Abstract
We have shown previously that H11, a serine/threonine kinase, is up-regulated in a heart subjected to ischaemia/reperfusion. In the present study, we have characterized the cellular function of H11, using neonatal rat cardiac myocytes. Although transduction of adenovirus harbouring H11 at low doses increased the cell size, at higher doses it induced apoptosis in cardiac myocytes. Apoptosis was not observed when adenovirus harbouring H11-KI (kinase-inactive mutant of H11) was used, suggesting that the proapoptotic effect of H11 is kinase-dependent. The hypertrophic effect of H11 at high doses was unmasked when apoptosis was inhibited by the caspase inhibitor DEVD-CHO, suggesting that H11 stimulates both hypertrophy and apoptosis in parallel. H11-KI induced hypertrophy even at high doses, indicating that H11 stimulates hypertrophy through kinase-independent mechanisms. H11-KI activated Akt, and cardiac hypertrophy induced by H11-KI was blocked by LY294002, an inhibitor of phosphoinositide 3-kinase. Co-immunoprecipitation analyses indicated that H11 interacts with the α subunit of CK2 (casein kinase 2). Overexpression of H11 decreased the kinase activity of CK2. DRB (5,6-dichloro-1-β-D-ribofuranosyl-benzimidazole), an inhibitor of CK2, mimicked the effect of H11, whereas DRB and H11 failed to exhibit additive effects on apoptosis, suggesting that H11 and DRB utilize a common mechanism to induce apoptosis, namely inhibition of CK2. In summary, H11 is a dual-function kinase in cardiac cells: it induces hypertrophy at low doses through kinase-independent activation of Akt, whereas it causes apoptosis at high doses through protein kinase-dependent mechanisms, in particular by physical interaction with and subsequent inhibition of CK2.
Keywords: cardiac myocyte, casein kinase 2, caspase 3, H11, hypertrophy, small heat-shock protein
Abbreviations: CK2, casein kinase 2; DRB, 5,6-dichloro-1-β-D-ribofuranosyl-benzimidazole; H11-KI, kinase-inactive mutant of H11; I/R, ischaemia/reperfusion; MOI, multiplicity of infection; sHsp, small heat-shock protein; XIAP, X-linked inhibitor of apoptosis
INTRODUCTION
An important therapeutic target in the treatment of heart diseases could be the protection of cardiac myocytes from cell death, such as apoptosis caused by myocardial I/R (ischaemia/reperfusion). Apoptosis has been implicated as an important mechanism promoting the loss of cardiac myocytes in the failing heart [1]. Myocyte apoptosis has been demonstrated in human myocardial infarction [2,3], as well as in animal models of I/R [4–7]. Although several components of the proapoptotic signalling mechanisms exist in cardiac myocytes [7–13], the signal-transduction mechanism through which I/R causes apoptosis in cardiac myocytes is not fully understood. To address this issue, we conducted subtraction hybridization analysis and identified several genes regulating the growth and death of the cardiac myocytes whose expressions are affected in a swine model of ischaemia.
One of the genes up-regulated in the ischaemic swine heart was H11 [14]. H11 is a 22 kDa protein abundantly expressed in skeletal muscles, placenta and heart [15], and shares homology with the protein kinase domain in the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) [16]. ICP10 phosphorylates RasGAP, thereby stimulating cell proliferation [17–19]. H11 also has protein kinase activity, and 293 cells transfected with H11 exhibit anchorage-independent growth in a kinase activity-dependent manner [16]. H11 was found by its homology with the C-terminal α-crystallin domain of the sHsp (small heat-shock protein) family and is referred to as HspB8 [20] or Hsp22 [21]. H11/HspB8/Hsp22 physically interacts with Hsp27 and other sHsps [21,22], thereby potentially affecting the function of the sHsp family, which generally has a cell-protective role in cardiac myocytes [23].
Although we and others have previously suggested that H11 has cell-growth-stimulatory effects [16,24], recent evidence suggests that H11 induces apoptosis in melanoma cells [15]. In our preliminary studies, we also found that high levels of H11 overexpression caused cell shrinkage in neonatal cardiac myocytes, despite the fact that moderate levels of H11 overexpression cause significant increases in protein/DNA content. This indicates that the cellular function of H11 is probably not only cell-type-specific but also dose-dependent. Thus the goal of the present study was to test our hypothesis that H11 has dose-dependent dual functions in cardiac myocytes: hypertrophy at moderate levels and apoptosis at high levels of expression. We also examined the signalling mechanism by which H11 mediates hypertrophy and apoptosis in cardiac myocytes. We provide evidence that a kinase-independent mechanism of H11 mediates hypertrophy, whereas kinase-dependent inhibition of an anti-apoptotic protein kinase, CK2 (casein kinase 2), promotes cardiac myocyte apoptosis.
MATERIALS AND METHODS
Materials
Rabbit polyclonal antibody was raised against the N-terminal rat H11 peptide CGESSFNNELPQDNQEVT (Biosource International, Camarillo, CA, U.S.A.) and affinity-purified. Anti-CK2α antibody (rabbit polyclonal) and anti-c-myc antibody (9E10, monoclonal) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Cleaved caspase 3 (Asp175) antibody was purchased from Cell Signaling Technology (Beverly, MA, U.S.A.). Myelin basic protein and casein were purchased from Sigma. DRB (5,6-dichloro-1-β-D-ribofuranosyl-benzimidazole), a specific CK2 inhibitor [25], LY294002 and DEVD-CHO were purchased from Biomol (Plymouth Meeting, PA, U.S.A.).
Primary culture of neonatal rat ventricular myocytes
Primary culture of cardiac ventricular myocytes was prepared from 1-day-old Crl: (WI) BR-Wistar rats (Charles River Laboratories, Wilmington, MA, U.S.A.). After enzymatic digestion of the hearts, a cardiac myocyte-enriched fraction was prepared using Percoll gradient centrifugation. Cells were cultured in the cardiac myocyte culture medium as described in [26]. Culture media were changed to serum-free media after exactly 24 h. Cardiac fibroblasts were prepared as described in [27]. Myocytes and fibroblasts (passage 3) were cultured under serum-free conditions for 48 h before experiments. Total protein content and DNA content of cardiac myocytes were obtained as described previously [26].
Immunostaining
Cells were fixed in PBS containing 4% (w/v) paraformaldehyde, permeabilized in PBS containing 0.3% Triton X-100 and blocked with 3% (v/v) BSA. Immunostaining was performed using anti-H11 antibody (1:100 dilution), FITC-conjugated anti-rabbit IgG (1:200; Jackson Immunoresearch Laboratories, West Grove, PA, U.S.A.) and Texas Red–phalloidin (Molecular Probes, Eugene, OR, U.S.A.).
Analysis of DNA fragmentation by ELISA
Histone-associated DNA fragments were quantified by the Cell Death Detection ELISA (Roche, Indianapolis, IN, U.S.A.) [7,11]. The values from triplicate measurements of absorbance A405 were averaged.
Immunoblot analysis
Cells were lysed in a lysis buffer containing 220 mmol/l mannitol, 20 mmol/l Hepes (pH 7.4), 68 mmol/l sucrose, 10 mmol/l KCl, 1 mmol/l EGTA, 2 mmol/l MgCl2, 1 mmol/l EDTA, 1 mmol/l dithiothreitol, 0.1 mmol/l [4-(2-aminoethyl)benzenesulphonyl fluoride, 10 μg/ml aprotinin and 10 μg/ml leupeptin. Samples containing equal amounts of protein (50 μg) were subjected to SDS/PAGE.
Immunocomplex protein kinase assays
Myocytes were lysed as described above. Immunoprecipitation was performed by incubating the samples with 1 μg/ml rabbit polyclonal antibody against H11 or CK2 for 2 h, followed by the addition of Protein A–Sepharose for 1 h at 4 °C. The immunoprecipitates were washed three times with lysis buffer and once with a kinase assay buffer containing 20 mmol/l Tris/HCl (pH 7.5), 5 mmol/l MgCl2 and 2 mmol/l MnCl2. Kinase reaction was initiated by the addition of 100 μM of unlabelled ATP, 5 μCi of [γ-32P]ATP (6000 Ci/mmol) and 10 μg of substrate. Myelin basic protein and casein were used as substrates for H11 and CK2 respectively. After 30 min of incubation at 30 °C, reactions were terminated by the addition of Laemmli sample buffer and boiling for 15 min. Samples were resolved bySDS/12.5% polyacrylamide gels. Phosphorylated substrates were detected by autoradiography.
Construction of the adenovirus vectors
Generation of Ad-H11 (adenovirus vectors harbouring H11) and Ad-XIAP (X-linked inhibitor of apoptosis) have been described in [11,24]. H11-KI (kinase-inactive mutant of H11) was generated by mutating Lys113 to a glycine residue [H11(K113G)] using Quik Change (Stratagene. La Jolla, CA, U.S.A.). Ad-H11, Ad-H11myc (H11 with myc-tag sequence in the C-terminus) and Ad-H11-KI were generated using the Adeno-X adenovirus construction kit (Clontech, Palo Alto, CA, U.S.A.). Ad-LacZ (adenovirus harbouring β-galactosidase) was used as the control.
Statistical analyses
Results are presented as means±S.E.M. Statistical analyses were performed using ANOVA. A post hoc test was performed by the Tukey method. P<0.05 was considered to be statistically significant.
RESULTS
Adenovirus-mediated transduction of H11 causes cardiac myocyte hypertrophy at low doses
Cardiac myocytes were transduced with Ad-H11 or Ad-LacZ and immunoblotting was performed using anti-H11 antibody. Cardiac myocytes expressed endogenous H11 and adenovirus transduction of H11 dose-dependently increased the expression of H11 (see the Supplementary Figures at http://www.BiochemJ.org/bj/388/bj3880475add.htm). At MOI (multiplicity of infection)=100, H11 expression was increased by 4.9±0.1-fold. A similar extent of up-regulation of endogenous H11 was induced in response to heat shock and pressure overload [15,24]. Transduction of Ad-LacZ failed to affect the expression of H11.
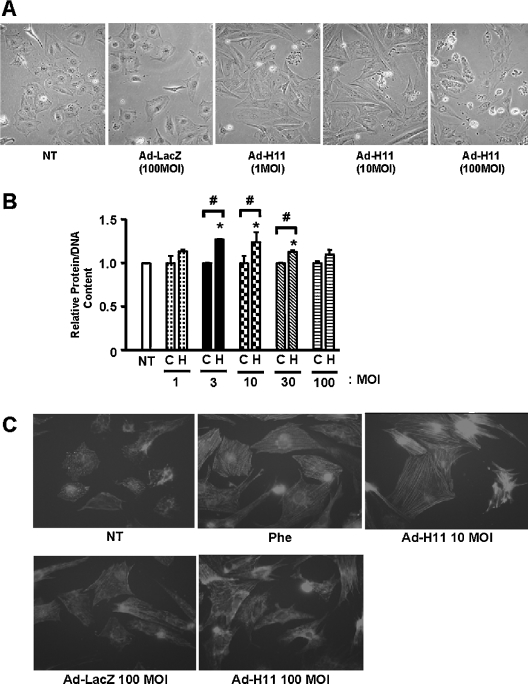
We next examined the effect of H11 overexpression on the morphology of cardiac myocytes. Transduction of Ad-H11 at MOI=1–10 increased the size of cardiac myocytes (Figure 1A), suggesting that H11 induces hypertrophy consistent with our previous observation [24]. Some myocytes exhibited shrinkage and death even when other myocytes showed enlargement (Figure 1A). Cell death became more prominent in many cells at MOI=100 of Ad-H11 (Figure 1A). Consistent with these observations, transduction of H11 at low doses (MOI=3–10) increased the total protein content/DNA content, reaching a peak at MOI=10. However, at high doses, increases in protein/DNA content became less prominent, and at MOI=100, they were not statistically significant (Figure 1B). Cardiac hypertrophy is also characterized by cytoskeletal organization. The extent of actin polymerization was evaluated by phalloidin staining. Although phalloidin staining exhibited a punctuated pattern in unstimulated or Ad-LacZ-transduced myocytes, it showed a striated pattern in myocytes treated with phenylephrine, an agonist for α1 adrenergic receptors, or transduced with Ad-H11 at MOI=10. Again, neither organization of actin nor increases in cell size were clearly observed in myocytes transduced with Ad-H11 at MOI=100 (Figure 1C).
Figure 1. H11 causes cell death and hypertrophy.
Myocytes were transduced with Ad-LacZ or Ad-H11; 12 h after transduction, they were incubated with fresh myocyte medium for 48 h. (A) Phase-contrast microscope images at low magnification. NT, no transduction. (B) Total protein content/DNA content was determined from three experiments performed in triplicate each. C, Ad-LacZ; H, Ad-H11; and NT, no transduction control. *P<0.001 versus NT, #P<0.001 versus respective controls. (C) Cells were stained with Texas Red–phalloidin. Phe, phenylephrine (10 μM) for 48 h.
Adenovirus-mediated transduction of H11 causes cardiac myocyte apoptosis at high doses
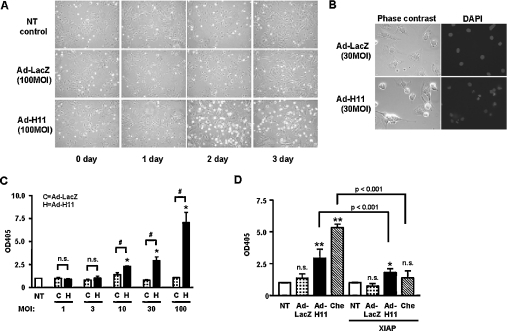
At lower magnification, phase-contrast microscopy showed that transduction of Ad-H11 at MOI=100 increased cell shrinkage on day 2 (Figure 2A). It is unlikely that this was caused by either serum-free culture or the toxicity of adenovirus transduction alone, since neither serum-free culture alone nor transduction of Ad-LacZ induced similar changes (Figure 2A). To examine whether the effect of H11 on cell shrinkage/death is cell-type-specific, we examined the effect of Ad-H11 transduction on cardiac fibroblasts. Ad-H11, but not Ad-LacZ, induced a marked increase in cell shrinkage/death in cardiac fibroblasts at a dose of MOI=100 (results not shown).
Figure 2. Expression of H11 induces apoptosis.
Neonatal cardiac myocytes were transduced with the indicated titres of adenovirus vectors. (A) Myocytes were incubated with fresh myocyte medium, 12 h after transduction, for the indicated durations. (B) Cells were stained with DAPI. (C, D) Results of Cell Death ELISAs. The A405 (OD405) in untransduced myocytes was designated as 1.0. (C) NT, no transduction; C, Ad-LacZ; and H, Ad-H11. Ad-H11 at MOI=100 caused a 7.1-fold increase in DNA fragmentation. (D) Myocytes were transduced with Ad-H11 or Ad-LacZ (MOI=30) in the presence or absence of Ad-XIAP (MOI=30). As a positive control of apoptosis, we treated myocytes with chelerythrine (Che; 10 μM) for 1 h [11]. NT, no transduction or transduction with Ad-XIAP alone. (C, D) Each bar represents the mean for three experiments performed in duplicate. (C) *P<0.001 versus NT, #P<0.001 versus respective controls. (D) *P<0.05, **P<0.001 versus respective NT. n.s., not significant.
Since cell death with shrinkage by H11 suggests apoptosis, we further examined whether cell death by H11 is apoptosis. DAPI (4,6-diamidino-2-phenylindole) staining indicated that transduction of Ad-H11, but not Ad-LacZ, induced nuclear fragmentation (Figure 2B). Transduction of Ad-H11 dose-dependently increased the cytoplasmic accumulation of mono- and oligo-nucleosomes, another important marker of apoptosis, whereas that of Ad-LacZ did not (Figure 2C). A significant increase in cytoplasmic accumulation of mono- and oligo-nucleosomes was observed when Ad-H11 was transduced at MOI>10 (Figure 2C). Similar effects were observed in cardiac fibroblasts transduced with Ad-H11 (results not shown). Increases in cytoplasmic accumulation of mono- and oligo-nucleosomes by Ad-H11 were significantly suppressed in the presence of XIAP, suggesting that cell death induced by H11 is mediated by a caspase-dependent mechanism (Figure 2D). Increases in DNA fragmentation and its suppression by XIAP were also observed in response to chelerythrine treatment used as a positive control of cardiac myocyte apoptosis [11] (Figure 2D). Taken together, these results suggest that cell shrinkage/death induced by H11 is mediated primarily by apoptosis.
H11-induced apoptosis is dependent on the protein kinase activity of H11
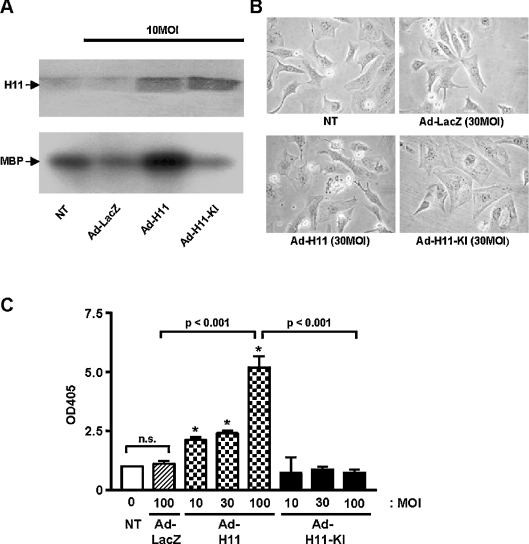
To examine whether the protein kinase activity of H11 is required to induce apoptosis in cardiac myocytes, we generated an adenovirus vector harbouring H11(K113G), a kinase-inactive mutant (Ad-H11-KI). Transduction of Ad-H11-KI in cardiac myocytes increased the expression of H11(K113G) (Figure 3A, upper panel). Although transduction of Ad-H11 increased the total cellular kinase activity of H11, that of Ad-H11-KI failed to increase it (Figure 3A, lower panel). Transduction of Ad-H11-KI at MOI=30–100 induced neither cell shrinkage/death (Figure 3B) nor cytoplasmic accumulation of mono- and oligonucleosomes (Figure 3C), but it increased the cell size (Figure 3B). These results suggest that the protein kinase activity of H11 is required for H11-induced cardiac myocyte apoptosis.
Figure 3. Transduction of Ad-H11-KI failed to induce apoptosis.
Myocytes were transduced with Ad-LacZ, Ad-H11 or Ad-H11-KI (MOI=10–100). (A) Cell lysates were subjected to immunoblot analyses with anti-H11 antibody (upper panel) and immunocomplex protein kinase assays using myelin basic protein (MBP) as a substrate (lower panel). (B) Phase-contrast microscope images of myocytes at 48 h. (C) At 48 h, apoptosis was quantified by Cell Death ELISAs. A405 (OD405) in untransduced myocytes was designated as 1.0. Each column represents the mean for three experiments performed in duplicate. NT, no transduction. *P<0.001 versus NT. n.s., not significant.
Hypertrophy at high doses of H11 is unmasked by caspase 3 inhibitor treatment
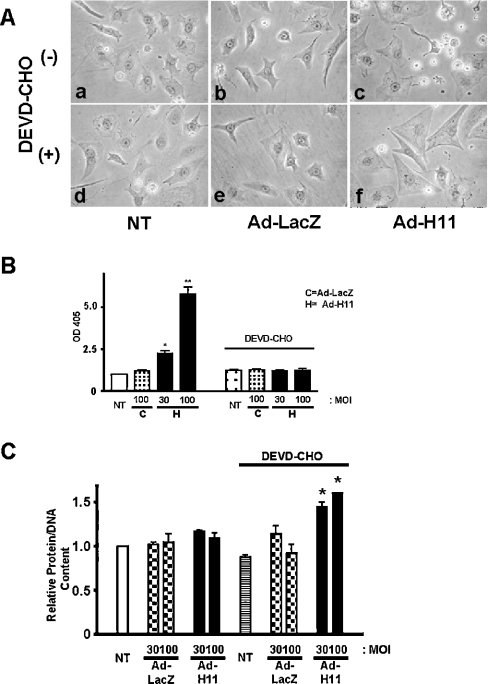
To test whether the hypertrophic effect of H11 is masked by concomitant apoptosis, myocytes were transduced with Ad-LacZ or Ad-H11 in the presence or absence of DEVD-CHO, a cell-permeant caspase 3 inhibitor. Although DEVD-CHO did not significantly affect the morphology of untransduced or Ad-LacZ-transduced myocytes, it inhibited cell shrinkage and actually increased the cell size in myocytes transduced with Ad-H11 (MOI=100; Figure 4A). We confirmed that DEVD-CHO treatment inhibits the cytoplasmic accumulation of mono- and oligo-nucleosomes by Ad-H11 (Figure 4B). Quantitative analyses indicated that DEVD-CHO treatment unmasked a significant increase in protein/DNA content in myocytes transduced with Ad-H11 at MOI=30 and 100 (Figure 4C). Treatment with DEVD-CHO alone did not affect protein/DNA content in control myocytes. These results are consistent with the notion that the mechanisms stimulating hypertrophy and apoptosis are co-activated, with apoptosis predominating at high levels of H11 overexpression.
Figure 4. Cardiac hypertrophy by high doses of H11 is unmasked by caspase 3 inhibitor treatment.
Myocytes were transduced with Ad-LacZ or Ad-H11 at MOI=100 (A) or at the indicated MOI (B, C); 12 h after transduction, myocytes were incubated with or without DEVD-CHO (150 μM) for 48 h. (A) Phase-contrast microscope images. NT, no transduction. (B) Results of Cell Death ELISAs are shown. The A405 (OD405) in untransduced myocytes was designated as 1.0. Results are representative of three experiments performed in duplicate. (C) Total protein content/DNA content was determined from three experiments performed in triplicate each. The data from myocytes without adenovirus transduction or DEVD-CHO (white bar) were designated as 1.0. NT, no transduction. *P<0.001 versus NT.
H11-induced cardiac hypertrophy is mediated by kinase-independent mechanisms
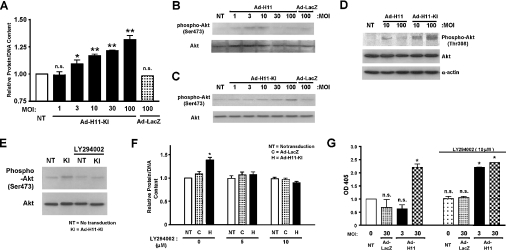
Since induction of apoptosis by H11 is mediated by kinase-dependent mechanisms, we hypothesized that cardiac hypertrophy by H11 may be mediated by a kinase-independent mechanism. Transduction of Ad-H11-KI dose-dependently increased protein/DNA content in cardiac myocytes (Figure 5A), whereas that of Ad-LacZ did not, suggesting that H11 has a kinase-independent prohypertrophic function.
Figure 5. H11-induced cardiac hypertrophy is mediated by a kinase-independent mechanism.
Myocytes were transduced with Ad-LacZ, Ad-H11 or Ad-H11-KI (MOI=1–100) for 48 h; (D–F) 24 h after transduction, myocytes were incubated with or without LY294002 (5 or 10 μM) for 48 h. (A) Total protein/DNA content without adenovirus transduction was designated as 1.0. Each column represents the mean for three experiments performed in duplicate. NT, no transduction. *P<0.05 versus NT, **P<0.001 versus NT. (B–E) The level of Ser473- or Thr308-phosphorylated Akt, total Akt and α-actin were evaluated using Western blotting. The result was representative of three experiments. (F) Cardiac myocytes were transduced with Ad-LacZ or Ad H11-KI (MOI=100). Mean protein/DNA content was obtained from three experiments, each performed in triplicate. The value from NT without LY294002 was designated as 1.0. NT, no transduction; C, Ad-LacZ; H, Ad-H11-KI. *P<0.001 versus NT. (G) Myocytes were transduced with Ad-H11 or Ad-LacZ (MOI=3 or 30). Some myocytes were incubated with LY294002 (10 μM) for 48 h. Apoptosis was quantified using Cell Death ELISA. A405 (OD405) in untransduced myocytes was designated as 1.0. Each column represents the mean for three experiments performed in triplicate each. NT, no transduction. *P<0.001 versus NT. n.s., not significant.
To elucidate the signalling mechanism mediating hypertrophy by H11-KI, we screened the activation of signalling molecules in response to overexpression of either wild-type H11 or H11-KI. Transduction of wild-type H11 moderately increased phospho-Akt (Ser473) at MOI=3–10, but not at higher doses (Figure 5B). In contrast, transduction of Ad-H11-KI dose-dependently (up to MOI=100) increased phospho-Akt (Figure 5C). Ad-H11-KI dose-dependently increased Thr308 phosphorylation of Akt, whereas Ad-H11 moderately increased Thr308 phosphorylation of Akt, reaching a peak at MOI=10 (Figure 5D). Increase in Akt phosphorylation by H11-KI was inhibited by LY294002, a specific inhibitor of phosphoinositide 3-kinase, which is an upstream regulator of Akt (Figure 5E). Hypertrophy caused by H11-KI was significantly suppressed in the presence of LY294002 (Figure 5F). These results suggest that activation of Akt may play an important role in mediating cardiac hypertrophy stimulated by kinase-independent mechanisms of H11. Since the hypertrophy arm of the H11 signalling mechanism involves Akt, a cell-survival kinase, we next examined whether inhibiting the signalling mechanism mediating hypertrophy manifests the proapoptotic effect of H11 at lower concentrations. In fact, proapoptotic mechanisms of H11 became apparent even at MOI=3 in the presence of LY294002 (Figure 5G). These results are consistent with the notion that the mechanisms stimulating hypertrophy and apoptosis are co-activated, with hypertrophy predominating at low levels of H11 overexpression.
CK2 interacts with H11
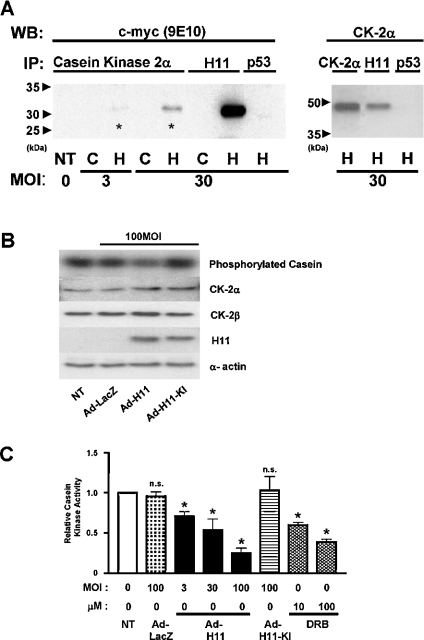
Several sHsps are phosphorylated by CK2 [28–30] and physical interaction between Hsp90 and CK2 has been demonstrated in [22,30]. We therefore examined whether H11 interacts with CK2. To this end, we overexpressed H11myc in myocytes, and CK2α (α subunit of CK2) was immunoprecipitated. Immunoblot analyses using anti-myc antibody indicated that a faint band co-migrating with H11myc was detected in the CK2α immunoprecipitates when myocytes were transduced with Ad-H11myc at MOI=3. A stronger H11 band was detected when CK2α immunoprecipitates were prepared from myocytes transduced with Ad-H11myc at MOI=30. H11 was not detected when myocyte lysates were subjected to immunoprecipitation with an irrelevant antibody, such as anti-p53 antibody (Figure 6A, left panel). To confirm physical interaction between H11 and CK2α, we conducted reciprocal co-immunoprecipitation experiments. Cell lysates from myocytes transduced with Ad-H11myc at MOI=30 were subjected to immunoprecipitation with anti-H11 antibody, followed by immunoblot analyses with anti-CK2α antibody. A band co-migrating with CK2α was detected in the immunoprecipitates with anti-H11 antibody, but not with anti-p53 antibody (Figure 6A, right panel).
Figure 6. H11 physically associates with CK2α.
(A) Cardiac myocytes were transduced with Ad-H11-myc (‘H’) or control Ad-LacZ (‘C’). Cell lysates were subjected to immunoprecipitation (IP) with an antibody against CK2α, H11 or p53. The immunoprecipitates were then subjected to immunoblot analysis with anti-c-myc (9E10) antibody (left panel) or anti-CK2α antibody (right). Asterisk indicates the H11myc co-immunoprecipitated with CK2α. NT, no transduction; WB, Western blot. (B, C) Myocytes were transduced with Ad-LacZ, Ad-H11 or Ad-H11-KI at MOI=100 for 48 h; 24 h after transduction, myocytes were treated with DRB at the indicated concentrations for 24 h. The kinase activity of CK2 was evaluated by immunocomplex kinase assays using casein as a substrate (B, top panel). A part of the cell lysate was subjected to immunoblot analyses with anti-CK2α, CK2β, H11 and α-actin antibody, showing that comparable levels of CK2 (α and β) are expressed and that H11 or H11-KI is overexpressed. (C) Phosphorylation of casein was quantified by densitometric analysis. The value from myocytes without adenovirus transduction or DRB was designated as 1.0. Each bar represents the mean for three experiments performed in duplicate. NT, no transduction. *P<0.001 versus NT. n.s., not significant.
To examine the functional consequence of the H11–CK2 interaction, we measured the kinase activity of CK2 in myocytes transduced with Ad-LacZ, Ad-H11 or Ad-H11-KI, using immunocomplex kinase assays. Overexpression of H11 or H11-KI did not affect the protein expression of CK2α or CK2β (Figure 6B). Transduction with Ad-H11 dose-dependently inhibited the activity of CK2, whereas transduction with Ad-LacZ or Ad-H11-KI failed to inhibit it (Figures 6B and 6C). Dose-dependent inhibition of CK2 activity was also observed in the presence of DRB, a potent and selective inhibitor of CK2, as expected (Figure 6C).
Inhibition of CK2 induces cardiac myocyte apoptosis
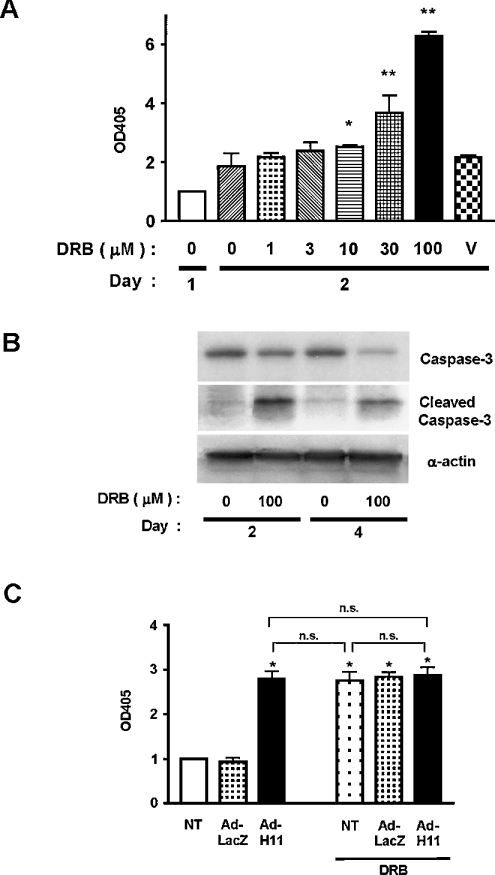
Since CK2 has anti-apoptotic effects in cardiac myocytes [10], inhibition of CK2 may mediate proapoptotic effects of H11. To determine whether inhibition of CK2 promotes apoptotic cell death under our experimental conditions, we examined the effect of DRB on cardiac myocyte death. DRB dose-dependently induced cytoplasmic accumulation of mono- and oligo-nucleosomes in cardiac myocytes (Figure 7A). DRB also induced cleavage of caspase 3 (Figure 7B), suggesting that DRB induces cardiac myocyte apoptosis. Owing to the fact that both transduction of H11 and treatment with DRB caused inhibition of CK2 and induction of apoptosis, we examined whether they have additive effects on cardiac myocyte apoptosis. Co-application of Ad-H11 and DRB failed to show additive effects on apoptosis (Figure 7C), suggesting that they induce apoptosis through a common mechanism, namely inhibition of CK2.
Figure 7. Inhibition of CK2α induces apoptosis in cardiac myocytes.
(A) Cardiac myocytes were treated with the indicated concentrations of DRB for 2 days. Apoptosis was determined by Cell Death ELISAs. Data were normalized by those obtained in control myocytes on day 0. V, myocytes treated with vehicle alone (0.1% ethanol). *P<0.05, **P<0.001 versus control (white bar). Results are the means for three experiments performed in triplicate. (B) Myocytes were treated with or without DRB for 2 or 4 days. Cleavage of caspase 3 was determined by immunoblotting with anti-(cleaved caspase 3) antibody. (C) Myocytes were transduced with Ad-LacZ or Ad-H11; 24 h after transduction, myocytes were treated with DRB (100 μM); 48 h after incubation with DRB, the extent of apoptosis was determined by Cell Death ELISAs. The data from myocytes without adenovirus transduction or DRB was designated as 1.0. Each bar represents the mean±S.E.M. for five experiments performed in duplicate. OD405, A405. n.s., not significant.
DISCUSSION
Our results suggest that moderate expression of H11 induces hypertrophy through a kinase-independent mechanism, whereas higher levels of H11 expression stimulate apoptosis through a kinase-dependent mechanism in cardiac myocytes. H11 physically interacts with CK2α, thereby inhibiting the kinase activity of CK2, which in part mediates cardiac myocyte apoptosis.
We have previously shown that the total protein content of cardiac myocytes was increased when Ad-H11 was transduced at MOI=3 [24]. Interestingly, an increase in the protein content, myocyte size or actin organization was not observed at higher doses of H11 and apoptosis became apparent instead. H11 has cell-proliferative effects in HEK-293 cells (human embryonic kidney 293 cells) [16], while it exhibits proapoptotic effects in melanoma cells [15]. Thus the effect of H11 on cell growth and death is cell-type-dependent. The effect of H11 on cell growth and death is also dose-dependent. Such a coexistence of dose-dependent dual functions of H11 within a single cell type has not been reported previously. Furthermore, no other kinases in the heart have dose-dependent and dual hypertrophic and proapoptotic functions. Interestingly, hypertrophic and proapoptotic actions of H11 are mediated by distinct signalling mechanisms. This may in part explain the complex outcome of H11 overexpression reported so far [15,21,24].
Although H11 was initially identified as an oncogenic serine/threonine kinase homologous with the protein kinase domain of ICP10 [16], H11 also has sequence similarity to sHsp family members, such as αB-crystallin and, thus, is also designated as HspB8 [20] or Hsp22 [21]. Although H11 is capable of phosphorylating a substrate in an Mn2+-dependent manner [16], protein phosphorylation may not be the primary function of H11 [21,31]. In the present study, we showed that H11-KI failed to stimulate cardiac myocyte apoptosis. Thus the proapoptotic effect of H11 depends on its protein kinase activity. Interestingly, however, H11-KI also dose-dependently stimulated cardiac hypertrophy. A higher level of hypertrophy is unmasked at high doses of H11 when apoptosis is inhibited. These results suggest that H11 does possess a protein kinase-independent function that induces cardiac hypertrophy.
We speculate that Akt may be involved in H11-induced cardiac hypertrophy since Thr308/Ser473 phosphorylation of Akt is enhanced when H11-KI is overexpressed. Cardiac hypertrophy induced by H11-KI is inhibited in the presence of LY294002, which also blocks the activation of Akt by H11. Association between Akt and Hsp27, another sHsp, enhances the activity of Akt [32]. Since H11 and Hsp27 interact with each other [32], the function of H11 as a chaperone for complex formation between Akt and sHsps may stimulate Akt.
Many members of the sHsp family, including Hsp27 and αB-crystallin, are phosphorylated by serine/threonine kinases, including MAPK (mitogen-activated protein kinase)-activated protein kinase 2, extracellular-signal-regulated kinase, protein kinase A and CK2 [33–35]. sHsps, including Hsp27, Hsp90 and Hsp105, are either co-activated with or directly phosphorylated by CK2 in response to stress [28–30,36] and physically associate with each other [30]. In the present study, we demonstrated that H11 and CK2 physically interact with each other. Overexpression of H11 dose-dependently inhibited the protein kinase activity of CK2, whereas that of H11-KI had no effect. Thus H11 inhibits CK2 through a kinase activity-dependent mechanism. Phosphatase treatment enhances the kinase activity of CK2 [37]. Whether or not H11 directly phosphorylates CK2 and, if so, whether such a direct phosphorylation of CK2 by H11 negatively regulates the kinase activity of CK2 remains to be elucidated.
CK2 plays a cell-protective role in several cell types [10]. Treatment of cardiac myocytes with DRB, a CK2-specific inhibitor, induced apoptosis. Thus inhibition of CK2 by H11 may induce cardiac myocyte apoptosis. In fact, DRB and H11 failed to exhibit additive effects on cardiac myocyte apoptosis, suggesting that H11 and DRB utilize a common mechanism for myocyte apoptosis, namely inhibition of CK2.
H11 interacts with Hsp27 and other sHsps [21,22] and anti-apoptotic molecules [23]. Thus H11 may promote apoptosis by negatively affecting the anti-apoptotic effects of other sHsps. Alternatively, hetero-oligomeric complex formation of sHsps can potentially be regulated by the phosphorylation of sHsps [21]. H11 may affect the oligomerization of sHsps by affecting the phosphorylation of sHsps through its regulation of CK2. Further investigation will be necessary to elucidate the mechanism of promotion of apoptosis by H11.
Attenuated increases in protein content or DNA content by high doses of wild-type H11 were accompanied not only by increases in apoptosis but also by cessation of the increases in cell size (Figure 1A). The cellular mechanism by which H11 fails to induce hypertrophy at high doses is currently unknown. Interestingly, high doses of wild-type H11 failed to enhance the phosphorylation of Akt (Thr308/Ser473). It is possible that the protein kinase activity of H11 negatively affects the chaperone activity of H11, thereby inhibiting Akt. Whether or not inhibition of CK2 and/or activation of the endogenous negative regulators of hypertrophy is involved in this process remains to be elucidated. We have shown that Mst1 (mammalian sterile 20-like kinase 1), a proapoptotic kinase, negatively regulates cardiac hypertrophy [7]. Thus direct coupling may exist between proapoptotic and antihypertrophic mechanisms in cardiac myocytes.
The mechanism of activation of H11 is not well understood. The specific protein kinase activity of H11 is not altered by stimulation with various hypertrophic or proapoptotic stimuli (M. Hase and J. Sadoshima, unpublished work). Protein expression of H11 is low in cancer cell lines [15], whereas H11 is up-regulated by myocardial ischaemia [14] and heat shock [15]. Although the low to moderate activity of H11 in cancer cells and cardiac myocytes subjected to mild stress may be growth-stimulatory, higher levels of H11 expression in response to some types of stress may stimulate cell death. It will be important to understand the mechanism regulating mRNA/protein expression of H11, which potentially plays an important role in determining whether cardiac myocytes should grow or die under various pathophysiological conditions.
Online data
Acknowledgments
We thank D. Zablocki (Department of Cell Biology and Molecular Medicine, University of Medicine and Dentistry of New Jersey) for a critical reading of this paper. This work was supported by grants from the National Institutes of Health (grant numbers HL59139, HL33107, HL33065, HL65182, HL65183, AG14121, HL69020, HL67724 and HL67727) and American Heart Association (grant numbers 9950673N, 0940123N and 0325409T).
References
- 1.Wencker D., Chandra M., Nguyen K., Miao W., Garantziotis S., Factor S. M., Shirani J., Armstrong R. C., Kitsis R. N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Invest. 2003;111:1497–1504. doi: 10.1172/JCI17664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh G., Tamura J., Suzuki M., Suzuki Y., Ikeda H., Koike M., Nomura M., Jie T., Ito K. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am. J. Pathol. 1995;146:1325–1331. [PMC free article] [PubMed] [Google Scholar]
- 3.Misao J., Hayakawa Y., Ohno M., Kato S., Fujiwara T., Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts with myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. [DOI] [PubMed] [Google Scholar]
- 4.Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kajstura J., Cheng W., Reiss K., Clark W. A., Sonnenblick E. H., Krajewski S., Reed J. C., Olivetti G., Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab. Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 6.Fliss H., Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ. Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto S., Yang G., Zablocki D., Liu J., Hong C., Kim S. J., Soler S., Odashima M., Thaisz J., Yehia G., et al. Activation of Mst1 causes dilated cardiomyopathy by stimulating apoptosis without compensatory ventricular myocyte hypertrophy. J. Clin. Invest. 2003;111:1463–1474. doi: 10.1172/JCI17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black S. C., Huang J. Q., Rezaiefar P., Radinovic S., Eberhart A., Nicholson D. W., Rodger I. W. Co-localization of the cysteine protease caspase-3 with apoptotic myocytes after in vivo myocardial ischemia and reperfusion in the rat. J. Mol. Cell. Cardiol. 1998;30:733–742. doi: 10.1006/jmcc.1998.0660. [DOI] [PubMed] [Google Scholar]
- 9.Reed J. C., Paternostro G. Postmitochondrial regulation of apoptosis during heart failure. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7614–7616. doi: 10.1073/pnas.96.14.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P. F., Li J., Muller E. C., Otto A., Dietz R., von Harsdorf R. Phosphorylation by protein kinase CK2: a signaling switch for the caspase-inhibiting protein ARC. Mol. Cell. 2002;10:247–258. doi: 10.1016/s1097-2765(02)00600-7. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S., Seta K., Morisco C., Vatner S. F., Sadoshima J. Chelerythrine rapidly induces apoptosis through generation of reactive oxygen species in cardiac myocytes. J. Mol. Cell. Cardiol. 2001;33:1829–1848. doi: 10.1006/jmcc.2001.1446. [DOI] [PubMed] [Google Scholar]
- 12.Yussman M. G., Toyokawa T., Odley A., Lynch R. A., Wu G., Colbert M. C., Aronow B. J., Lorenz J. N., Dorn, II G. W. Mitochondrial death protein Nix is induced in cardiac hypertrophy and triggers apoptotic cardiomyopathy. Nat. Med. 2002;8:725–730. doi: 10.1038/nm719. [DOI] [PubMed] [Google Scholar]
- 13.Sanchis D., Mayorga M., Ballester M., Comella J. X. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–986. doi: 10.1038/sj.cdd.4401267. [DOI] [PubMed] [Google Scholar]
- 14.Depre C., Tomlinson J. E., Kudej R. K., Gaussin V., Thompson E., Kim S. J., Vatner D. E., Topper J. N., Vatner S. F. Gene program for cardiac cell survival induced by transient ischemia in conscious pigs. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9336–9341. doi: 10.1073/pnas.171297498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gober M. D., Smith C. C., Ueda K., Toretsky J. A., Aurelian L. Forced expression of the H11 heat shock protein can be regulated by DNA methylation and trigger apoptosis in human cells. J. Biol. Chem. 2003;278:37600–37609. doi: 10.1074/jbc.M303834200. [DOI] [PubMed] [Google Scholar]
- 16.Smith C. C., Yu Y. X., Kulka M., Aurelian L. A novel human gene similar to the protein kinase (PK) coding domain of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) codes for a serine-threonine PK and is expressed in melanoma cells. J. Biol. Chem. 2000;275:25690–25699. doi: 10.1074/jbc.M002140200. [DOI] [PubMed] [Google Scholar]
- 17.Smith C. C., Luo J. H., Aurelian L. The protein kinase activity of the large subunit of herpes simplex virus type 2 ribonucleotide reductase (ICP10) fused to the extracellular domain of the epidermal growth factor receptor is ligand-inducible. Virology. 1996;217:425–434. doi: 10.1006/viro.1996.0136. [DOI] [PubMed] [Google Scholar]
- 18.Peng T., Hunter J. R., Nelson J. W. The novel protein kinase of the RR1 subunit of herpes simplex virus has autophosphorylation and transphosphorylation activity that differs in its ATP requirements for HSV-1 and HSV-2. Virology. 1996;216:184–196. doi: 10.1006/viro.1996.0045. [DOI] [PubMed] [Google Scholar]
- 19.Nelson J. W., Zhu J., Smith C. C., Kulka M., Aurelian L. ATP and SH3 binding sites in the protein kinase of the large subunit of herpes simplex virus type 2 of ribonucleotide reductase (ICP10) J. Biol. Chem. 1996;271:17021–17027. doi: 10.1074/jbc.271.29.17021. [DOI] [PubMed] [Google Scholar]
- 20.Kappe G., Verschuure P., Philipsen R. L., Staalduinen A. A., Van de Boogaart P., Boelens W. C., De Jong W. W. Characterization of two novel human small heat shock proteins: protein kinase-related HspB8 and testis-specific HspB9. Biochim. Biophys. Acta. 2001;1520:1–6. doi: 10.1016/s0167-4781(01)00237-8. [DOI] [PubMed] [Google Scholar]
- 21.Benndorf R., Sun X., Gilmont R. R., Biederman K. J., Molloy M. P., Goodmurphy C. W., Cheng H., Andrews P. C., Welsh M. J. HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 ((3D)HSP27) J. Biol. Chem. 2001;276:26753–26761. doi: 10.1074/jbc.M103001200. [DOI] [PubMed] [Google Scholar]
- 22.Sun X., Fontaine J. M., Rest J. S., Shelden E. A., Welsh M. J., Benndorf R. Interaction of human HSP22 (HSPB8) with other small heat shock proteins. J. Biol. Chem. 2004;279:2394–2402. doi: 10.1074/jbc.M311324200. [DOI] [PubMed] [Google Scholar]
- 23.Christians E. S., Yan L. J., Benjamin I. J. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit. Care Med. 2002;30:S43–S50. [PubMed] [Google Scholar]
- 24.Depre C., Hase M., Gaussin V., Zajac A., Wang L., Hittinger L., Ghaleh B., Yu X., Kudej R. K., Wagner T., et al. H11 kinase is a novel mediator of myocardial hypertrophy in vivo. Circ. Res. 2002;91:1007–1014. doi: 10.1161/01.res.0000044380.54893.4b. [DOI] [PubMed] [Google Scholar]
- 25.Wada T., Takagi T., Yamaguchi Y., Kawase H., Hiramoto M., Ferdous A., Takayama M., Lee K. A., Hurst H. C., Handa H. Copurification of casein kinase II with transcription factor ATF/E4TF3. Nucleic Acids Res. 1996;24:876–884. doi: 10.1093/nar/24.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita H., Nazmy M., Kajimoto K., Yehia G., Molina C. A., Sadoshima J. Inducible cAMP early repressor (ICER) is a negative-feedback regulator of cardiac hypertrophy and an important mediator of cardiac myocyte apoptosis in response to beta-adrenergic receptor stimulation. Circ. Res. 2003;93:12–22. doi: 10.1161/01.RES.0000079794.57578.F1. [DOI] [PubMed] [Google Scholar]
- 27.Sadoshima J., Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 28.Ishihara K., Yasuda K., Hatayama T. Phosphorylation of the 105-kDa heat shock proteins, HSP105alpha and HSP105beta, by casein kinase II. Biochem. Biophys. Res. Commun. 2000;270:927–931. doi: 10.1006/bbrc.2000.2541. [DOI] [PubMed] [Google Scholar]
- 29.Lees-Miller S. P., Anderson C. W. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J. Biol. Chem. 1989;264:2431–2437. [PubMed] [Google Scholar]
- 30.Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J. Biol. Chem. 1992;267:7042–7047. [PubMed] [Google Scholar]
- 31.Kim M. V., Seit-Nebi A. S., Marston S. B., Gusev N. B. Some properties of human small heat shock protein Hsp22 (H11 or HspB8) Biochem. Biophys. Res. Commun. 2004;315:796–801. doi: 10.1016/j.bbrc.2004.01.130. [DOI] [PubMed] [Google Scholar]
- 32.Konishi H., Matsuzaki H., Tanaka M., Takemura Y., Kuroda S., Ono Y., Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 33.Gaestel M., Schroder W., Benndorf R., Lippmann C., Buchner K., Hucho F., Erdmann V. A., Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J. Biol. Chem. 1991;266:14721–14724. [PubMed] [Google Scholar]
- 34.Beall A., Bagwell D., Woodrum D., Stoming T. A., Kato K., Suzuki A., Rasmussen H., Brophy C. M. The small heat shock-related protein, HSP20, is phosphorylated on serine 16 during cyclic nucleotide-dependent relaxation. J. Biol. Chem. 1999;274:11344–11351. doi: 10.1074/jbc.274.16.11344. [DOI] [PubMed] [Google Scholar]
- 35.Kato K., Ito H., Kamei K., Inaguma Y., Iwamoto I., Saga S. Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J. Biol. Chem. 1998;273:28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- 36.Kim S. O., Baines C. P., Critz S. D., Pelech S. L., Katz S., Downey J. M., Cohen M. V. Ischemia induced activation of heat shock protein 27 kinases and casein kinase 2 in the preconditioned rabbit heart. Biochem. Cell Biol. 1999;77:559–567. [PubMed] [Google Scholar]
- 37.Agostinis P., Goris J., Pinna L. A., Merlevede W. Regulation of casein kinase 2 by phosphorylation/dephosphorylation. Biochem. J. 1987;248:785–789. doi: 10.1042/bj2480785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.