed with the indicated primary antibodies. The leftmost lane of each blot in panels (A)–(C) represents the basal level of ERK2 activation in serum-starved cells. The pan ERK panel indicates the level of total endogenous ERK proteins. (D) FLAG-tagged Spred constructs were transfected into HEK-293 cells with Elk-1 reporter plasmids and stimulated with (+) or without (−) 10% serum for 6 h, then lysed and analysed by the CAT ELISA. The results are the means+S.D. for three independent experiments.
Following mitogenic stimulation, there is a rapid intracellular redistribution of ERK proteins with an accumulation of phosphorylated ERK proteins in the nucleus of NIH-3T3 cells within 10 min of stimulation [23,24]. Activation of the Ras/MAPK pathway is essential for this translocation, as blocking MEK (MAPK/ERK kinase) activity prevents ERK enrichment in the nucleus [23]. We tested whether Spred-ΔC mutant proteins could also block the nuclear enrichment of phosphorylated ERK by transiently expressing them in NIH-3T3 cells and observing the nuclear accumulation of phosphorylated ERK following serum stimulation. Removal of the C-terminal Sprouty domain of both proteins had very different effects on ERK translocation. Spred-2ΔC lost the ability to block ERK translocation, which full-length Spred-2 possessed. However, Spred-1ΔC retained the ability to inhibit the nuclear accumulation of endogenous ERK as effectively as full-length Spred-1 (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/388/bj3880445add.htm).
Spred proteins specifically block the RAS/MAPK pathway
The MAPKs comprise three closely related kinase pathways, the ERKs, JNKs and p38 MAPKs. We wanted to determine whether the Spred proteins could also affect these related pathways or whether they are specific for the ERK pathway. We co-transfected HEK-293 cells with FLAG-tagged JNK and HA-tagged p38 together with Spred constructs, and determined the activation of these kinases with phospho-specific antibodies. As shown in Figures 3(A) and 3(B), neither Spred-1 nor Spred-2, or their C-terminal mutants, was able to block activation of these kinases by sorbitol-induced osmotic stress. We confirmed this result in another cell line, NIH-3T3 cells, where transfection of the same Spred constructs failed to repress the nuclear accumulation of endogenous activated JNK and activated p38, but was able to repress nuclear accumulation of activated ERK (Figures 4C, 4E and 4G, and see Supplementary Figure 2A at http://www.BiochemJ.org/bj/388/bj3880445add.htm). The nuclear accumulation of the activated MAPK proteins was dependent on serum stimulation (Figures 4C, 4E and 4G, and see Supplementary Figure 2B at http://www.BiochemJ.org/bj/388/bj3880445add.htm). The same trend was observed using non-activation specific (pan) anti-MAPK antibodies; however, nuclear accumulation was, in this case, more difficult to determine clearly (Figures 4D, 4F and 4H, and see Supplementary Figure 2C at http://www.BiochemJ.org/bj/388/bj3880445add.htm). Spred-1 and Spred-2 were similarly unable to interfere with signalling mediated by the phosphoinositide 3-kinase pathway, with EGF-mediated phosphorylation of the protein kinase Akt and the transcription factor FKHR unaffected by co-expression of either Spred protein (Figures 3C and 3D).
Figure 3. Spred proteins specifically block the RAS/MAPK pathway, but not other related pathways.
(A) and (B) HEK-293 cells were transfected with 2 μg of either vector alone (v: pEFBOS), FLAG-tagged hSpred-1 or -2 (S1, S2) or FLAG-tagged C-terminal Sprouty domain deletion mutants of hSpred-1 or -2 (S1ΔC, S2ΔC), and with (+) or without (−) FLAG-tagged JNK (A) or HA-tagged p38 (B). At 24 h post-transfection, the cells were treated for 10 min with (+) or without (−) 0.5M sorbitol, then lysed in RIPA buffer. Equal lysate volumes were loaded on an SDS/polyacrylamide gel, and the immunoblot (IB) was probed with the indicated primary antibodies. (C) and (D) HEK-293 cells were transfected with (C) 2 μg of FLAG-tagged hSpred-1 or -2 (S1, S2) and HA-tagged Akt (0.4 μg) or (D) 4 μg of FLAG-tagged hSpred-1 or -2 (S1, S2) and GFP–FKHR (0.8 μg). Cells were serum-starved for 16 h, then treated with (+) EGF (100 ng/ml) for either 10 (C) or 90 (D) min. Equal lysate volumes were loaded on an SDS/polyacrylamide gel, and the immunoblot was probed with the indicated primary antibodies.
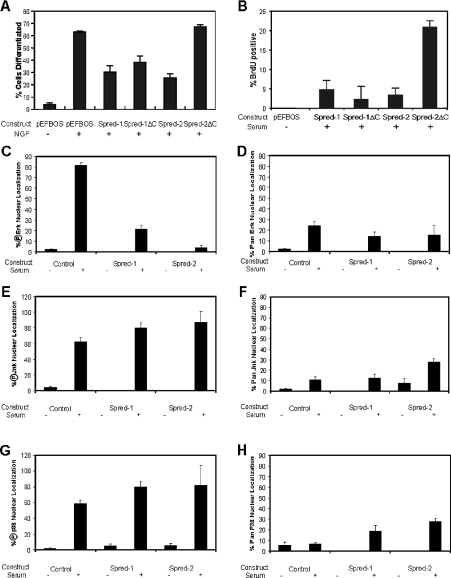
Figure 4. Compilation of Spred protein effects on cell differentiation, cell cycle, and ERK, JNK and p38 nuclear translocation.
PC12 cells (A) or NIH-3T3 cells (B)–(H) were transfected with the indicated Spred constructs and serum-starved for 12 h. Cells were then (A) stimulated (+) with NGF (100 ng/ml) for 36 h to induce neurite formation. Transfected, differentiated PC12 cells assessed as having neurites 1.5× longer than the cell body were scored and compiled from the results of three independent experiments, (B) stimulated with (+) 10% serum for 16 h, then fixed and processed for BrdU incorporation into dividing S-phase-positive cells, and (C)–(H) stimulated with (+) or without (−) 10% serum for 15 min, then fixed and stained with anti-FLAG and (C) phospho-ERK (℗Erk), (D) pan ERK, (E) phospho-JNK (℗Jnk) (F) pan JNK, (G) phospho-p38 (℗p38) or (H) pan p38 antibodies. In all panels, the results are the means+S.D. for at least three independent experiments.
The Sprouty domain of Spred-1 is not necessary to block neurite formation, while that of Spred-2 is essential
NGF-mediated differentiation of PC12 cells is critically dependent on sustained activation of the RAS/MAPK pathway [25]. mSpred-1 has been shown to block NGF-mediated PC12 differentiation [15], and we wished to determine whether the C-terminal truncated mutant of hSpred-1 could also block in this functional differentiation assay. As demonstrated in Figure 4(A), both wild-type Spred proteins efficiently hindered NGF-dependent neurite outgrowth in these cells. However, in this assay, the stark difference in the functional activity of Sprouty-domain-deficient Spred-1 and Spred-2 was again borne out, with hSpred-1ΔC able to repress neurite formation, yet hSpred-2ΔC was unable to do so (Figure 4A, and see Supplementary Figure 3 at http://www.BiochemJ.org/bj/388/bj3880445add.htm).
Loss of the Sprouty domain does not affect the ability of Spred-1 to block mitogen-stimulated S-phase progression
Activation of the ERKs is essential for cell growth and cell-cycle progression [26–29]. Hence, we reasoned that expression of Spred proteins might interfere with cellular proliferation. We synchronized Spred-transfected NIH-3T3 cells in G0 by overnight serum deprivation then restimulated with serum for 16 h, then checked by immunofluorescence for BrdU-positive nuclei. Under these conditions, there was a large increase in S-phase cells, as indicated by BrdU-positive cells following mitogenic stimulation (see Supplementary Figure 4A at http://www.BiochemJ.org/bj/388/bj3880445add.htm). However, both full-length Spred proteins, as well as the Spred-1ΔC mutant, were able to clearly reduce the number of BrdU-positive cells in comparison with the Spred-2ΔC mutant (Figure 4B, and see Supplementary Figure 4B at http://www.BiochemJ.org/bj/388/bj3880445add.htm).
Spred proteins inhibit Ras and Rap1 GTP levels without affecting receptor phosphorylation
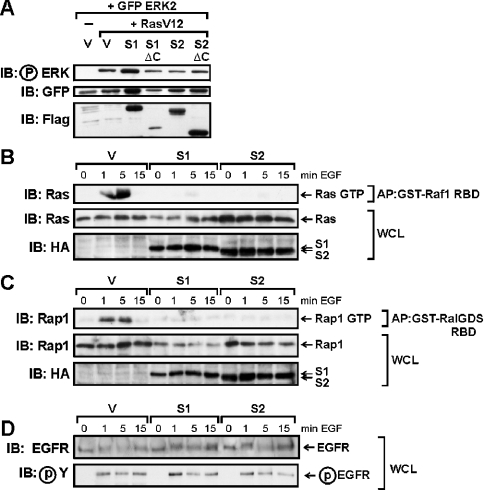
Ras and Rap1 are members of the Ras family of small GTPases that are activated by diverse extracellular stimuli and which play vital signal-relay roles in growth and differentiation [30]. We wished to determine at which level Spred proteins act/function in the RTK-mediated signalling cascade to inhibit ERK activation, and specifically whether Spred proteins interfere with activation of these two members of the Ras family of small GTPases. We first determined whether Spred proteins could inhibit ERK activation downstream of constitutively activated Ras (RasV12). As shown in Figure 5(A), neither Spred-1 nor Spred-2 was able to interfere with the strong ERK activation induced by RasV12, suggesting their mode of action was upstream of Ras. In order to measure the activation of endogenous Ras and Rap1 following RTK activation in the presence or absence of Spred expression, we generated stable isogenic HEK-293 cell lines with tetracycline-inducible expression of HA-tagged Spred-1 and Spred-2, using the Flp-In™ T-REx™ system. These cell lines permit real comparisons between Spred proteins, as the single-copy Spred constructs are incorporated at the identical genetic locus. These cells were transfected with DNA encoding the EGFR and, 24 h post-transfection, expression of Spred-1 or Spred-2 was induced for 8 h, and then the cells were serum-starved for a further 16 h while induction of Spred-1 or Spred-2 expression was maintained. The cells were then stimulated with EGF for 0, 1, 5 and 15 min to activate GTP loading of Ras and Rap1. Active GTP-bound forms of Ras and Rap1 were affinity-purified from cell lysates using specific GST-fusion proteins containing either the Ras-binding domain of the Ala85→Lys mutant of Raf1 (residues 51–131), which exhibits 5-fold higher binding affinity for Ras-GTP than that of wild-type Raf [20] or the Rap1-binding domain of RalGDS respectively. As shown in Figures 5(B) and 5(C), induction of either Spred-1 or Spred-2 decreased the proportion of both Ras and Rap1 that was in its GTP-bound form following EGF stimulation, relative to the whole-cell levels of total Ras and Rap1. Next, we investigated whether Spred-1 and Spred-2 could exert this effect by interfering with activation of the RTK itself by examining the tyrosine phosphorylation state of the EGFR following EGF stimulation. Blots of the whole-cell lysates used for the Ras-affinity purification (Figure 5B) were probed with anti-EGFR and anti-phosphotyrosine antibodies. As shown in Figure 5(D), neither Spred-1 nor Spred-2 had any discernible effect on the overall tyrosine phosphorylation of the EGFR following EGF stimulation.
Figure 5. Spred proteins inhibit Ras and Rap1 activation, but not receptor phosphorylation.
(A) HEK-293 cells were transfected with GFP–ERK (0.5 μg), RasV12 (1 μg) and Spred-1, Spred-2 or C-terminal mutants (5 μg), then serum-starved for 16 h, and whole-cell lysates were probed with the indicated antibodies. (B)–(D) Tetracycline-inducible Spred-1- and Spred-2-expressing cell lines (based on Flp-In™ T-REx™ 293 cell line), or control Flp-In™ T-REx™ 293 cells containing vector alone (V), were transfected with EGFR (0.2 μg). At 24 h post-transfection, all cells were treated with tetracycline (1 μg/ml) for 8 h to induce expression of Spred-1 or Spred-2 (or not), and then the cells were serum-starved for a further 16 h, during which time tetracycline induction was maintained. Cells were then stimulated with EGF (100 ng/ml) for the indicated times, and the whole-cell lysates were immediately affinity-purified using GST–Raf1 RBD (B) or GST–RalGDS RBD (C) fusion proteins. Replicate blots were run of the whole-cell lysates (WCL) used for the GST–Raf1 RBD affinity-purifications (D). Whole-cell lysates (WCL) and GTP-bound Ras and Rap1 affinity-precipitated proteins (AP) were subjected to SDS/PAGE, transferred on to membranes and probed with the indicated antibodies. IB, immunoblot.
Forced membrane localization enhances/restores the functional activity of Sprouty-less Spred mutants
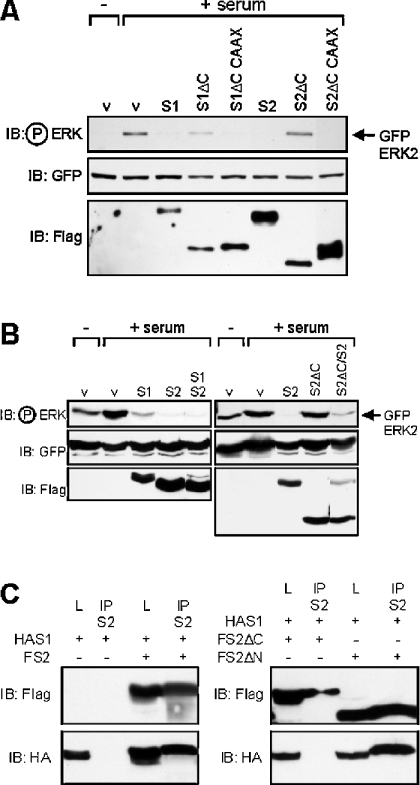
Spred-1ΔC and Spred-2ΔC have differing abilities to block ERK activation. We wondered what effect forced localization of the Spred-ΔC mutants at the membrane would have on their ability to block downstream ERK activation. Accordingly, we attached the membrane-targeting Cys-Ala-Ala-Xaa (CAAX) motif of Ras to the C-termini of the Spred-ΔC mutants, and tested their ability to block ERK activation. Both Spred-ΔC CAAX proteins were membrane-localized (results not shown) and were able to strongly repress serum-mediated ERK activation (Figure 6A).
Figure 6. Spred proteins heterodimerize via their Sprouty domain.
(A) and (B) HEK-293 cells were transfected with the indicated FLAG-tagged Spred constructs including Spred-ΔC CAAX vectors comprising the Spred-ΔC constructs fused with the C-terminal 17 residues of Ras. Cells were serum-starved overnight, then treated with 10% serum for 20 min, and lysates were probed with the indicated antibodies. (C) HEK-293T cells were transfected with FLAG-tagged Spred-2 (FS2, FS2ΔN, FS2ΔC) and HA-tagged Spred-1 (HAS1) constructs. Lysates (L) or immunoprecipitates (IP) were treated with the anti-Spred-2 antibody (S2) run out on the gel and probed with the indicated antibodies. IB, immunoblot.
Spred proteins can heterodimerize via their Sprouty domains
We wondered whether co-expression of these proteins modified their functions and whether they might physically interact. Co-expression of the Spred proteins has no obvious modifying effect on their inhibitory activity (Figure 6B). However, the Spred-2ΔC mutant appeared to have a dominant-negative effect on Spred-2 function, with the inhibitory activity of the protein slightly impaired in the presence of the truncated C-terminal mutant (Figure 6B). Both Spred-1 and Spred-2 are able to form stable heterodimers when co-expressed in cells, with HA-tagged Spred-1 able to co-immunoprecipitate with FLAG-tagged Spred-2 using an anti-Spred-2 antibody (Figure 6C). This interaction was mediated via the Sprouty domain, as a N-terminal deletion mutant of Spred-2 was able to interact with Spred-1, but the C-terminal deletion mutant lacking the Sprouty domain was unable to do so (Figure 6C).
DISCUSSION
Spred proteins were first identified in a screen for proteins able to interact with the intracellular tyrosine kinase catalytic domain of the c-kit and c-fms receptors [15]. We screened the human database for cDNAs homologous with mSpred proteins, and identified a number of possible candidates for which we generated PCR primers and subsequently amplified full-length variants of both hSpred cDNAs. Like the mSpreds, the human counterparts have a tripartite domain structure with an N-terminal EVH1 domain, a central KBD, which, in the case of mSpred-1, mediates binding to the c-kit receptor [15,22], and a cysteine-rich C-terminal Sprouty domain. mSpred-1 was phosphorylated on tyrosine in response to SCF (stem cell factor), and reportedly also by PDGF and EGF, and this was most efficient when the KBD region was present [15]. Both mSpred-1 and mSpred-2 are tyrosine phosphorylated by a constitutively active mutant of the c-kit receptor [22]. However, for functional suppression of the Ras/MAPK pathway, as measured by abrogation of ERK activation, the KBD region was not essential [15]. In our studies, we have determined that hSpred-1 and hSpred-2 have different requirements for the C-terminal Sprouty domain in blocking ERK activation. However, we have not been able to detect, either by immunoblotting with a phosphotyrosine-specific antibody or by phospho-amino acid analysis, any tyrosine phosphorylation of hSpred proteins in HEK-293T cells under conditions in which both hSpred proteins readily block ERK activation. Therefore it seems likely that tyrosine phosphorylation is not a requirement for Spred inhibitory activity.
Our finding that Spred-1 and Spred-2 can be phosphorylated on serine and threonine residues by one or more unknown endogenous protein kinases in HEK-293T cells raises the intriguing possibility that such phosphorylation could play a role in Spred-1 or Spred-2 regulation. However, serum stimulation itself does not appear to affect Spred-1 or -2 serine/threonine phosphorylation levels, since we did not observe any marked changes in the phospho-amino acid content of the Spreds in response to serum stimulation. The status of Spry protein phosphorylation and its role in Ras/MAPK suppression is also controversial. Spry phosphorylation has been reported to occur exclusively on serine residues [7], while a recent study has demonstrated that tyrosine phosphorylation of Spry is required for its inhibitory activity [21]. It is noteworthy that the tyrosine phosphorylation observed for Spry occurred in a cell-type-dependent manner. A comparison of the hSpred and mSpred protein sequences reveals five invariant tyrosine residues conserved among all proteins. However, it seems that phosphorylation of these or any other tyrosine residues is not required for functional suppression of the Ras/MAPK pathway, and the full significance of the direct interaction with the c-kit receptor and the role of tyrosine phosphorylation of the Spred proteins remains to be ascertained.
The hSpred-1 and mSpred-1 proteins seem to strongly diverge with respect to the functional activity of their respective N-terminal domains. In contrast with the activity reported with the mouse N-terminal domain [15], we find that this domain in hSpred-1 is able to block ERK activation downstream of different stimuli. Furthermore, this activity was maintained in the context of a PC12 differentiation assay and in a growth-factor-dependent cell-cycle progression assay. In contrast, the hSpred-2 N-terminal domain, as has been reported with the mouse variant has no such activity [15].
The C-terminal Sprouty domain of Spred proteins has been shown to be necessary for their membrane translocation following growth factor stimulation [15], and the analogous domain is responsible for movement of Spry proteins to the plasma membrane [21,31]. Indeed, there appears to be a very good correlation between the ability of Spry proteins to translocate to the membrane and to inhibit ERK activation [21]. In the present paper, we show that forced translocation of both Spred-ΔC mutants to the membrane using a CAAX motif enhances/restores their ability to block ERK activation. This suggests that the inhibitory function of the Spred proteins appears to reside in their N-terminal EVH1/KBD domains, and, in the case of Spred-1, the Sprouty domain is not absolutely necessary for membrane translocation and function. It would appear then that some Spred proteins may differ in their mode of inhibitory action from the Spry proteins. In this regard, it is of note that the Spred and Spry protein families have very different N-terminal domains. The Spry proteins are poorly conserved at their N-termini, yet it has been demonstrated that this region has a conserved tyrosine residue, Tyr55, which is phosphorylated in response to growth factors and which mediates binding to the SH2 domain of Grb2 [12,21]. Spred proteins do not have this conserved tyrosine residue nor do they share any homology with the N-termini of Spry proteins, but instead have an EVH1 domain. The EVH1 domain is a protein–protein interaction domain that directs binding to specific polyproline motifs in target proteins [32]. It is therefore possible that the targets of Spred action may be distinct from those of the Spry proteins.
In this regard, it is of interest that both Spred-1 and Spred-2 were able to interfere with NGF-mediated neuronal differentiation of PC12 cells. NGF-mediated PC12 differentiation involves activation of the Rap1/B-Raf/ERK pathway [33,34], a pathway distinct from the Ras/c-Raf/ERK cascade induced by most mitogenic growth factors and which the Spred proteins can also clearly inhibit. These findings prompted the question of where in these pathways the Spred proteins exert their inhibitory effects. Surprisingly, we have found that both Spred-1 and Spred-2 are able to reduce levels of GTP-loaded Ras and Rap1 without having an observable effect on receptor activation. This suggests that the Spred proteins may target quite distinct receptor-activated guanine nucleotide-exchange factor complexes as the basis of their inhibitory activity, or possibly enhance the GTPase activity of Ras or Rap1. Spred proteins then appear to be able to modulate ERK activity, potentially via regulating the levels of the active forms of members of the Ras GTPase family. These results are in direct contrast with previous reported findings where transfected active Ras GTP levels, induced by EGF, were unaffected by Spred-1 co-expression, but transfected active c-Raf levels were reduced [15]. These discrepancies serve to underline that Spred proteins, analogous to Sprouty family members, may act at different levels in the Ras/Raf/MAPK pathway, the mechanistic basis of which is still to be determined.
There are a number of non-conservative residue variances in the EVH1 domains of Spred-1 and Spred-2 which may account for the functional differences between these two N-terminal regions that we have observed. However, the constructs we used extended up to the KBD regions of the Spred proteins, and we note that there is an insert of some 32 residues in the EVH1/KBD inter region in Spred-1 that is not present in Spred-2. This sequence may possibly have a role in the functional variation between Spred-1 and Spred-2.
Expression of Spred proteins in NIH-3T3 fibroblasts can prevent cell-cycle progression of G0-synchronized cells in response to serum stimulation, as detected by S-phase BrdU-incorporation. It will now be of great interest to determine if induction or action of the Spred proteins is influenced by the cell cycle.
Spred-1 and Spred-2 inhibit mitogenic signalling induced by a range of growth factors, but conversely appear to have a focused action on Ras GTPase family members' mediated activation of the MAPK pathway. We detected no significant effect on either of the two other MAPK pathways, namely the JNK and p38 kinase pathways. mSpred proteins also do not affect Akt or phospholipase Cγ activation, or Rac-induced membrane ruffling [15], and hence share with the Spry protein family an inhibitory activity which appears to be restricted to that of the Ras/Raf/MAPK pathway.
Spry proteins have recently been shown to form homodimers, an activity mediated by the C-terminal Spry domain [21]. We have now demonstrated that Spred proteins can also form heterodimers, and that this association is mediated by the Sprouty domain. It appears that different Spred proteins can interact, raising the possibility that they may also heterodimerize with Spry proteins. Such complexes could serve specific inhibitory functions in response to different stimuli in a spatial and temporal fashion.
Multimedia adjunct
Acknowledgments
We thank Dr Marie Bogoyevitch (University of W.A. Australia) for the p38/JNK constructs, Professor Rony Seger (Weizmann Institute, Israel) for the GFP–ERK2 construct, Dr David Huang for the pEF-BOS vector, Dr Suzy Orchard (Ludwig Institute for Cancer Research) for the anti-EGFR antibody, Dr Bill Sellers (Dana-Farber Cancer Institute, Harvard Medical School) for the GFP–FKHRwt construct and Dr Rick Pearson (Peter MacCallum Cancer Institute) for the HA-Akt1construct. J.A.J.K. is thankful to the Victor Hurley Medical Research Fund, RMH, the Royal Australasian College of Surgeons and the Melville Hughes Scholarship for support. A.F.L.S. is a recipient of a James and Linda Wang Fellowship. This work was supported by grants from the NHMRC (National Health and Medical Research Council), Australia, the RMH (Royal Melbourne Hospital) Neuroscience Foundation, the Acoustic Neuroma Association of Australasia Inc. and the Roche Foundation, Switzerland.
References
- 1.Ghiglione C., Carraway K. L., 3rd, Amundadottir L. T., Boswell R. E., Perrimon N., Duffy J. B. The transmembrane molecule kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell. 1999;96:847–856. doi: 10.1016/s0092-8674(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 2.Hacohen N., Kramer S., Sutherland D., Hiromi Y., Krasnow M. A. sprouty encodes a novel antagonist of FGF signaling that patterns apical branching of the Drosophila airways. Cell. 1998;92:253–263. doi: 10.1016/s0092-8674(00)80919-8. [DOI] [PubMed] [Google Scholar]
- 3.Casci T., Vinos J., Freeman M. Sprouty, an intracellular inhibitor of Ras signaling. Cell. 1999;96:655–665. doi: 10.1016/s0092-8674(00)80576-0. [DOI] [PubMed] [Google Scholar]
- 4.Kramer S., Okabe M., Hacohen N., Krasnow M. A., Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–2525. doi: 10.1242/dev.126.11.2515. [DOI] [PubMed] [Google Scholar]
- 5.Reich A., Sapir A., Shilo B. Sprouty is a general inhibitor of receptor tyrosine kinase signaling. Development. 1999;126:4139–4147. doi: 10.1242/dev.126.18.4139. [DOI] [PubMed] [Google Scholar]
- 6.Taguchi A., Sawamoto K., Okano H. Mutations modulating the Argos-regulated signaling pathway in Drosophila eye development. Genetics. 2000;154:1639–1648. doi: 10.1093/genetics/154.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Impagnatiello M. A., Weitzer S., Gannon G., Compagni A., Cotten M., Christofori G. Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J. Cell Biol. 2001;152:1087–1098. doi: 10.1083/jcb.152.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Maximy A. A., Nakatake Y., Moncada S., Itoh N., Thiery J. P., Bellusci S. Cloning and expression pattern of a mouse homologue of Drosophila sprouty in the mouse embryo. Mech. Dev. 1999;81:213–216. doi: 10.1016/s0925-4773(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 9.Chambers D., Mason I. Expression of sprouty2 during early development of the chick embryo is coincident with known sites of FGF signalling. Mech. Dev. 2000;91:361–364. doi: 10.1016/s0925-4773(99)00288-9. [DOI] [PubMed] [Google Scholar]
- 10.Gross I., Bassit B., Benezra M., Licht J. D. Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J. Biol. Chem. 2001;276:46460–46468. doi: 10.1074/jbc.M108234200. [DOI] [PubMed] [Google Scholar]
- 11.Minowada G., Jarvis L. A., Chi C. L., Neubuser A., Sun X., Hacohen N., Krasnow M. A., Martin G. R. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki A., Taketomi T., Wakioka T., Kato R., Yoshimura A. Identification of a dominant negative mutant of Sprouty that potentiates fibroblast growth factor- but not epidermal growth factor-induced ERK activation. J. Biol. Chem. 2001;276:36804–36808. doi: 10.1074/jbc.C100386200. [DOI] [PubMed] [Google Scholar]
- 13.Tefft J. D., Lee M., Smith S., Leinwand M., Zhao J., Bringas P., Jr, Crowe D. L., Warburton D. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr. Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- 14.Lee S. H., Schloss D. J., Jarvis L., Krasnow M. A., Swain J. L. Inhibition of angiogenesis by a mouse sprouty protein. J. Biol. Chem. 2001;276:4128–4133. doi: 10.1074/jbc.M006922200. [DOI] [PubMed] [Google Scholar]
- 15.Wakioka T., Sasaki A., Kato R., Shouda T., Matsumoto A., Miyoshi K., Tsuneoka M., Komiya S., Baron R., Yoshimura A. Spred is a Sprouty-related suppressor of Ras signalling. Nature (London) 2001;412:647–651. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 16.Mizushima S., Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fridman M., Maruta H., Gonez J., Walker F., Treutlein H., Zeng J., Burgess A. Point mutants of c-raf-1 RBD with elevated binding to v-Ha-Ras. J. Biol. Chem. 2000;275:30363–30371. doi: 10.1074/jbc.M003193200. [DOI] [PubMed] [Google Scholar]
- 18.van Triest M., de Rooij J., Bos J. L. Measurement of GTP-bound Ras-like GTPases by activation-specific probes. Methods Enzymol. 2001;333:343–348. doi: 10.1016/s0076-6879(01)33068-9. [DOI] [PubMed] [Google Scholar]
- 19.Taylor S. J., Resnick R. J., Shalloway D. Nonradioactive determination of Ras-GTP levels using activated ras interaction assay. Methods Enzymol. 2001;333:333–342. doi: 10.1016/s0076-6879(01)33067-7. [DOI] [PubMed] [Google Scholar]
- 20.Campbell D. H., Sutherland R. L., Daly R. J. Signaling pathways and structural domains required for phosphorylation of EMS1/cortactin. Cancer Res. 1999;59:5376–5385. [PubMed] [Google Scholar]
- 21.Hanafusa H., Torii S., Yasunaga T., Nishida E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 2002;4:850–858. doi: 10.1038/ncb867. [DOI] [PubMed] [Google Scholar]
- 22.Kato R., Nonami A., Taketomi T., Wakioka T., Kuroiwa A., Matsuda Y., Yoshimura A. Molecular cloning of mammalian Spred-3 which suppresses tyrosine kinase-mediated Erk activation. Biochem. Biophys. Res. Commun. 2003;302:767–772. doi: 10.1016/s0006-291x(03)00259-6. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda M., Gotoh Y., Nishida E. Interaction of MAP kinase with MAP kinase kinase: its possible role in the control of nucleocytoplasmic transport of MAP kinase. EMBO J. 1997;16:1901–1908. doi: 10.1093/emboj/16.8.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pouyssegur J., Volmat V., Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem. Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 25.Marshall C. J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 26.Pages G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenormand P., Pages G., Sardet C., L'Allemain G., Meloche S., Pouyssegur J. MAP kinases: activation, subcellular localization and role in the control of cell proliferation. Adv. Second Messenger Phosphoprotein Res. 1993;28:237–244. [PubMed] [Google Scholar]
- 28.Pouyssegur J., L'Allemain G., Lenormand P., Pages G., Pavirani A., Rasmussen U., Van Obberghen-Schilling E., Vouret-Craviari V. α-Thrombin receptor and MAP kinases in the control of cell growth. Nouv. Rev. Fr. Hematol. 1993;35:263–264. [PubMed] [Google Scholar]
- 29.Lenormand P., Sardet C., Pages G., L'Allemain G., Brunet A., Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J. Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattori M., Minato N. Rap1 GTPase: functions, regulation, and malignancy. J. Biochem. (Tokyo) 2003;134:479–484. doi: 10.1093/jb/mvg180. [DOI] [PubMed] [Google Scholar]
- 31.Lim J., Wong E. S., Ong S. H., Yusoff P., Low B. C., Guy G. R. Sprouty proteins are targeted to membrane ruffles upon growth factor receptor tyrosine kinase activation: identification of a novel translocation domain. J. Biol. Chem. 2000;275:32837–32845. doi: 10.1074/jbc.M002156200. [DOI] [PubMed] [Google Scholar]
- 32.Renfranz P. J., Beckerle M. C. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr. Opin. Cell Biol. 2002;14:88–103. doi: 10.1016/s0955-0674(01)00299-x. [DOI] [PubMed] [Google Scholar]
- 33.Kao S., Jaiswal R. K., Kolch W., Landreth G. E. Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 2001;276:18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 34.York R. D., Yao H., Dillon T., Ellig C. L., Eckert S. P., McCleskey E. W., Stork P. J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature (London) 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.