Abstract
XOR (xanthine oxidoreductase) purified from human milk was shown to contain 0.04 atom of Mo and 0.09 molecule of molybdopterin/subunit. On the basis of UV/visible and CD spectra, the human enzyme was approx. 30% deficient in iron-sulphur centres. Mo(V) EPR showed the presence of a weak rapid signal corresponding to the enzyme of low xanthine oxidase activity and a slow signal indicating a significant content of desulpho-form. Resulphuration experiments, together with calculations based on enzymic activity and Mo content, led to an estimate of 50–60% desulpho-form. Fe/S EPR showed, in addition to the well-known Fe/S I and Fe/S II species, the presence of a third Fe/S signal, named Fe/S III, which appears to replace partially Fe/S I. Comparison is made with similarly prepared bovine milk XOR, which has approx. 15-fold higher enzymic activity and Mo content. Taken along with evidence of low Mo content in the milk of other mammals, these findings add further support to the idea that XOR protein plays a physiological role in milk (e.g. in secretion) equal in importance to its catalytic function as an enzyme.
Keywords: bovine enzyme, iron-sulphur centre, molybdenum, molybdopterin, xanthine oxidase, xanthine oxidoreductase
Abbreviations: ROS, reactive oxygen species; XO, xanthine oxidase; XOR, xanthine oxidoreductase
INTRODUCTION
XOR (xanthine oxidoreductase) is a complex molybdoflavoprotein. The fully constituted enzyme is a dimer, each subunit of which contains one Mo, one FAD and two non-identical ironsulphur redox centres [1–3]. Although XOR interacts with a wide range of both reducing and oxidizing substrates, its conventionally accepted role is in purine catabolism, catalysing the oxidation of hypoxanthine to xanthine and xanthine to uric acid. Mammalian XOR exists in two interconvertible forms, xanthine dehydrogenase (EC 1.1.1.204), which predominates in vivo, and XO (xanthine oxidase; EC 1.1.3.22). Although both forms of the enzyme reduce molecular oxygen, only xanthine dehydrogenase can reduce NAD+, which is its preferred electron acceptor. Reduction of oxygen leads to superoxide anion and H2O2 and it is the potential to generate these ROS (reactive oxygen species) that has led to widespread interest in the enzyme as a pathogenic agent in many forms of ischaemia-reperfusion injury [3,4]. ROS are also increasingly cited as intermediates in normal signal transduction pathways [5]. More recently, XOR has been shown to catalyse the reduction of nitrates and nitrites to NO [3], an important signalling molecule in its own right and the source of other, potentially destructive reactive nitrogen species, such as peroxynitrite. On the basis of the above properties, a role for XOR has been proposed in innate immunity [6–9].
Proposed mechanisms of pathophysiological involvement of XOR are largely based on the well known properties of the bovine milk [1,2] and rat liver [10] enzymes, and although results obtained in experimental animal systems are commonly extrapolated, at least implicitly, to humans, remarkably little is known about the human enzyme. Krenitsky et al. [11] reported a preparation from post-mortem human liver with properties essentially similar to those of the bovine milk enzyme. More recently, purification of XOR from human milk has been described in [12]. Human milk XOR, although showing physicochemical properties very similar to those of the bovine milk enzyme, has only approx. 5% of the activity of the latter towards xanthine and related substrates [12,13] suggesting dissimilarities between the bovine and human enzymes at the Mo and iron-sulphur centres. However, human milk XOR exhibits NADH oxidase activity that is fully equivalent to that of the bovine milk enzyme [13], demonstrating the integrity of the FAD centre of the human enzyme as compared with that of its bovine counterpart. Clearly, there are significant differences between the enzymic activities of human and bovine milk XORs, particularly in their potential for generating ROS and reactive nitrogen species, which are of major clinical interest. We now present evidence for the molecular basis of these differences, namely that human milk XOR is not only grossly deficient in Mo but is also substantially lacking in iron-sulphur centres. Preliminary reports of these deficiencies have been presented elsewhere [14,15].
EXPERIMENTAL
Purification and characterization of XOR
Human breast milk was donated by mothers who had that in excess at the Intensive Care Baby Units of the Bristol Royal Infirmary and Southmead Hospital, Bristol, U.K.
XOR was purified from pooled samples of frozen human milk essentially as described by Sanders et al. [13] with minor modifications. Frozen milk (1500–2000 ml) was allowed to thaw and then centrifuged (2000 g for 30 min) at 4 °C to separate the cream. All subsequent operations were performed at 4 °C. The cream was resuspended in 5 vol. of 0.2 M K2HPO4, containing 1 mM EDTA and 5.0 mM dithiothreitol, with stirring for 90 min. The suspension was centrifuged (3000 g for 30 min) and the subnatant collected, filtered through glass wool and mixed slowly with 15% (v/v) cold butanol (−20 °C). Ammonium sulphate (15 g/100 ml) was added slowly with stirring, which was continued for a further 50 min, before centrifugation (8000 g for 20 min) and filtration of the supernatant through glass wool. Ammonium sulphate (20 g/100 ml) was then added slowly with mixing and the suspension was stirred for a further 30 min before centrifuging (9500 g for 30 min). The brown precipitate was suspended in a small volume of buffer A (25 mM sodium phosphate, pH 7.4, containing 1 mM EDTA) and dialysed against the same buffer (3.5 litres) overnight. The precipitate was removed by centrifugation (10000 g for 60 min) and the supernatant was filtered through a 0.22 μm membrane, before applying to a column (1 cm×9 cm) of heparin immobilized on cross-linked 4% beaded agarose (Sigma, type 1), previously equilibrated in buffer A. The column was washed with 0.1 M NaCl in buffer A (20 ml) and XOR was eluted with 0.4 M NaCl in the same buffer.
Further purification was effected by ion-exchange chromatography. Eluate from the heparin column was dialysed into 50 mM Na/Bicine, pH 8.3 (buffer B), containing 50 mM NaCl, filtered through a 0.22 μm syringe filter and applied to an equilibrated Hi-Trap Mono Q ion exchange column (Pharmacia, Milton Keynes, U.K.) for FPLC. The column was eluted with buffer B, containing 50 mM NaCl, followed by an increasing salt gradient (0.05–1.0 M NaCl) in buffer B, when enzyme-containing fractions were pooled and concentrated. The enzyme-containing eluate from either heparin or Mono Q was dialysed overnight into buffer B, frozen dropwise in liquid nitrogen and stored at −80 °C. Typical yields after heparin chromatography were 15 mg/l enzyme protein, with a A280/A450 ratio of 5.0–6.0. Mono Q chromatography yielded enzyme with A280/A450 ratios of 5.0–5.2 in yields of approx. 85%. Enzyme purified to either stage was typically 75% in the oxidase form. Unless otherwise stated, human enzyme used in these studies was purified on Mono Q.
Bovine XOR was purified from fresh cows' milk and stored at −80 °C as described by Godber et al. [16]. The enzyme showed an A280/A450 value of 5.0–5.2 and, after thawing, contained >97% oxidase activity as determined by the method described below. Commercially available bovine milk XOR (Biozyme, Blaenavon, Wales, U.K.), used in some of the EPR studies had very similar properties. Bovine XOR, containing essentially stoichiometric amounts of Mo and Fe/S, was represented either by that affinity-purified on folate (see below) or by the extensively characterized preparation of Hart et al. [17], obtained by proteolytic digestion and denaturation with sodium salicylate.
The oxidase activity of XOR was determined by measuring the rate of oxidation of xanthine to uric acid spectrophotometrically at 295 nm in a Cary 100 spectrophotometer, using a molar absorption coefficient (ε) of 9.6 mM−1·cm−1 [18]. Assays were performed at 25.0±0.2 °C in air-saturated buffer B, containing 100 μM xanthine. Total (oxidase plus dehydrogenase) activity was determined as above but in the presence of 0.5 mM NAD+. Dehydrogenase content of an enzyme sample was determined from the ratio of oxidase and total activities.
The FAD content of XOR was determined by comparison of its fluorescence with that of standard flavin solutions. XOR in 50 mM potassium phosphate buffer, pH 7.4, containing 5% (w/v) trichloroacetic acid, was incubated at 4 °C for 30 min before centrifugation (13000 g for 10 min) at 4 °C. Fluorescence at 525 nm was measured with excitation at 450 nm in a PerkinElmer LS-5B Luminescence Spectrometer and FAD was quantified by comparison with standard solutions of FAD. The latter were quantified by absorbance spectra (ε450=11300 M−1·cm−1 [19]).
Folate affinity chromatography
This was based on the method of Nishino et al. [20] and Ventom et al. [21]. AH-Sepharose 4B (4 g) was allowed to swell and washed with 0.5 M NaCl. A solution of folic acid (2 mM) and 1-ethyl-3-(3-dimethylpropyl)carbodi-imide (200 mg) in 30 ml of 50% (w/v) N,N′-dimethylformamide was added and the slurry was incubated with gentle agitation at room temperature (22 °C) for 3 h. The gel was removed by centrifugation (3000 g for 10 min) and the process was repeated with fresh reagents, twice or until the supernatant retained some yellow colour. The gel was then washed sequentially with 50% N,N′-dimethylformamide, 10 mM NaOH and 0.1 M sodium pyrophosphate buffer (pH 8.5). A column (1 cm×6 cm) was packed with the gel, covered with aluminium foil and stored at 4 °C until use.
The column was equilibrated in buffer C (80% 50 mM Tris/HCl, pH 7.8, and 20% 0.1 M sodium pyrophosphate buffer, pH 8.5). XOR in buffer C was then loaded on to the column at a flow rate of 0.5 ml/min until the brown colouration extended approx. one third of the way down. The column was then washed with buffer C until A280 had returned to baseline values, after which XOR was eluted with the buffer consisting of 70% 50 mM Tris/HCl (pH 7.8) and 30% 0.1 M sodium pyrophosphate buffer (pH 8.5) containing 100 mM hypoxanthine. The column was finally washed with 0.1 M sodium pyrophosphate buffer. After two successive runs, bovine XOR had activity/flavin ratio 210, consistent with that of the fully functional enzyme and Mo content 0.92±0.05/subunit [1].
Spectrometry
UV/visible spectra of enzyme samples in 50 mM potassium phosphate buffer (pH 7.4), were recorded using a Cecil CE 6600 Multimode Computing UV Spectrometer. UV/visible CD spectra of enzyme samples in 25 mM potassium phosphate (pH 7), were recorded using a Jasco J-700 spectrophotometer.
EPR spectra were obtained using a modified ER200SRC spectrometer (Bruker Spectrospin, Bruker, Karlsruhe, Germany) with an ESR 900 cryostat (Oxford Instruments, Oxford, U.K.) using liquid helium cooling. Samples were reduced anaerobically, as described in the Results section, in buffer B. Simulations were performed as described previously [22] assuming that the g- and linewidth tensors were colinear.
Determination of Mo content
Colorimetric assay of Mo in purified XOR was performed after wet-ashing, using a scaled-down method of Hart et al. [17]. Known amounts of XOR (containing ∼1 nmol of Mo) in 50–100 μl of buffer B were mixed with 50 μl of a solution of concentrated H2SO4 containing 70% HClO4 (2:3, v/v) in glass sample tubes and heated at 250–260 °C for 3 h, after which time all samples became colourless. The tubes were stoppered and mixed between sequential additions of 1.5 ml of 5 M HCl, 50 μl of FeSO4 reagent [20 ml of 5% (w/v) FeSO4·7H2O plus 0.4 ml of concentrated H2SO4] and 50 μl of 33% (w/v) NaI. They were kept for 10 min at room temperature during which time colour developed. This colour was then discharged by sequential additions of 10 μl of 2.5% (w/v) Na2SO3, 200 μl of 10% (w/v) thiourea, 50 μl of 43% (w/v) L-(+)-tartaric acid and 200 μl of dithiol reagent [0.2% (w/v) 3,4-dimercaptotoluene in 1% (w/v) NaOH, containing 1.4% (v/v) mercaptoacetic acid]. The tubes were then thoroughly mixed by 100 inversions and allowed to stand for 30 min, after which amyl acetate (200 μl) was added; the tubes were again mixed as above and left to stand to allow separation of the organic layer. This was removed, centrifuged to separate any remaining aqueous layer, and absorbance spectra were recorded. Standard samples of ammonium molybdate and molybdenum trioxide were carried through the process each time to provide an ε. All assays were performed in triplicate. For all samples (controls and standards), the absorbance A540 was corrected by subtraction of A680 and used to construct a standard curve from which the Mo contents of the samples were estimated.
Determination of molybdopterin content
Total molybdopterin content was determined as described previously [23]. Different amounts of XOR (1–65 μg) were oxidized in 1% I2, 2% (w/v) KI, 1 M HCl for 30 min at 95 °C, neutralized, dephosphorylated and purified using QAE columns yielding form A dephospho, which was subsequently analysed and quantified by HPLC. According to the linear range of the detection, the obtained form A fluorescence was used to calculate the amount of total molybdopterin present in XOR. It should be noted that molybdopterin so quantified may or may not contain Mo.
Desulphuration and resulphuration
Desulphuration of XOR was performed by treatment with KCN, by the procedure of Massey and Edmondson [24] as described by Godber et al. [16]. Resulphuration was performed by anaerobic incubation of reduced XOR with sodium sulphide, with a modification [16] of the method described by Wahl and Rajagopalan [25].
Unless otherwise stated, experimental values are quoted as means±S.D.
RESULTS
Mo and molybdopterin content, XO activity and relative molecular mass
Purified human milk XOR was compared with bovine milk XOR, similarly prepared without folate affinity chromatography (see the Experimental section). The latter enzyme was essentially identical with that obtained commercially (prepared without proteolysis) and the bovine preparations were used interchangeably in the subsequent assays. The Mo contents of purified human XOR and bovine milk XOR were 0.04±0.01 (n=8) and 0.61±0.04 (n=4) atom/subunit respectively. Corresponding molybdopterin contents of human and bovine milk XOR were 0.09±0.03 (n=10) and 0.62±0.07 (n=4) molecule/subunit respectively.
Total XO activities of the above preparations were 87.2±4.4 nmol·min−1·mg−1 (n=10) for human milk XOR and 1377±23.5 nmol·min−1·mg−1 (n=5) for the bovine enzyme. Mr values for human and bovine milk XOR, determined by MALDI–TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS), were 148000±700 (n=12) and 148300±500 (n=6) respectively.
UV/visible spectra
Using the values from FAD analysis, the ε450 value was calculated from the UV/visible spectrum of human milk XOR to be 28500±1300 M−1·cm−1 (n=3). The corresponding value for bovine milk XOR, affinity-purified on folate, was 34800±1200 M−1·cm−1 (n=3), consistent with the value (36000 M−1·cm−1) for bovine milk enzyme having a full complement of cofactors [1].
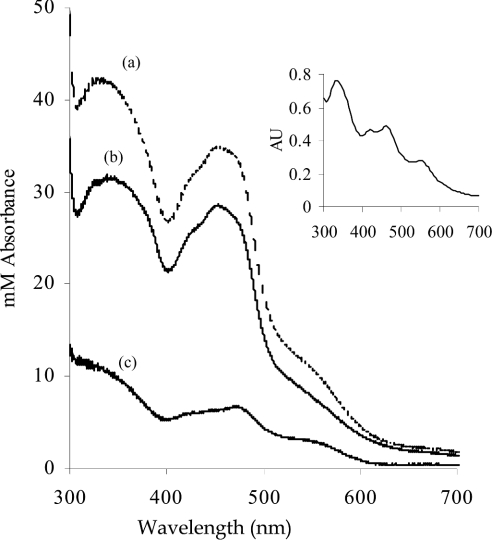
Figure 1 shows the UV/visible spectra of the above samples of human and bovine XOR normalized to identical molarities according to their FAD contents. Subtraction of the spectrum of human XOR from that of bovine XOR gives a difference spectrum similar to those of non-haem iron-sulphur-containing proteins [26] (Figure 1, inset), suggesting that human XOR lacks iron-sulphur. The apparent iron-sulphur deficiency of human XOR was estimated from ε, by using equation (1), based on the assumptions that the enzyme with an ε450 36000 M−1·cm−1 has 100% iron-sulphur content [1], that the A450 is solely attributable to FAD and iron-sulphur, and that the spectrum of FAD remains unchanged [27]:
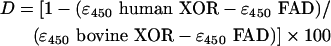 |
(1) |
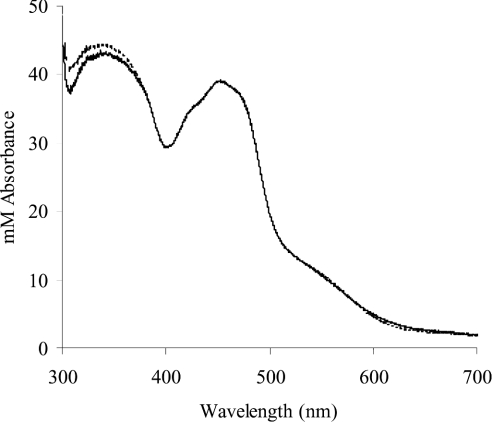
where D is the percentage deficiency, ε450 (bovine XOR)=36000 M−1·cm−1 [1] and ε450 (FAD)=11300 M−1·cm−1 [19]. On this basis, human XOR was estimated to be 30.4% deficient in iron-sulphur. In support of this finding, the UV/visible spectrum of human XOR was simulated by the addition of the spectrum of FAD to that of bovine XOR to create the ratio of iron-sulphur/FAD absorbance as calculated for the human enzyme. As can be seen in Figure 2, the simulated spectrum agrees well with the original.
Figure 1. Comparison of the near-UV/visible spectrum of human milk XOR with that of bovine milk XOR, containing a full complement of cofactors.
Human milk XOR was prepared as described in the Experimental section. Bovine milk XOR was affinity-purified on folate as described in the same section. The spectra are normalized on the basis of FAD content to a subunit concentration of 1 mM, as described in the Results section. (a) Bovine XOR; (b) human XOR; (c) difference spectrum of bovine minus human XOR. Inset: typical spectrum of non-haem iron-sulphur protein. Reprinted from Biochemical and Biophysical Reseach Communications, 31, Y. I. Shethna, D. V. DerVartanian and H. Beinert, 'Non heme (iron-sulfur) proteins of Azotobacter vinelandii, pp. 862–868, © 1968, with permission from Elsevier.
Figure 2. Comparison of the near-UV/visible spectrum of human milk XOR with that simulated by manipulation of the iron-sulphur/FAD ratios of the bovine spectrum.
The spectrum of native human enzyme is shown together with the spectrum simulated as described in the Results section. The two spectra are coincident above 400 nm, and nearly so from 300 nm. In the 300–400 nm range, the spectrum showing the higher absorbances is the simulated spectrum.
An alternative approach to the determination of the iron-sulphur deficiency of human XOR involved the measurement of A450/A550 ratios. Because both the flavin and the iron-sulphur centres of XOR contribute to A450 but only the latter contributes at A550 [27], it has long been recognized (e.g. [28]) that the A450/A550 ratio will be an indicator of the relative contents of FAD and iron-sulphur. The percentage of iron-sulphur deficiency, D, may be calculated from the observed ratio of absorbance, R, using
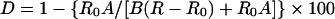 |
(2) |
where R0 and B are A450/A550 and ε450 respectively for bovine XOR in which the iron-sulphur and FAD sites are fully occupied and A stands for ε450 FAD. Using R0=3.13 [26], A=11300 M−1·cm−1 [19] and B=36000 M−1·cm−1 [1], percentage deficiencies of iron-sulphur in eight individual preparations of human XOR were calculated to be 29±4%, in good agreement with the result obtained above from simulation of the combined A450.
CD spectra
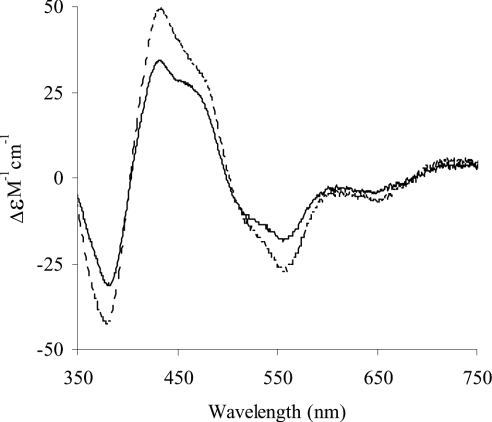
Spectra of human milk XOR and of folate affinity-purified bovine milk XOR were measured and replotted at identical molarities according to their ε450 values, as for the UV/visible spectra. As can be seen in Figure 3, the two spectra have essentially the same form, albeit with different intensities. The bovine enzyme shows markedly greater Cotton effects compared with the human enzyme. These CD spectra derive very largely from the iron-sulphur centres [29] and provide further evidence of a deficiency of the latter in human, compared with fully active bovine XOR.
Figure 3. Comparison of the near-UV/visible CD molar spectra of human milk XOR with that of bovine milk XOR, containing a full complement of cofactors.
Human milk XOR was prepared as described in the Experimental section. Bovine milk XOR was affinity-purified on folate as described in the same section. The spectra are normalized on the basis of FAD content to a subunit concentration of 1 mM, as described in the Results section. - - - -, bovine XOR; ——, human XOR.
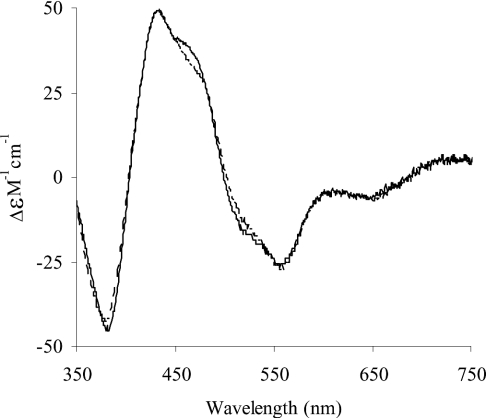
CD ε450 values for human (34000 M−1·cm−1) and bovine (49500 M−1·cm−1) XOR were determined from the spectra and used to calculate an iron-sulphur deficiency of 31.3% for the human compared with the bovine enzyme, assuming that iron-sulphur is the sole contributor to the CD spectrum at 450 nm. Normalization on the basis of this iron-sulphur deficiency led to spectra (Figure 4) that are remarkably similar above 350 nm.
Figure 4. Comparison of the near-UV/visible CD molar spectrum of human milk XOR with that of bovine XOR after normalization for iron sulphur content.
The spectrum of the bovine enzyme is shown, together with that of the human enzyme, after correction for iron sulphur deficiency, as described in the Results section. - - - -, bovine XOR simulated; ——, human XOR.
EPR spectra
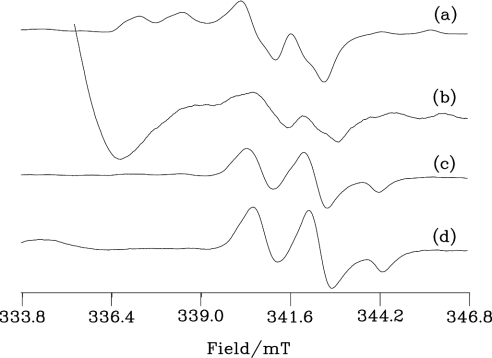
Figure 5 shows Mo(V) EPR spectra from bovine and human milk XOR. Both bovine and human enzymes show rapid signals after reduction by purine. These are given by the actively oxidizing enzyme substrate. The height of this signal from the human enzyme is 0.58% of that from the bovine enzyme reflecting their relative specific activities. An apparently strong FADH radical spectrum is observed at the low-field end of the human enzyme spectrum (Figure 5b), although, after correcting for the difference in the enzyme concentration and the number of scans, its concentration is similar to that of the signal in the bovine sample. Reduction by dithionite gives the slow EPR signal, from desulpho enzyme and, as shown in Figures 5(c) and 5(d), this is evident in both the bovine and human proteins. After correcting for the number of scans and the difference in protein concentration, the slow signal from the human protein is 16% of that in the bovine enzyme; it is thus a major Mo(V) species, consistent with the suggestion that much of this enzyme is in the desulpho form.
Figure 5. Mo(V) EPR spectra of human and bovine milk XOR.
Samples (a) and (c) are bovine (38.5 μM) and (b) and (d) human (132 μM) XO. (a) and (b) were reduced by 9.6 and 9.9 mM purine respectively in an anaerobic glove box before freezing in liquid nitrogen. Samples (c) and (d) are (a) and (b) further reduced by 5 mM sodium dithionite to give final enzyme concentrations of 37.4 and 128 μM respectively before freezing in liquid nitrogen. The samples were in 50 mM bicine (pH 8.3) containing 1 mM EDTA. EPR was run at 150 K, with microwave power 10 mW at 9.401 GHz using 0.39 mT field modulation at 100 kHz; the conversion time was 81.92 ms with a time constant of 1.31 s. The numbers of scans for each spectrum were (a) 2, (b) 60, (c) 4 and (d) 10.
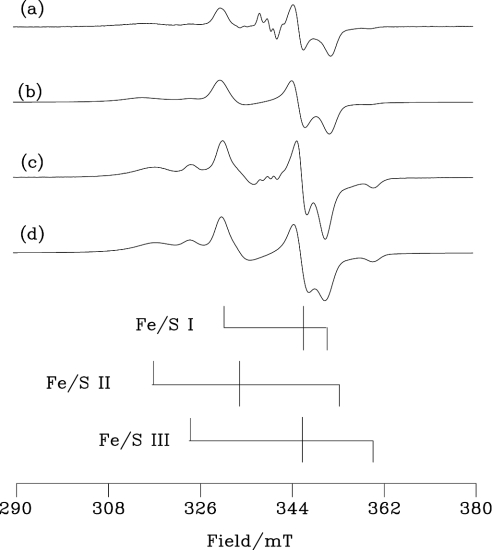
Fe/S EPR parameters for bovine and human enzymes are shown in Table 1 and the spectra, together with simulations based on these parameters, in Figure 6. Spectra from the bovine enzyme are consistent with previous work [30,31]. Although the signals are similar, the g-values of the human protein are shifted relative to the bovine. These small differences in g-values from the two sources no doubt reflect real, albeit minor, differences in the environments of the Fe/S clusters. There is a considerably higher proportion of Fe/S III [32] in the human protein, estimated from the simulated fits shown in Figure 6 to be 20% of the Fe/S II concentration. Although the inherent inaccuracies in the simulation procedures mean that it would be optimistic to hope for more than 10% accuracy for our measurements of this ratio, the results in Table 1 indicate a reasonably close approximation to the expected 1:1 value for the Fe/S I to Fe/S II ratio for the bovine enzyme, but, in contrast, a substantial excess of Fe/S II over Fe/S I in the human enzyme.
Table 1. EPR parameters of the Fe/S EPR signals from bovine and human milk XOR.
The g-values, line widths (Δi, mT) and occupancies are those used in the simulations of Figure 6. Gaussian line shapes were assumed for Fe/S I and III, and Lorenzian for Fe/S II. Occupancies of the centres correspond to molar ratios of the different signal-giving species relative to that of Fe/S II, which is taken as 1.00 for each enzyme. Errors in g-values were estimated from repetitive measurements.
| Enzyme | Signal | g1 | Δ1 | g2 | Δ2 | g3 | Δ3 | Occupancy |
|---|---|---|---|---|---|---|---|---|
| Bovine | Fe/S I | 2.0230±001 | (1.3) | 1.933±0.001 | (1.0) | 1.899±0.001 | (1.2) | 0.85 |
| Fe/S II | 2.122±0.005 | (4.5) | 2.005±0.003 | (2.5) | 1.888±0.005 | (3.8) | 1.00 | |
| Fe/S III | 2.060±0.001 | (1.2) | 1.930* | (1.2) | 1.857±0.001 | (1.2) | 0.08 | |
| Human | Fe/S I | 2.021±0.001 | (1.1) | 1.930±0.001 | (1.1) | 1.903±0.001 | (1.3) | 0.51 |
| Fe/S II | 2.105±0.005 | (4.7) | 2.000±0.005 | (2.5) | 1.89* | (4.2) | 1.00 | |
| Fe/S III | 2.059±0.001 | (1.2) | 1.93* | (1.2) | 1.854±0.001 | (1.2) | 0.20 |
* Errors not calculated.
Figure 6. Fe/S EPR spectra of human and bovine milk XOR.
(a) The bovine XOR sample of Figure 5(c) and (c) the human enzyme of Figure 5(d). The stick diagrams indicate the g1, g2 and g3 positions for Fe/S I, II and III (data from Table 1) of the human enzyme. EPR was run at 15 K, with 5 mW microwave power at 9.355 GHz using 1.0 mT field modulation at 100 kHz; the conversion time was 40.96 ms with a time constant of 82 ms. Each spectrum is the sum of two scans. (b, d) Simulations of the bovine and human enzymes respectively using the g-values, line widths and occupancies from Table 1.
Resulphuration
To quantify the desulpho-content of Mo-containing human XOR, the following experiments were conducted. Purified human XOR was subjected to desulphuration by incubation with KCN for 2 h; this led to total loss of XO activity. Samples of untreated and of inactivated human XOR were separately resulphurated over a period of 5 h, by the time XO activity had risen to plateau values of 147±3% and 127±3% respectively of initial values (before any treatment). On the assumption that resulphuration is only 50% efficient [33], this leads to contents of active enzyme of 51 and 39% from experiments involving untreated and cyanide-treated enzymes respectively.
DISCUSSION
The molecular properties of human and bovine milk XOR are compared in Table 2. The most striking difference is seen in the Mo content. Purified human milk XOR contains only 4% of the theoretical maximum, compared with approx. 60% for the bovine milk enzyme prepared under similar conditions. The molybdopterin content of human milk XOR is also much less than that of the bovine enzyme. Moreover, our results suggest that some of the cofactor in human milk XOR could lack Mo.
Table 2. Comparison of molecular properties of human and bovine milk XORs.
| Molecular property | Human | Bovine |
|---|---|---|
| Mr | 148000* | 148300* |
| Amino acid composition | ||
| Chain length | 1333 [40] | 1332 [34] |
| Identity (%) | 90 | 90 |
| Highly conserved (%) | 4.8 | 4.8 |
| Semi-conserved (%) | 4.0 | 4.0 |
| Unconserved (%) | 1.2 | 1.2 |
| Xanthine oxidase activity (μmol·min−1·mg−1) | 0.09* | 1.4* |
| NADH oxidase activity (μmol·min−1·mg−1) | 0.33 [13] | 0.28 [13] |
| FAD content (mol/subunit) | 1* | 1* |
| Mo content (atom/subunit) | 0.04* | 0.61* |
| Molybdopterin content (mol/subunit) | 0.09* | 0.62* |
| Mo=S (sulpho content) (% theoretical based on available Mo) | 40–50* | 45*† |
| FeS centres | See text | See text |
* The present study.
† Based on Mo content and activity.
Although the UV/visible spectrum of human milk XOR is similar in form to that of folate-affinity-purified bovine milk enzyme, its ε450 is clearly lower. The difference spectrum resembles those of proteins containing non-haem iron-sulphur, suggesting deficiency of the latter in human milk XOR. Comparison of ε450 values estimated this deficiency to be approx. 30%, and the spectrum of the human enzyme was essentially simulated by addition of the spectrum of FAD to that of bovine XOR, so as to recreate the iron-sulphur/FAD ratio of the human enzyme. A similar extent of iron-sulphur deficiency was also estimated by comparison of A450/A550 ratios of human and bovine XOR; in this case using results from the extensively characterized preparation of Hart et al. [17], known to contain stoichiometric amounts of Mo and iron-sulphur. Because CD spectra of XOR preparations are largely determined by their iron-sulphur contents, they allow direct comparison of the latter. By these means also, the human enzyme was shown to be approx. 30% deficient in iron-sulphur and again, the human spectrum could be simulated on this basis.
Two Fe/S clusters commonly occur in XOR and related mononuclear molybdenum hydroxylases [2]. Termed Fe/S I and Fe/S II, they are indistinguishable in their UV/visible absorption spectra but differ in their EPR properties, which have been well characterized for the bovine milk enzyme [30,31]. A third Fe/S signal was first described by Rajagopalan and co-workers [32] who observed it in demolybdo-fractions of bovine milk XOR that were also deficient in Fe/S. This signal is present in human milk XOR and, in preliminary studies, we named it Fe/S III [15]. It has subsequently been detected in recombinant rat [33] and Drosophila [35] enzymes. The nature of the Fe/S III sites is of considerable interest. They seem to occur primarily under conditions of Fe/S deficiency and they might, in principle, replace either Fe/S I or Fe/S II centres, or conceivably both of these, in some of the enzyme molecules. To obtain information on the occupancy of the different sites in our samples, we have to combine data on Fe/S deficiency with the EPR results. By using the spectroscopic methods described above, the bovine and human samples used in the EPR experiments were found to have deficiencies of 0.06 and 0.32 respectively (results not shown). Assuming that all three kinds of Fe/S cluster have the same UV/visible and CD spectra, in line with work that suggests close similarities between such clusters in other systems [36,37], we can scale the occupancy ratios for the EPR samples from Table 1 for the three EPR-signal-giving species to make allowances for the Fe/S deficiencies. It is also necessary to assume that there are only two sites/half molecule, each of which might be empty or occupied by any of the three cluster types. On this basis, the calculated occupancies of the two Fe/S sites/half molecule by Fe/S I, II, III and nothing respectively are: 0.83, 0.97, 0.08 and 0.12 for bovine; 0.41, 0.80, 0.16 and 0.64 for human. The observation that Fe/S I is more depleted compared with Fe/S II in both our work and that of Gardlik et al. [32] implies that, in Fe/S deficiency, Fe/S III replaces Fe/S I. This indicates that the post-translational modification that is required for the formation of fully functional Fe/S I centres [35] is incomplete in the human enzyme.
Note that it is not certain which cysteine ligands are associated with Fe/S III. Some of those from Fe/S I are presumably involved, although others may also take part (cf. Martin et al. [38]) as a consequence of conformational change resulting from lack of molybdopterin. Some of the enzyme half molecules, at least for the human protein, must contain only one Fe/S cluster, and some probably no clusters at all. Somewhat analogously, Nishino et al. [39] describe a recombinant XOR species having only one Fe/S cluster. It may be asked why Fe/S III signals were not observed in extensive studies of bovine milk XOR before they were reported by Gardlik et al. [32]. In particular, Fe/S III signals were clearly absent from the demolybdo samples studied by Ventom et al. [21] despite their seeming to be generally analogous to the preparations of Gardlik et al. [32]. An answer may be that, in contrast with earlier studies, the latter workers did not use proteases in their enzyme preparation, and the discrepancies might be explained if the Fe/S III-containing enzyme is especially sensitive to proteolysis.
Comparison of the Mo contents and XO activities of human and bovine XOR (see the Results section) allowed estimation of activities corresponding to 100% Mo content. The percentage of inactive Mo-containing enzyme could then, in each case, be calculated by using a theoretical maximum activity/flavin ratio of 210 [1]. This gave estimates of 59 and 55% content of inactive Mo-containing enzyme for human and bovine XOR respectively. It was assumed that the inactive enzyme was most probably of the desulpho-form and this was further investigated by resulphuration experiments. Resulphuration of human milk XOR and of cyanide-treated human enzyme yielded activities consistent with contents of desulpho-forms of 49 and 61% respectively. The higher value for cyanide-treated enzyme could reflect some inactivation during the desulphuration process and the final values obtained are, in any case, necessarily approximate. It can, however, be concluded that the desulpho-content of the Mo-containing human enzyme is approx. 50–60%. That human XOR contains only a low proportion of active enzyme and that this is largely the desulpho-form was confirmed by EPR. However, absolute quantification on this basis is barely meaningful, as conversion into Mo(V) is very much dependent on preparation conditions, relative redox potentials and populations of all the centres [35].
On the basis of the present study and also other studies [32,35] it seems clear that bovine and human XORs contain similar demolybdo-forms of the enzyme that are deficient in Fe/S and in which Fe/S I is partially replaced by Fe/S III. The essential difference is that the content of this demolybdo form is much higher in the human case and an important question is why should this be so? It seems unlikely that the deficit is a consequence of differences in primary structure as these are highly homologous. The relative molecular masses of the two enzymes are experimentally indistinguishable from each other and correspond to the values (including all cofactors) derived from the deduced amino acid sequences. The latter have been reported for XOR from bovine mammary gland, human liver and human intestine [3]. Despite initial discrepancies, a single sequence has now been agreed for the human liver and intestinal enzymes [GenBank® accession number D11456 (updated 2000)], and it has also recently been shown to be identical with that of human mammary cells [40].
The agreed sequence of human XOR, from liver, intestine or mammary tissue, is 90% identical with that of bovine mammary gland. Of the 10% of differing residues, only 12% (17 residues) are completely unconserved. Of the rest, 40% of the changes are semi-conservative and 48% are highly conservative [40]. In this context, it seems unlikely that differences in the primary sequences of the bovine and human milk enzymes dictate their widely different Mo contents. This conclusion is supported by the apparent identity of the molybdopterin binding clefts in XOR from human and bovine milk, as shown by crystal structures [40]. Further support is provided by the similar values of the Michaelis constant Km for human and bovine milk XORs [13]. Most probably, differences in Mo contents of human and bovine milk XOR reflect different activities of the molybdopterin synthesis and incorporation pathways [41] in the respective mammary cells. It is unlikely that our human milk samples are unusual in that the donors came from a Mo-deficient area: similarly low specific activities have been reported for XOR purified from breast milk in California [42] and Finland [43].
The question arises as to the extent to which human milk XOR is typical of the human enzyme in general. As already noted, Krenitsky et al. [11] described a preparation of XOR from human liver with activity comparable with that of bovine milk XOR. However, recent immunoaffinity purification of human liver XOR [44] gave an enzyme with much lower specific activity (200 nmol·min−1·mg−1). The preparation of Krenitsky et al. [11] depends on affinity purification using a guanine residue analogue, which may be expected to select for the active enzyme, and so not to represent the true situation in liver tissue.
With regard to mammalian milk XOR generally, unoccupied Mo sites are not confined to the human enzyme. Preparations of XOR from goat [45] and sheep [46] milk contain only 0.09 and 0.18 atom of Mo/subunit respectively and, although purified bovine milk XOR is clearly much richer in Mo, it is still 40% deficient. It is far from clear what advantage might derive from this. It is of interest that, although XOR plays a key role in the process of milk secretion [47,48], this does not require the active enzyme, depending rather on XOR protein [48]. Moreover, the specific activity of human XOR protein has been shown to reach a peak in the first few weeks post-partum [49], possibly to coincide with an antimicrobial role in the neonatal gut [7–9,50]. Thereafter, specific activity rapidly falls to consistently low levels [49] and it is from human milk collected during this latter phase that XOR is usually (also in the present study) prepared. A consequence of such a variation may be to spare the metabolically expensive process of MoCo incorporation in the later stages of milk secretion, when an antimicrobial function of milk is less critical.
Acknowledgments
We are grateful to Dr G. Siligardi at the EPSRC National Chiroptical Spectroscopy and ULIRS Optical Spectroscopy Centres, Kings College, London, for determination of CD spectra. D.J.L. acknowledges support from the BBSRC by its Core Strategic Grant to John Innes Centre, Norwich, U.K.
References
- 1.Bray R. C. Molybdenum iron-sulfur flavin hydroxylases and related enzymes. In: Boyer P. D., editor. The Enzymes, vol. 12, 3rd edn. New York: Academic Press; 1975. pp. 299–419. [Google Scholar]
- 2.Hille R. The mononuclear molybdenum enzymes. Chem. Rev. 1996;96:2757–2816. doi: 10.1021/cr950061t. [DOI] [PubMed] [Google Scholar]
- 3.Harrison R. Structure and function of xanthine oxidoreductase: where are we now? Free Radical Biol. Med. 2002;33:774–797. doi: 10.1016/s0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 4.Meneshian A., Bulkley G. B. The physiology of endothelial xanthine oxidase: from urate catabolism to reperfusion injury to inflammatory signal transduction. Microcirculation. 2002;9:161–175. doi: 10.1038/sj.mn.7800136. [DOI] [PubMed] [Google Scholar]
- 5.Hancock J. T., Desikan R., Neill S. J. Role of reactive oxygen species in cell signalling pathways. Biochem. Soc. Trans. 2001;29:345–350. doi: 10.1042/0300-5127:0290345. [DOI] [PubMed] [Google Scholar]
- 6.Vorbach C., Harrison R., Capecchi M. Xanthine oxidoreductase is central to the evolution and function of the innate immune system. Trends Immunol. 2003;24:512–517. doi: 10.1016/s1471-4906(03)00237-0. [DOI] [PubMed] [Google Scholar]
- 7.Harrison R. Physiological roles of xanthine oxidoreductase. Drug Metab. Rev. 2004;36:363–375. doi: 10.1081/dmr-120037569. [DOI] [PubMed] [Google Scholar]
- 8.Martin H. M., Hancock J. T., Salisbury V., Harrison R. Role of xanthine oxidoreductase as an antimicrobial agent. Infect. Immun. 2004;72:4933–4939. doi: 10.1128/IAI.72.9.4933-4939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin H. M., Moore K. P., Bosmans E., Davies S., Burroughs A. K., Dhillon A. P., Tosh D., Harrison R. Xanthine oxidoreductase is present in bile ducts of normal and cirrhotic liver. Free Radical Biol. Med. 2004;37:1214–1223. doi: 10.1016/j.freeradbiomed.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Amaya Y., Yamazaki K., Sato M., Noda K., Nishino T., Nishino T. Proteolytic conversion of xanthine dehydrogenase from the NAD-dependent type to the O2-dependent type. J. Biol. Chem. 1990;265:14170–14175. [PubMed] [Google Scholar]
- 11.Krenitsky T. A., Spector T., Hall W. W. Xanthine oxidase from human liver: purification and characterisation. Arch. Biochem. Biophys. 1986;247:108–119. doi: 10.1016/0003-9861(86)90539-4. [DOI] [PubMed] [Google Scholar]
- 12.Abadeh S., Killacky J., Benboubetra M., Harrison R. Purification and partial characterization of xanthine oxidase from human milk. Biochim. Biophys. Acta. 1992;1117:25–32. doi: 10.1016/0304-4165(92)90157-p. [DOI] [PubMed] [Google Scholar]
- 13.Sanders S. A., Eisenthal R., Harrison R. NADH oxidase activity of human xanthine oxidoreductase. Generation of superoxide anion. Eur. J. Biochem. 1997;245:541–548. doi: 10.1111/j.1432-1033.1997.00541.x. [DOI] [PubMed] [Google Scholar]
- 14.Godber B., Sanders S., Harrison R., Eisenthal R., Bray R. C. > or =95% of xanthine oxidase in human milk is present as the demolybdo form, lacking molybdopterin. Biochem. Soc. Trans. 1997;25:519S. doi: 10.1042/bst025519s. [DOI] [PubMed] [Google Scholar]
- 15.Bray R. C., Lowe D., Godber B., Harrison R., Eisenthal R. Properties of xanthine oxidase from human milk: the enzyme is grossly deficient in molybdenum and substantially deficient in iron-sulfur centres. In: Ghisla S., Kroneck P. M. H., Macheroux P., Sund H., editors. Flavins and Flavoproteins, Proceedings of the 13th International Symposium, Konstanz, Germany. Berlin: Agency for Scientific Publications; 1999. pp. 775–778. [Google Scholar]
- 16.Godber B. L. J., Doel J. J., Sapkota J. P., Blake D. R., Stevens C. R., Eisenthal R., Harrison R. Reduction of nitrite to nitric oxide catalysed by xanthine oxidoreductase. J. Biol. Chem. 2000;275:7757–7763. doi: 10.1074/jbc.275.11.7757. [DOI] [PubMed] [Google Scholar]
- 17.Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem. J. 1970;116:851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avis P. G., Bergel F., Bray R. C. Cellular constituents. The chemistry of xanthine oxidase. Part III. Estimations of the cofactors and the catalytic functions of enzyme fractions of cows' milk. J. Chem. Soc. 1956:1219–1226. [Google Scholar]
- 19.Beaucamp K., Bergmeyer H.-U., Beutler H.-O. Coenzymes, metabolites and other biochemical reagents. In: Bergmeyer H.-U., editor. Methods of Enzymatic Analysis. New York: Verlag Chemie, Weinheim and Academic Press; 1974. p. 532. [Google Scholar]
- 20.Nishino T., Nishino T., Tsushima K. Purification of highly active milk xanthine oxidase by affinity chromatography on Sepharose 4B/folate gel. FEBS Lett. 1981;131:369–372. doi: 10.1016/0014-5793(81)80406-1. [DOI] [PubMed] [Google Scholar]
- 21.Ventom A. M., Deistung J., Bray R. C. The isolation of demolybdo xanthine oxidase from bovine milk. Biochem. J. 1988;255:949–956. doi: 10.1042/bj2550949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bray R. C., George G. N. Electron-paramagnetic-resonance studies using pre-steady-state kinetics and substitution with stable isotopes on the mechanism of action of molybdoenzymes. Biochem. Soc. Trans. 1985;13:560–567. doi: 10.1042/bst0130560. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz G., Boxer D. H., Mendel R. R. Molybdenum cofactor biosynthesis – the plant protein Cnx1 binds molybdopterin with high affinity. J. Biol. Chem. 1997;272:26811–26814. doi: 10.1074/jbc.272.43.26811. [DOI] [PubMed] [Google Scholar]
- 24.Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J. Biol. Chem. 1970;245:6595–6598. [PubMed] [Google Scholar]
- 25.Wahl R. C., Rajagopalan K. V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J. Biol. Chem. 1982;257:1354–1359. [PubMed] [Google Scholar]
- 26.Shethna Y. I., DerVartanian D. V., Beinert H. Non heme (iron-sulfur) proteins of Azotobacter vinelandii. Biochem. Biophys. Res. Commun. 1968;31:862–868. doi: 10.1016/0006-291x(68)90531-7. [DOI] [PubMed] [Google Scholar]
- 27.Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J. Biol. Chem. 1969;244:1692–1700. [PubMed] [Google Scholar]
- 28.Kanda M., Rajagopalan K. V. Nonequivalence of the flavin adenine dinucleotide moieties of chicken liver xanthine dehydrogenase. J. Biol. Chem. 1972;247:2177–2182. [PubMed] [Google Scholar]
- 29.Bayer E., Bacher A., Krauss P., Woelter W., Barth G., Bunnenberg E., Djerassi C. Investigation of xanthine oxidase. Magnetic circular dichroism studies. Eur. J. Biochem. 1971;22:580–584. doi: 10.1111/j.1432-1033.1971.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 30.Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin–spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem. J. 1972;130:239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hille R., Hagen W. R., Dunham W. R. Spectroscopic studies on the iron-sulfur centers of milk xanthine oxidase. J. Biol. Chem. 1985;260:10569–10575. [PubMed] [Google Scholar]
- 32.Gardlik S., Barber M. J., Rajagopalan K. V. A molybdopterin-free form of xanthine oxidase. Arch. Biochem. Biophys. 1987;259:363–371. doi: 10.1016/0003-9861(87)90502-9. [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki T., Okamoto K., Nishino T., Mizushima J., Hori H., Nishino T. Sequence motif-specific assignment of two [2Fe-2S] clusters in rat xanthine oxidoreductase studied by site-directed mutagenesis. J. Biochem. (Tokyo) 2000;127:771–778. doi: 10.1093/oxfordjournals.jbchem.a022669. [DOI] [PubMed] [Google Scholar]
- 34.Berglund L., Rasmussen J. T., Anderson M. D., Rasmussen M. S., Petersen T. E. Purification of the bovine xanthine oxidoreductase from milk fat globule mem-branes and cloning of complementary deoxyribonucleic acid. J. Dairy Sci. 1996;79:198–204. doi: 10.3168/jds.S0022-0302(96)76351-8. [DOI] [PubMed] [Google Scholar]
- 35.Adams B., Lowe D. J., Smith A. T., Scazzocchio C., Demais S., Bray R. C. Expression of Drosophila melanogaster xanthine dehydrogenase in Aspergillus nidulans and some properties of the recombinant enzyme. Biochem. J. 2002;362:223–229. doi: 10.1042/0264-6021:3620223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu. Rev. Biochem. 1973;42:159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- 37.Palmer G. Iron-sulfur proteins. In: Boyer P. D., editor. The Enzymes, vol. 12, 3rd edn. New York: Academic Press; 1975. pp. 1–56. [Google Scholar]
- 38.Martin A. E., Burgess B. K., Stout C. D., Cash V. L., Dean D. R., Jensen G. M., Stephens P. J. Site-directed mutagenesis of azotobacter-vinelandii ferredoxin-1-[FE-S] cluster-driven protein rearrangement. Proc. Natl. Acad. Sci. U.S.A. 1990;87:598–602. doi: 10.1073/pnas.87.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishino T., Amaya Y., Kawamoto S., Kashima Y., Okamoto K., Nishino T. Purification and characterization of multiple forms of rat liver xanthine oxidoreductase expressed in baculovirus-insect cell system. J. Biochem. (Tokyo) 2002;132:597–606. doi: 10.1093/oxfordjournals.jbchem.a003262. [DOI] [PubMed] [Google Scholar]
- 40.Pearson A. Ph.D. dissertation. Scotland, U.K.: University of St Andrews, St Andrews; 2001. Solution of the crystal structure of human milk xanthine oxidoreductase. [Google Scholar]
- 41.Garattini E., Mendel R., Romao M. J., Wright R., Terao M. Mammalian molybdo-flavoenzymes, an expanding family of proteins: structure, genetics, regulation, function and pathophysiology. Biochem. J. 2003;372:15–32. doi: 10.1042/BJ20030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Graham K., Fleming J. E., Young R., Bensch K. G. Preparation of antibodies against xanthine oxidase from human milk. Int. J. Biochem. 1989;21:715–722. doi: 10.1016/0020-711x(89)90201-2. [DOI] [PubMed] [Google Scholar]
- 43.Sarnesto A., Linder N., Raivio K. O. Organ distribution and molecular forms of human xanthine dehydrogenase/xanthine oxidase protein. Lab. Invest. 1996;74:48–56. [PubMed] [Google Scholar]
- 44.Choudhury S. Ph.D. dissertation. Bath, U.K.: University of Bath; 2001. Purification and characterisation of xanthine oxidoreductase from liver. [Google Scholar]
- 45.Atmani D., Benboubetra M., Harrison R. Goats' milk xanthine oxidoreductase is grossly deficient in molybdenum. J. Dairy Res. 2004;71:7–13. doi: 10.1017/s0022029903006514. [DOI] [PubMed] [Google Scholar]
- 46.Benboubetra M., Baghiani A., Atmani D., Harrison R. Physicochemical and kinetic properties of purified sheep's milk xanthine oxidoreductase. J. Dairy Sci. 2004;87:1580–1584. doi: 10.3168/jds.S0022-0302(04)73311-1. [DOI] [PubMed] [Google Scholar]
- 47.McManaman J. L., Palmer C. A., Wright R. M., Neville M. C. Functional regulation of xanthine oxidoreductase expression and localization in the mouse mammary gland: evidence of a role in lipid secretion. J. Physiol. (Cambridge, Mass.) 2002;545:567–579. doi: 10.1113/jphysiol.2002.027185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorbach C., Scriven A., Capecchi M. R. The housekeeping gene xanthine oxidoreductase is necessary for milk fat droplet secretion: gene sharing in the lactating mammary gland. Genes Dev. 2002;16:3223–3235. doi: 10.1101/gad.1032702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown A.-M., Benboubetra M., Ellison M., Powell D., Reckless J. D., Harrison R. Molecular activation-deactivation of xanthine oxidase in human milk. Biochim. Biophys. Acta. 1995;1245:248–254. doi: 10.1016/0304-4165(95)00093-q. [DOI] [PubMed] [Google Scholar]
- 50.Hancock J. T., Salisbury V., Ovejero-Boglione M. C., Cherry R., Hoare C., Eisenthal R., Harrison R. Antimicrobial properties of milk: dependence on presence of xanthine oxidase and nitrite. Antimicrob. Agents Chemother. 2002;46:3308–3310. doi: 10.1128/AAC.46.10.3308-3310.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]