Abstract
Cannabinoids are broadly immunosuppressive, and anti-inflammatory properties have been reported for certain marijuana constituents and endogenously produced cannabinoids. The CB2 cannabinoid receptor is an established constituent of immune system cells, and we have recently established that the CB1 cannabinoid receptor is expressed in mast cells. In the present study, we sought to define a role for CB1 in mast cells and to identify the signalling pathways that may mediate the suppressive effects of CB1 ligation on mast cell activation. Our results show that CB1 and CB2 mediate diametrically opposed effects on cAMP levels in mast cells. The observed long-term stimulation of cAMP levels by the Gαi/o-coupled CB1 is paradoxical, and our results indicate that it may be attributed to CB1-mediated transcriptional regulation of specific adenylate cyclase isoenzymes that exhibit superactivatable kinetics. Taken together, these results reveal the complexity in signalling of natively co-expressed cannabinoid receptors and suggest that some anti-inflammatory effects of CB1 ligands may be attributable to sustained cAMP elevation that, in turn, causes suppression of mast cell degranulation.
Keywords: anti-inflammatory potential, cAMP, cannabinoids, immunosuppressive, mast cell, methanandamide
Abbreviations: AC, adenylate cyclase; AD, average difference; BMMC, bone marrow-derived mast cell; CNS, central nervous system; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; FSK, forskolin; GPCR, G-protein-coupled receptor; IBMX, isobutylmethylxanthine; MA, methanandamide; poly(A)+ RNA, polyadenylated RNA; PDE, phosphodiesterase; PTX, pertussis toxin; RT–PCR, reverse transcriptase PCR
INTRODUCTION
By virtue of their presence in the gastrointestinal and respiratory mucosa, mast cells are points of immunological contact for inhaled or ingested marijuana constituents [1–5]. Moreover, mast cells are sources of endo-cannabinoids, which are putative immunomodulators [6–9]. Cannabinoids are broadly immunosuppressive, and their potential as anti-inflammatory agents is supported by in vitro and in vivo studies. In vitro, marijuana constituents and endo-cannabinoids can suppress the release of mast cell pro-inflammatory mediators [10,11]. In vivo, the marijuana constituent tetrahydrocannabinol (Δ9-THC) is highly suppressive in models of mast cell pro-inflammatory function including passive cutaneous anaphylaxis and substance P/carageenan-induced hyperalgesia and oedema [7,12,13]. The mechanisms by which cannabinoids suppress mast cell activation have not been extensively explored.
CB2 cannabinoid receptors are well-established components of immune tissues, whereas a CNS (central nervous system)-restricted distribution has been proposed for CB1 [6,8,14]. However, an increasing amount of evidence suggests that CB1 is present in peripheral contexts, including immune system cells [10,12,15,16]. In the present study, in addition to CB2, we confirm the presence of the CB1 isoform in primary mast cells. Mast cells are a native context in which the individual and combined contributions of coexpressed CB1 and CB2 can be modelled [10,17]. We have previously shown that exposure of mast cells to cannabinoids that bind to both CB1 and CB2 cause a net suppression in the pro-inflammatory responses. However, the individual contributions of the two receptor isoforms in these cells have not been dissected.
The contribution of CB1 to mast cell biology has not been studied. In the present report, we explored CB1 signalling in this newly defined expression context. Since there is an intimate relationship between cAMP levels and the secretory status of mast cells, we explored CB1-mediated regulation of cAMP. Increased cAMP levels tend to suppress secretion in mast cells [18–20]. We hypothesized that CB1 may increase cAMP using a specific mechanism that is associated with certain Gαi/o-coupled GPCR (G-protein-coupled receptor) [17,21,22]. Gαi/o-coupled GPCRs are conventionally regarded as inhibitory receptors, since they down-regulate AC (adenylate cyclase) activity and hence decrease intracellular cAMP [14,22,23]. However, several Gαi/o-coupled GPCR (e.g. opioid receptors) and CB1 (in an over-expression system) have been shown, counter-intuitively, to induce sustained AC activation [17,22–25], termed AC ‘superactivation’. This superactivation results in chronic up-regulation in cAMP, despite an initial Gαi/o-coupled stimulus [24,26].
In the present study, we provide evidence that, although both CB1 and CB2 ligands induce marked acute decreases in cytosolic cAMP [27], the results of long-term stimulation of the two receptor isoforms are diametrically opposed. Over a 60–120 min time course, CB1 ligands cause a net increase, whereas CB2 ligands cause a net suppression, in cytosolic cAMP. These disparate effects on cAMP levels do not reflect coupling of CB1 and CB2 to stimulatory (Gαs) and inhibitory (Gαi/o) heterotrimeric G-proteins respectively. Rather, our results confirm that both receptor isoforms are Gαi/o coupled in RBL2H3 mast cells. The CB1-induced chronic cAMP increase is AC-dependent in RBL2H3. We suggest that differential coupling of CB1 and CB2 to the AC isoforms that exhibit superactivation is responsible for their differing long-term effects on cAMP. We document the rapid transcriptional up-regulation of AC isoforms V and VI after CB1 receptor ligation, providing a potential superactivation mechanism. This increase in transcript levels is shown to specifically translate to an increase in the protein representation for the relevant AC isoenzymes in mast cells. Taken together, these results reveal complexity in the signalling of natively co-expressed cannabinoid receptors, and suggest that some anti-inflammatory effects of CB1 ligands may be attributable to cAMP increase and hence suppression of mast cell secretion.
EXPERIMENTAL
Cell lines and culture
RBL2H3 mast cells were cultured in DMEM (Dulbecco's modified Eagle's medium; Cambrex Bio Science, Walkersville, MD, U.S.A.) supplemented with 10% (v/v) heat-inactivated FBS (fetal bovine serum; Cambrex Bio Science) with 2 mM L-glutamine in a 5% CO2/90% humidity/37 °C atmosphere. Primary murine BMMCs (bone marrow-derived mast cells) was a gift from Dr A. Fleig (Queen's Medical Center, Honolulu, HI, U.S.A.). BMMCs were expanded in media supplemented with recombinant interleukin-3 (Sigma, St. Louis, MO, U.S.A.).
Reagents and stimulations
Cannabinoid compounds AM281, JWH015 and MA (methanandamide) were obtained from Tocris Cookson (Ellisville, MO, U.S.A.). SR144528 [28] was obtained from the National Institute on Drug Abuse (National Institutes of Health, Bethesda, MD, U.S.A.). PTX (pertussis toxin) and FSK (forskolin) were obtained from Sigma. Cannabinoids were freshly dissolved for each experiment from concentrated stocks stored at −80 °C for <3 months. Stimulations were performed on 80% confluent RBL2H3 in 1.0 ml of DMEM/10% FBS at 37 °C. Matched vehicle controls, using either 0.1% DMSO or 0.1% ethanol in distilled water were performed. Antibodies to cannabinoid receptors and Grb2 were from Affinity Bioreagents (Golden, CO, U.S.A.). Antibodies to AC isoforms were from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.).
Human cell preparation and gene chip analysis
Blood cell subsets were prepared as described in [29], and total RNA was prepared from 107 cells. Human genome-wide gene expression was examined using the human genome U133A probe array (GeneChip; Affymetrix, Santa Clara, CA, U.S.A.), which contains the oligonucleotide probe set for 22000 full-length genes. The expression level of single mRNAs was determined as the average fluorescence intensity among the intensities obtained by 11-paired (perfect-matched and single nucleotide-mismatched) probes. If the intensities of mismatched probes were very high, gene expression was judged to be absent, even if a high average fluorescence was obtained with the GeneChip Analysis Suite 5.0 program. The level of gene expression was determined as the AD (average difference) using the GeneChip software. Each AD level was then normalized by dividing it with the median value of 22283 AD levels obtained in an experiment (‘normalized AD’ level).
Western-blot analysis
Cells were washed with PBS before lysis (ice-cold, 30 min) in 350 μl of lysis buffer (50 mM Hepes, pH 7.4, 75 mM NaCl, 20 mM NaF, 10 mM iodoacetamide, 0.5%, w/v, Triton X-100 and 1 mM PMSF). After clarification (10000 g, 5 min), lysates were acetone-precipitated at −20 °C for 1 h, and the protein precipitate was harvested (13000 g, 5 min). Samples were resolved by reducing SDS/PAGE in 25 mM Tris, 192 mM glycine and 0.05% (w/v) SDS (pH 8.8). Resolved proteins were electro-transferred on to a PVDF membrane. For Western blotting, membranes were blocked using 5% (w/v) non-fat milk [1 h at room temperature (18–23 °C)]. Primary antibodies were dissolved in PBS/0.05% (v/v) Tween 20/0.05% NaN3 and incubated with the membrane for 16 h at 4 °C. Developing antibodies (anti-rabbit IgGs conjugated to horseradish peroxidase) were diluted to 0.1 μg/ml in PBS/0.05% Tween 20 and incubated with membranes for 45 min at room temperature. Standard washes (4× 5 min in 50 ml of PBS/0.1% Tween 20 at room temperature) were performed between application of primary and secondary antibodies. Western blots were visualized using ECL® (enhanced chemiluminescence; Amersham Biosciences, Piscataway, NJ, U.S.A.).
Cell surface immunostaining and FACS
Cannabinoid receptor antibodies (anti-CB1R and anti-CB2R from Affinity Bioreagents) raised to extracellular portions containing the first 77 or 32 N-terminal amino acids of each receptor respectively were used for cell surface immunostaining. RBL2H3 mast cells were fixed in 4% (w/v) paraformaldehyde for 2 h at 4 °C. The cells used for immunostaining were not permeabilized. Paraformaldehyde-fixed cells were blocked in 0.5% FBS for 20 min at room temperature, then incubated with a 1:100 dilution of either anti-CB1R or anti-CB2R in 0.5% FBS for 30 min at room temperature. Cells were rinsed twice in PBS and then exposed to Alexa™ Fluor 488 conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR, U.S.A.) in 0.5% FBS/PBS (room temperature, 30 min). Control cells were exposed to secondary antibody alone. Cells were rinsed twice in PBS after the secondary antibody exposure. FACS analysis was performed on a FACScan™ (Becton Dickinson, Franklin Lakes, NJ, U.S.A.).
Direct cAMP assay
Cells were stimulated as described above. Reactions were halted by cell lysis in 0.1 M HCl/0.5% Triton X-100 (37 °C, 10 min). Cell lysates were centrifuged (room temperature, 10000 g, 1 min) and cAMP levels were measured from these cell lysates using Correlate-EIA™ Direct cAMP assays (Assay Designs, Ann Arbor, MI, U.S.A.) [30]. Absorbance A405 for each sample was converted to pmol cAMP, relative to a cAMP standard curve that was obtained for each run of the experiment. Data are presented as a percentage of the maximal attained FSK response from the internal positive control in each experiment.
5-Hydroxytryptamine release assay
BMMCs were incubated with 1 μCi/ml [3H]hydroxytryptamine (NEN Life Science Products, Boston, MA, U.S.A.) for 16 h at 37 °C [31]. During this time period, the FcεRI receptors were primed with 1 μg/ml IgE anti-DNP. Monolayers were then washed once with Tyrode's buffer (10 mM Hepes, pH 7.4, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1 mg/ml glucose and 1 mg/ml BSA) at 37 °C and cells were incubated with the indicated stimuli or vehicle in 250 μl/cm2 Tyrode's buffer (37 °C, 45 min). Reactions were quenched in ice-cold PBS and radioactivity was counted in liquid scintillation cocktail.
RT–PCR (reverse transcriptase PCR)
Poly(A)+ (polyadenylated) mRNA was isolated from RBL2H3 cells using an Oligotex Direct mRNA purification kit (Qiagen, Valencia, CA, U.S.A.). Two sets of isoform-specific primers were tested in RT–PCR for each of the nine characterized AC isoenzymes. RT–PCR reactions were performed three times at varying annealing temperatures to optimize the probability of obtaining products. Gel-purified RT–PCR products were sequenced.
Quantitative RT–PCR
Total RNA samples were prepared from adherent RBL2H3 mast cells using a Nucleospin RNA II kit (BD Biosciences-Clontech, Palo Alto, CA, U.S.A.). Total RNA samples were reverse transcribed using an Advantage RT-for-PCR kit (BD Biosciences-Clontech) and the resulting cDNA was analysed by real-time PCR, using AC isoform-specific primers. A single pool of first-strand material was used for all of the analyses shown. Real-time PCR reactions were performed with SYBR Green I chemistry on an iCycler machine (Bio-Rad Laboratories, Hercules, CA, U.S.A.). Authenticity of the PCR products was verified by melting curve analysis. Quantities were calculated using a standard curve based on an external reference vector. This pGEM3ZF(+) amplicon was selected to have a similar amplicon size (158 bp) to the experimental primer pairs. RAB13 was used as an internal reference, but values were not normalized to RAB13 levels.
Analysis
Results are shown as the means±S.D. Statistical significance was determined based on a two-way analysis of variance (Student's t test). Adjacent to data points in the respective graphs, significant differences were recorded as follows: *P<0.05; **P<0.01; ***P<0.001; and no symbol, P>0.05.
RESULTS
CB1 expression in primary and transformed mast cells
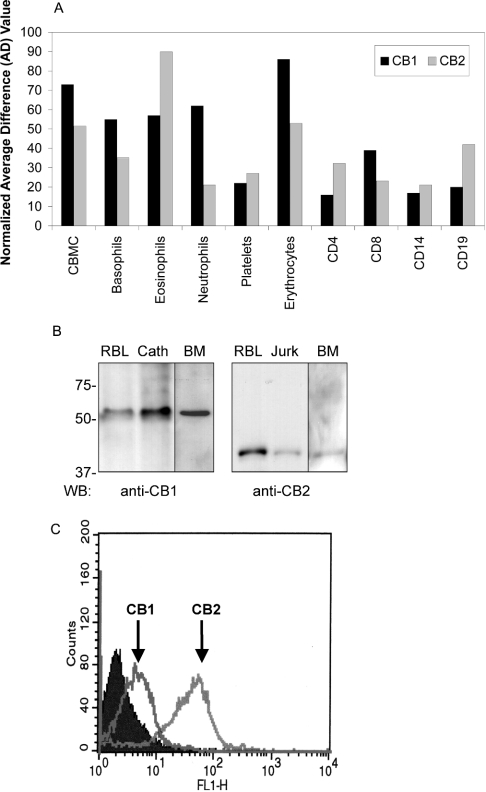
RBL2H3 and P815 mast cell lines express transcripts and protein for both CB1 and CB2 [10]. We questioned in this study whether CB1 and CB2 co-expression is a physiological feature of mast cell and/or basophil lineages. The extracted human genome array data in Figure 1(A) suggest that mRNA for both CB1 and CB2 is a feature of primary human mast cells and basophils. Other human haematopoietic lineages express comparable levels of CB1 mRNA, including erythrocytes, neutrophils and eosinophils. If confirmed at the protein level, these results indicate that CB1 and CB2 expressions may be widespread in human immune effector cells. Figure 1(B) suggests that CB1 and CB2 proteins are indeed present in primary murine BMMCs. Taken together, these results show that mast cells are probably a native co-expression context for CB1 and CB2. Moreover, we used a flow cytometry approach to show that the subcellular localization of cannabinoid receptors in mast cells is consistent with probable functionality. Figure 1(C) shows, using antisera raised against the extracellular portions of the receptors, that CB1, like CB2, localizes to the plasma membrane of intact (non-permeabilized) mast cells.
Figure 1. Cannabinoid receptor subtype 1 (CB1) expression in primary and transformed mast cells.
(A) Transcript levels for CB1 and CB2 in human haematopoietic cells. Hybridization data were extracted from human genome-wide expression analysis (Affymetrix U133A) array and probed with cDNA derived from the indicated cell sources. CBMCs (cord blood derived mast cells) were derived from human CD34+ cord blood progenitor cells. Basophil populations were isolated from peripheral human blood buffy coat by Percoll gradient separation and negative selection. CD14+ monocyte/macrophages were purified from peripheral blood using positive selection. Black bars indicate hybridization to hCB1 (NM016083) and grey bars indicate hybridization to hCB2 (NM001841) gene spots. Data are expressed as fold increases in hybridization relative to normalized background hybridization levels from one representative experiment. (B) Western-blot analysis of CB1 and CB2 protein expression in RBL2H3 mast cells and primary BMMCs. Left panel: post-nuclear lysates from 2×106 RBL2H3 (RBL), Cath.a cells (neuronal positive control for CB1 expression), and 1×107 murine primary BMMCs (BM) were acetone precipitated to recover protein, and resolved by SDS/PAGE (10% polyacrylamide). After electrotransfer to PVDF, Western blotting was performed using 0.1 μg/ml rabbit polyclonal anti-CB1. Predicted molecular mass of CB1 is 53 kDa. Right panel: post-nuclear lysates from 2×106 RBL2H3 (RBL) or Jurkat (lymphocyte positive control for CB2 expression) cells were acetone precipitated to recover protein, and resolved by SDS/PAGE (10% polyacrylamide). After electrotransfer to PVDF, Western blotting was performed using 0.5 μg/ml rabbit polyclonal anti-CB2. The predicted molecular mass of CB2 is 38 kDa. Molecular mass markers are shown in kDa. n=5 for RBL2H3 and Cath.a and n=2 for BMMC. (C) Flow cytometry analysis of CB1 and CB2 surface expression in RBL2H3 mast cells. Intact RBL2H3 were stained using anti-CB1 or anti-CB2 antibodies as described in the Experimental section. Antibody epitopes are peptides derived from the extreme amino-terminal (extracellular) domains of CB1 and CB2 respectively. Positive staining in intact, non-permeabilized cells therefore represents possible surface localization of CB1 and CB2. Staining was visualized using an Alexa-488 conjugated donkey anti-mouse IgG secondary antibody. Filled area represents secondary antibody staining control. Open areas represent cell samples stained, separately, for CB1 or CB2 as indicated (n=2).
CB1 ligand stimulates cAMP production in mast cells
Co-expression of two native cannabinoid receptors within one cell type raises intriguing questions as to whether these receptors are likely to signal redundantly. Suppression of cytosolic cAMP is a major consequence of cannabinoid exposure in neuronal and peripheral contexts [14,16,21]. We questioned how ligands that specifically activate either CB1 or CB2, regulate cAMP levels in mast cells. We used a competitive ELISA to measure cytosolic cAMP. In preliminary experiments, we established the validity of the assay in both RBL2H3 mast cells and BMMCs using FSK [32] and compound 48/80 [33], which caused marked increase and suppression respectively in cytosolic cAMP levels (results not shown).
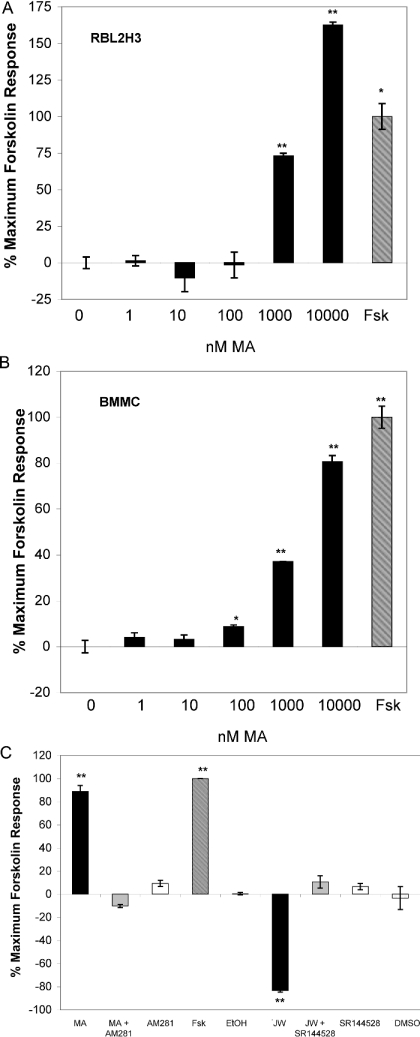
We used an eicosanoid cannabinoid, MA, to probe CB1 effects on cAMP in mast cells. Figures 2(A) and 2(B) show that, when compared with vehicle effects, MA exposure causes a time- and dose-dependent increase in cAMP levels in RBL2H3 and primary BMMCs. The magnitude of this effect is similar to the FSK response. These results are paradoxical, since CB1 is well established as a Gαi/o-coupled GPCR, and would be predicted to have a suppressive effect on cAMP following ligand binding to this receptor. Figure 2(C) shows that, in contrast, CB2 ligation has the predicted consequence in mast cells. Application of the CB2 ligand JWH015 causes a marked suppression of cAMP levels. Figure 2(C) also shows that antagonists of CB1 and CB2 established that both CB2-mediated cAMP suppression and CB1-mediated cAMP increase are specifically achieved through the correct receptor–ligand interactions. The CB1 antagonist/inverse agonist AM281 blocks MA-induced cAMP increase, whereas the CB2 antagonist SR144528 blocks JW-induced cAMP suppression. In control experiments (results not shown), we verified that the CB1 antagonist, AM281, had no effect on CB2-mediated cAMP responses. Conversely, we also verified that the CB2 antagonist, SR144528, did not affect CB1-mediated cAMP responses. These results indicate that CB2 ligands act as predicted in mast cells. However, the CB1-mediated increase in cAMP levels that occurs in mast cells is inconsistent with the established coupling of CB1 to Gαi/o G-proteins.
Figure 2. CB1 ligands increase intracellular cAMP levels in mast cells.
(A) Dose–response analysis of cAMP responses to CB1-selective ligand in RBL2H3 mast cells. Adherent RBL2H3 cells were incubated with 25 μM FSK or with the indicated concentrations of the CB1 agonist (MA) for 120 min at 37 °C. Baseline [cAMP] was 12.6 pmoles/1×104 cells, representing vehicle stimulated levels. Data are expressed as percentage of the maximal attained FSK response in the experiment (n=5). (B) Dose–response analysis of cAMP responses to CB1-selective ligand in primary murine BMMCs. Primary BMMCs were prepared from mouse femurs and expanded in interleukin-3-containing media until a homogeneous mast cell population was obtained. BMMCs were incubated with 25 μM FSK, or with the indicated concentrations of the CB1 agonist (MA) for 120 min at 37 °C. Baseline [cAMP] was 1.9 pmol/104 cells, representing vehicle stimulated levels. Data are represented as percentage of the maximal attained FSK response in the experiment (n=3). (C) Receptor-specific antagonism of MA and JWH015-induced cAMP responses in mast cells. Primary BMMCs were prepared from mouse femurs and expanded in interleukin-3-containing media until a homogeneous mast cell population was obtained. Adherent BMMCs were incubated with 25 μM FSK, or with 10 μM MA or 10 μM JWH015. These stimulations were performed in the absence or in the presence of preapplied (10 min at 37 °C) AM281 (10 μM, CB1 antagonist) or SR144528 (10 μM, CB2 antagonist). Levels of cAMP at 120 min after stimulation were measured as described above (n=3).
CB1-induced cAMP elevation in mast cells involves Gαi/o and does not involve PDE (phosphodiesterase) inhibition
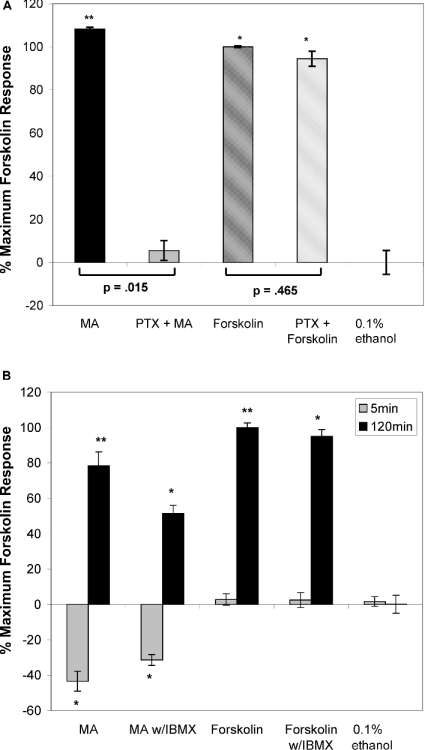
As described above, Gαi/o-mediated signals are conventionally regarded as having a suppressive effect on cytosolic [cAMP]. Our results suggest that CB1 is coupled to stimulatory heterotrimeric G-proteins in RBL2H3, and that MA ligation to the CB1 receptor is therefore causing a direct enhancement of cAMP levels by Gαs activation of AC. We investigated this potential mechanism for MA effects on cytosolic [cAMP]. We used PTX, which inactivates Gαi through ADP-riboxylation of the G-protein. Figure 3(A) demonstrates the effect of PTX on cannabinoid-induced cAMP signals in RBL2H3. In the present study, PTX has no effect on the ability of FSK to mobilize cAMP, reflecting the fact that FSK does not act through a heterotrimeric G-protein. In this experiment, we observed that 120 min exposure to MA causes an increase in cytosolic [cAMP], as described above. However, in cells preincubated with PTX, levels of cAMP were only slightly stimulated by MA. This effect of PTX suggests that the increase in cAMP that follows MA exposure is dependent on Gαi/o and is therefore unlikely to reflect CB1 coupling to Gαs.
Figure 3. CB1-mediated signalling requires Gαi/o.
(A) CB1-mediated increase in cAMP levels is attributable to signalling through Gαi/o. Adherent RBL2H3 cells were preincubated with 100 ng/ml PTX, or vehicle, for 16 h at 37 °C. Cells were then incubated with 20 μM MA (CB1-selective ligand), or 25 μM FSK (positive control) for 120 min at 37 °C. cAMP levels were assayed as described above. Baseline cytosolic cAMP level in untreated control cells was 11.8 pmol/105 cells (n=2). (B) Preblockade of PDE activity using IBMX does not prevent MA-induced increase in cytosolic cAMP levels. Adherent RBL2H3 cells were preincubated with 0.5 μM IBMX, or vehicle, for 30 min at 37 °C. Cells were then incubated with 20 μM MA for 0, 5 and 120 min at 37 °C. cAMP levels were assayed as described in the Experimental section (n=2).
We hypothesized that the observed chronic increase in cAMP could be attributable to a CB1-mediated signal that causes inhibition of PDE-mediated cAMP degradation. We assayed the effect of complete blockade of PDE activity, using IBMX (isobutylmethylxanthine) before MA application. Figure 3(B) shows that, although preincubation with IBMX does increase the basal cytosolic levels of cAMP before MA exposure (results not shown), the nature of the response to MA is unchanged. MA-induced increase in cAMP is therefore independent of the presence of IBMX. If MA causes PDE blockade, we would not predict that MA could generate this response in cells where the PDE is already effectively inactive. Taken together, the data presented in Figure 3 indicate that MA regulates cAMP through Gαi/o, and independently of IBMX-sensitive PDEs.
Superactivation kinetics of CB1-mediated cAMP response in mast cells
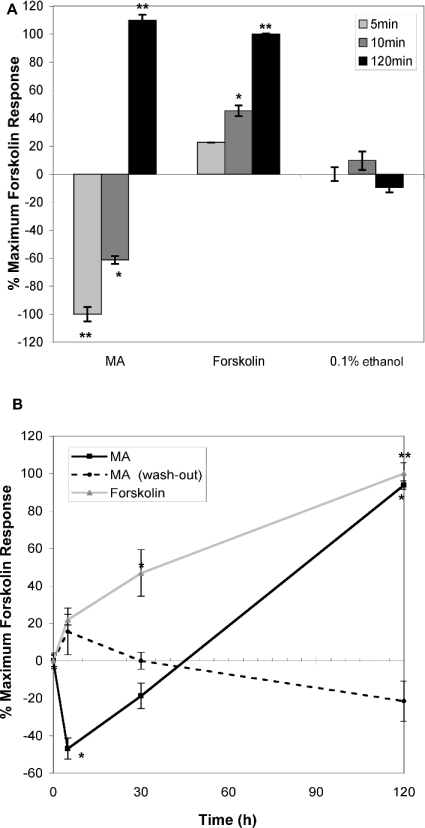
The phenomenon of AC superactivation has been reported in several cell types following chronic exposure to agonists of Gαi/o-coupled GPCR [21,24,26,32]. In the present study, a diphasic regulation of cytosolic cAMP levels occurs, where an acute suppression is followed by chronic activation, or ‘overshoot’. Super-activation of AC has been suggested for CB1 receptors in a heterologous over-expression system [21], but it has not yet been demonstrated as a feature of a native CB1 expression system. We asked whether CB1/MA-induced cAMP increase in mast cells should be attributed to AC superactivation. Figures 4(A) and 4(B) show that the CB1-induced cAMP response in mast cells meets three key criteria for classification as a superactivation response [21,24,26,32].
Figure 4. CB1-mediated cAMP response in mast cells exhibits adenylate cyclase superactivation kinetics.
(A) Acute versus chronic effects of CB1 ligand on cytosolic cAMP levels in RBL2H3. Adherent RBL2H3 cells were incubated with 25 μM FSK, 20 μM MA and vehicle for 5, 10 or 120 min at 37 °C. cAMP levels were assayed as above. Baseline cAMP level in untreated cells was 12.8 pmol/105 cells (n=5). (B) Persistent ligand application is required for stimulation of cAMP levels in response to MA. Adherent RBL2H3 cells were incubated with 20 μM MA for 0, 5, 30 or 120 min at 37 °C and assayed as described above (black line) or with 25 μM FSK (grey line). Alternatively, RBL2H3 cells were incubated with 20 μM MA for 0, 5, 30 or 120 min, then washed three times with culture media and incubated in the fresh media for 120 min before lysis for the cAMP assay (broken line). cAMP levels were assayed as described in the Experimental section. Baseline cAMP level in untreated cells was 10.67 pmol/1×104 cells (n=2).
First, early and late kinetics of CB1-induced cAMP responses are markedly different. Figure 4(A) shows that exposure to MA initially causes a sharp decrease in cAMP levels (5–10 min) that is completely inverted by 120 min. We also noted that both the suppressive and increased phases of the cAMP response to MA are PTX-sensitive (Figure 3 and results not shown), strongly implicating Gαi/o in their development. The second feature of superactivation responses to Gαi/o-coupled GPCR is their extended time course. We have shown that cAMP levels remain high up to 18 h after exposure to MA, but not JHW015. At 18 h after exposure to MA, RBL2H3 cAMP levels remained at 60% of the maximal FSK response, which is significantly (P<0.01) greater than the response in vehicle-treated cells (results not shown). Thirdly, AC superactivation has been shown to depend on the continuous presence of the Gαi/o receptor ligand [21]. The present study also attempted to determine whether the withdrawal of ligand prevents the chronic increase in cAMP levels. We found (Figure 4B) that MA must be consistently present for a sustained increase in cytosolic [cAMP] to be achieved over a 120 min period.
Representation and transcriptional regulation of superactivatable AC isoforms in mast cells
Our results suggest that co-expressed CB1 and CB2 both couple to Gαi/o in RBL2H3. At short time points, ligation of either CB1 or CB2 leads to a suppression of cAMP levels. However, over a longer exposure time course, the cAMP responses that follow ligation of the two receptors are diametrically opposed. These results allow us to draw two conclusions. First, the results imply that CB1 and CB2 receptors are not redundant when co-expressed in mast cells. Secondly, the results suggest that the two receptors couple to distinct signalling pathways that diverge downstream of the Gαi/o proteins to which they are both coupled. Since superactivation is not a universal feature of AC isoenzymes [21,24,26,32], we can propose that differential coupling to distinct AC isoenzymes may form the mechanistic basis for the distinctive long-term signalling of CB1 and CB2.
This differential coupling may operate at two levels. First, differential post-translational modification (e.g. phosphorylation of AC isoenzymes) may contribute to their propensity for superactivation. Secondly, the lengthy kinetics of superactivation is consistent with the possible transcriptional/translational up-regulation of AC isoenzymes that are associated with superactivation.
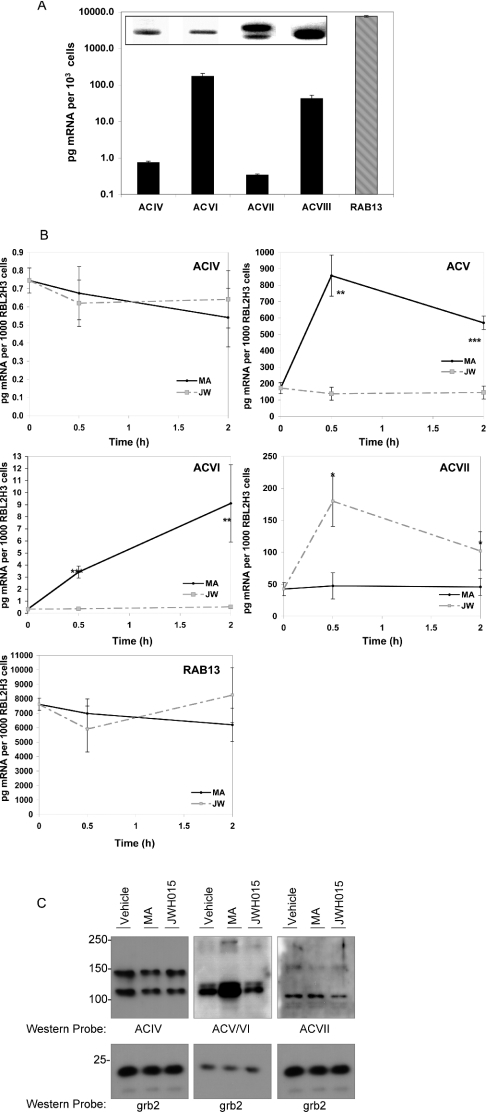
We examined the representation of AC isoform transcripts in RBL2H3 using diagnostic RT–PCR, and we quantified their relative amounts using quantitative (real-time) RT-PCR. Figure 5(A) shows that transcripts corresponding to AC isoforms ACIV, ACV, ACVI and ACVII are detectable in RBL2H3. Under resting conditions, each AC isoenzyme transcript is present at a different level (Figure 5A), with the order of abundance being ACV>ACVII>ACIV>ACVI. These results suggest that mast cells may express protein for the superactivatable AC isoenzymes ACV and ACVI [21,24,26,32]. In addition, proteins for AC isoenzymes ACIV and ACVII, which do not exhibit superactivation, are also likely to be present in mast cells. Thus, in RBL2H3 mast cells, there is sufficient diversity in the representation of AC isoenzymes to allow differential coupling of CB1 and CB2 receptors to superactivatable and non-superactivatable AC isoenzymes respectively.
Figure 5. Representation and transcriptional regulation of superactivatable adenylate cyclase isoforms in mast cells.
(A) Relative mRNA levels of AC isoenzymes in RBL2H3 cells. Quantitative RT–PCR was used to assess the relative quantities of mRNA for the AC isoforms (ACIV–ACVII) present in resting RBL2H3. Results are given in pg of mRNA/1×103 cells, based on an external standard curve. RAB13 was used as an internal control gene; however, the AC isoenzyme values were not normalized to this reference value (n=4). Inset: RT–PCR analysis of AC isoenzyme expression in resting RBL2H3. Two sets of specific primers were designed for each of the nine isoforms of AC. Poly(A)+ mRNA was amplified with the relevant primer pairs at optimized temperatures. Products were resolved by 1.5% agarose electrophoresis. This gel depicts the single products (all ∼150 bp) that were observed in reactions in which ACIV, ACV, ACVI and ACVII primers were
We compared AC isoform transcript levels between resting mast cells and cells exposed to either CB1 or CB2 ligand. These experiments used quantitative PCR and the results are shown in Figure 5(B). Figure 5(B) shows the effect of exposure to MA, the CB1 ligand, on AC isoenzyme transcript levels in RBL2H3 mast cells. In the present study, we observe significant up-regulation of transcript levels for the two superactivatable isoenzymes ACV and ACVI, over a 2 h time period. In contrast, exposure to the CB2 ligand JWH015 induces significant regulation of only ACVII, which is not documented to display superactivation. Hence, CB1, but not CB2, ligands apparently cause specific transcriptional up-regulation of superactivatable AC isoenzymes. We proposed that, if translated to the protein level, this up-regulation may account for the sustained increase in cAMP levels that is observed after CB1 treatment of mast cells.
As shown in Figure 5(C), we examined the levels of AC isoenzyme protein in RBL2H3 mast cells following treatment with either MA or JWH015. Mast cells were exposed to either MA or JWH015, or vehicle, for 18 h at 37 °C. Cell lysates were prepared and protein levels were normalized using a Bradford assay. As an assessment of this normalization, membranes were probed with an irrelevant antibody directed against the Grb2 adapter protein, which confirms equivalent loading in each lane (Figure 5C, lower panels). The upper panels of Figure 5(C) show the levels of AC isoenzymes ACIV, ACV, ACVI and ACVII. ACIV protein levels appear unaffected by cannabinoid treatment (upper left panel), whereas ACVII levels (upper right panel) may decrease slightly with JWH015 exposure. Interestingly, ACV levels (predicted molecular mass 129 kDa) increase markedly with MA treatment but are unaffected by exposure to JWH015. The ACV antibody does cross-react with ACVI, which would be predicted to migrate at approx. 212 kDa. These results suggest that the CB1-mediated up-regulation in transcript levels for the superactivatable AC isoenzymes ACV and VI does result in increased protein levels for these enzymes.
Sustained cAMP elevation is functionally relevant in mast cells
Our results suggest that the sustained up-regulation of cAMP levels is a major consequence of CB1 ligand exposure in mast cells, and that this up-regulation may be attributable to CB1-induced up-regulation of certain AC isoenzymes. We speculated whether CB1-induced cAMP increase has functional consequences for mast cell activation.
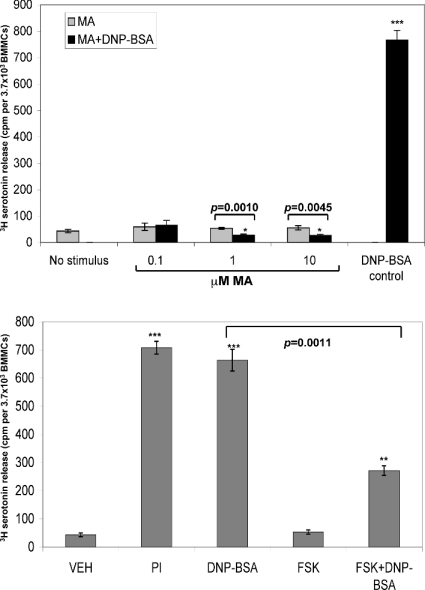
Degranulation of preformed inflammatory mediators is a major consequence of mast cell activation [1,4,34]. Secretory events are antagonized by high levels of cytosolic cAMP [18,19], and we have shown that exposure to a CB1/CB2 co-agonist, CP55940, suppresses mast cell degranulation [10]. We measured the accumulated amount of a degranulation marker in the extracellular milieu after 1 h stimulation of BMMCs through the FcεRI immunoreceptor. This stimulation was performed in the absence and presence of the CB1 ligand MA. Figure 6(A) shows that the presence of the CB1 ligand dominantly interferes with the FcεRI-induced secretory response in BMMCs. Interestingly, the secretory response is apparently highly sensitive to MA, with doses as low as 100 nM, which cause a small but significant (P<0.05) rise in cAMP (see Figure 2B), resulting in a marked suppression in 5-hydroxytryptamine release. We questioned whether cAMP increase was sufficient to block FcεRI-induced degranulation. Figure 6(B) shows that treatment with FSK also blocks secretory responses initiated by FcεRI cross-linking. These results suggest that the sustained effects of CB1 ligands on cAMP levels are sufficient to suppress mast cell secretory responses.
Figure 6. Sustained cAMP elevation suppresses secretion responses in mast cells.
(A) CB1 mediated suppression of mast cell degranulation. BMMCs were plated at a density of 1×105 cells/cm2 and loaded with [3H]-5-hydroxytryptamine. All cells were primed with 1 μg/ml IgE anti-DNP for 16 h before the start of the experiment. Cells were then exposed to the indicated stimuli for 60 min. IgE/DNP stimulation comprises antigenic cross-linking of the FcεRI using 200 ng/ml DNP/BSA. MA (at the indicated doses) was applied for 45 min before the addition of DNP/BSA. After 60 min at 37 °C, supernatants were harvested and the secreted 5-hydroxytryptamine levels were assayed by liquid scintillation counting. Data are expressed as c.p.m./3.7×103 BMMCs (n=3). (B) FSK treatment suppresses mast cell degranulation. BMMCs were plated at a density of 1×105 cells/cm2 and loaded with [3H]-5-hydroxytryptamine. All cells were primed with 1 μg/ml IgE anti-DNP for 16 h before the start of the experiment. Cells were then exposed to the indicated stimuli for 60 min. IgE/DNP stimulation comprises antigenic cross-linking of the FcεRI using 200 ng/ml DNP/BSA. PI [(1 μM phorbol ester)/(500 nM ionomycin)] treatment to elicit maximal secretion. FSK (25 μM) was applied for 45 min before the addition of DNP/BSA. After 60 min at 37 °C, supernatants were harvested and secreted 5-hydroxytryptamine levels were assayed by liquid scintillation counting. Data are expressed as c.p.m./3.7×103 BMMCs (n=2).
DISCUSSION
Cannabinoids exert diverse physiological effects on cells of the CNS, peripheral tissues and components of the immune system. In certain cells, such as keratinocytes, adipocytes, microglia, dendritic cells and lymphocytes, added complexity in cannabinoid signalling apparently arises from the co-expression of two separate receptors for cannabinoid compounds [6,15,16,35–37]. In the present study, we demonstrate that primary and immortalized mast cells both represent a native co-expression context for CB1 and CB2 cannabinoid receptors [10]. In this cell system, CB1 and CB2 are not functionally redundant.
Broadly speaking, cannabinoids are considered to be immunosuppressive [6,8]. In the context of the pro-inflammatory function of mast cells, cannabinoids act as potential anti-inflammatory agents [38,39]. Application of CP55940 or MA suppresses 5-hydroxytryptamine release, leukotriene C4 production and tryptase release ([10]; A. L. Small-Howard and H. Turner, unpublished work) when these are induced by ligation of the FcεRI immunoreceptor. Taken together, these results indicate that exposure to cannabinoids would markedly suppress the acute, proinflammatory functions of mast cells. This suppressive effect is apparently limited to CB1-selective ligands, such as MA, and CB1/CB2 co-ligands. Ablation of CB1 signalling using the antagonist/inverse agonist AM281 prevented the suppression of secretion by CB1/CB2 co-ligands [10]. We proposed that, since increases in cytosolic cAMP have been described to prevent mast cell secretion [18,19], the mechanism for CB1-mediated suppression of secretion could be by a CB1-induced elevation in cAMP levels. Results of the present study are consistent with this model.
Our results support a model in RBL2H3, where ligation of CB1, but not CB2, leads to superactivation of AC. Numerous studies of Gαi/o-coupled GPCR, including the opioid receptors, and heterologously expressed CB1 and CB2, have shown a paradoxical increase in cAMP levels following chronic receptor ligation [21,24,26,32]. This increase in cAMP has been attributed to AC ‘superactivation’, and has been shown, importantly, to be PDE-independent using inhibitors such as IBMX. Thus, the process of superactivation seems to be intrinsic to AC itself. In the present study, we provide two novel findings. First, we show that the phenomenon of superactivation follows ligand binding to CB1 in a native expression system (rather than a heterologous over-expression system). Secondly, we show superactivation of AC within an immune system cell type.
The mechanistic aspects of AC superactivation are not well defined. Not all AC isoenzymes exhibit superactivation [24,26,40], but it is not clear whether superactivation represents an increase in the activity of existing AC enzymes, or whether it reflects de novo synthesis of AC. Our present results are consistent with the latter model. We show that CB ligands cause rapid (30 min) transcriptional up-regulation of superactivatable AC isoenzymes ACV and ACVI. To attribute the superactivation phenomenon to this up-regulation, it was necessary to show that synthesis and trafficking of new AC protein is completed within a time period that would allow it to contribute to a chronic (2–18 h) enhancement of cAMP levels. Our results suggest that the superactivatable ACV isoform, and possibly ACVI, is indeed up-regulated at the protein level following CB1 ligand application. Future studies will define the exact relationship between the magnitude of increase in cellular AC levels and the kinetics/magnitude of cAMP responses. Transcriptional up-regulation is unlikely to be the sole mechanistic basis for AC superactivation, in view of reports that ACVI phosphorylation is kinetically consistent with superactivation, and that ACVI superactivation is sensitive to protein kinase inhibitors [41]. ACVI phosphorylation may result in increased activity of the existing enzyme, whereas up-regulation in AC levels proceeds concurrently to allow for chronic increase in cAMP. Both of these mechanisms may contribute, with sequential kinetics, to an increase in cAMP, which we suggest suppresses secretory responses in mast cells.
Cannabinoids clearly exert marked regulatory effects on mast cell function. The model developed by us suggests that CB1 ligation (for example through exposure to the endo-cannabinoid anandamide or the marijuana constituent Δ9-THC) would tend to suppress the responses of ongoing mast cell activation. Recent reports suggesting that various parasites produce CB1-binding endo-cannabinoids are intriguing in view of the centrality of FcεRI/mast cell responses to anti-parasite inflammatory responses [6,9,42–44]. CB2 ligands, which cause a chronic suppression in cytosolic cAMP levels, as well as extracellular-signal-regulated kinase and AKT (v-akt murine thymoma viral oncogene homologue) activation [10] in mast cells, apparently do not affect secretion but do induce a significant transcriptional programme (A. L. Small-Howard and H. Turner, unpublished work). We are yet to determine a clear picture of the overall consequences of CB2 ligation for mast cell function and hence inflammation.
Our results show that the functional consequences of mast cell exposure to cannabinoids depend on the receptor selectivity of the applied ligand, and, critically, on the time course of exposure. CB2 ligation results in sustained suppression in cAMP levels. In contrast, long-term exposure to CB1 ligands reverses their acute effects, resulting in sustained increases in cytosolic [cAMP]. In the CNS, chronic cAMP mobilization is proposed as a key tolerization mechanism to long-term cannabinoid, or opioid, exposure [21,24,26]. Tolerance arises from the fact that chronic increments in cAMP oppose the acutely suppressive effects of continued agonist binding to Gαi/o GPCR. AC superactivation is thought to contribute to the neurochemical and behavioural alterations that result from extended cannabinoid, or opioid, exposure [45]. Our results suggest that a parallel mechanism for cAMP compensation, tolerance and eventual hyper-elevation is present in immune system cells that bear receptors for cannabinoids. The corresponding long-term phenotypic changes in the immune system, that result from chronic cannabinoid exposure, remain to be elucidated.
Acknowledgments
We acknowledge the support of the Victoria and Bradley Geist Foundation (H.T.). C. Wakano and L. T. Doescher provided excellent technical assistance, and C. Rillero aided in manuscript preparation. We thank A. Stokes for helpful discussions of the data. We thank Dr A. Fleig for primary murine bone marrow derived mast cells. DNA sequencing and qRT–PCR were performed at the Greenwood Molecular Biology Facility (University of Hawaii), with the valuable help of Dr P. Armstrong. FACS facilities were provided by the Cancer Research Center of Hawaii.
References
- 1.Galli S. J. Mast cells and basophils. Curr. Opin. Hematol. 2000;7:32–39. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Galli S. J., Wedemeyer J., Tsai M. Analyzing the roles of mast cells and basophils in host defense and other biological responses. Int. J. Hematol. 2002;75:363–369. doi: 10.1007/BF02982125. [DOI] [PubMed] [Google Scholar]
- 3.Roth M. D., Arora A., Barsky S. H., Kleerup E. C., Simmons M., Tashkin D. P. Airway inflammation in young marijuana and tobacco smokers. Am. J. Respir. Crit. Care Med. 1998;157:928–937. doi: 10.1164/ajrccm.157.3.9701026. [DOI] [PubMed] [Google Scholar]
- 4.Sharma B. B., Apgar J. R., Liu F. T. Mast cells. Receptors, secretagogues, and signaling. Clin. Rev. Allergy Immunol. 2002;22:119–148. doi: 10.1385/CRIAI:22:2:119. [DOI] [PubMed] [Google Scholar]
- 5.Wedemeyer J., Tsai M., Galli S. J. Roles of mast cells and basophils in innate and acquired immunity. Curr. Opin. Immunol. 2000;12:624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
- 6.Berdyshev E. V. Cannabinoid receptors and the regulation of immune response. Chem. Phys. Lipids. 2000;108:169–190. doi: 10.1016/s0009-3084(00)00195-x. [DOI] [PubMed] [Google Scholar]
- 7.Hollister L. E. Marijuana and immunity. J. Psychoactive Drugs. 1992;24:159–164. doi: 10.1080/02791072.1992.10471635. [DOI] [PubMed] [Google Scholar]
- 8.Klein T. W., Newton C., Friedman H. Cannabinoid receptors and immunity. Immunol. Today. 1998;19:373–381. doi: 10.1016/s0167-5699(98)01300-0. [DOI] [PubMed] [Google Scholar]
- 9.Salzet M., Breton C., Bisogno T., Di Marzo V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur. J. Biochem. 2000;267:4917–4927. doi: 10.1046/j.1432-1327.2000.01550.x. [DOI] [PubMed] [Google Scholar]
- 10.Samson M. T., Small-Howard A., Shimoda L. M., Koblan-Huberson M., Stokes A. J., Turner H. Differential roles of CB1 and CB2 cannabinoid receptors in mast cells. (2003) J. Immunol. 2003;170:4953–4962. doi: 10.4049/jimmunol.170.10.4953. [DOI] [PubMed] [Google Scholar]
- 11.Vannacci A., Passani M. B., Pierpaoli S., Giannini L., Mannaioni P. F., Masini E. Nitric oxide modulates the inhibitory effect of cannabinoids on the immunological activation of guinea pig mast cells. Inflamm. Res. 2003;52:S07–S08. doi: 10.1007/s000110300029. [DOI] [PubMed] [Google Scholar]
- 12.Waksman Y., Olson J. M., Carlisle S. J., Cabral G. A. The central cannabinoid receptor (CB1) mediates inhibition of nitric oxide production by rat microglial cells. J. Pharmacol. Exp. Ther. 1992;288:1357–1366. [PubMed] [Google Scholar]
- 13.Faure M., Voyno-Yasenetskaya T. A., Bourne H. R. cAMP and beta gamma subunits of heterotrimeric G proteins stimulate the mitogen-activated protein kinase pathway in COS-7 cells. J. Biol. Chem. 1994;269:7851–7854. [PubMed] [Google Scholar]
- 14.Howlett A. C., Barth F., Bonner T. I., Cabral G., Casellas P., Devane W. A., Felder C. C., Herkenham M., Mackie K., Martin B. R., et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 15.Daaka Y., Klein T. W., Friedman H. Expression of cannabinoid receptor mRNA in murine and human leukocytes. Adv. Exp. Med. Biol. 1995;373:91–96. doi: 10.1007/978-1-4615-1951-5_13. [DOI] [PubMed] [Google Scholar]
- 16.Schatz A. R., Lee M., Condie R. B., Pulaski J. T., Kaminski N. E. Cannabinoid receptors CB1 and CB2: a characterization of expression and adenylate cyclase modulation within the immune system. Toxicol. Appl. Pharmacol. 1997;142:278–287. doi: 10.1006/taap.1996.8034. [DOI] [PubMed] [Google Scholar]
- 17.Rhee M. H., Bayewitch M., Avidor-Reiss T., Levy R., Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J. Neurochem. 1998;71:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- 18.Weston M. C., Peachell P. T. Regulation of human mast cell and basophil function by cAMP. Gen. Pharmacol. 1998;31:715–719. doi: 10.1016/s0306-3623(98)00080-9. [DOI] [PubMed] [Google Scholar]
- 19.Penner R., Neher E. Stimulus-secretion coupling in mast cells. Soc. Gen. Physiol. Ser. 1989;44:295–310. [PubMed] [Google Scholar]
- 20.Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc. Natl. Acad. Sci. U.S.A. 1988;85:9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee M. H., Nevo I., Avidor-Reiss T., Levy R., Vogel Z. Differential superactivation of adenylyl cyclase isozymes after chronic activation of the CB(1) cannabinoid receptor. Mol. Pharmacol. 2000;57:746–752. doi: 10.1124/mol.57.4.746. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz J. H. The many dimensions of cAMP signaling. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13482–13484. doi: 10.1073/pnas.251533998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Childers S. R., Pacheco M. A., Bennett B. A., Edwards T. A., Hampson R. E., Mu J., Deadwyler S. A. Cannabinoid receptors: G-protein-mediated signal transduction mechanisms. Biochem. Soc. Symp. 1993;59:27–50. [PubMed] [Google Scholar]
- 24.Nevo I., Avidor-Reiss T., Levy R., Bayewitch M., Heldman E., Vogel Z. Regulation of adenylyl cyclase isozymes on acute and chronic activation of inhibitory receptors. Mol. Pharmacol. 1998;54:419–426. doi: 10.1124/mol.54.2.419. [DOI] [PubMed] [Google Scholar]
- 25.Childers S. R., Deadwyler S. A. Role of cyclic AMP in the actions of cannabinoid receptors. Biochem. Pharmacol. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- 26.Ammer H., Christ T. E. Identity of adenylyl cyclase isoform determines the G protein mediating chronic opioid-induced adenylyl cyclase supersensitivity. J. Neurochem. 2002;83:818–827. doi: 10.1046/j.1471-4159.2002.01188.x. [DOI] [PubMed] [Google Scholar]
- 27.Alfonso A., Cabado A. G., Vieytes M. R., Botana L. M. Functional compartments in rat mast cells for cAMP and calcium on histamine release. Cell. Signal. 2000;12:343–350. doi: 10.1016/s0898-6568(00)00070-x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin G., Wray E. J., Tao Q., McAllister S. D., Rorrer W. K., Aung M. M., Martin B. R., Abood M. E. Evaluation of the cannabinoid CB2 receptor-selective antagonist, SR144528: further evidence for cannabinoid CB2 receptor absence in the rat central nervous system. Eur. J. Pharmacol. 1999;377:117–125. doi: 10.1016/s0014-2999(99)00402-1. [DOI] [PubMed] [Google Scholar]
- 29.Nakajima T., Iikura M., Okayama Y., Matsumoto K., Uchiyama C., Shirakawa T., Yang X., Adra C. N., Hirai K., Saito H. Identification of granulocyte subtype-selective receptors and ion channels by using a high-density oligonucleotide probe array. J. Allergy Clin. Immunol. 2004;113:528–535. doi: 10.1016/j.jaci.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 30.Kingan T. G. A competitive enzyme linked immunosorbent assay: applications in the assay of peptides, steroids and cyclic nucleotides. Anal. Biochem. 1989;183:283–289. doi: 10.1016/0003-2697(89)90481-8. [DOI] [PubMed] [Google Scholar]
- 31.Mazingue C., Dessaint J. P., Capron A. 3H serotonin release: an improved method to measure mast cell degranulation. J. Immunol. Methods. 1978;21:67–77. doi: 10.1016/0022-1759(78)90224-7. [DOI] [PubMed] [Google Scholar]
- 32.Sunahara R. K., Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol. Interv. 2002;2:168–184. doi: 10.1124/mi.2.3.168. [DOI] [PubMed] [Google Scholar]
- 33.Shefler I., Seger R., Sagi-Eisenberg R. Gi-mediated activation of mitogen-activated protein kinase (MAPK) pathway by receptor mimetic basic secretagogues of connective tissue-type mast cells: bifurcation of arachidonic acid-induced release upstream of MAPK. J. Pharmacol. Exp. Ther. 1999;289:1654–1661. [PubMed] [Google Scholar]
- 34.Turner H., Kinet J. P. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature (London) 1999;402:B24–B30. doi: 10.1038/35037021. [DOI] [PubMed] [Google Scholar]
- 35.Galiegue S., Mary S., Marchand J., Dussossoy D., Carriere D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 36.Nieri P., Greco R., Adinolfi B., Breschi M. C., Martinotti E., Nannetti C., Podesta A. CB1- and CB2-cannabinoid receptor-independent lipolysis induced by WIN 55,212-2 in male rat adipocytes. Naunyn Schmiedebergs Arch. Pharmacol. 2003;368:352–359. doi: 10.1007/s00210-003-0831-3. [DOI] [PubMed] [Google Scholar]
- 37.Matias I., Pochard P., Orlando P., Salzet M., Pestel J., Di Marzo V. Presence and regulation of the endocannabinoid system in human dendritic cells. Eur. J. Biochem. 2002;269:3771–3778. doi: 10.1046/j.1432-1033.2002.03078.x. [DOI] [PubMed] [Google Scholar]
- 38.De Petrocellis L., Melck D., Bisogno T., Di Marzo V. Endocannabinoids and fatty acid amides in cancer, inflammation and related disorders. Chem. Phys. Lipids. 2000;108:191–209. doi: 10.1016/s0009-3084(00)00196-1. [DOI] [PubMed] [Google Scholar]
- 39.Bisogno T., Maurelli S., Melck D., De Petrocellis L., Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J. Biol. Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- 40.Taussig R. Assays of recombinant adenylyl cyclases expressed in Sf9 cells. Methods Mol. Biol. 2004;237:77–85. doi: 10.1385/1-59259-430-1:77. [DOI] [PubMed] [Google Scholar]
- 41.Chen Y., Harry A., Li J., Smit M. J., Bai X., Magnusson R., Pieroni J. P., Weng G., Iyengar R. Adenylyl cyclase 6 is selectively regulated by protein kinase A phosphorylation in a region involved in Galphas stimulation. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14100–14104. doi: 10.1073/pnas.94.25.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matias I., Bisogno T., Melck D., Vandenbulcke F., Verger-Bocquet M., De Petrocellis L., Sergheraert C., Breton C., Di Marzo V., Salzet M. Evidence for an endocannabinoid system in the central nervous system of the leech Hirudo medicinalis. Brain Res. Mol. Brain Res. 2001;87:145–159. doi: 10.1016/s0169-328x(00)00290-4. [DOI] [PubMed] [Google Scholar]
- 43.Salzet M., Capron A., Stefano G. B. Molecular crosstalk in host-parasite relationships: schistosome- and leech-host interactions. Parasitol. Today. 2000;16:536–540. doi: 10.1016/s0169-4758(00)01787-7. [DOI] [PubMed] [Google Scholar]
- 44.Fezza F., Dillwith J. W., Bisogno T., Tucker J. S., Di Marzo V., Sauer J. R. Endocannabinoids and related fatty acid amides, and their regulation, in the salivary glands of the lone star tick. Biochim. Biophys. Acta. 2003;1633:61–67. doi: 10.1016/s1388-1981(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 45.Nestler E. J. Molecular neurobiology of addiction. Am. J. Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]