Abstract
Background
Dyslipidemia represents an important risk factor for cardiovascular diseases, although its optimal management after kidney transplantation remains unclear. The present meta-analysis aimed to shed light on the efficacy and safety of statins among kidney transplant recipients, evaluating their potential effects on the risk of cardiovascular events, mortality and graft survival.
Methods
Medline, Scopus, Web of Science, CENTRAL, Clinicaltrials.gov and Google Scholar were systematically searched from their inception through April 20, 2024. Both randomized controlled trials and observational studies evaluating the effects of statin administration after kidney transplantation were held eligible. Random-effects models were fitted using the maximum likelihood method, while the certainty of evidence was appraised following the GRADE (Grading of Recommendations, Assessment, Development, and Evaluations) approach.
Results
Overall, 27 studies (10 randomized controlled trials and 17 observational studies) were included. Statin use compared to no use was associated with a lower risk of major adverse cardiovascular events [Relative risk (RR): 0.87, 95% confidence interval (CI): 0.67–0.96, moderate certainty] and overall mortality (RR: 0.84, 95% CI: 0.74–0.94, low certainty). The risk of graft loss did not differ between the compared groups (RR: 0.72, 95% CI: 0.48–1.08, very low certainty). Regarding safety endpoints, statin use was associated with a lower risk of hepatotoxicity (RR: 0.81, 95% CI: 0.70–0.93, moderate certainty), but with a greater risk of rhabdomyolysis (RR: 1.37, 95% CI: 1.10–1.70, low certainty) and cataract (RR: 1.22, 95% CI: 1.14–1.31, moderate certainty). No statistically significant differences between the compared groups with and without statin use were observed concerning the risk of creatine kinase elevation, post-transplant diabetes mellitus, hip fracture, venous thromboembolism, or cancer.
Conclusions
Among kidney transplant recipients, statin use is associated with a lower risk of cardiovascular events and better patient survival, presenting an acceptable safety profile. Further large-scale studies are needed to determine the optimal statin dosing strategy and lipid-lowering goals, depending on comorbidities and immunosuppression regimens.
Registration
10.17504/protocols.io.5qpvok3yzl4o/v1.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02276-w.
Keywords: Statin, Kidney transplantation, Cardiovascular, Dyslipidemia, Systematic review
Introduction
Cardiovascular disease constitutes a major source of morbidity after kidney transplantation, representing the leading cause of mortality with a functioning graft [1, 2]. Cardiovascular care accounts for one-third of hospitalizations among kidney transplant recipients and is associated with high healthcare costs [3]. The high incidence of cardiovascular events is based on the interplay of traditional and non-traditional risk factors [4]. Specifically, kidney failure patients who develop de novo disease or who have comorbid hypertension, diabetes mellitus or dyslipidemia can have increased cardiovascular risk. In addition, several factors unique to transplant recipients may also contribute to cardiovascular morbidity, such as oxidative stress, anemia, hyperhomocysteinemia and immunosuppression effects. The spectrum of cardiovascular disease, including ischemic heart disease, valvular disease, heart failure and pulmonary hypertension, is wide [5]. However, kidney transplant recipients are typically excluded from major cardiovascular outcome randomized controlled trials and are thus less likely to receive evidence-based goal-directed interventions [6].
Dyslipidemia is prevalent after kidney transplantation, as it is potentiated by the combined effects of various contributing factors, such as post-transplant diabetes mellitus, obesity, impaired renal function, proteinuria and glucocorticoid administration [7]. In addition, cyclosporine may exacerbate hyperlipidemia by reducing hepatocellular levels of low-density lipoprotein receptor [8], while mammalian target of rapamycin (mTOR) inhibitors have been linked to enhanced lipogenesis [9] and impaired catabolism of apolipoprotein B100-containing lipoproteins [10]. Currently, no established lipid targets exist for the kidney transplant population, but it is estimated that a significant proportion of kidney transplant recipients may be undertreated, not achieving the proposed targets for the general population [11].
Hydroxymethylglutaryl-CoA reductase inhibitors or statins are the most commonly prescribed hypolipidemic agents, as they potently reduce low-density lipoprotein levels and exert pleiotropic cardioprotective effects [12]. Although the 2013 Kidney Disease: Improving Global Outcomes (KDIGO) guidelines provide a weak recommendation for the administration of statins in all adult kidney transplant recipients [13], concerns about toxicity and interactions with immunosuppressive agents may impede their widespread use. Previous meta-analyses [14–16] have suggested that statin use may be linked to cardiovascular risk reduction and survival improvement, although evidence regarding statin safety remains scattered. The present meta-analysis aimed to systematically accumulate the existing evidence in the field and shed more light on the association of statin use with the risk of cardiovascular events, mortality and adverse effects among kidney transplant recipients. An updated systematic literature review was conducted including both observational studies and randomized controlled trials in order to provide a critical evaluation of evidence regarding statin efficacy and safety following kidney transplantation.
Materials and methods
Study design
The meta-analysis protocol was prospectively registered and made publicly available (10.17504/protocols.io.5qpvok3yzl4o/v1). The study was reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [17]. No ethical approval was needed as the meta-analysis was exclusively based on previously published data.
Eligibility criteria
The study population consisted of adult kidney transplant recipients. The intervention of interest was statin administration after kidney transplantation, given for primary or secondary cardiovascular prevention. The intervention was compared to placebo or standard care. The primary outcome of interest was the occurrence of major adverse cardiovascular events (MACE). The endpoint of MACE was a composite one and could include a combination of cardiovascular mortality, acute coronary syndrome, cerebrovascular accident, peripheral artery disease requiring revascularization and congestive heart failure. Secondary efficacy outcomes included patient overall survival and kidney allograft survival. The safety endpoints included the following: hepatotoxicity, rhabdomyolysis, creatine kinase elevation, post-transplant diabetes mellitus, cataract, venous thromboembolic events, hip fracture and cancer. Randomized controlled trials and observational (cohort and case-control) studies were considered potentially eligible. Studies evaluating only the effects of statins on blood lipids or on acute/chronic allograft rejection risk were not included. Pre-transplant statin use was not evaluated as the study focused only on the effects of statin therapy following kidney transplantation. Uncontrolled trials, cross-sectional and descriptive studies, review articles and in vitro studies were also excluded.
Literature search
The literature search was conducted by systematically searching Medline (via PubMed), Scopus, Web of Science, CENTRAL (Cochrane Central Register of Controlled Trials) and Clinicaltrials.gov. In addition, Google Scholar was screened to provide grey literature coverage, while the “snowball” method [18] was applied by examining the reference lists of the studies included in the review. All searches were performed from inception till June 20, 2024, without any language restrictions. The search algorithm was constructed using both MeSH terms and key-words. The main search algorithm was as follows: “(“Hydroxymethylglutaryl-CoA Reductase Inhibitors“[Mesh] OR statin* OR atorvastatin OR rosuvastatin OR fluvastatin OR lovastatin OR pitavastatin OR pravastatin OR simvastatin OR cerivastatin) AND (“Kidney Transplantation“[Mesh] OR “kidney transplant*” OR “renal transplant*”)”.
Study selection
The evaluation of studies as eligible or not eligible followed three consecutive stages. Firstly, the titles and abstracts of all identified electronic articles were screened for potential eligibility. Secondly, all studies that were considered to fulfil the inclusion criteria were retrieved in full-text form. Subsequently, studies that examined different populations, did not report any of the outcomes of interest or met any of the exclusion criteria were excluded. The process of study selection was independently carried out by two authors, resolving any discrepancies regarding the eligibility of the articles through the consensus of all the authors.
Data extraction
The following data were extracted from the included studies: year of publication, country, eligibility criteria, sample size, study design, type of statin, statin dose, time from transplantation, median age, body mass index, estimated glomerular filtration rate (eGFR), low-density lipoprotein cholesterol, percentage of male sex, diabetes mellitus status, hypertension status, history of cardiovascular disease, type of immunosuppression (calcineurin inhibitors/mTOR inhibitors) and all the necessary information regarding the outcomes of interest. Specifically, relative risks (RR) along with their 95% confidence intervals were extracted. In observational studies, adjusted effect sizes were extracted from multivariate regression models. In case relative risks were not reported, the number of events in the total of patients was extracted. Data extraction was independently performed by two authors, who resolved any disagreements through their consensus.
Quality assessment
The risk of bias in randomized controlled trials was judged by applying the RoB-2 tool [19]. Specifically, low risk, some concerns or high risk of bias were assigned in the domains of randomization, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported results. The quality of cohort studies was assessed with the ROBINS-I tool [20], which considers the following domains: confounding, selection of participants, classification of interventions, departures from intended interventions, missing data, measurement of outcomes and selection of the reported results. Risk of bias evaluation was performed by two authors and any potential discrepancies were resolved after discussion with all the authors.
Statistical analysis
Pooling of studies was performed by fitting random-effects meta-analysis models, due to the expected existence of methodological heterogeneity across studies. The restricted maximum likelihood method was used for between-study variance estimation (τ2) [21]. The weight assigned to each included study was the inverse of the sum of the within-study variance (σi2) and the estimated between-study variance (τ2). The confidence intervals (CI) were set at 95%. Inter-study heterogeneity was quantified by the inconsistency index (I2), with values greater than 50% indicating remarkable heterogeneity [22]. The 95% prediction intervals were estimated to evaluate the effects to be expected by future studies in the field [23]. Publication bias was assessed by the trim-fill method [24], while the statistical significance (P < 0.10) of Egger’s regression and Begg & Mazumdar’s rank correlation tests was taken into account in the case of 10 or more studies [23]. Subgroup analysis was conducted based on study design (randomized controlled trial or observational study), location (Europe, North America, South America Asia or Oceania), sample size (< 400 or ≥ 400 patients), risk of bias (low, moderate or high) and type of calcineurin inhibitor (cyclosporine or tacrolimus). Meta-regression analysis was performed for endpoints with 4 or more studies, based on the following variables: age, sex, BMI, eGFR, diabetes mellitus and history of cardiovascular disease. All analyses were performed in R-4.4.0 (package “metafor” [25]).
Certainty of evidence
The certainty of the existing evidence was appraised following the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) approach [26]. Specifically, the quality of evidence could be classified as very low, low, moderate or high by evaluating the domains of study limitations, inconsistency, indirectness, imprecision and publication bias.
Results
Study selection
The PRISMA flowchart depicts the process of study selection (Fig. 1). The database search identified 1952 electronic articles; after deduplication, a total of 1068 articles were screened for potential eligibility, 45 of which were retrieved as full-texts. Subsequently, 18 studies [11, 27–43] were excluded because they did not report the outcomes of interest (n = 7), lacked a control group (n = 4), were partial duplicates of studies already included in the review, resulting in population overlap (n = 3), evaluated different populations (n = 3) or assessed the effects of pre-transplant statin use (n = 1) (Suppl. Table 1, Appendix 1). Overall, 27 studies [44–70] (10 randomized controlled trials and 17 observational studies) were finally included in the meta-analysis.
Fig. 1.
PRISMA search plot diagram
Included studies
Table 1 presents the methodological characteristics of the included studies. Eleven studies were conducted in North America, 7 in Europe, 3 in Asia (South Korea), 1 in South America (Brazil), 1 in Oceania (Australia) and 4 were international ones. Three studies presented analyses from the ALERT (Assessment of Lescol in Renal Transplant) trial, while 3 studies derived their data from the U.S. Renal Data System. In 2 studies, statins were exclusively administered for primary cardiovascular prevention. The median participant age was 48.4 years, while the median percentage of males was 60%. In cohort studies, patients could have been treated with more than one statin at different doses and intensities. All studies used calcineurin inhibitors as maintenance immunosuppression, with cyclosporine being exclusively administered in 11 studies. On the other hand, mTOR inhibitors were exclusively used in only 1 study. The median follow-up of studies was 60 months (range: 3 to 180 months). The exclusion criteria, outcomes of interest and definitions of MACE in the included studies are shown in Suppl. Table 2 (Appendix 2). Suppl. Table 3 (Appendix 2) presents details about matching and covariate adjustment in the included cohort studies.
Table 1.
Methodological characteristics of the included studies
| Study | Design | Country | Sample size | Statin | Time from Tx † | Age (years)† | Male sex (%) | DM (%) | HTN (%) | BMI (kg/m2) † | eGFR (ml/min/ 1.73 m2) † |
LDL-C (mmol/L) † |
CVD (%) |
CNI (%) |
mTORi (%) | Follow-up (months) † |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024; Nazoiri [54] | RC | France | 318 | Low-intensity: 58.3%; Moderate-intensity: 68.6%; High-intensity: 11.5% | 12.1 months | 45.7 | 64.2 | 7.5 | 88.1 | 23.8 | 52 | 2.60 | 0 |
CsA: 23.3 Tacrolimus: 76.4 |
1.9 | 72 |
| 2024; Yim [55] | PC | South Korea | 714 | Any | Up to 12 months | 45.6 | 64.1 | 25.5 | 92.4 | 22.8 | 72.6 | 2.16 | 8.4 |
CsA: 5.3 Tacrolimus: 94.7 |
7.1 | 84 |
| 2023b; Bae-USRDS [53] | RC | USA | 58,264 | Simvastatin: 46.9%; Atorvastatin: 45.2%; Pravastatin: 14.5%; Rosuvastatin: 9.7% | NR | 51.4 | 60.3 | 35.3 | NR | 27.5 | NR | NR | NR |
CsA: 4.4 Tacrolimus: 91.8 |
3.1 | 36 |
| 2023a; Bae-USRDS [52] | RC | USA | 57,699 | Simvastatin: 46.9%; Atorvastatin: 45.2%; Pravastatin: 14.5%; Rosuvastatin: 9.7% | NR | 51.1 | 60.4 | 35.3 | NR | 27.5 | NR | NR | NR |
CsA: 4.3 Tacrolimus: 90.2 |
3.0 | 36 |
| 2021; Anderson [51] | RC | Germany | 622 | Atorvastatin: 65.2%; Simvastatin: 20.8%; Fluvastatin: 5.2%; Rosuvastatin: 4.8%; Pravastatin: 4.4% | > 12 months | 53.9 | 57.2 | 23.2 | NR | 26.6 | NR | 2.98 | 14.8 |
CsA: 39.5 Tacrolimus: 18.8 |
0 | 64.8 |
| 2022; Frasco [50] | RC | USA | 1384 | Atorvastatin: 54.3%; Rosuvastatin: 18.8%; Simvastatin: 12.1%; Pravastatin: 12.1% | Post-transplant | 54 | 57.9 | 37.1 | NR | 27.5 | NR | NR | NR | Tacrolimus: 100 | 1.2 | 12 |
| 2020; Szili-Torok [49] | PC | Netherlands | 190 | Atorvastatin: 52.6%; Fluvastatin: 27.4%; Simvastatin: 14.7%; Pravastatin: 5.3% | 78 months | 50.3 | 50.2 | 0 | 82 | 24.9 | 50.8 | 3.5 | NR |
CsA: 66.4 Tacrolimus: 8.2 |
0 | 115.2 |
| 2017; Han [48] | RC | South Korea | 165 | Any | 6.7 months | 47.4 | 55.2 | 17.6 | 92.7 | 21.0 | NR | NR | 15.8 | Tacrolimus: 100 | 0 | 60 |
| 2017; Vangala-USRDS [4] |
Nested C-C |
USA | 15,806 | Any | 55.6 months | 51.2 | 59.6 | 74.6 | NR | 27.3 | NR | NR | 75.3 |
CsA: 18.5 Tacrolimus: 62.8 |
9.9 | 12 |
| 2014; Choe [46] | RC | South Korea | 394 |
Atorvastatin (20 mg):45.3%; Fluvastatin (80 mg): 54.7 |
NR | 40.1 | 58.4 | 0 | 72.1 | 22.0 | 62.5 | NR | NR |
CsA: 34.8 Tacrolimus: 67.0 |
0 | 60 |
| 2010; Younas [70] | RC | USA | 615 | Any | Up to 12 months | 50 | 63 | 16.9 | NR | 26 | NR | 2.65 | 7.2 |
CsA: 0.3 Tacrolimus: >90% |
8.5 | 79.7 |
| 2008; Wiesbauer [69] | RC | Austria | 2041 | Any | NR | 48 | 59.9 | 18.8 | 81.1 | NR | NR | NR | 67.1 | NR | NR | 144 |
| 2008; Seron [68] | RCT | Spain | 89 | Fluvastatin (80 mg) | Post-transplant | 42 | 57.3 | 0 | NR | NR | NR | NR | 0 | CsA: 100 | 0 | 72 |
| 2007; Lisik [67] | RC | USA | 325 | Pravastatin: 73.3%; Atorvastatin: 25%; Simvastatin: 2% | Up to 3 months | 44.9 | 60 | NR | NR | NR | NR | NR | NR | CsA: 100 | 100 | 75 |
| 2007; Lopau [66] | C-C | Germany | 150 | Pravastatin (29.7 mg†) | 4 days | 48.5 | 66.7 | 18 | NR | NR | 62.9 | 3.75 | NR | CsA: 100 | 0 | 48 |
| 2004; Prasad [65] | RC | Canada | 314 | Atorvastatin: 85%; Pravastatin: 7%; Simvastatin: 4%; Fluvastatin: 4% | NR | 44.6 | 56.8 | 0 | NR | NR | NR | 2.24 | NR |
CsA: 58.7 Tacrolimus: 41.3 |
0 | 36 |
| 2004; Fellström-ALERT [60] | RCT |
Inter- national |
2102 | Fluvastatin (40 mg) | 62.4 | 49.8 | 66 | 18.8 | 74.9 | 25.8 | 60.3 | 4.1 | 23.5 | CsA: 100 | NR | 61.2 |
| 2001; Holdaas [57] | RCT |
Inter- national |
364 | Fluvastatin (40 mg) | Post-transplant | 48.4 | 71.4 | 12.4 | NR | NR | NR | 2.98 | NR | CsA: 100 | 0 | 3 |
| 1999; Lepre [45] | RCT | Australia | 49 | Simvastatin (10 mg) | > 12 months | 51.4 | 34.7 | 14.3 | 77.6 | NR | NR | 4.63 | NR | CsA: 57.1 | 0 | 9 |
| 1996; Katznelson [40] | RCT | USA | 48 | Pravastatin (20 mg) | 7 days | 47.3 | 69 | NR | NR | NR | NR | NR | NR | CsA: 100 | 0 | 4 |
| 2002; Cosio [63] | RC | USA | 1574 | Any |
< 24 months: 32.4% 24–60 months: 20% |
42 | 59 | 22 | NR | 26 | NR | NR | NR | CsA: 100 | 0 | 98.4 |
| 2010; Moreso [62] | RC | Spain | 3682 | Any | NR | 46.5 | 63.1 | 5.3 | NR | NR | NR | NR | NR |
CsA: 70 Tacrolimus: 20.9 |
NR | 180 |
| 1994; Arnadottir [61] | RCT | USA | 40 | Simvastatin (10 mg) | 50 | 51.5 | 65 | 20 | NR | 25.3 | NR | 5.45 | NR | CsA: 100 | 0 | 4 |
| 2005; Holdaas-ALERT [58] | RCT |
Inter- national |
1652 | Fluvastatin (40 mg) | 62.4 | 48.5 | 66.3 | 16.9 | 74.6 | 26 | 60.3 | 4.1 | 23.5 | CsA: 100 | NR | 72 |
| 2003; Holdaas-ALERT [56] | RCT |
Inter- national |
2102 | Fluvastatin (40 mg) | 62.4 | 49.8 | 66 | 18.8 | 74.9 | 25.8 | 60.3 | 4.1 | 23.5 | CsA: 100 | NR | 72 |
| 2001; Santos [60] | RCT | Brazil | 67 | Simvastatin (10 mg) | 60.5 | 43.3 | 44.8 | NR | NR | NR | 65.9 | 4.67 | NR | CsA: 77.6 | 0 | 57.5 |
| 2001; Kasiske [59] | RCT | USA | 105 | Simvastatin (10 mg) | Post-transplant | 46.5 | 59 | NR | NR | NR | NR | NR | NR | CsA: 100 | 0 | 3 |
†Median values. RC: retrospective cohort; PC: prospective cohort; C-C: case-control; RCT: randomized controlled trial; DM: diabetes mellitus; HTN: hypertension; BMI: body mass index; CNI: calcineurin inhibitor; mTORi: mammalian target of rapamycin inhibitor; CsA: cyclosporine; eGFR: estimated glomerular filtration rate; LDL-C: low-density lipoprotein cholesterol; CVD: cardiovascular disease; Tx: transplantation; ALERT: Assessment of Lescol in Renal Transplant; USRDS: United States Renal Data System; NR: not reported
Risk of bias
The outcomes of the RoB-2 evaluation of randomized controlled trials are illustrated in Suppl. Figure 1 (Appendix 3). The ALERT trial was evaluated to be at low risk of bias, while in the remaining trials, some concerns of bias were raised in the domain of randomization, due to the lack of adequate information regarding allocation sequence generation and concealment. Suppl. Table 4 presents the outcomes of the ROBINS-I assessment of observational studies. Overall, 14 studies were judged to be at moderate risk of bias and 3 studies were judged to be at serious risk of bias. No critical risk of bias was identified in any study. The main source of potential bias was considered to be confounding, due to concerns of inadequate adjustment for important covariates that could affect the association between statin use and the outcomes of interest.
Outcomes
Table 2 presents the meta-analysis outcomes for the primary and secondary endpoints. The stratified analysis based on the type of calcineurin inhibitor co-administered is shown in Table 3, while the outcomes of the subgroup analysis based on sample size, location, study design and risk of bias are shown in Table 4. Appendix 4 (Suppl. Figures 2–11) includes the forest plots of efficacy and safety outcomes, while the respective funnel plots are displayed in Appendix 5 (Suppl. Figures 12–22). The outcomes of meta-regression analyses are presented in Appendix 6 (Suppl. Table 5).
Table 2.
Summary of findings table regarding the association of statin use with primary and secondary endpoints
| Endpoint | Studies no. | Sample size | Follow-up (months)† | RR (95% CI) | 95% prediction interval | I2 | Trim-fill method | GRADE assessment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Missing studies | New RR (95% CI) |
Certainty of evidence | Downgrading | |||||||
| Efficacy | ||||||||||
| MACE | 6 | 3621 |
68.4 (48–72) |
0.87 (0.67–0.96) * | 0.67–0.96 * | 0% | 0 | 0.87 (0.67–0.96) * | Moderate | Study limitations |
| Mortality | 15 | 70,750 |
72 (3-180) |
0.84 (0.74–0.94) * | 0.65–1.08 | 40.4% | 1 | 0.84 (0.75–0.94) * | Low | Study limitations, inconsistency |
| Graft loss | 9 | 10,255 |
72 (3-180) |
0.72 (0.48–1.08) | 0.23–2.24 | 90.2% | 0 | 0.72 (0.48–1.08) | Very low | Study limitations, imprecision, inconsistency |
| Safety | ||||||||||
| Hepatotoxicity | 6 | 60,641 |
54 (3–72) |
0.81 (0.70–0.93) * | 0.70–0.93 * | 0% | 2 | 0.80 (0.70–0.92) * | Moderate | Study limitations |
| Creatine kinase elevation | 4 | 2624 |
40.5 (3–72) |
0.97 (0.50–1.89) | 0.50–1.89 | 0% | 1 | 1.02 (0.53–1.96) | Low | Study limitations, imprecision |
| Rhabdomyolysis | 2 | 59,801 |
54 (36–72) |
1.37 (1.10–1.70) * | 1.10–1.70 * | 0% | NA | NA | Low | Study limitations, imprecision |
| Diabetes mellitus | 4 | 58,597 |
48 (36-115.2) |
1.11 (0.38–3.27) | 0.11–11.07 | 92.4% | 1 | 0.79 (0.26–2.39) | Very low | Study limitations, imprecision, inconsistency |
| Cataract | 1 | 57,699 | 36 | 1.22 (1.14–1.31) * | NA | NA | NA | NA | Moderate | Study limitations |
| Hip fracture | 2 | 15,846 |
8 (4–12) |
0.90 (0.68–1.20) | 0.68–1.20 | 0% | NA | NA | Low | Study limitations, imprecision |
| Venous thromboembolism | 1 | 1384 | 12 | 0.92 (0.39–2.19) | NA | NA | NA | NA | Low | Study limitations, imprecision |
| Cancer | 1 | 2102 | 72 | 0.94 (0.82–1.07) | NA | NA | NA | NA | Moderate | Imprecision |
Asterisks denote statistically significant results
†Median (range); MACE: major adverse cardiovascular events; RR: relative risk; CI: confidence intervals: I2: inconsistency index; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; NA: not applicable
Table 3.
Association of statin use with primary and secondary endpoints, stratified by the type of the co-administered calcineurin inhibitor
| Endpoint | Calcineurin inhibitor | P | ||||
|---|---|---|---|---|---|---|
| Cyclosporine | Tacrolimus | |||||
| Studies no. | RR (95% CI) | Studies no. | RR (95% CI) | |||
| MACE | 3 | 1.46 (0.38–5.60) | 2 | 0.52 (0.14–1.88) | 0.344 | |
| Mortality | 3 | 0.96 (0.89–1.03) | 9 | 0.82 (0.67-1.00) | 0.393 | |
| Graft loss | 7 | 0.83 (0.43–1.58) | 1 | 0.98 (0.82–1.18) | 0.812 | |
| Hepatotoxicity | 4 | 0.64 (0.44–0.92) * | 1 | 0.84 (0.72–0.98) * | 0.178 | |
| Creatine kinase elevation | 3 | 1.17 (0.56–2.42) | 0 | - | NA | |
| Rhabdomyolysis | 2 | 1.09 (0.57–2.10) | 1 | 1.41 (1.10–1.81) * | 0.478 | |
| Diabetes mellitus | 1 | 1.11 (0.89–1.37) | 1 | 1.13 (1.07–1.20) * | NA | |
| Cataract | 1 | 1.22 (0.93–1.62) | 1 | 1.22 (1.14–1.31) * | NA | |
| Hip fracture | 1 | 3.00 (0.13–69.5) | 0 | - | NA | |
| Venous thromboembolism | 0 | - | 1 | 0.92 (0.39–2.19) | NA | |
| Cancer | 1 | 0.94 (0.82–1.07) | 0 | - | NA | |
Asterisks denote statistically significant results. P-values correspond to the significance of the interaction between the type of calcineurin inhibitor and statin effects
RR: relative risk; CI: confidence intervals; NA: not applicable
Table 4.
Subgroup analyses based on study sample size, location, design and risk of bias
| Endpoint | Sample size | Location | Study design | Risk of bias | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| < 400 | ≥ 400 | P | Europe | North America | Asia | P | RCT | Observational | P | Low | Moderate | Serious | P | |
| MACE |
0.60 (0.28–1.32) |
0.83 (0.68-1.00) |
0.647 |
0.84 (0.59–1.20) |
- |
0.73 (0.14–3.92) |
0.975 |
0.79 * (0.63–0.99) |
0.80 (0.48–1.34) |
0.971 |
0.79 * (0.63–0.99) |
0.81 (0.45–1.47) |
0.73 (0.17–3.15) |
0.994 |
| Mortality |
0.66 (0.30–1.46) |
0.86 * (0.78–0.96) |
0.194 |
0.79 * (0.67–0.93) |
0.83 * (0.70–0.99) |
0.28 * (0.11–0.71) |
0.108 |
1.02 (0.83–1.24) |
0.80 * (0.70–0.92) |
0.164 |
1.02 (0.83–1.25) |
0.80 * (0.70–0.92) |
2.92 (0.12–70.6) |
0.284 |
| Graft loss |
0.76 (0.33–1.75) |
0.64 (0.36–1.15) |
0.766 |
0.75 * (0.65–0.85) |
0.67 (0.27–1.67) |
0.23 * (0.13–0.42) |
< 0.001 |
1.10 (0.88–1.37) |
0.54 * (0.32–0.90) |
0.039 |
1.05 (0.83–1.33) |
0.70 (0.43–1.14) |
0.39 (0.08–1.95) |
0.681 |
| Hepatotoxicity |
0.66 (0.35–1.24) |
0.82 * (0.71–0.94) |
0.456 |
1.33 (0.26–6.87) |
0.82 * (0.71–0.95) |
- | 0.656 |
0.65 (0.40–1.06) |
0.82 * (0.71–0.95) |
0.377 |
0.71 (0.34–1.47) |
0.81 * (0.70–0.93) |
- | 0.720 |
| Creatine kinase elevation |
0.76 (0.26–2.20) |
1.20 (0.37–3.93) |
0.512 |
0.33 (0.01–7.80) |
- | - | 0.322 |
0.97 (0.50–1.89) |
- | NA |
1.20 (0.37–3.93) |
0.76 (0.26–2.20) |
- | 0.632 |
| Rhabdomyolysis | - |
1.37 (1.10–1.70) |
NA | - |
1.37 (1.10–1.70) |
- | NA |
1.00 (0.06-16.0) |
1.37 (1.10–1.70) |
NA |
1.00 (0.06-16.0) |
1.37 * (1.10–1.71) |
- | NA |
| Diabetes mellitus |
1.22 (0.22–5.61) |
1.12 * (1.07–1.18) |
0.998 |
3.86 * (1.21–12.29) |
0.54 (0.12–2.47) |
1.70 (0.85–3.40) |
0.357 | - |
1.11 (0.38–3.27) |
NA | - |
1.81 (0.55–5.88) |
0.64 (0.09–4.42) |
0.348 |
| Cataract | - |
1.22 * (1.14–1.31) |
NA | - |
1.22 * (1.14–1.31) |
- | NA | - |
1.22 * (1.14–1.31) |
NA | - |
1.22 * (1.14–1.31) |
- | NA |
| Hip fracture |
0.89 (0.67–1.19) |
3.00 (0.13–69.5) |
NA | - |
0.90 (0.68–1.20) |
- | NA |
0.89 (0.67–1.19) |
3.00 (0.13–69.5) |
NA | - |
0.90 (0.68–1.20) |
- | NA |
| Venous thromboembolism | - |
0.92 (0.39–2.19) |
NA | - |
0.92 (0.39–2.19) |
- | NA | - |
0.92 (0.39–2.19) |
NA | - |
0.92 (0.39–2.19) |
- | NA |
| Cancer | - |
0.94 (0.82–1.07) |
NA | - | - | - | NA |
0.94 (0.82–1.07) |
- | NA |
0.94 (0.82–1.07) |
- | - | NA |
Asterisks denote statistical significance. P-values correspond to the significance of the difference of statin effects depending on study sample size, location, design and risk of bias
RR: relative risk; CI: confidence intervals; NA: not applicable; MACE: major adverse cardiovascular events; RCT: randomized controlled trials
Cardiovascular events
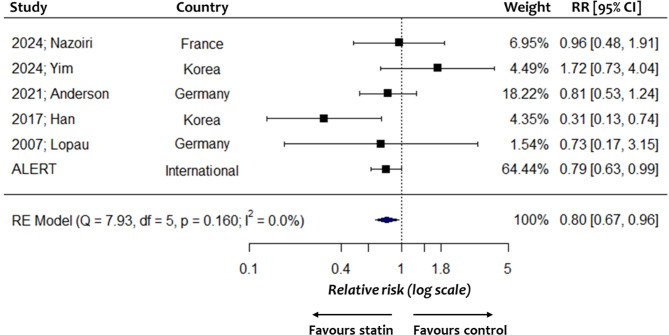
The association between statin use and MACE risk was examined in 6 studies (1 randomized controlled trial and 5 cohort studies). The administration of statins was associated with a significantly lower risk of MACE [Relative risk (RR): 0.87, 95% CI: 0.67 to 0.96, 3621 participants] (Fig. 2). No statistical heterogeneity was observed (I2: 0%) and thus the 95% prediction intervals were identical to the confidence intervals. No missing studies were identified by the trim-fill method (Suppl. Figure 12, Appendix 5). No statistically significant interaction with the type of calcineurin inhibitor was noted (P = 0.344) (Table 3). Similarly, the meta-analysis outcome was not significantly influenced by study sample size (P = 0.647), location (P = 0.975), design (P = 0.971) or risk of bias (P = 0.994) (Table 4). The meta-regression analysis showed no significant effects of age, sex, BMI, eGFR, history of cardiovascular disease and diabetes mellitus (Suppl. Table 5). The certainty of evidence was evaluated as moderate, downgrading for study limitations as most studies were observational ones with a moderate risk of bias.
Fig. 2.
Forest plot illustrating the association between statin use and risk of major adverse cardiovascular events (3621 participants). RR: relative risk; CI: confidence intervals; RE: random-effects; df: degrees of freedom
Patient survival
Overall patient survival was assessed in 15 studies (6 randomized controlled trials and 9 cohort studies). Statin use was associated with a significantly lower mortality risk (RR: 0.84, 95% CI: 0.74 to 0.94, 70,750 participants). Moderate statistical heterogeneity was observed (I2: 40.4%), while the 95% prediction intervals ranged from 0.65 to 1.08. After imputing potentially missing studies, the new trim-fill estimate remained statistically significant. No significant funnel plot asymmetry was present (Egger’s P = 0.113, Begg & Mazumdar’s P = 0.923) (Suppl. Figure 13). Subgroup analyses indicated no significant influence of calcineurin inhibitor type, study sample size, location, design or risk of bias (P > 0.05) (Tables 3 and 4). The meta-regression analysis suggested that the observed association was more pronounced in studies with participants of younger age and high eGFR on average (Suppl. Table 5). The certainty of evidence was judged to be low. Concerns about study limitations were raised as the majority of studies were to be at moderate risk of bias. In addition, the certainty of evidence was downgraded due to inconsistency, as statistical heterogeneity was moderate and the 95% prediction intervals crossed the null hypothesis.
Graft survival
The endpoint of graft survival was evaluated in 9 studies (5 randomized controlled trials and 4 cohort studies). The risk of graft loss was not significantly different between the statin group and the control group (RR: 0.72, 95% CI: 0.48 to 1.08, 10,255 participants). The level of statistical heterogeneity was remarkable (I2: 90.2%), and the 95% prediction intervals ranged from 0.23 to 2.24. No missing studies were identified by the trim-fill method (Suppl. Figure 14). Subgroup analysis indicated that the meta-analysis estimates significantly differed depending on the study location and design (Table 4). Specifically, statin use was linked to a significantly lower graft loss risk when separately pooling studies conducted in Asia (RR: 0.23, 95% CI: 0.13 to 0.42) or observational studies (RR: 0.54, 95% CI: 0.32 to 0.90). The meta-regression analysis suggested that participants’ BMI may have affected the pooled estimate (Suppl. Table 5). The certainty of evidence was assessed to be very low, downgrading for study limitations, imprecision and inconsistency. In particular, in most studies, the risk of bias was evaluated as moderate, while both 95% CI and prediction intervals were wide.
Hepatotoxicity
The risk of liver toxicity was examined in 4 randomized controlled trials and 2 cohort studies. A pooled analysis of the studies indicated that statin use was associated with a significantly lower risk of liver injury (RR: 0.81, 95% CI: 0.70 to 0.93, 60,641 participants). No statistical heterogeneity was noted (I2: 0%), and the 95% prediction intervals were similar to the confidence intervals. After the imputation of 2 potentially missing studies by the trim-fill method, the new estimate remained statistically significant (new RR: 0.80, 95% C: 0.70 to 0.93) (Suppl. Figure 15). The meta-analysis outcome was not significantly influenced by calcineurin inhibitor type, study sample size, location, design or risk of bias (P > 0.05) (Tables 3 and 4). The meta-regression analysis showed no significant effect of the examined covariates (Suppl. Table 5). The certainty of evidence was evaluated as moderate due to concerns of study limitations (moderate risk of bias in 5 out of 6 studies).
Creatine kinase elevation
The endpoint of creatine kinase elevation was reported by 4 randomized controlled trials. The risk of creatine kinase elevation was similar between statin-treated and nontreated patients (RR: 0.97, 95% CI: 0.50 to 1.89). No statistical heterogeneity was present (I2: 0%); hence, the 95% prediction intervals were identical to the confidence intervals. The trim-fill method identified 1 potentially missing study (new RR: 1.02, 95% CI: 0.53 to 1.96) (Suppl. Figure 16). The outcome did not differ among cyclosporine or tacrolimus users and was not significantly affected by study sample size, location, design or risk of bias (P > 0.05) (Tables 3 and 4). The meta-regression analysis proposed that the outcome was not significantly influenced by age, sex, BMI, eGFR, history of cardiovascular disease and diabetes mellitus (Suppl. Table 5). The certainty of evidence was appraised as low, downgrading for study limitations (moderate risk of bias in 3 out of 4 trials) and imprecision (wide 95% CI).
Rhabdomyolysis
The potential link between statin administration and rhabdomyolysis was investigated in 2 studies (1 randomized controlled trial and 1 retrospective cohort study). Bae et al [52]. identified rhabdomyolysis cases through Medicare Parts A and B claims using International Classification of Diseases (ICD) codes (ICD-9: 728.88 and ICD-10: M62.82). In the ALERT trial [56], rhabdomyolysis was developed in 2 patients (1 in each group) and was attributed to severe trauma. Overall, statin use was associated with a significantly greater risk of rhabdomyolysis (RR: 1.37, 95% CI: 1.10 to 1.70). No statistical heterogeneity was observed (I2: 0%), and thus, the 95% prediction intervals did not differ from the estimated confidence intervals. The stratified analysis indicated that the association remained significant only among tacrolimus users, although no significant subgroup effect could be ascertained (Table 3). Study sample size, location, design and risk of bias did not significantly influence the outcome (Table 4). The certainty of evidence was evaluated to be low, downgrading for study limitations and imprecision, reflecting the width of the estimated 95% CI.
Post-transplant diabetes mellitus
The potential association between statin use and the development of post-transplant diabetes mellitus was examined in 4 studies (1 prospective cohort study and 3 retrospective cohort studies). Two of them [49, 52] indicated that statin administration was linked to a significantly greater risk of diabetes mellitus, 1 study showed no significant association [46], while a significantly lower diabetes mellitus risk was suggested by Prasad et al. [65]. The pooling of all studies demonstrated no significant association between statin intake and post-transplant diabetes mellitus risk (RR: 1.11, 95% CI: 0.38 to 3.27, 58,597 participants), with high statistical inter-study heterogeneity (I2: 92.4%). The 95% prediction intervals ranged from 0.11 to 11.07. The trim-fill method identified 1 potentially missing study (new RR: 0.79, 95% CI: 0.26 to 2.39) (Suppl. Figure 18). Concerning the type of calcineurin inhibitor administered, the available data suggested a significant association between statins and diabetes mellitus among tacrolimus-treated patients (1 study, RR: 1.13, 95% CI: 1.07 to 1.20) (Table 3). The stratified analyses showed a positive association between statin use and diabetes mellitus development when studies conducted in Europe and those with a large sample size (≥ 400 participants) were pooled, although no statistically significant subgroup effects were calculated (Table 4). The certainty of evidence was appraised to be very low, downgrading for study limitations, imprecision and inconsistency. Specifically, the endpoint of post-transplant diabetes mellitus was evaluated only by observational studies, judged to be at moderate to serious risk of bias. Both methodological and statistical heterogeneity were noted, while the 95% CI and prediction intervals were wide, including both negative and positive effects.
Hip fracture
The endpoint of hip fracture was examined in 2 studies (1 randomized controlled trial and 1 nested case-control study). No significant association was observed between the administration of statins and hip fracture after kidney transplantation (RR: 0.90, 95% CI: 0.68 to 1.20, 15,846 participants). No statistical heterogeneity was noted (I2: 0%). Subgroup analysis indicated no significant effects of sample size or study design (Table 4). The certainty of evidence was appraised as low, downgrading for study limitations (2 studies at a moderate risk of bias) and imprecision (wide 95% CI).
Cataract, venous thromboembolism and cancer
The endpoint of cataract was assessed in 1 retrospective cohort study [52]. Cataract was identified through Medicare Parts A and B claims (ICD-9: 366.1x, 366.3x, 366.4x, 366.5x, 366.8, 366.9 or ICD-10: H25.x, H26.2x, H26.3x, H26.4x, H26.8, H26.9), which indicated that the use of statins may be significantly associated with cataract risk after kidney transplantation (RR: 1.22, 95% CI: 1.14 to 1.31, 57,699 participants). In particular, the estimated incidence of cataract was 22% at 5 years following statin use compared to 12% among statin non-users. The association remained statistically significant only among tacrolimus users (RR: 1.22, 95% CI: 1.14 to 1.31) (Table 3). The certainty of evidence was evaluated to be moderate, due to concerns about study limitations, as the endpoint was only evaluated by 1 observational study at a moderate risk of bias. One retrospective study [50] examined the risk of venous thromboembolism, and suggested no statistically significant association with the use of statins (RR: 0.92, 95% CI: 0.39 to 2.19, 1384 participants). Specifically, within 1 year, a venous thromboembolic event occurred in 9 out of 223 statin users and in 44 out of 1161 statin non-users. All patients were treated with tacrolimus. The certainty of evidence was judged as low, due to concerns of study limitations (1 observational study at a moderate risk of bias) and imprecision (wide 95% CI). Cancer risk was investigated in the ALERT trial [58], which indicated no statistically significant association with statin administration (RR: 0.94, 95% CI: 0.82 to 1.07, 2102 participants). In particular, malignancies were detected in 296 out of 1045 statin users compared to 316 out of 1049 patients treated with placebo. All kidney transplant recipients were treated with cyclosporine. The certainty of evidence was evaluated as moderate, downgrading for imprecision, as reflected by the width of the 95% CI.
Discussion
Based on 27 studies, the present systematic review and meta-analysis collected the available literature and evaluated the efficacy and safety of statins in kidney transplant recipients. Current evidence suggests that statin therapy may be associated with a cardiovascular benefit, reflected by the significant reduction in MACE risk among statin-treated patients. Statin use after transplantation was also linked to a significant improvement in overall survival, although graft survival may not be affected. The safety profile of statins appeared to be acceptable since no increased risk of severe adverse effects, especially liver toxicity, was detected. The rate of creatine kinase elevation was not associated with statin administration, although a minor increase in the risk of rhabdomyolysis was observed among statin-treated kidney transplant recipients.
The benefits of statins in the prevention of cardiovascular events after kidney transplantation may be mediated by their lipid-lowering action in conjunction with potential pleiotropic effects. A recent meta-analysis confirmed that statin therapy may effectively enhance the lipid profile of kidney transplant recipients by significantly decreasing serum triglyceride and low-density lipoprotein cholesterol levels [71]. Statin administration in kidney transplant recipients has been proposed to improve endothelial function not only through blood lipid reduction but also via an increase in nitric oxide bioavailability [72]. In addition, experimental data have shown that after kidney transplantation, statins may reduce the generation of reactive oxygen species, exerting antioxidant effects, as reflected by increased levels of glutathione peroxidase [73]. The beneficial impact of statins on cardiovascular health after kidney transplantation may also be promoted by their anti-inflammatory properties, resulting in lower levels of peptides involved in vascular inflammation [74].
Despite the cardiovascular benefits of statins, hesitancy in their prescription may arise due to concerns about possible interactions with calcineurin inhibitors that may increase the risk of toxicity. Specifically, most statins are metabolized by cytochrome P450 3A4, which is known to be inhibited by cyclosporine [75]. Moreover, cyclosporine may inhibit the hepatocellular uptake of statins via P-glycoprotein and organic anion-transporting polypeptides (OATP1B1), leading to increased statin exposure [76]. However, the present study raised no concerns about liver damage, as statin therapy was even linked to a significantly lower risk of hepatotoxicity. As in the general population, statin prescription may be limited by concerns about temporary transaminasemia, although the incidence of idiosyncratic drug-induced liver injury is low (1.2/100,000 statin users) [77]. The outcome of the present meta-analysis is in accordance with recent research evidence supporting the potential protective effects of statin against liver disease [78]. In particular, the possible hepatoprotective properties of statins are based on the inhibition of the prenylation of small guanosine triphosphate hydrolases, leading to reduced inflammation and oxidative stress [79].
On the other hand, rhabdomyolysis was more common among statin-treated kidney transplant recipients, although the increase in the risk was comparable to that estimated in studies conducted in the general population [80]. It should also be noted that the link between statin therapy and elevated rhabdomyolysis risk was based on the retrospective study of Bae et al [52]. , in which simvastatin, a statin that has been associated with the highest rhabdomyolysis risk in pharmacovigilance studies, was used by the majority of patients [81].
Remarkable interstudy heterogeneity exists concerning the risk of post-transplant diabetes mellitus. Studies in the general population have suggested a modest increase in impaired glucose metabolism among statin-treated individuals [82], which is based on the statin-induced increase in pancreatic low-density lipoprotein receptor expression, leading to beta-cell lipotoxicity and reduced insulin secretion [83]. In this context, Szili-Torok et al [49]. have indicated that the majority (73.3%) of post-transplant diabetes cases occurred among statin-treated patients, while Bae et al [52]. reported an increase in posttransplant diabetes risk of 13% among statin users receiving tacrolimus as maintenance immunosuppression. In contrast, the opposite outcome was suggested by the study of Prasad et al. [65] although baseline differences in the type of calcineurin inhibitor between the compared groups may have confounded the estimated effects. As a result, further evidence is needed to reach firm conclusions regarding the true impact of statin therapy on diabetes mellitus risk, depending on maintenance immunosuppression and comorbidities.
Current evidence concerning cataract comes from 1 retrospective study, which revealed that the risk of cataract increased by 22% among statin-treated kidney transplant recipients. A similar outcome has been estimated in the general population [84], while animal studies have confirmed the dose-dependent cataractogenic effects of statins [85]. It has been hypothesized that the statin-induced inhibition of cholesterol biosynthesis may affect the development of the lens epithelium, which requires a high cholesterol content to preserve its transparency [86]. Consequently, cataract may be considered as an adverse effect that should be expected but may not limit the prescription of statins in kidney transplant recipients.
Evidence regarding statin type, intensity and dosing after kidney transplantation remains limited. According to the KDIGO guidelines, all kidney transplant recipients should be evaluated for statin treatment, although follow-up blood lipid measurements are discouraged. As a result, a “fire and forget” strategy is recommended and no specific low-density lipoprotein cholesterol goals have been established [13]. A retrospective cohort study has indicated that the use of higher-intensity statins before kidney transplantation is associated with the greatest posttransplant survival benefit [36]. However, given the increased risk of adverse events among kidney transplant recipients and the lack of adequate safety data, lower statin doses are recommended. Specifically, for patients with eGFR below 60 ml/min/1.73 m2, an equivalent atorvastatin dose of 20 mg is suggested [13]. The choice of statin type may be influenced by concerns about potential drug interactions. In this context, fluvastatin has been widely studied in the kidney transplant population due to the lack of significant interaction with cyclosporine. Specifically, fluvastatin is not a P-glycoprotein substrate, while it is metabolized not only by P450 3A4, but also by multiple cytochrome enzymes, such as 2C9 and 2C8 [87]. On the other hand, different pharmacokinetics are anticipated in patients receiving maintenance immunosuppression with cyclosporine and tacrolimus. In particular, tacrolimus has been proposed to have weaker inhibitory effects against cytochrome P450 and P-glycoprotein activity [88] and thus its combination with atorvastatin has been shown to be both safe and efficacious [89].
Strengths and limitations
The present study has several strengths. A comprehensive literature search with strict selection criteria was applied, limiting the possibility of any article loss. Inter-study heterogeneity was both quantified and explored, using various subgroup analyses. The risk of bias in the included studies has been evaluated using validated tools, while the certainty of evidence per outcome has been critically appraised, allowing a realistic assessment of existing data in the field. Downgrading of the quality of evidence occurred mainly due to study limitations since well-designed randomized controlled trials are currently limited and data are mainly derived from small trials with short follow-up periods or cohort studies prone to selection biases and residual confounding. Although meta-regression analysis was performed to assess the effects of potentially important covariates, it should be acknowledged that ecological fallacy may complicate the interpretability of meta-regression analyses examining individual patient-level characteristics that were reported as aggregate data in the original studies. Meta-regression analysis should be also interpreted with caution due to missing data and the small number of included studies. Heterogeneity may have also arisen from differenced in the definitions of endpoints across the included studies, especially concerning the definition of MACE. It should be also highlighted that the outcomes of some serious adverse effects, such as cataract, venous thromboembolism and cancer were only evaluated by one study and thus further validation is necessary. Furthermore, limited evidence was available regarding the potential differential statin effects among cyclosporine or tacrolimus users, while only few patients were treated with mTOR inhibitors. It is also important to note that statin dosing was not consistently reported in the included studies and thus the optimal prescriptions strategy remains unclear.
Implication for current clinical practice and future research
This meta-analysis suggests that clinicians should be encouraged to evaluate all kidney transplant recipients for statin therapy, given the potentially important clinical benefits in regards to cardiovascular risk reduction and improvement of overall survival. Nonetheless, statin prescription requires individualization in order to minimize the risk of toxicity and drug-drug interactions. Therefore, further large-scale research is needed to enable decision-making concerning statin intensity, dosing and treatment goals. In particular, it remains to be clarified whether the administration of high-intensity statins may be associated with more pronounced clinical benefits in terms of cardiovascular disease and patient survival, without increasing significantly the risk of adverse events. In addition, since most clinical trials have included patients receiving cyclosporin, future studies should focus on tacrolimus-treated kidney transplant recipients, aiming to shed more light on the actual risk of statin interaction with tacrolimus blood levels. This would also enable the drawing of clinical decisions about how early statin therapy could be initiated following kidney transplantation, especially in patients requiring statins for secondary cardiovascular prevention. The clinical utility of follow-up low-density lipoprotein cholesterol is currently unknown in the kidney transplant population and thus further high-quality evidence is needed to clarify whether the attainment of specific lipid targets exerts significant prognostic effects.
Conclusions
Among kidney transplant recipients, statin therapy is associated with significant benefits in terms of cardiovascular event reduction and survival improvement. Statin administration is well-tolerated, being associated with minor increases in the risk of rhabdomyolysis and cataract. Further research in large scale is needed to establish the favorable cardiovascular effects of statins and determine the subpopulation of kidney transplant recipients that may be safely targeted for higher-intensity statin treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
None.
Author contributions
Conception and design: I. Bellos. Analysis and interpretation of the data: I. Bellos, V. Benetou. Drafting of the article: I. Bellos, V. Benetou. Critical revision of the article for important intellectual content: P. Lagiou, S. Marinaki. Final approval of the article: I. Bellos, V. Benetou, P. Lagiou, S. Marinaki. Statistical expertise: I. Bellos, V. Benetou. Collection and assembly of data: I. Bellos, V. Benetou, S. Marinaki.
Funding
None.
Data availability
The data extracted from the original studies are available in the supplementary appendix. All data are available from the corresponding author upon request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vassiliki Benetou and Smaragdi Marinaki contributed equally to this work.
References
- 1.Andersson C, Hansen D, Sørensen SS, McGrath M, McCausland FR, Torp-Pedersen C, et al. Long-term cardiovascular events, graft failure, and mortality in kidney transplant recipients. Eur J Intern Med Elsevier. 2024;121:109–13. 10.1016/j.ejim.2023.10.026 [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK. Long-term survival in renal transplant recipients with graft function. Kidney Int [Internet]. Kidney Int; 2000 [cited 2024 Jun 10];57:307–13. https://pubmed.ncbi.nlm.nih.gov/10620213/ [DOI] [PubMed]
- 3.Mathur AK, Chang YH, Steidley DE, Heilman R, Khurmi N, Wasif N et al. Patterns of Care and Outcomes in Cardiovascular Disease After Kidney Transplantation in the United States. Transplant Direct [Internet]. Wolters Kluwer Health; 2017 [cited 2024 Jun 10];3:E126. https://www.pmc/articles/PMC5367743/ [DOI] [PMC free article] [PubMed]
- 4.Birdwell KA, Park M. Post-Transplant Cardiovascular Disease. Clin J Am Soc Nephrol [Internet]. American Society of Nephrology; 2021 [cited 2024 Jun 10];16:1878. https://www.pmc/articles/PMC8729500/ [DOI] [PMC free article] [PubMed]
- 5.Rangaswami J, Mathew RO, Parasuraman R, Tantisattamo E, Lubetzky M, Rao S et al. Cardiovascular disease in the kidney transplant recipient: epidemiology, diagnosis and management strategies. Nephrol Dial Transplant [Internet]. Nephrol Dial Transplant; 2019 [cited 2024 Jun 10];34:760–73. https://pubmed.ncbi.nlm.nih.gov/30984976/ [DOI] [PubMed]
- 6.Rossignol P, Agarwal R, Canaud B, Charney A, Chatellier G, Craig JC et al. Cardiovascular outcome trials in patients with chronic kidney disease: challenges associated with selection of patients and endpoints. Eur Heart J [Internet]. Oxford University Press; 2019 [cited 2024 Jun 10];40:880. Available from: /pmc/articles/PMC6657268/. [DOI] [PMC free article] [PubMed]
- 7.Chmielnicka K, Heleniak Z, Dębska-Ślizień A. Dyslipidemia in Renal Transplant Recipients. Transplantology. 2022, Vol 3, Pages 188–199 [Internet]. Multidisciplinary Digital Publishing Institute; 2022 [cited 2024 Jun 10];3:188–99. https://www.mdpi.com/2673-3943/3/2/20/htm
- 8.Kockx M, Kritharides L. Cyclosporin A-Induced Dyslipidemia and LDL Receptors. Mol Nutr Fats. Academic Press; 2019;323–33.
- 9.Holdaas H, Potena L, Saliba F. mTOR inhibitors and dyslipidemia in transplant recipients: a cause for concern? Transplant Rev. Volume 29. W.B. Saunders; 2015. pp. 93–102. [DOI] [PubMed]
- 10.Hoogeveen RC, Ballantyne CM, Pownall HJ, Opekun AR, Hachey DL, Jaffe JS et al. Effect of sirolimus on the metabolism of apoB100- containing lipoproteins in renal transplant patients. Transplantation [Internet]. Transplantation; 2001 [cited 2024 Jun 10];72:1244–50. https://pubmed.ncbi.nlm.nih.gov/11602850/ [DOI] [PubMed]
- 11.Arabi Z, Tawhari M, Alghamdi AA, Alnasrullah A. Lipid Management in Kidney Transplant Recipients Per KDIGO and American Heart Association Guidelines: A Single-Center Experience. Saudi J Med Med Sci [Internet]. Wolters Kluwer -- Medknow Publications; 2024 [cited 2024 Jun 10];12:47. https://www.pmc/articles/PMC10866382/ [DOI] [PMC free article] [PubMed]
- 12.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis Elsevier. 2009;203:325–30. 10.1016/j.atherosclerosis.2008.08.022 [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Tonelli M. KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int [Internet]. Kidney Int; 2014 [cited 2024 Jun 10];85:1303–9. https://pubmed.ncbi.nlm.nih.gov/24552851/ [DOI] [PubMed]
- 14.Hwang SD, Lee JH, Jhee JH, Kim YJ, Park KM, Kim JK et al. Effect of Fluvastatin on Cardiovascular Complications in Kidney Transplant Patients: A Systemic Review and Meta-analysis. Transplant Proc [Internet]. Transplant Proc; 2019 [cited 2024 Aug 14];51:2710–3. https://pubmed.ncbi.nlm.nih.gov/31447193/ [DOI] [PubMed]
- 15.Ghayda RA, Lee JY, Yang JW, Han CH, Jeong GH, Yoon S et al. The effect of statins on all-cause and cardiovascular mortality in patients with non-dialysis chronic kidney disease, patients on dialysis, and kidney transplanted recipients: an umbrella review of meta-analyses. Eur Rev Med Pharmacol Sci [Internet]. Eur Rev Med Pharmacol Sci; 2021 [cited 2024 Aug 14];25:2696–710. https://pubmed.ncbi.nlm.nih.gov/33829456/ [DOI] [PubMed]
- 16.Rostami Z, Arani M, Salesi M, Safiabadi M, Einollahi B. Effect of statins on patients and graft survival in kidney transplant recipients: a Survival Meta-analysis. Iran J Kidney Dis. 2017;11:329–38. [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ [Internet]. British Medical Journal Publishing Group; 2021 [cited 2021 Jul 20];372. https://www.bmj.com/content/372/bmj.n71 [DOI] [PMC free article] [PubMed]
- 18.Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. BMJ [Internet]. BMJ Publishing Group; 2005 [cited 2020 Feb 28];331:1064–5. http://www.ncbi.nlm.nih.gov/pubmed/16230312 [DOI] [PMC free article] [PubMed]
- 19.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ [Internet]. BMJ Publishing Group; 2019 [cited 2021 Apr 22];366. 10.1136/bmj.l4898http://www.bmj.com/ [DOI] [PubMed]
- 20.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ [Internet]. British Medical Journal Publishing Group; 2016 [cited 2019 Sep 26];355:i4919. http://www.ncbi.nlm.nih.gov/pubmed/27733354 [DOI] [PMC free article] [PubMed]
- 21.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods [Internet]. John Wiley & Sons, Ltd; 2016 [cited 2021 Aug 30];7:55–79. https://onlinelibrary.wiley.com/doi/full/10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed]
- 22.Higgins JPT, Thompson SG, Deeks JJ, Altman DG, Publishing Group. Measuring inconsistency in meta-analyses. BMJ [Internet]. BMJ ; 2003 [cited 2018 Aug 5];327:557–60. http://www.ncbi.nlm.nih.gov/pubmed/12958120 [DOI] [PMC free article] [PubMed]
- 23.Ioannidis JPA, Trikalinos TA. The appropriateness of asymmetry tests for publication bias in meta-analyses: a large survey. CMAJ [Internet]. 2007 [cited 2018 Aug 1];176:1091–6. http://www.cmaj.ca/cgi/doi/10.1503/cmaj.060410 [DOI] [PMC free article] [PubMed]
- 24.Shi L, Lin L. The trim-and-fill method for publication bias. Medicine (Baltimore) [Internet]. 2019 [cited 2020 Jan 21];98:e15987. http://insights.ovid.com/crossref?an=00005792-201906070-00070 [DOI] [PMC free article] [PubMed]
- 25.Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Softw [Internet]. 2010 [cited 2018 Aug 21];36:1–48. http://www.jstatsoft.org/v36/i03/
- 26.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol [Internet]. Elsevier; 2011 [cited 2021 Jul 20];64:383–94. http://www.jclinepi.com/article/S0895435610003306/fulltext [DOI] [PubMed]
- 27.Heleniak ZT, Illersperger S, Brakemeier S, Dębska-ślizień A, Bach P, Budde K et al. Influence of lipid profile and statin administration on arterial stiffness in renal transplant recipients. Cardiol J [Internet]. Cardiol J; 2022 [cited 2024 Jun 12];29:263–71. https://pubmed.ncbi.nlm.nih.gov/32329037/ [DOI] [PMC free article] [PubMed]
- 28.Baigent C, Landray M, Leaper C, Altmann P, Armitage J, Baxter A et al. First United Kingdom Heart and Renal Protection (UK-HARP-I) study: Biochemical efficacy and safety of simvastatin and safety of low-dose aspirin in chronic kidney disease. Am J Kidney Dis [Internet]. W.B. Saunders; 2005 [cited 2024 Jun 12];45:473–84. https://pubmed.ncbi.nlm.nih.gov/15754269/ [DOI] [PubMed]
- 29.Holdaas H, Fellström B, Jardine AG, Nyberg G, Grönhagen-Riska C, Madsen S et al. Beneficial effect of early initiation of lipid-lowering therapy following renal transplantation. Nephrol Dial Transplant [Internet]. Nephrol Dial Transplant; 2005 [cited 2024 Jun 12];20:974–80. https://pubmed.ncbi.nlm.nih.gov/15784644/ [DOI] [PubMed]
- 30.Imamura R, Ichimaru N, Moriyama T, Shi Y, Namba Y, Nonomura N et al. Long term efficacy of simvastatin in renal transplant recipients treated with cyclosporine or tacrolimus. Clin Transplant [Internet]. Clin Transplant; 2005 [cited 2024 Jun 12];19:616–21. https://pubmed.ncbi.nlm.nih.gov/16146552/ [DOI] [PubMed]
- 31.Cofan F, Zambon D, Laguna JC, Casals E, Ros E, Cofan M et al. Pravastatin improves low-density lipoprotein oxidation in renal transplantation. Transplant Proc [Internet]. Transplant Proc; 2002 [cited 2024 Jun 12];34:389–91. https://pubmed.ncbi.nlm.nih.gov/11959339/ [DOI] [PubMed]
- 32.Ichimaru N, Takahara S, Kokado Y, Wang JD, Hatori M, Kameoka H et al. Changes in lipid metabolism and effect of simvastatin in renal transplant recipients induced by cyclosporine or tacrolimus. Atherosclerosis [Internet]. Atherosclerosis; 2001 [cited 2024 Jun 12];158:417–23. https://pubmed.ncbi.nlm.nih.gov/11583721/ [DOI] [PubMed]
- 33.Li PKT, Mak TWL, Chan TH, Wang A, Lam CWK, Lai KN. Effect of fluvastatin on lipoprotein profiles in treating renal transplant recipients with dyslipoproteinemia. Transplantation [Internet]. Transplantation; 1995 [cited 2024 Jun 12];60:652–6. https://pubmed.ncbi.nlm.nih.gov/7570971/ [DOI] [PubMed]
- 34.Martinez Hernandez B, Persaud J, Varghese Z, Moorhead J. Low-dose simvastatin is safe in hyperlipidaemic renal transplant patients. Nephrol Dial Transpl. 1993;8:637–41. [PubMed] [Google Scholar]
- 35.Tuncer M, Süleymanlar G, Ersoy FF, Yakupoǧlu G. Comparison of the effects of simvastatin and pravastatin on acute rejection episodes in renal transplant patients. Transplant Proc [Internet]. Transplant Proc; 2000 [cited 2024 Jun 12];32:622–5. https://pubmed.ncbi.nlm.nih.gov/10812143/ [DOI] [PubMed]
- 36.Co MLF, Agdamag AC, Co MZ, Hertl M, Mohamedali B. Intensity-Dependent Benefit of Statins in Survival Among Prospective Kidney Transplant Patients. Am J Cardiol [Internet]. Am J Cardiol; 2019 [cited 2024 Jun 12];123:254–9. https://pubmed.ncbi.nlm.nih.gov/30442361/ [DOI] [PubMed]
- 37.Yazbek DC, De Carvalho AB, Barros CS, Pestana JOM, Canziani MEF. Effect of Statins on the Progression of Coronary Calcification in Kidney Transplant Recipients. PLoS One [Internet]. PLOS; 2016 [cited 2024 Jun 12];11. https://www.pmc/articles/PMC4839705/ [DOI] [PMC free article] [PubMed]
- 38.Amirzargar MA, Hosseini AT, afresh., Gholiaf M, Dadras F, Khoshjoo F. Long-term efficacy of atorvastatin in allograft rejection following renal transplantation: A randomized clinical trial. Saudi J Kidney Dis Transpl [Internet]. Saudi J Kidney Dis Transpl; 2015 [cited 2024 Jun 12];26:953–7. https://pubmed.ncbi.nlm.nih.gov/26354567/ [DOI] [PubMed]
- 39.Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A et al. Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol [Internet]. J Am Soc Nephrol; 2014 [cited 2024 Jun 12];25:1825–33. https://pubmed.ncbi.nlm.nih.gov/24790178/ [DOI] [PMC free article] [PubMed]
- 40.Hariparshad SP, Madala ND, Assounga AGH. Statin therapy ameliorates renal allograft function. Transplant Proc [Internet]. Transplant Proc; 2009 [cited 2024 Jun 12];41:4178–80. https://pubmed.ncbi.nlm.nih.gov/20005363/ [DOI] [PubMed]
- 41.Norby GE, Holme I, Fellström B, Jardine A, Cole E, Abedini S et al. Effect of fluvastatin on cardiac outcomes in kidney transplant patients with systemic lupus erythematosus: a randomized placebo-controlled study. Arthritis Rheum [Internet]. Arthritis Rheum; 2009 [cited 2024 Jun 12];60:1060–4. https://pubmed.ncbi.nlm.nih.gov/19333947/ [DOI] [PubMed]
- 42.Soveri I, Abedini S, Holdaas H, Jardine A, Eriksson N, Fellström B. Metabolic syndrome and cardiovascular risk in renal transplant recipients: effects of statin treatment. Clin Transplant [Internet]. Clin Transplant; 2009 [cited 2024 Jun 12];23:914–20. https://pubmed.ncbi.nlm.nih.gov/19594771/ [DOI] [PubMed]
- 43.Pazik J, Ostrowska J, Lewandowski Z, Mróz A, Perkowska-Ptasińska A, Baczkowska T, et al. Renin-angiotensin-aldosterone system inhibitors and statins prolong graft survival in post-transplant glomerulonephritis. Ann Transpl. 2008;13:41–5. [PubMed] [Google Scholar]
- 44.Katznelson S, Wilkinson AH, Kobashigawa JA, Wang XM, Chia D, Ozawa M et al. The effect of pravastatin on acute rejection after kidney transplantation–a pilot study. Transplantation [Internet]. Transplantation; 1996 [cited 2024 Jun 12];61:1469–74. https://pubmed.ncbi.nlm.nih.gov/8633373/ [DOI] [PubMed]
- 45.Lepre F, Rigby R, Hawley C, Saltissi D, Brown A, Walsh ZA. A double-blind placebo controlled trial of simvastatin for the treatment of dyslipidaemia in renal allograft recipients. Clin Transplant [Internet]. Clin Transplant; 1999 [cited 2024 Jun 12];13:520–5. https://pubmed.ncbi.nlm.nih.gov/10617243/ [DOI] [PubMed]
- 46.Choe EY, Wang HJ, Kwon O, Cho Y, Huh KH, Kim MS et al. HMG CoA reductase inhibitor treatment induces dysglycemia in renal allograft recipients. Transplantation [Internet]. Transplantation; 2014 [cited 2024 Jun 12];97:419–25. https://pubmed.ncbi.nlm.nih.gov/24285338/ [DOI] [PubMed]
- 47.Vangala C, Lenihan CR, Montez-Rath ME, Nair SS, Navaneethan SD, Ramanathan V et al. Statin use and hip fractures in U.S. kidney transplant recipients. BMC Nephrol [Internet]. BMC Nephrol; 2017 [cited 2024 Jun 12];18. https://pubmed.ncbi.nlm.nih.gov/28460645/ [DOI] [PMC free article] [PubMed]
- 48.Han N, Han SH, Song YK, Kim MG, Kim YS, Kim IW et al. Statin therapy for preventing cardiovascular diseases in patients treated with tacrolimus after kidney transplantation. Ther Clin Risk Manag [Internet]. Ther Clin Risk Manag; 2017 [cited 2024 Jun 12];13:1513–20. https://pubmed.ncbi.nlm.nih.gov/29200861/ [DOI] [PMC free article] [PubMed]
- 49.Szili-Torok T, Bakker SJL, Tietge UJF. Statin Use Is Prospectively Associated With New-Onset Diabetes After Transplantation in Renal Transplant Recipients. Diabetes Care [Internet]. Diabetes Care; 2020 [cited 2024 Jun 12];43:1945–7. https://pubmed.ncbi.nlm.nih.gov/32444455/ [DOI] [PubMed]
- 50.Frasco PE, Rosenfeld DM, Jadlowiec CC, Zhang N, Heilman RL, Bauer IL et al. Postoperative statin therapy is not associated with reduced incidence of venous thromboembolic events following kidney transplantation. Clin Transplant [Internet]. Clin Transplant; 2022 [cited 2024 Jun 12];36. https://pubmed.ncbi.nlm.nih.gov/36065684/ [DOI] [PubMed]
- 51.Anderson JLC, van der Giet M, Gomes Neto AW, Bakker SJL, Tietge UJF. Statin use and incident cardiovascular events in renal transplant recipients. Eur J Clin Invest [Internet]. Eur J Clin Invest; 2021 [cited 2024 Jun 12];51. https://pubmed.ncbi.nlm.nih.gov/34042174/ [DOI] [PMC free article] [PubMed]
- 52.Bae S, Ahn JB, Joseph C, Whisler R, Schnitzler MA, Lentine KL et al. Incidence of Statin-Associated Adverse Events in Kidney Transplant Recipients. Clin J Am Soc Nephrol [Internet]. Clin J Am Soc Nephrol; 2023 [cited 2024 Jun 12];18:626–33. https://pubmed.ncbi.nlm.nih.gov/36800538/ [DOI] [PMC free article] [PubMed]
- 53.4 S, Ahn JB, Joseph C, Whisler R, Schnitzler MA, Lentine KL et al. Statins in Kidney Transplant Recipients: Usage, All-Cause Mortality, and Interactions with Maintenance Immunosuppressive Agents. J Am Soc Nephrol [Internet]. J Am Soc Nephrol; 2023 [cited 2024 Jun 12];34:1069–77. https://pubmed.ncbi.nlm.nih.gov/36890643/ [DOI] [PMC free article] [PubMed]
- 54.Nazoiri C, Liabeuf S, Brazier F, Nowak A, Bennis Y, Laville SM et al. Statin therapy and the incidence of atherosclerotic cardiovascular events after kidney transplantation. Nephrol Dial Transplant [Internet]. Nephrol Dial Transplant; 2024 [cited 2024 Jun 12];39:818–29. https://pubmed.ncbi.nlm.nih.gov/37791395/ [DOI] [PubMed]
- 55.Yim SH, Kim HJ, Ro H, Ryu JH, Kim MG, Park JB et al. Benefits of statin therapy within a year after kidney transplantation. Sci Rep [Internet]. Sci Rep; 2024 [cited 2024 Jun 12];14. https://pubmed.ncbi.nlm.nih.gov/38263253/ [DOI] [PMC free article] [PubMed]
- 56.Holdaas H, Fellström B, Jardine AG, Holme I, Nyberg G, Fauchald P et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet (London, England) [Internet]. Lancet; 2003 [cited 2024 Jun 12];361:2024–31. https://pubmed.ncbi.nlm.nih.gov/12814712/ [DOI] [PubMed]
- 57.Holdaas H, Jardine AG, Wheeler DC, Brekke IB, Conlon PJ, Fellstrøm B et al. Effect of fluvastatin on acute renal allograft rejection: a randomized multicenter trial. Kidney Int [Internet]. Kidney Int; 2001 [cited 2024 Jun 12];60:1990–7. https://pubmed.ncbi.nlm.nih.gov/11703619/ [DOI] [PubMed]
- 58.Holdaas H, Fellström B, Cole E, Nyberg G, Olsson AG, Pedersen TR et al. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant [Internet]. Am J Transplant; 2005 [cited 2024 Jun 12];5:2929–36. https://pubmed.ncbi.nlm.nih.gov/16303007/ [DOI] [PubMed]
- 59.Kasiske BL, Heim-Duthoy KL, Singer GG, Watschinger B, Germain MJ, Bastani B. The effects of lipid-lowering agents on acute renal allograft rejection. Transplantation [Internet]. Transplantation; 2001 [cited 2024 Jun 12];72:223–7. https://pubmed.ncbi.nlm.nih.gov/11477342/ [DOI] [PubMed]
- 60.Santos A, Keitel E, Bittar A, Neumann J, Fuchs F, Goldani J et al. Safety and efficacy of simvastatin for hyperlipidemia in renal transplant recipients: a double-blind, randomized, placebo-controlled study. Transplant Proc [Internet]. Transplant Proc; 2001 [cited 2024 Jun 12];33:1194–5. https://pubmed.ncbi.nlm.nih.gov/11267254/ [DOI] [PubMed]
- 61.Arnadottir M, Eriksson LO, Germerhausen JI, Thysell H. Low-dose simvastatin is a well-tolerated and efficacious cholesterol-lowering agent in ciclosporin-treated kidney transplant recipients: double-blind, randomized, placebo-controlled study in 40 patients. Nephron [Internet]. Nephron; 1994 [cited 2024 Jun 12];68:57–62. https://pubmed.ncbi.nlm.nih.gov/7991041/ [DOI] [PubMed]
- 62.Moreso F, Calvo N, Pascual J, Anaya F, Jiménez C, Del Castillo D et al. Early statin use is an independent predictor of long-term graft survival. NDT Plus [Internet]. NDT Plus; 2010 [cited 2024 Jun 12];3. https://pubmed.ncbi.nlm.nih.gov/20508861/ [DOI] [PMC free article] [PubMed]
- 63.Cosio FG, Pesavento TE, Pelletier RP, Henry M, Ferguson RM, Kim S et al. Patient survival after renal transplantation III: the effects of statins. Am J Kidney Dis [Internet]. Am J Kidney Dis; 2002 [cited 2024 Jun 12];40:638–43. https://pubmed.ncbi.nlm.nih.gov/12200817/ [DOI] [PubMed]
- 64.Fellström B, Holdaas H, Jardine AG, Holme I, Nyberg G, Fauchald P et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney Int [Internet]. Kidney Int; 2004 [cited 2024 Jun 12];66:1549–55. https://pubmed.ncbi.nlm.nih.gov/15458450/ [DOI] [PubMed]
- 65.Prasad GVR, Kim SJ, Huang M, Nash MM, Zaltzman JS, Fenton SSA et al. Reduced incidence of new-onset diabetes mellitus after renal transplantation with 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors (statins). Am J Transplant [Internet]. Am J Transplant; 2004 [cited 2024 Jun 12];4:1897–903. https://pubmed.ncbi.nlm.nih.gov/15476492/ [DOI] [PubMed]
- 66.Lopau K, Spindler K, Wanner C. Effects of pravastatin treatment on blood pressure regulation after renal transplantation. Kidney Blood Press Res [Internet]. Kidney Blood Press Res; 2006 [cited 2024 Jun 12];29:329–37. https://pubmed.ncbi.nlm.nih.gov/17124431/ [DOI] [PubMed]
- 67.Lisik W, Schoenberg L, Lasky RE, Kahan BD. Statins benefit outcomes of renal transplant recipients on a sirolimus-cyclosporine regimen. Transplant Proc [Internet]. Transplant Proc; 2007 [cited 2024 Jun 12];39:3086–92. https://pubmed.ncbi.nlm.nih.gov/18089328/ [DOI] [PubMed]
- 68.Serón D, Oppenheimer F, Pallardó LM, Lauzurica R, Errasti P, Gomez-Huertas E et al. Fluvastatin in the prevention of renal transplant vasculopathy: results of a prospective, randomized, double-blind, placebo-controlled trial. Transplantation [Internet]. Transplantation; 2008 [cited 2024 Jun 12];86:82–7. https://pubmed.ncbi.nlm.nih.gov/18622282/ [DOI] [PubMed]
- 69.Wiesbauer F, Heinze G, Mitterbauer C, Harnoncourt F, Hörl WH, Oberbauer R. Statin use is associated with prolonged survival of renal transplant recipients. J Am Soc Nephrol [Internet]. J Am Soc Nephrol; 2008 [cited 2024 Jun 12];19:2211–8. https://pubmed.ncbi.nlm.nih.gov/18650477/ [DOI] [PMC free article] [PubMed]
- 70.Younas N, Wu CM, Shapiro R, McCauley J, Johnston J, Tan H et al. HMG-CoA reductase inhibitors in kidney transplant recipients receiving tacrolimus: statins not associated with improved patient or graft survival. BMC Nephrol [Internet]. BMC Nephrol; 2010 [cited 2024 Jun 12];11. https://pubmed.ncbi.nlm.nih.gov/20359353/ [DOI] [PMC free article] [PubMed]
- 71.Huang X, Jia Y, Zhu X, Zhang Y, Jiang L, Wei X et al. Effects of Statins on Lipid Profile of Kidney Transplant Recipients: A Meta-Analysis of Randomized Controlled Trials. Biomed Res Int [Internet]. Hindawi Limited; 2020 [cited 2024 Jun 11];2020. https://www.pmc/articles/PMC7212277/ [DOI] [PMC free article] [PubMed]
- 72.Åsberg A, Hartmann A, Fjeldså E, Holdaas H. Atorvastatin improves endothelial function in renal-transplant recipients. Nephrol Dial Transplant [Internet]. Nephrol Dial Transplant; 2001 [cited 2024 Jun 11];16:1920–4. https://pubmed.ncbi.nlm.nih.gov/11522880/ [DOI] [PubMed]
- 73.Ruiz MC, Moreno JM, Ruiz N, Vargas F, Asensio C, Osuna A. Effect of statin treatment on oxidative stress and renal function in renal transplantation. Transplant Proc [Internet]. Transplant Proc; 2006 [cited 2024 Jun 11];38:2431–3. https://pubmed.ncbi.nlm.nih.gov/17097958/ [DOI] [PubMed]
- 74.Pérez V, Navarro-Muñoz M, Mas S, Bayés B, Pastor MC, Martínez-Cáceres E et al. Proteomic approach to the study of statin pleiotropy in kidney transplant patients. Pharmacology [Internet]. Pharmacology; 2011 [cited 2024 Jun 11];87:161–8. https://pubmed.ncbi.nlm.nih.gov/21372619/ [DOI] [PubMed]
- 75.Amundsen R, Åsberg A, Ohm IK, Christensen H. Cyclosporine A- and tacrolimus-mediated inhibition of CYP3A4 and CYP3A5 in vitro. Drug Metab Dispos [Internet]. Drug Metab Dispos; 2012 [cited 2024 Aug 10];40:655–61. https://pubmed.ncbi.nlm.nih.gov/22205779/ [DOI] [PubMed]
- 76.Åsberg A. Interactions between cyclosporin and lipid-lowering drugs: implications for organ transplant recipients. Drugs [Internet]. Drugs; 2003 [cited 2024 Jun 11];63:367–78. https://pubmed.ncbi.nlm.nih.gov/12558459/ [DOI] [PubMed]
- 77.Björnsson E, Jacobsen EI, Kalaitzakis E. Hepatotoxicity associated with statins: reports of idiosyncratic liver injury post-marketing. J Hepatol [Internet]. J Hepatol; 2012 [cited 2024 Aug 11];56:374–80. https://pubmed.ncbi.nlm.nih.gov/21889469/ [DOI] [PubMed]
- 78.Vell MS, Loomba R, Krishnan A, Wangensteen KJ, Trebicka J, Creasy KT et al. Association of Statin Use With Risk of Liver Disease, Hepatocellular Carcinoma, and Liver-Related Mortality. JAMA Netw Open [Internet]. American Medical Association; 2023 [cited 2024 Aug 11];6:e2320222–e2320222. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2806370 [DOI] [PMC free article] [PubMed]
- 79.Schierwagen R, Maybüchen L, Hittatiya K, Klein S, Uschner FE, Braga TT et al. Statins improve NASH via inhibition of RhoA and Ras. Am J Physiol Gastrointest Liver Physiol [Internet]. Am J Physiol Gastrointest Liver Physiol; 2016 [cited 2024 Aug 11];311:G724–33. https://pubmed.ncbi.nlm.nih.gov/27634010/ [DOI] [PubMed]
- 80.Hippisley-Cox J, Coupland C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ [Internet]. British Medical Journal Publishing Group; 2010 [cited 2024 Jun 11];340:1232. https://www.bmj.com/content/340/bmj.c2197 [DOI] [PMC free article] [PubMed]
- 81.Montastruc JL. Rhabdomyolysis and statins: A pharmacovigilance comparative study between statins. Br J Clin Pharmacol [Internet]. John Wiley & Sons, Ltd; 2023 [cited 2024 Jun 11];89:2636–8. https://onlinelibrary.wiley.com/doi/full/10.1111/bcp.15757 [DOI] [PubMed]
- 82.Reith C, Preiss D, Blackwell L, Emberson J, Spata E, Davies K et al. Effects of statin therapy on diagnoses of new-onset diabetes and worsening glycaemia in large-scale randomised blinded statin trials: an individual participant data meta-analysis. Lancet Diabetes Endocrinol [Internet]. Elsevier Ltd; 2024 [cited 2024 Jun 11];12:306–19. http://www.thelancet.com/article/S2213858724000408/fulltext [DOI] [PMC free article] [PubMed]
- 83.Laakso M, Fernandes Silva L. Statins and risk of type 2 diabetes: mechanism and clinical implications. Front Endocrinol (Lausanne) [Internet] Front Media SA; 2023 [cited 2024 Jun 11];14. Available from: /pmc/articles/PMC10546337/. [DOI] [PMC free article] [PubMed]
- 84.Yu S, Chu Y, Li G, Ren L, Zhang Q, Wu L. Statin Use and the Risk of Cataracts: A Systematic Review and Meta-Analysis. Wiley; 2017. [cited 2024 Jun 11];6. Available from: /pmc/articles/PMC5523994/. J Am Hear Assoc Cardiovasc Cerebrovasc Dis [Internet]. [DOI] [PMC free article] [PubMed]
- 85.Zakrzewski P, Milewska J, Czerny K. The eye lens evaluation of the atorvastatin-treated white rat. Ann Univ Mariae Curie Sklodowska Med [Internet]. 2002;57:165–71. https://pubmed.ncbi.nlm.nih.gov/12898835/ [PubMed]
- 86.Widomska J, Subczynski WK. Why Is Very High Cholesterol Content Beneficial for the Eye Lens but Negative for Other Organs? Nutrients [Internet]. Nutrients; 2019 [cited 2024 Jun 11];11. https://pubmed.ncbi.nlm.nih.gov/31096723/ [DOI] [PMC free article] [PubMed]
- 87.Jardine A, Holdaas H. Fluvastatin in combination with cyclosporin in renal transplant recipients: a review of clinical and safety experience. J Clin Pharm Ther [Internet]. J Clin Pharm Ther; 1999 [cited 2024 Jun 12];24:397–408. https://pubmed.ncbi.nlm.nih.gov/10651972/ [DOI] [PubMed]
- 88.Migliozzi DR, Asal NJ. Clinical Controversy in Transplantation: Tacrolimus Versus Cyclosporine in Statin Drug Interactions. Ann Pharmacother [Internet]. Ann Pharmacother; 2020 [cited 2024 Jun 12];54:171–7. https://pubmed.ncbi.nlm.nih.gov/31441337/ [DOI] [PubMed]
- 89.Skalicka B, Kubanek M, Malek I, Vymetalova Y, Hoskova L, Podzimkova M et al. Conversion to tacrolimus and atorvastatin in cyclosporine-treated heart transplant recipients with dyslipidemia refractory to fluvastatin. J Heart Lung Transplant [Internet]. J Heart Lung Transplant; 2009 [cited 2024 Jun 12];28:598–604. https://pubmed.ncbi.nlm.nih.gov/19481021/ [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data extracted from the original studies are available in the supplementary appendix. All data are available from the corresponding author upon request.