Abstract
The phagocytic NADPH oxidase generates superoxide by transferring electrons from cytosolic NADPH to extracellular O2. The activity of the oxidase at the plasma membrane can be measured as electron current (Ie), and the voltage dependence of Ie was recently reported to exhibit a strong rectification in human eosinophils, with the currents being nearly voltage independent at negative potentials. To investigate the underlying mechanism, we performed voltage-clamp experiments on inside-out patches from human eosinophils activated with PMA. Electron current was evoked by bath application of different concentrations of NADPH, whereas slow voltage ramps (0.8 mV/ms), ranging from −120 to 200 mV, were applied to obtain ‘steady-state’ current–voltage relationships (I–V). The amplitude of Ie recorded at −40 mV was minimal at 8 μM NADPH and saturated above 1 mM, with half-maximal activity (Km) observed at approx. 110 μM NADPH. Comparison of I–V values obtained at different NADPH concentrations revealed that the voltage-dependence of Ie is strongly influenced by the substrate concentration. Above 0.1 mM NADPH, Ie was markedly voltage-dependent and steeply decreased with depolarization within the physiological membrane potential range (−60 to 60 mV), the I–V curve strongly rectifying only below −100 mV. At lower NADPH concentrations the I–V curve was progressively shifted to more positive potentials and Ie became voltage-independent also within the physiological range. Consequently, the Km of the oxidase decreased by approx. 40% (from 100 to 60 μM) when the membrane potential increased from −60 to 60 mV. We concluded that the oxidase activity depends on both membrane potential and [NADPH], and that the shape of the Ie–V curve is influenced by the concentration of NADPH in the submillimolar range. The surprising voltage-independence of Ie reported in whole-cell perforated patch recordings was most likely due to substrate limitation and is not an intrinsic property of the oxidase.
Keywords: cytochrome, diphenylene iodonium, electron, current, eosinophil, NADPH oxidase, patch-clamp experiment
Abbreviations: CGD, chronic granulomatous disease; DPI, diphenylene iodonium; I–V, current-to-voltage relationship; GTP[S], guanosine S′-[γ-[35S]thio]triphosphate; Ie, electron current; phox, phagocytic NADPH oxidase
INTRODUCTION
Phagocytic leucocytes, such as neutrophils and eosinophils, are key effectors of our innate immune system, being the first line of defence against bacterial, fungal and parasitic infections. Their microbicidal activity is due in large part to the phox (phagocytic NADPH oxidase), a transmembrane enzyme complex expressed in phagocytes. This remarkable enzyme transports electrons from cytoplasmic NADPH to extracellular oxygen, thereby generating high amounts of superoxide radicals (O2•−). The relatively harmless superoxide anion (O2−) is then converted into more reactive oxygen species which are able to kill invading pathogens [1]. The putative electron transport chain comprises a FAD and two haem molecules bound within the core gp91phox subunit [2]. The importance of a functional oxidase is underscored by CGD (chronic granulomatous disease), an inherited disease associated with mutations within one of the phox proteins, predominantly within the core subunit of the oxidase, gp91phox. CGD patients have impaired phagocytic NADPH oxidase activity and suffer from severe and recurrent infections (reviewed in [3]).
The oxidase contributes to microbial killing not only by initiating the generation of different toxic free radicals, but also, indirectly, by changing the electromotive force of important ions, such as Ca2+ [4] or K+ [5]. Electron transport by the phox is electrogenic [6] and is associated with a depolarization of the plasma membrane, to values ranging from −40 to 60 mV, depending on the technique used for determining the membrane potential [7]. Sustained electron transport thus requires the movement of a compensating charge, either H+ [6] or K+ [5,8]. The activity of the phagocytic NADPH oxidase also has a major impact on the local pH. Activation of the oxidase causes a cytosolic acidification, because substantial amounts of intracellular acid are generated during the conversion of NADPH into NADP+ and H+ [9]. In contrast, the production of superoxide within the phagocytic vacuole generates a local transient alkalinization of the phagosome [5,10]. The activity of phox also depends on the local concentration of NADPH, and oscillations in cytoplasmic [NADPH] have been reported in activated neutrophils [11,12]. Propagating NADPH waves were observed accompanied by pulsatile superoxide release at the leading edge of polarized neutrophils [13], indicating that fluctuations in substrate availability might regulate the spatial and temporal profile of the oxidase activity at the cellular level.
Many of the parameters that influence the activity of phox, such as the membrane potential, the local pH or the NADPH concentration, are affected by the activity of the oxidase itself. Given the importance of this enzyme in the host defence against infections, a detailed biochemical and biophysical characterization of the electron transport process is essential to validate our present models of oxidase function. Electron currents carried by the oxidase can be directly measured with the patch-clamp technique [14], but the data on the electrophysiological properties of the phagocytic NADPH oxidase are very limited. The voltage-dependence of electron current was investigated in only two studies that used either the whole-cell broken patch [14] or the perforated patch approach [15]. In both cases, electron current was defined by its sensitivity to DPI (diphenylene iodonium), a potent inhibitor of flavoproteins. The steady-state (or near steady-state) voltage-dependence of electron current was assessed using voltage steps or voltage ramps recorded before and after the application of DPI. These studies thus rely on the assumption that the block of electron transport by DPI is specific and voltage-independent. DPI was found to non-specifically inhibit different cation currents [8,16], and the voltage-dependence of DPI block has not been tested experimentally.
To study the activity of the oxidase under more controlled conditions, we have used inside-out patches excised from PMA-activated human eosinophils. Using this model, we have characterized the voltage-dependence of the phox-mediated electron current at different concentrations of NADPH.
EXPERIMENTAL
Cell preparation and culture
Preparation of human eosinophils was performed as described previously [17], except for minor modifications as follow. Cells were prepared from venous blood drawn from non-atopic healthy adult men, after obtaining their informed consent. Cells were kept on 4 °C in airtight, closed, polypropylene Eppendorf tubes (Treff AG, Degersheim, Switzerland) at approx. 500 cells/μl density in culture medium (for composition, see below). Eosinophils stored this way were able to respond to PMA and produced electron current in inside-out patches even after 96 h of storage. The studies conformed to the standards set by the Declaration of Helsinki, and the procedures were approved by the ethics committee of the University of Geneva.
Patch-clamp measurements
For inside-out patch-clamp experiments, cells were placed on a glass coverslip (diameter, 25 mm) that was attached (with vacuum grease) to the bottom of a 0.5-mm-thick polypropylene disc (diameter, 30 mm). A rhombus-shaped hole in the middle of the plastic disc served as recording chamber. The longer and shorter diameters of the rhombus-shaped hole were 10 and 9 mm respectively. Cells and pipette electrode were visualized using an inverted microscope (Axiovert 10, Zeiss, Germany). The pipette electrode tip was positioned after patch excision in the centre of the chamber, within 150 μm from the bottom. The initial volume of the bath solution was 50 μl and drugs, such as NADPH and DPI, were added directly into the bath with a 20 μl pipette and thoroughly mixed (pipetting 10 times). Drug applications caused at most a 3% cumulative error (dilution) in the ATP and GTP[S] (guanosine S′-[γ-[35S]thio]triphosphate) concentration, except for DPI application at the end of the experiment that caused an additional 6% error. Experiments were finished within 30 min after the chamber was filled with the bath solution, and the chamber was surrounded by wet tissue to avoid any significant effect of evaporation. Voltage-clamp experiments were performed using an Axoptach-1D patch-clamp amplifier (Axon Instruments, Foster City, CA, U.S.A.) equipped with CV-4-1/100U headstage. The headstage gain was set to 100 (50 GΩ feedback resistor, suitable for single-channel measurements) in all cases. Pipettes were pulled from borosilicate glass tubing (type GC150F-10, Harvard Apparatus, Kent, U.K.) using a P-87 puller (Sutter Instrument Co., CA, U.S.A.). After fire polishing, the pipette resistance was 10–15 MΩ when filled with the recording solution (for composition, see below). The resistance of the same pipettes ranged between 2–4 MΩ when filled with 150 mM KCl solution, indicating that the pipette tip had a size commonly used in whole-cell experiments. The bath was grounded using an Ag/AgCl pellet. Current signals were low-pass filtered at 1 kHz or 1 Hz (−3 dB, 8-pole Bessel filter) and digitally sampled at 8 kHz or 4 Hz respectively. For data analysis and illustration, further software-based noise reduction was performed offline. The frequency of the ∼50 Hz line noise was determined offline and notch filtered by the built-in algorithm of pClamp 8 software (Axon Instruments). Current traces illustrated in the Figures were smoothed before analysis with a 9-point running average and, when indicated in the Figure legend, further smoothed with the origin software (version 6.0, Microcal Software, Northampton, MA, U.S.A.). Data acquisition was performed on a PC using the pClamp 6 software, and pClamp 8 was used for data analysis. Online compensation for the full electrode capacitance (C0) was not performed, thus the influence of small changes in electrode capacitance (ΔC, due to evaporation or solution mixing) on the time constant (τ) of voltage clamping was negligible, assuming that the access resistance (R) remained stable [Δτ=R(C0+ΔC)−RC0]. This was beneficial for minimizing distortion of the shape of leak corrected curves by changes in the slow component (τ∼10 ms) of the pipette capacitance. After subtraction of leak (or background) I–V (current-to-voltage relationship), electrode capacitance changes mainly caused a parallel shift of the current curves recorded during hyper-(Ihyper) and de-polarizing (Idepol) ramps, as shown in Supplementary Figure 1 (http://www.BiochemJ.org/bj/388/bj3880485add.htm). To correct for this shift, the amplitude of the offset (Ioff) was calculated from the current amplitudes measured at identical voltages during hyper- and de-polarizing ramps [Ioff=(Ihyper−Idepol)/2), and subtracted from the depolarizing ramps currents. Background current was recorded in the nominal absence of NADPH, after proton current was inhibited by Zn2+, and was <0.2 pA at −40 mV in all cases. The stability of the background current I–V during 200 s after Zn2+ inhibition and the effect of drift correction on the electron current I–V is shown in Supplementary Figure 2 (http://www.BiochemJ.org/bj/388/bj3880485add.htm).
Solutions
The cell storage medium was a 2:1 mixture of medium 199 with L-glutamine and L-amino acids containing 25 mM Hepes (Gibco™, Invitrogen Corporation) and L-glutamine-free RPMI 1640 Medium (Gibco™) supplemented with 5 mM Na2-EDTA and 2% fetal calf serum (BioConcept, Allschwil, Switzerland). The recording solutions contained (in mM): CsCl, 1; tetraethylammonium chloride, 1; MgCl2, 2; EGTA, 1; citrate, 1; N-methyl-D-glucamine base, 60; Hepes acid, 240; pH 7.05. The bath solution was supplemented with 4 mM MgATP and 25 μM Li4-GTP[S] to hamper electron current run-down [18]. The pipette solution was supplemented with 3 mM ZnCl2 giving an estimated free [Zn2+] of 1.07 mM. Free [Zn2+] was calculated using MaxChelator software (version 2.10, http://www.stanford.edu/~cpatton/maxc.html). Li4-GTP[S] was dissolved in water at 10 mM. DPI was first dissolved in DMSO to give a 10 mM stock, which was then further diluted in pH 7.05 solution to 200 μM. PMA was dissolved in DMSO at 1 mM. MgATP and Na4NADPH were dissolved in pH 7.05 solution to give a stock solution of 30 mM and 0.4–80 mM respectively. ZnCl2 was dissolved at 0.5 M in water containing 40 mM HCl. Na2-EDTA was dissolved in water at 250 mM. All chemicals were obtained from Sigma–Aldrich unless otherwise specified. All manipulations were performed at room temperature (22–25 °C).
Conventions
The sign of the originally recorded inside-out patch current signal is inverted in the Figures, thus positive current value indicates outward current to comply with classical whole-cell experiments.
Data analysis
The number of cells (n) indicates the cumulative number of measurements performed on at least two independent cell preparations. The results are presented as the means±S.E.M. For statistical analysis paired Student's t test or one-way ANOVA (for post-hoc comparison Tukey honest-significant-difference test was used) was applied using Statistica software (version 4.5, Statsoft, Tulsa, OK, U.S.A.). A value of P<0.05 was considered statistically significant. Sigmoidal (modified Boltzmann, see below) and hyperbolic (Michaelis–Menten) fits were calculated using the built-in algorithm of the Origin software. For all fits the sum of squared error minimization method was applied.
For sigmoidal fits of I–Vs of Ie (electron current), the following equation was applied:
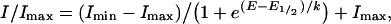 |
where E1/2 is the membrane potential (E) at which the electron current amplitude (I) is half-maximal (Imax/2) and k is the slope factor. In corrected I–Vs, Imax was constrained to 1 and in Figure 3 Imin was constrained to 0. Changes in background current (during 200 s; Supplementary Figure 2, http://www.BiochemJ.org/bj/388/bj3880485add.htm) caused less than 10% error in E1/2 and k of the sigmoidal fit of the mean electron current I–V at all NADPH concentrations.
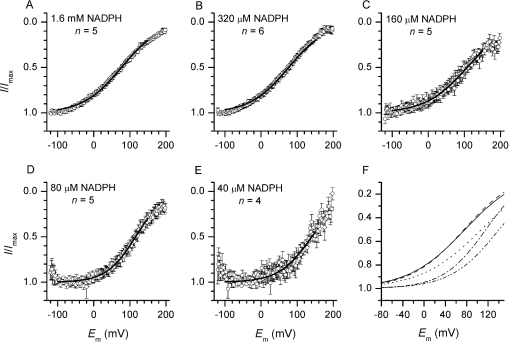
Figure 3. I–V of electron currents recorded at different concentrations of NADPH.
Electron currents were evoked by bath application of different concentration of NADPH. (A–E) Averaged Ie–Vs recorded at different [NADPH] and normalized to the maximal current amplitude. Traces are smoothed with a 19-point running average and only 160 of the 3200 data points are shown. Sigmoidal fits of the averaged Ie–Vs between −100 and 150 mV are shown as black lines. (F) Superimposed fits from (A–E). A progressive shift of the half-maximal electron current amplitude to positive voltages occurs with decreasing substrate concentration. Below 320 μM NADPH, a voltage-independent section becomes visible. NADPH concentrations are: 1.6 mM (——), 320 μM (– –), 160 μM (- - -), 80 μM (-·-·) and 40 μM (-· ·-).
RESULTS
NADPH dependence of electron currents
To determine the relationship between the amplitude of electron currents and the concentration of the oxidase substrate, NADPH, we used inside-out patches excised from human eosinophils pretreated with supramaximal doses of PMA (100–400 nM for 5–10 min). Electron currents were continuously recorded at −40 mV, and the pipette solution contained 3 mM Zn2+ to minimize proton channel activity [15]. As illustrated in Figure 1(A), application of 1.6 mM NADPH to the cytosolic side of the patch elicited robust Ies, which averaged 2.35±0.39 pA at −40 mV (n=32; range, 0.28–9.1 pA). Run-down of electron currents was minimal in the presence of ATP and GTP[S], as reported previously [18], the amplitude of Ie decreasing by 8.4±0.75% during a 2 min period (n=4). Ie was not affected by mixing of the bath solution, but was completely inhibited by the addition of 12 μM DPI, a known blocker of the NADPH oxidase (Figure 1A). In some recordings, the activity of a single channel was apparent (e.g. Figure 1B, from t∼3 min), but the single channel currents were of very small amplitude and did not interfere with Ie measurements.
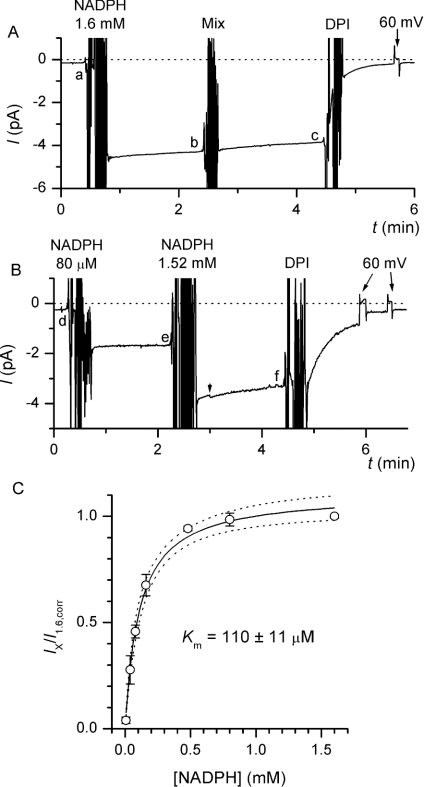
Figure 1. NADPH-dependence of electron current in inside-out patches.
(A) Inside-out patches were excised into a NADPH-free bath solution and held at −40 mV. After Zn2+ in the pipette had blocked outward H+ current activation at 60 mV (results not shown), electron current was evoked by application of 1.6 mM NADPH to the bath. After 2 min, the bath solution was mixed with a pipette to test the effect of bath manipulation on Ie, and 2 min later 12 μM DPI was added to inhibit Ie. The average current amplitude during the last 10 s preceding each intervention was determined to assess the extent of Ie run-down (c−a)/(b−a). (B) To obtain the NADPH dose–response curve of Ie, different concentrations of NADPH (80 μM in this representative trace) were applied to the bath, and 2 min later the [NADPH] was increased to 1.6 mM. Ie amplitudes were determined as described above (Ix=e−d and I1.6=f−d). The arrowhead indicates the beginning of burst-like activity of an undefined single channel. (C) Fractional Ie amplitudes corrected for mean run-down (Ix/I1.6,corr) are plotted against the corresponding NADPH concentrations. Solid line is the result of fitting the Michaelis–Menten equation to the data (broken lines show the 95% confidence interval), which gave a half-saturating [NADPH] of ∼110 μM. Points are the means of 4 or 5 independent experiments for each [NADPH].
To establish the NADPH-dependence of Ie, different concentrations of NADPH were applied to the bath (intracellular) solution and Ie was recorded for 2 min (Figure 1B). Then, the concentration of NADPH was increased to 1.6 mM, and Ie recorded for a further 2 min before adding 12 μM DPI to inhibit the oxidase. The Ie amplitude recorded during the first NADPH addition was normalized to the current amplitude recorded subsequently in the same patch with 1.6 mM NADPH, to correct for the differences in the number of active oxidases between patches. The concentration curve derived from these experiments is shown in Figure 1(C) and fitted well to the Michaelis–Menten equation. Ie was minimal at 8 μM NADPH, Km was observed at ∼110 μM NADPH and millimolar NADPH concentrations were required to evoke maximal oxidase activity.
Voltage-dependence of electron currents and DPI effects
To establish the I–V of Ie, we applied voltage ramps ranging from −120 mV to 200 mV during activation and block of electron currents (Figure 2A; ramp speed, 0.8 mV/ms). Electron currents were elicited by bath application of near-saturating [NADPH] (1.6 mM) and subsequently blocked by 12 μM DPI, as in Figure 1(A). The pipette solution contained ∼1 mM free Zn2+ (3 mM total), a concentration that blocks voltage-gated H+ currents at all potentials [15]. Figure 2(B) shows typical ramp currents recorded before drug addition (a), after NADPH application (b) and after DPI block (c). In all cases, a rapid current deflection followed by a slower current increase was observed during initiation and reversal of the voltage ramps (Figure 2B), reflecting the charge of the capacitance of the patch-clamp circuit. The capacity currents were essentially eliminated by subtracting consecutive ramp currents (Supplementary Figure 1, http://www.BiochemJ.org/bj/388/bj3880485add.htm) and the background ramp currents varied by less than 0.1 pA within 200 s in the −100 to +150 mV voltage range (Supplementary Figure 2, http://www.BiochemJ.org/bj/388/bj3880485add.htm). The ramp currents were subtracted to determine the NADPH-induced (b–a) and DPI-sensitive (b–c) current components, which were plotted against the corresponding ramp voltage to establish the Ie–V (Figure 2C).
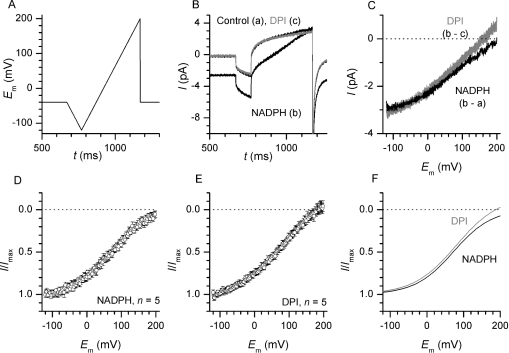
Figure 2. I–V of electron currents and of DPI inhibition.
(A) Voltage-ramp protocol. Every 10 s, a series of fast (8 mV/ms, see Supplementary Figure 1, http://www.BiochemJ.org/bj/388/bj3880485add.htm) and slow (0.8 mV/ms) voltage ramps were applied from the −40 mV holding potential. For clarity, only the slow ramp waveform is shown. (B) Ramp currents recorded before and after bath application of 1.6 mM NADPH and after DPI inhibition (grey line). Traces are an average of three contiguous recordings. Current deflections during initiation and reversal of the voltage ramps reflect the charge and discharge of the patch-clamp circuit capacitance. (C) The NADPH-induced (black) and DPI-sensitive (grey) currents, derived by subtracting the traces shown in (B), are plotted against the corresponding ramp voltage. Currents recorded during hyperpolarizing ramps were used to correct for minor current offsets arising from electrode capacitance changes (see the Experimental section and Supplementary Figure 1, http://www.BiochemJ.org/bj/388/bj3880485add.htm). (D, E) Averaged I–Vs of five patches measured during sequential application of 1.6 mM NADPH (D) and 12 μM DPI (E). For clarity, only 100 of the 3200 data points are shown on the I–V curves. (F) Sigmoidal fit of the mean results reveals only minor difference between the two approaches used to obtain electron current I–V.
As shown in Figures 2(C)–2(F), the I–V of DPI-inhibited and NADPH-induced currents recorded in the same patches were very similar. When normalized to the maximal current amplitude (Figures 2D–2E), both I–Vs displayed a moderate sigmoidicity with flattening sections below 0 mV and at large positive potentials. DPI-sensitive currents were somewhat less sigmoidal and tended to reverse sign at very large positive potentials, but analysis of the fits performed on the individual traces revealed no significant difference in the half-maximal potential or in the slope factor of the I–V curves (E1/2, 75.0±4.62 versus 81.5±3.18 mV for NADPH and DPI, respectively, n=5, P>0.2; k, −56.6±3.91 versus −61.7±4.18 mV for NADPH and DPI respectively, n=5, P>0.47). After correction for the drift in background current, the differences between the NADPH-induced and DPI-inhibited I–Vs became even smaller (Supplementary Figure 2, http://www.BiochemJ.org/bj/388/bj3880485add.htm), confirming that the two approaches used to determine the Ie–V were equivalent.
Effect of NADPH concentration on the I–V of electron currents
The I–V curves measured in excised patches did not display the pronounced rectification and large cell-to-cell variability reported by DeCoursey et al. [15] in whole-cell perforated patches. To test whether differences in the concentration of NADPH could explain the variability of I–V shapes reported in perforated patches, we applied voltage ramps to patches exposed to different [NADPH]. As shown in Figure 3, reducing the NADPH concentration below half-saturating doses led to substantial change in the shape of the Ie–V. At concentrations exceeding 110 μM NADPH, the electron current amplitude decreased monotonically with membrane depolarization, following a sigmoidal pattern (Figures 3A–3C). Below 100 μM NADPH, the I–Vs were U-shaped, rather than sigmoid; the current displayed a voltage-independent section within the physiologically relevant potential range and decreased at very negative voltages (Figures 3D and 3E). Sigmoidal fit of the averaged traces (Figure 3F) revealed a progressive positive shift in E1/2 with decreasing [NADPH] (76, 75, 107, 116 and 141 mV with 1.6 mM, 320, 160, 80 and 40 μM NADPH respectively). Sigmoidal fits performed on the individual traces between 320 and 40 μM NADPH revealed that E1/2 was significantly higher at 80 μM NADPH (122.6±15.3 mV, n=4, P<0.03) and at 40 μM NADPH (146.3±19.9 mV, n=5, P<0.03) than at 320 μM NADPH (74.5±14.1 mV, n=6).
Effect of membrane potential on the Km of the NADPH oxidase
By combining the dose–response curve obtained at a constant voltage (Figure 1C) with the I–Vs recorded at the different NADPH concentrations (Figure 3), we could determine the Km of the NADPH oxidase at different membrane potentials (Figure 4). To validate this approach, we compared the electron current amplitude measured at the holding voltage of −40 mV with the Ie amplitude measured in the same patch, at the same voltage, during subsequent voltage ramps. The Ie amplitudes, measured at constant voltage and during subsequent voltage ramps, were identical for all NADPH concentrations tested. Furthermore, in five patches the Ie amplitude measured during ramps at intermediate [NADPH] (Ix) could be normalized for the maximal Ie amplitude measured with 1.6 mM NADPH (I1.6, corr), as in Figure 1. All five Ix/I1.6, corr ramp values fell into the mean±S.D. range of the corresponding steady-state values shown in the dose–response curve of Figure 1(C), thus confirming that the voltage ramp was slow enough to reach steady-state conditions.
Figure 4. Effect of membrane potential on the Km of oxidase.
(A) Relative oxidase activity as a function of membrane potential and [NADPH] (the latter on logarithmic scale). The fits of the I–Vs obtained at different [NADPH] (Figure 3F) were scaled using the dose–response curve obtained at −40 mV (Figure 1C) to determine the fractional oxidase activity during ramps. (B) Km values derived from fitting the Michaelis–Menten equation to the five iso-potential data sets shown in (A) are plotted against the membrane potential.
To express the oxidase activity as a function of both membrane potential and [NADPH], the Ie–V fits obtained at different [NADPH] (Figure 3) were scaled according to the dose–response curve of Figure 1(C), using −40 mV as common reference voltage (Figure 4A). The relative oxidase activity was determined at five different potentials ranging from −80 to +80 mV for each [NADPH], and the dose–response curves were fitted by the Michaelis–Menten equation to determine the Km. As shown in Figure 4(B), the results of the fits indicate that the apparent Km of the NADPH oxidase gradually decreases with membrane depolarization.
DISCUSSION
This is the first study to use excised patches to explore the voltage- and NADPH-dependence of the phagocytic NADPH oxidase. To date, the voltage-dependence of Ie has been investigated in only two patch-clamp studies, performed either under whole-cell broken patch [14] or perforated patch conditions [15]. Our observations extend these pioneering studies in two respects. Firstly, we could establish the voltage-dependence of the electron transport process in the absence of the oxidase inhibitor DPI. In whole-cell studies, electron currents were defined by their sensitivity to DPI, whose effects might be voltage-dependent and not specific [8,16]. Our voltage ramps obtained in the presence and absence of DPI indicate that the block of electron currents by DPI is indeed voltage-independent (Figure 2 and Supplementary Figure 2, http://www.BiochemJ.org/bj/388/bj3880485add.htm). Secondly, and more importantly, the use of excised patches allowed us to control the substrate concentration on the cytosolic side of the membrane and to impose rapid changes in [NADPH].
NADPH dependence of electron currents
The activity of the oxidase depends on the concentration of NADPH, and Km values for NADPH ranging from 26 to 150 μM have been reported in cell-free essays using different phagocyte membrane preparations [19–23]. We have now demonstrated that the activity of the oxidase in excised patches from human eosinophils is steeply dependent on the concentration of NADPH in the submillimolar range. According to our concentration curve, the activity of the NADPH oxidase is minimal below 10 μM, half maximal at ∼110 μM (apparent Km at −40 mV), whereas supramillimolar substrate concentrations are needed for maximal activity (Figure 1). We also demonstrated that the Km of the oxidase decreases considerably with membrane depolarization (by ∼40% between −60 and 60 mV, Figure 4). Quantitative NADPH measurements performed in human phagocytes indicated that the cellular NADPH content during phagocytosis is 33.2±5.9 amol/cell [24]. As the mean corpuscular volume of human phagocytes is 334±32 fl [25], this would correspond to a ∼100 μM average NADPH concentration if NADPH was evenly distributed throughout the cell volume. In a model simulating the oscillatory behaviour of the oxidase in neutrophils in which the Km for NADPH was set to 60 μM, the estimated average cytoplasmic NADPH concentration oscillated between 170–220 μM [12]. Importantly, travelling NAD(P)H waves were shown to correlate temporally and spatially with oxidant release at the lamellipodium of neutrophils [13]. This indicates that the average cytosolic concentration of NADPH is below saturating levels, and might reach saturating levels only at the peak of the oscillations. Taken together, these observations and our own data suggest that the average cytosolic NADPH concentration is slightly above the Km of the oxidase, and that the activity of the enzyme might be limited by substrate availability in intact cells.
Voltage dependence of electron currents
Our results obtained in excised patches confirm that the steady-state activity of the NADPH oxidase is strongly voltage-dependent and is abolished around 200 mV [15]. At saturating concentrations of [NADPH], the electron current amplitude varied linearly with voltage in the range −60 to +120 mV and decreased asymptotically to zero at higher potentials. Reversal of electron current is thermodynamically predicted when the electrical component of the driving force for e− (the membrane potential) exceeds the chemical component (the redox potential difference between the two redox pairs, NADPH:NADP+ and O2:O2•−). The asymptotic decline suggests that the concentration of superoxide at the extracellular side of the patch was either very low at all membrane potentials or gradually decreased during slow depolarization. The latter scenario is more likely as: (i) the superoxide concentration in the patch was high enough to precipitate Nitro Blue Terazolium [18] and (ii) a more linear I–V, and occasionally a reversal, of the electron current were observed when faster voltage ramps were applied (8 mV/ms, from −160 to 200 mV; results not shown). Thus the simplest explanation for the observed voltage-dependence is that the equilibrium potential for e− continuously shifts to more positive values as the production of superoxide gradually decreases during slow voltage ramps. The negatively charged superoxide anion might be retained within the membrane at positive membrane potentials and decrease the accessibility to oxygen, accounting for the current decrease at high voltages. In addition, superoxide might diffuse back through anion channels [26] and distort the electron current I–V.
Unfortunately, we could not establish a stable concentration of superoxide within the patch pipette, despite numerous attempts with scavengers and with superoxide generating systems (see supplementary information at http://www.BiochemJ.org/bj/388/bj3880485add.htm). In the absence of stable driving force for e−, a possible built-in voltage-dependence of the oxidase cannot be excluded, and whether electrons can be captured from superoxide and flow backward across the oxidase remains to be demonstrated. However, the voltage-dependence observed in excised patches is mainly due to changes in the driving force for electrons during voltage ramps, and not to conformational changes in the oxidase protein.
Effect of NADPH concentration on the voltage-dependence of the electron current
The I–Vs measured in excised patches differed in several respects from the ones obtained in the perforated patch configuration. In perforated patches, the voltage-dependence of the oxidase was found to be highly non-linear. The NADPH oxidase activity was steeply voltage-dependent at large positive voltages, but nearly independent of membrane potential at negative voltages. The voltage-independent region was postulated to be beneficial for phagocytes, because it encompassed the physiological range of membrane potentials observed during phagocyte activation. It was thus proposed that voltage-gated proton channels, by limiting oxidase-induced membrane depolarization, ensure that the oxidase does not inhibit itself [15].
Such a strongly rectifying pattern was not observed in excised patches at high [NADPH] (320 μM–1.6 mM). Instead, the oxidase activity was steeply voltage-dependent throughout the whole physiologically relevant voltage range (−60 to 60 mV). Remarkably, we could recapitulate the whole-cell phenotype by decreasing the NADPH concentration. At low [NADPH] (40–80 μM) we observed a strong rectification with a clear voltage-independent section at negative voltages. This indicates that, when the availability of substrate is limited, the oxidase activity saturates at a given current amplitude. The e− current amplitude even decreased at very negative voltages, as observed in some traces in the whole-cell study. This pattern might reflect an intrinsic voltage-dependence of the oxidase, which may contribute to the sigmoidicity of the Ie–V observed at high [NADPH]. Thus the simplest explanation for the strong rectification reported in some perforated patch recordings is that the cytosolic NADPH levels were low in those cells.
Using our dose–response curve and electron current I–Vs, one can infer from the data published by DeCoursey et al. [15] the range of the intracellular NADPH concentration in their perforated patch recordings. In inside-out patches, the oxidase activity varied by ∼40% between −60 and 60 mV, at concentrations of NAPDH exceeding 320 μM. In contrast, with 40 μM NADPH the current varied by less than 10% in this range. DeCoursey et al. [15] reported a 24±15% variation in this parameter (mean±S.D., between −60 and +58 mV), corresponding to a ∼40–300 μM concentration range of intracellular NADPH in the cells tested.
In conclusion, the activity of the NADPH oxidase is highly dependent on the concentration of NADPH in the submillimolar range. The voltage-dependence of electron currents is influenced by the concentration of NADPH, suggesting that the strong rectification reported in whole-cell perforated patch recordings was due to substrate limitation and is not an intrinsic property of the oxidase. The observation that the apparent Km of the oxidase decreases with membrane depolarization is important to fine tune the models describing the oscillatory behaviour of the oxidase activity in phagocytes.
Online data
Acknowledgments
We thank Dr Maud Frieden for her useful comments. This work was supported by grant number 31-068317.02 from the Swiss National Science Foundation.
References
- 1.Babior B. M. Phagocytes and oxidative stress. Am. J. Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 2.Vignais P. V. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol. Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldblatt D., Thrasher A. J. Chronic granulomatous disease. Clin. Exp. Immunol. 2000;122:1–9. doi: 10.1046/j.1365-2249.2000.01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geiszt M., Kapus A., Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? J. Leukoc. Biol. 2001;69:191–196. [PubMed] [Google Scholar]
- 5.Reeves E. P., Lu H., Jacobs H. L., Messina C. G., Bolsover S., Gabella G., Potma E. O., Warley A., Roes J., Segal A. W. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature (London) 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 6.Henderson L. M., Chappell J. B., Jones O. T. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 1987;246:325–329. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jankowski A., Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J. Biol. Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 8.Ahluwalia J., Tinker A., Clapp L. H., Duchen M. R., Abramov A. Y., Pope S., Nobles M., Segal A. W. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature (London) 2004;427:853–858. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Grinstein S., Furuya W., Biggar W. D. Cytoplasmic pH regulation in normal and abnormal neutrophils. Role of superoxide generation and Na+/H+ exchange. J. Biol. Chem. 1986;261:512–514. [PubMed] [Google Scholar]
- 10.Segal A. W., Geisow M., Garcia R., Harper A., Miller R. The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature (London) 1981;290:406–409. doi: 10.1038/290406a0. [DOI] [PubMed] [Google Scholar]
- 11.Petty H. R. Neutrophil oscillations: temporal and spatiotemporal aspects of cell behavior. Immunol. Res. 2001;23:85–94. doi: 10.1385/IR:23:1:85. [DOI] [PubMed] [Google Scholar]
- 12.Olsen L. F., Kummer U., Kindzelskii A. L., Petty H. R. A model of the oscillatory metabolism of activated neutrophils. Biophys. J. 2003;84:69–81. doi: 10.1016/S0006-3495(03)74833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kindzelskii A. L., Petty H. R. Apparent role of traveling metabolic waves in oxidant release by living neutrophils. Proc. Natl. Acad. Sci. U.S.A. 2002;99:9207–9212. doi: 10.1073/pnas.1004434107. [DOI] [PubMed] [Google Scholar]
- 14.Schrenzel J., Serrander L., Banfi B., Nusse O., Fouyouzi R., Lew D. P., Demaurex N., Krause K. H. Electron currents generated by the human phagocyte NADPH oxidase. Nature (London) 1998;392:734–737. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 15.DeCoursey T. E., Morgan D., Cherny V. V. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature (London) 2003;422:531–534. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 16.Weir E. K., Wyatt C. N., Reeve H. L., Huang J., Archer S. L., Peers C. Diphenyleneiodonium inhibits both potassium and calcium currents in isolated pulmonary artery smooth muscle cells. J. Appl. Physiol. 1994;76:2611–2615. doi: 10.1152/jappl.1994.76.6.2611. [DOI] [PubMed] [Google Scholar]
- 17.Schrenzel J., Lew D. P., Krause K. H. Proton currents in human eosinophils. Am. J. Physiol. 1996;271:C1861–C1871. doi: 10.1152/ajpcell.1996.271.6.C1861. [DOI] [PubMed] [Google Scholar]
- 18.Petheo G. L., Maturana A., Spat A., Demaurex N. Interactions between electron and proton currents in excised patches from human eosinophils. J. Gen. Physiol. 2003;122:713–726. doi: 10.1085/jgp.200308891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burritt J. B., Foubert T. R., Baniulis D., Lord C. I., Taylor R. M., Mills J. S., Baughan T. D., Roos D., Parkos C. A., Jesaitis A. J. Functional epitope on human neutrophil flavocytochrome b558. J. Immunol. 2003;170:6082–6089. doi: 10.4049/jimmunol.170.12.6082. [DOI] [PubMed] [Google Scholar]
- 20.McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J. Clin. Invest. 1985;75:1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H., Pabst M. J., Johnston, Jr R. B. Enhancement by Ca2+ or Mg2+ of catalytic activity of the superoxide-producing NADPH oxidase in membrane fractions of human neutrophils and monocytes. J. Biol. Chem. 1985;260:3635–3639. [PubMed] [Google Scholar]
- 22.Ezekowitz R. A., Orkin S. H., Newburger P. E. Recombinant interferon-γ augments phagocyte superoxide production and X-chronic granulomatous disease gene expression in X-linked variant chronic granulomatous disease. J. Clin. Invest. 1987;80:1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki Y., Lehrer R. I. NAD(P)H oxidase activity in human neutrophils stimulated by phorbol myristate acetate. J. Clin. Invest. 1980;66:1409–1418. doi: 10.1172/JCI109994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aellig A., Maillard M., Phavorin A., Frei J. The energy metabolism of the leukocyte. IX. Changes in the concentration of the coenzymes NAD, NADH, NADP, and NADPH in polymorphonuclear leukocytes during phagocytosis of Staphylococcus albus and due to the action of phospholipase C. Enzyme. 1977;22:207–212. [PubMed] [Google Scholar]
- 25.Nibbering P. H., Zomerdijk T. P., Corsel-Van Tilburg A. J., Van Furth R. Mean cell volume of human blood leucocytes and resident and activated murine macrophages. J. Immunol. Methods. 1990;129:143–145. doi: 10.1016/0022-1759(90)90432-u. [DOI] [PubMed] [Google Scholar]
- 26.Terada L. S. Hypoxia-reoxygenation increases O2•− efflux which injures endothelial cells by an extracellular mechanism. Am. J. Physiol. 1996;270:H945–H950. doi: 10.1152/ajpheart.1996.270.3.H945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.