Abstract
Alterations in proliferation and hypertrophy of renal mesangial cells are typical features of diabetic nephropathy. The HP (hexosamine pathway) has been proposed as a biochemical hypothesis to explain microvascular alterations due to diabetic nephropathy; however, involvement of HP in the regulation of mesangial cell growth or hypertrophy has been poorly studied. Although gangliosides are known to regulate cell proliferation, their potential role in mesangial cell-growth perturbations has hardly been explored. In the present study, we investigated the effects of the HP activation, mimicked by GlcN (glucosamine) treatment, on mesangial cell growth and hypertrophy and the potential implication of gangliosides in these processes. Our results indicate that GlcN induced hypertrophy of mesangial cells, as measured by an increase in the protein/cell ratio, and it caused cell-cycle arrest by an increase in the expression of cyclin-dependent kinase inhibitor p21Waf1/Cip1. Furthermore, GlcN treatment resulted in a massive increase in the levels of gangliosides GM2 and GM1. Treatment of cells with exogenous GM2 and GM1 reproduced the effects of 0.5 mM GlcN on p21Waf1/Cip1 expression, cell-cycle arrest and hypertrophy, suggesting that gangliosides GM2 and GM1 are probably involved in mediating GlcN effects. These results document a new role of the HP in the regulation of mesangial cell growth and hypertrophy. They also suggest a potential new mechanism of action of the HP through modulation of ganglioside levels.
Keywords: diabetic nephropathy, ganglioside, growth arrest, hexosamine pathway, hypertrophy, mesangial cell
Abbreviations: CDKI, cyclin-dependent kinase inhibitor; DN, diabetic nephropathy; GFAT, glutamine:fructose 6-phosphate amidotransferase; GlcN, glucosamine; HP, hexosamine pathway; HPTLC, high-performance TLC; RMC, renal mesangial cells; TGFβ, transforming growth factor β
INTRODUCTION
Diabetic nephropathy (referred to as DN) is the most common cause of end-stage renal disease. DN was found to affect approx. 30% of patients who have suffered from diabetes for 20 years and exposes them to a higher rate of mortality. The pathology is characterized by complex structural changes including renal hypertrophy, basement membrane thickening and progressive accumulation of extracellular matrix components. Combined with haemodynamic disturbances, these changes lead to glomerulosclerosis, microalbuminuria and, finally, to a decrease in renal function [1]. Hypertrophy of mesangial cells and increased secretion of matrix proteins contribute to glomerular enlargement, which is considered as one of the earliest alterations of DN. Mesangial cell growth during the diabetic state consists in an early, transient and limited proliferation, followed by growth arrest and hypertrophy. For example, it has been shown that, in kidneys of streptozotocin-induced diabetic animals, mesangial cells undergo arrest at the G1 phase and undergo sustained hypertrophy associated with an increase in synthesis and deposition of the extracellular matrix proteins [2]. The CDKIs (cyclin-dependent kinase inhibitors) p21Waf1/Cip1 and p27Kip1 have been implicated in this blockade, as shown in mesangial cells treated with high glucose concentrations [3,4]. Increased expression of p21Waf1/Cip1 and p27Kip1 has also been observed in mesangial cells of streptozotocin-induced diabetic rats and in diabetic db/db mice [3,5].
Numerous hypotheses have been proposed to explain glucose toxicity and diabetic microvascular alterations. In 1991, Marshall et al. [6] first reported a new pathway mediating glucose effects, namely the HP (hexosamine pathway) [6]. After entering cells, glucose is rapidly phosphorylated and converted into fructose 6-phosphate, which is mainly catabolized by the glycolytic pathway. Under hyperglycaemic conditions, however, a greater part of fructose 6-phosphate can be converted into GlcN (glucosamine) 6-phosphate by the action of GFAT (glutamine:fructose 6-phosphate amidotransferase), the first and rate-limiting enzyme of the hexosamine biosynthetic pathway. GlcN 6-phosphate is then metabolized into various hexosamine products, including UDP-GlcNAc (UDP-N-acetylglucosamine). Thus the HP exerts its effects by providing glycosidic precursors for glycoproteins, glycolipids and proteoglycans. Additionally, the HP could mediate its effects through a single O-linked glycosylation. In fact, GlcNAc, a product of the HP, can covalently modify and affect the activities of proteins involved in the control of gene expression, cell growth and division, enzyme activity or structural integrity of the cytoskeleton [7,8].
The HP was first implicated in glucose-induced desensitization of the insulin response [6]. In vivo, acute GlcN infusion induces insulin resistance in normoglycaemic rats [9]. Moreover, overexpression of GFAT in mouse skeletal muscles [10] or in β cells [11] has proven the role of the HP in providing insulin resistance to animals and highlighted the importance of this pathway. Recent reports also suggested a role for the HP in the development of diabetic microvascular complications such as nephropathy. Thus the HP has been shown to activate through a mechanism involving the transcription factor Sp1, the promoter of the plasminogen activator inhibitor-1 gene, a regulator of extracellular matrix production [12,13]. Moreover, HP activation has been largely described to induce TGFβ (transforming growth factor β) production and matrix protein synthesis in mesangial cells [14–17]. However, the involvement of the HP in the regulation of mesangial cell growth or hypertrophy has been poorly studied.
Gangliosides are glycosphingolipids characterized by the presence of sialic acid in their oligosaccharidic part. They are concentrated in the plasma membrane with a cell-specific pattern and found in most tissues of the body, especially in brain and nervous tissues [18]. Gangliosides play major roles in cell–cell and cell–matrix recognition through interactions with adhesion receptors such as integrins and matrix proteins (collagen and fibronectin) or with other glycosphingolipids [19]. On the other hand, gangliosides are known to regulate transmembrane signalling by modulating functional membrane proteins. Thus they have been reported to regulate the proliferation of different cell types through the modulation of growth factor receptor-associated kinases such as epidermal growth factor and platelet-derived growth factor receptor [20–23]. Other than these known effects of gangliosides in regulating cell proliferation, their role in mesangial cell-growth perturbations occurring during DN has not been studied so far.
The aim of the present study was to investigate the effect of HP activation, mimicked by GlcN treatment, on mesangial cell growth and hypertrophy, and to determine the potential role of gangliosides in mediating the GlcN effects. Our results show that 0.5 mM GlcN inhibited mesangial cell growth and induced hypertrophy by increasing p21Waf1/Cip1 expression and arresting cells at the G0/G1 phase. Moreover, GlcN treatment significantly increased the levels of GM2 and GM1 gangliosides, and treatment with exogenous GM2 and GM1 reproduced GlcN effects on mesangial cell hypertrophy, cell–cycle arrest at the G0/G1 phase and p21Waf1/Cip1 expression. These results suggest that GlcN induces cell-cycle arrest and hypertrophy of mesangial cells, at least in part, through the production of GM2 and GM1 gangliosides.
EXPERIMENTAL
Materials
Animals were procured from Charles River (L'Arbresle, France) and fetal bovine serum was purchased from Gibco BRL (Invitrogen, New York, NY, U.S.A.). Other cell-culture products, such as RNase and resorcinol, were obtained from Sigma (St Quentin-Fallavier, France). D-[U-14C]galactose and D-[1-14C]GlcN hydrochloride were from Amersham Biosciences (Little Chalfont, Bucks., U.K.). HPTLC (high-performance TLC) plates were bought from Merck (Darmstadt, Germany), C18 silica gel column from Waters (Milford, MA, U.S.A.), gangliosides from Matreya (Biovalley, Marne la Vallée, France), fluorescein-labelled cholera toxin B subunit from Molecular Probes (Leiden, The Netherlands), Nonidet P40 (1%) from Pierce, Perbio Science (Brebières, France) and monoclonal antibodies were from BD Biosciences (Le Pont de Claix, France).
METHODS
Mesangial cell isolation and culture
RMC (renal mesangial cells) were obtained from glomeruli of young male Wistar rat kidneys isolated by differential sieving, as described previously [24]. RMC were characterized by morphological criteria (stellate, spindle-shaped when confluent) and by negative staining for Factor VIII and cytokeratin and their prominent cytoskeletal staining for actin. Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 15% (v/v) fetal bovine serum, 1% glutamine and 1% penicillin/streptomycin and used between passages 7 and 15. Under these conditions, cells reached confluency after approx. 4 days; therefore, treatments were performed for a maximum of 4 days.
Total protein/cell number ratio
RMC were treated with 0.5 or 5 mM GlcN for 1–4 days. At the end of the treatment period, cells were trypsinized and washed twice with ice-cold PBS. For each sample, an aliquot of cells was counted using a haemocytometer to determine the cell number. Another aliquot was lysed and used to measure the total protein content by the Bradford method. The total protein/cell number ratio expressed as μg/105 cells was used as a hypertrophy index.
Cell-cycle analysis
RMC were harvested with trypsin after various treatments, washed twice with PBS, fixed in 70% (v/v) ethanol and stored at 4 °C until use. Before flow-cytometric analysis, cells were washed with PBS, centrifuged and the cell pellets were resuspended in RNase (1 mg/ml) for 20 min. Cells were then stained for 15 min with propidium iodide in PBS (final concentration 40 μg/ml) before analysis with FACSCAN (Becton Dickinson, Franklin Lakes, NJ, U.S.A.) using CellQuest software (Becton Dickinson).
Ganglioside analysis
After 48 h of treatment, RMC (1–4×106 cells) were collected by trypsinization and washed twice with PBS. For metabolic labelling of gangliosides, 0.2 μCi/ml D-[U-14C]galactose (303 mCi/mmol) were added to the medium overnight before harvesting the cells, as galactose is incorporated into all the gangliosides. Cell pellets were dispersed into 2 ml of chloroform/methanol (1:1, v/v), mixed thoroughly and extracted overnight at 4 °C. After centrifugation, the residues were extracted twice with 2 ml of the same solvent. The pooled extracts of total lipids were evaporated to dryness and submitted to partition with chloroform/methanol/PBS (1 mM) (10:10:7, by vol.). The upper phases containing gangliosides were next desalted on a C18 silica gel column and analysed by HPTLC. Plates were developed in chloroform/methanol/0.2% CaCl2 (55:45:10, by vol.) and the gangliosides were visualized by autoradiography using phosphor screen and Storm 820 (Molecular Dynamics, Amersham Biosciences) and by resorcinol staining [gangliosides-specific stain: 0.3% resorcinol (Sigma), 0.03% CuSO4 and 30% HCl] with Image Master VDS-CL (Amersham Biosciences). Gangliosides were identified by co-migration with standards. GM2 and GM1 identities were confirmed after HPTLC separation using anti-GM2 antibody (a gift from Dr J. Portoukalian, Institut National de la Sante et de la Recherche Medicale, Hopital E. Herriot, Lyon, France) and fluorescein-labelled choleratoxin B subunit that interact specifically with GM1. Quantification was performed by densitometric analysis using Image Quant (Molecular Dynamics). As GT1b is absent from the RMC ganglioside profile, it was added as an internal standard in the samples before lipid extraction. Results were then adjusted for extraction efficiency and total protein in the sample.
Radiolabelling with [14C]GlcN
To verify the hypothesis that GlcN treatment can affect the ganglioside profile by providing substrates for the ganglioside biosynthesis, cells were labelled with [14C]GlcN. Control cells were incubated with 0.2 μCi/ml D-[1-14C]GlcN for 48 h. GlcN-treated cells were incubated with 2 mM GlcN at a much higher specific activity of 0.5 mCi/ml D-[1-14C]GlcN. After the incubation, cells were washed and the radioactivity associated with gangliosides was then analysed to test the ability of GlcN to incorporate into ganglioside structures.
Treatment with exogenous gangliosides
Exogenous gangliosides GM2 and GM1 were added to the complete culture medium at a final concentration of 60 μM. This concentration was used since it induces inhibition of RMC proliferation similar to the effects observed with 0.5 mM GlcN treatment. Gangliosides were added in the form of complexes with BSA in the ratio 1:1 in a mixture of Dulbecco's modified Eagle's medium and 10 mM Hepes (pH 7.4) to facilitate their incorporation into the cell.
Western-blot analysis
At the end of the treatment period, cells were washed and collected in Ripa lysis buffer (10 mM PBS, 1% Nonidet P40, 0.5% sodium deoxycholate, 0.1% SDS and 10 μl/ml protease inhibitors). To remove the insoluble materials, lysates were centrifuged at 10000 g for 5 min. Samples (20 μg of lysate) were then boiled for 5 min, loaded on to a SDS/12% polyacrylamide gel, electrophoresed and transferred on to a PVDF membrane. The membranes were blocked with 5% (w/v) milk in PBST (PBS containing 0.05% Tween) and blotted with monoclonal antibodies (p21, 1 μg/ml; p27, 1:2000) in the blocking solution. After extensive washing, the membranes were blotted with secondary anti-mouse antibody at a dilution of 1:2000. The signal was visualized by enhanced chemiluminescence.
Statistical analysis
Results are expressed as means±S.E.M. The Wilcoxon signed-rank test was used to define the significance of the difference between groups. P<0.05 was considered to be statistically significant.
RESULTS
GlcN decreases mesangial cell proliferation and induces hypertrophy
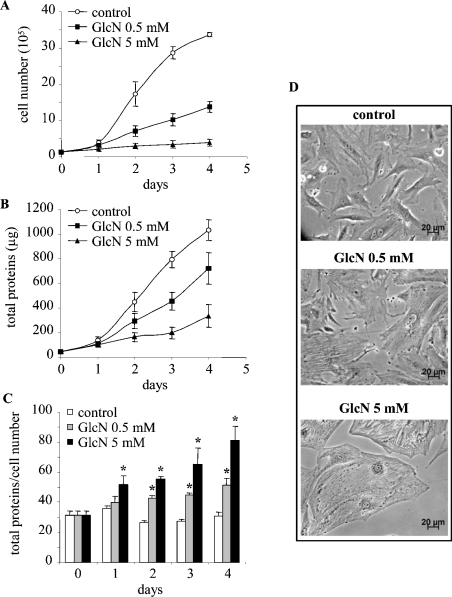
The effect of GlcN on RMC proliferation and hypertrophy was investigated in the present study. GlcN was used to mimic HP activation because of its demonstrated ability to enter selectively the biosynthetic HP and to bypass the rate-limiting enzyme GFAT [25]. To this end, mesangial cells were treated with either 0.5 or 5 mM GlcN, collected and the cell number and total protein content were measured. As shown in Figure 1, time-course experiments revealed that GlcN potently decreases proliferation. The effect of GlcN was evident starting from 48 h and, at the end of the treatment period, the number of cells in RMC treated with 0.5 and 5 mM glucosamine was only 41 and 12% of control cells respectively (Figure 1A). This adverse effect on cell proliferation was also measurable by a decrease in the total protein content (Figure 1B). In addition, since the total protein/cell number ratio is a well-established measurement of cellular hypertrophy, this parameter was used to determine whether the alteration of cell growth was accompanied by cell hypertrophy. As shown in Figure 1(C), GlcN indeed induced an increase in the protein/cell number ratio. At the end of the treatment period, the protein/cell number ratio increased by 1.6- and 2-fold for cells treated with 0.5 and 5 mM GlcN respectively. An increase in cell size, which is characteristic of cellular hypertrophy, was also obvious by microscopy, as illustrated in Figure 1(D). These observations indicate that GlcN triggers a decrease in proliferation as well as hypertrophy of mesangial cells. Since these effects were evident at 0.5 mM GlcN and to avoid potential cytotoxicity with the relatively high concentration of 5 mM, the following experiments were performed using 0.5 mM GlcN. Moreover, since the GlcN effects were evident at 48 h, the subsequent experiments were performed at this time of treatment, thus avoiding contact inhibition at confluence (4 days).
Figure 1. GlcN decreases RMC growth and induces hypertrophy.
Mesangial cells were exposed to 0.5 or 5 mM GlcN for 4 days. On each day, the cells were collected, cell number was counted and total protein content was measured. Growth curves corresponding to cell number (expressed in 105 cells) (A) and total protein content (expressed in μg) (B) are shown. The total protein/cell number ratio is presented in (C), expressed in μg of protein/105 cells. Results are the means±S.E.M. for four independent experiments. (D) Microscopy phase images of cells under control conditions or cells treated with GlcN. *P<0.05 versus control.
GlcN arrests cell-cycle progression
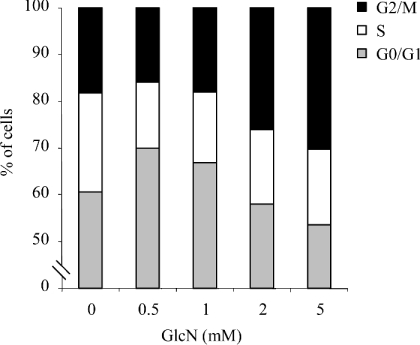
Results of our previous experiments showed that GlcN treatment blocks very early stage cell proliferation and may thus contribute to cellular hypertrophy. Therefore the mechanisms responsible for cell-growth inhibition in response to GlcN were investigated next. Cell-cycle analysis by flow cytometry was performed on control cells and cells treated with either 0.5 or 5 mM GlcN. The results, summarized in Table 1, indicate that 0.5 mM GlcN increased the proportion of cells in the G0/G1 phase of the cell cycle compared with control cells. This suggests that 0.5 mM GlcN blocks cell-cycle progression of RMC by inhibiting the G1–S transition phase, arresting cells at G0/G1 phase. Surprisingly, treatment of cells with a higher GlcN concentration (5 mM) resulted in cell-cycle blockade at the G2/M phase. To investigate further this discrepancy, a dose–response study was performed. As shown in Figure 2, GlcN concentrations <1 mM arrested cells in G0/G1 phase, whereas higher concentrations of GlcN (2–5 mM) induced G2/M phase arrest. These observations suggest that, at high GlcN concentrations (>1 mM), additional pathways are involved.
Table 1. GlcN and gangliosides GM2 and GM1 block the cell-cycle progression of mesangial cells.
RMC were treated with GlcN (0.5 or 5 mM) or cultured in the presence of GM2 or GM1 (60 μM) for 48 h. Cells were then collected and analysed by flow cytometry as described in the Experimental section. Results are expressed as percentage of cells in each phase of the cell cycle: G0/G1, S and G2/M. Values are the means±S.E.M. for five independent experiments.
| G0/G1 phase | S phase | G2/M phase | |
|---|---|---|---|
| Control | 58±2 | 22±1 | 19±1 |
| GlcN | |||
| 0.5 mM | 71±1 | 13±1 | 16±1 |
| 5 mM | 54±1 | 15±1 | 29±2 |
| GM2 | 79±2 | 9±1 | 12±1 |
| GM1 | 79±1 | 8±1 | 12±1 |
Figure 2. Dose-dependent effects of GlcN on cell-cycle arrest.
RMC were treated with increasing concentrations of GlcN for 48 h. Cells were then collected and analysed by flow cytometry as described in the Materials and methods section. Results are expressed as percentage of cells in G0/G1, S and G2/M phases.
GlcN increases p21Waf1/Cip1 expression
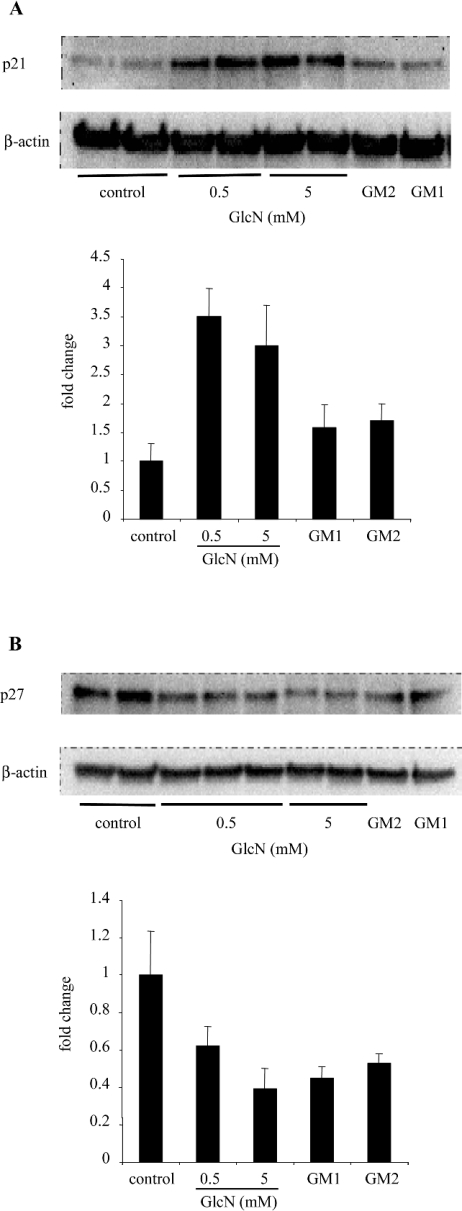
P21Waf1/Cip1 and p27Kip1 are CDKIs known to inhibit the G1–S phase transition. Moreover, it has been shown that p21Waf1/Cip1 and p27Kip1 proteins are involved in growth arrest at the G1–S phase transition and hypertrophy of RMC in response to high glucose concentrations [2]. Therefore the expression of p21Waf1/Cip1 and p27Kip1 proteins was measured. As shown in Figure 3, p21Waf1/Cip1 expression in cells treated with GlcN was approx. 3-fold higher than in control cells. These results are consistent with the cell-cycle arrest shown by flow-cytometric analysis. In contrast, however, p27Kip1 expression was decreased by GlcN treatment. These results suggest that, unlike the effects of high ambient glucose concentrations on RMC, GlcN-induced cell-cycle arrest is probably p21Waf1/Cip1-dependent.
Figure 3. GlcN, GM2 and GM1 increase p21Waf1/Cip1 expression.
RMC were exposed to GlcN (0.5 and 5 mM), GM2 (60 μM) and GM1 (60 μM) for 48 h. Cells were then lysed and equal amounts of protein were immunoblotted with monoclonal anti-p21Waf1/Cip1 (A) or anti-p27Kip1 (B) antibodies. Membranes were immunoblotted with anti-β-actin to control for equal protein loading and transfer. Representative immunoblots and quantification obtained by densitometric analysis of the bands are shown. Results were corrected to β-actin signal and are expressed as fold change compared with control. Results are the means±S.E.M. for four independent experiments.
GlcN increases GM2 and GM1 levels
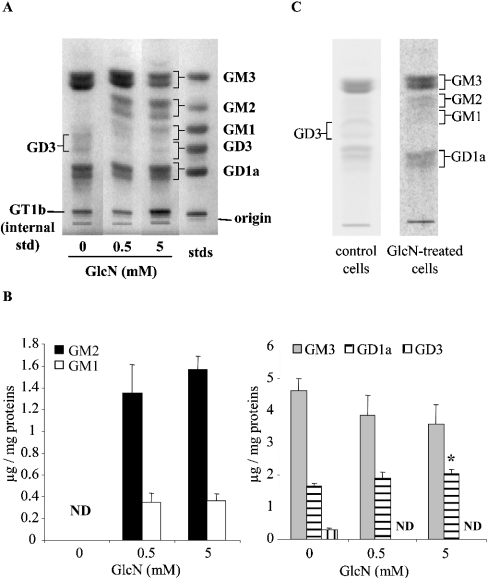
The HP has been implicated in the regulation of numerous enzyme and protein activities and, as a consequence, may regulate several pathways. Moreover, because the HP provides intermediates for the synthesis of glycoconjugates such as glycolipids, GlcN treatment could affect the ganglioside pattern of mesangial cells. To investigate this possibility, the ganglioside pattern was analysed by HPTLC in control cells and cells treated with either 0.5 or 5 mM GlcN for 48 h. Under control conditions, the main gangliosides observed in RMC were GM3 (70% of all gangliosides detected), GD1a (24%) and GD3 (5%). When cells were treated with GlcN, important modifications of the ganglioside profile were observed (Figure 4A). Thus the levels of gangliosides GM2 and GM1, hardly detectable in control cells, were strongly increased to reach approx. 1.5 and 0.4 μg/mg of protein, which corresponded to 20 and 5% of all gangliosides detected respectively (Figure 4B). Similar results were obtained by autoradiography after galactose labelling (results not shown). GM3 and GD1a levels were only slightly affected, whereas GD3 could not be detected in the GlcN-treated cells. These results suggest a shift into a-series ganglioside biosynthesis in response to GlcN. To investigate further whether HP affects ganglioside biosynthesis, control cells were labelled with exogenous radioactive GlcN and gangliosides were extracted and analysed for radioactivity content. Results show that indeed GlcN or its metabolites incorporate into the ganglioside biosynthesis pathway (Figure 4C). Next, cells were treated for 48 h with 2 mM GlcN at a specific activity of 0.5 mCi/ml. Ganglioside analysis revealed radioactive GM3, GM2, GM1 and GD1a (Figure 4C). These results support the hypothesis of a connection between the HP and ganglioside biosynthesis pathways.
Figure 4. Effect of GlcN on ganglioside profile.
(A, B) RMC were treated with GlcN (0.5 and 5 mM) for 48 h and then collected. Gangliosides were extracted, purified and then analysed by HPTLC and resorcinol staining. Representative HPTLC (A) and densitometric quantification (B) are shown. Results are expressed as μg of gangliosides/mg of protein and are the means±S.E.M. for four independent experiments each performed in duplicate. Under control conditions, ganglioside quantities were 4.62±0.37, 1.65±0.11 and 0.30±0.05 μg/mg of protein for GM3, GD1a and GD3 respectively. ND, not detectable. *P<0.05 versus control. (C) RMC were labelled for 48 h in the presence of 0.2 μCi/ml [14C]GlcN for control cells and 2 mM GlcN at a specific activity of 0.5 mCi/ml for treated cells. At the end of incubation, cells were washed extensively and gangliosides were extracted, purified and separated by HPTLC as described in the Experimental section. Radioactivity associated with gangliosides was then analysed by autoradiography of the HPTLC plates.
GM2 and GM1 gangliosides induce mesangial cell hypertrophy
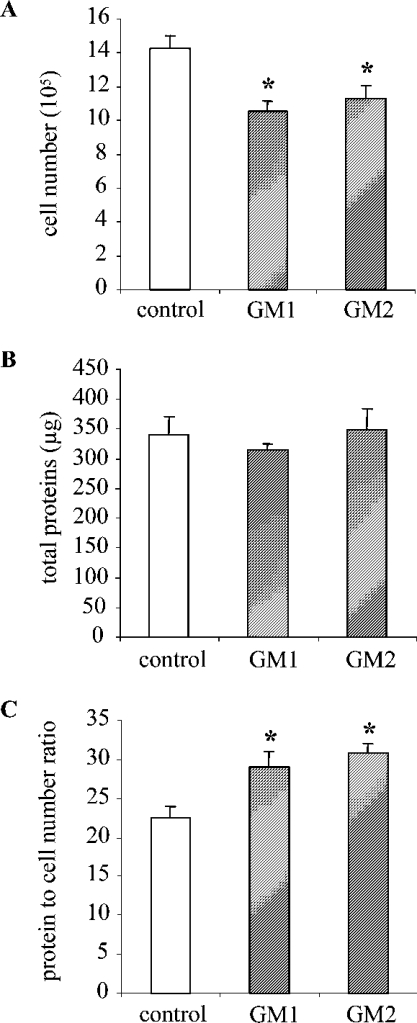
Gangliosides are known to play a role in a variety of important cellular functions including proliferation and differentiation. Since GM2 and GM1 were massively increased concomitantly to RMC hypertrophy in response to GlcN, we examined further the possibility of involvement of gangliosides in mediating the GlcN effects. Protein/cell number ratio was measured in control cells and in cells treated with exogenous gangliosides. Results presented in Figure 5 show that exogenous addition of GM2 and GM1 decreases mesangial cell proliferation as measured by cell number counting and it increases the protein/cell number ratio by approx. 30%, suggesting that RMC undergo hypertrophy in response to GM2 and GM1 treatment. GM3, GD3, GD1a and lactosylceramide, a non-sialylated precursor of gangliosides, were also tested. GM3, GD3 and GD1a induced mesangial cell hypertrophy similar to GM2 and GM1, whereas lactosylceramide had no effect (results not shown). These results suggest that sialic acid is a part of the active structure.
Figure 5. GM2 and GM1 gangliosides induce mesangial cell hypertrophy.
RMC were cultured in the presence of GM2 or GM1 (60 μM) for 48 h. Control conditions were complete medium with a mixture of BSA and 10 mM Hepes (pH 7.4). Cells were then harvested and counted using a haemocytometer and total protein content was measured. Cell number (expressed for 105 cells) (A) and total protein content (expressed in μg) (B) are presented. The total protein/cell number ratio used to assess hypertrophy is expressed in μg of protein/105 cells (C). Results are the means±S.E.M. for 4–6 independent experiments. *P<0.05 versus control.
GM2 and GM1 gangliosides block cell-cycle progression by increasing p21Waf1/Cip1 expression
Since GM2 and GM1 induced RMC hypertrophy as observed for GlcN, we next investigated whether similar mechanisms were involved. First, the cell cycle of cells treated with exogenous GM2 and GM1 was analysed by flow cytometry. As shown in Table 1, gangliosides induced an accumulation of cells in the G0/G1 phase, suggesting a blockade in cell-cycle progression at the G1–S transition. Next, p21Waf1/Cip1 and p27Kip1 expressions were measured. Results indicate that p21Waf1/Cip1 expression tended to increase in response to GM2 and GM1 treatment, whereas p27Kip1 expression decreased (Figure 3). Altogether, these results suggest that GlcN and exogenous gangliosides (GM2 and GM1) inhibit RMC proliferation probably through similar mechanisms, by increasing p21Waf1/Cip1 expression and blocking cells at the G0/G1 phase. They also suggest that gangliosides GM2 and GM1 are, at least partly, mediators of the GlcN effects.
DISCUSSION
Several lines of evidence indicate that the flux through the HP may be causally involved in the development of DN. Activation of the HP has been implicated in signalling pathways that lead to an overproduction of matrix proteins [25], a major cause of glomerular enlargement in the early stages of DN. For example, GlcN, in particular, has been shown to increase the production of the known matrix-stimulating growth factor, TGFβ [26–28]. However, the potential role of the HP in growth perturbation and hypertrophy of mesangial cells has been poorly studied. Therefore these hypotheses have been investigated in the present study.
Treatment of RMC with GlcN potently inhibited cell growth and induced hypertrophy as assessed by the protein/cell number ratio, in a time-dependent manner. Hypertrophy is the consequence of an increase in protein content combined with the absence of cell division. Therefore the effect of GlcN treatment on cell-cycle progression of RMC was investigated next. Flow-cytometric analyses indicated that a low concentration of GlcN (0.5 mM) arrested RMC cycle progression at the G0/G1 phase. On the other hand, 5 mM GlcN caused an accumulation of cells at the G2/M phase, suggesting that high concentrations block mesangial cell cycle at the G2/M transition. These observations clearly show that low and high concentrations of GlcN exert different actions on RMC. These differences could be explained by taking into account the recent observations of Marshall et al. [29] in adipocytes. Indeed, these authors showed that a high concentration of GlcN (2 mM) causes massive accumulation of the hexosamine intermediate product GlcN 6-phosphate, which was accompanied by ATP depletion. In contrast, these effects were not observed in response to low concentrations of GlcN (0.25 mM) or to high glucose concentrations (20 mM). In RMC treated with GlcN, we found that ATP depletion or, alternatively, high GlcN concentrations, through other unknown mechanisms, could result ultimately in DNA damage, leading to growth arrest of cells during G2/M. Thus treatment of RMC with a low GlcN concentration (0.5 mM) is probably more relevant physiologically.
To gain better insight into the mechanisms responsible for the cell-cycle arrest caused by GlcN, the expression of the CDKIs p21Waf1/Cip1 and p27Kip1 was studied. Our studies focused on p21Waf1/Cip1 and p27Kip1 since these proteins are known to be involved in the G0/G1-phase growth arrest of mesangial cells in different diabetic animal models as well as of cultured mesangial cells exposed to high ambient glucose concentrations [2]. Results indicated that p21Waf1/Cip1 expression was increased by approx. 3-fold by GlcN treatment when compared with control levels, suggesting that p21Waf1/Cip1 is involved in blocking cell-cycle progression observed in RMC exposed to GlcN. In contrast, p27Kip1 expression was found to be rather diminished in response to GlcN treatment. A decreased or unchanged p27Kip1 expression has also been observed in glomeruli of diabetic animal models [3,30] and in RMC treated with high glucose concentrations [3]. Thus the effect of GlcN on RMC cell-cycle arrest is most probably p21Waf1/Cip1-dependent. In addition, p21Waf1/Cip1 has been described as a pivotal regulator of hypertrophy. Fan and Weiss [31] showed that exogenous attenuation of p21Waf1/Cip1 using antisense oligonucleotides decreased the hypertrophy of mesangial cells induced by hyperglycaemia and insulin growth factor-1. Moreover, in vivo, p21Waf1/Cip1 knockout diabetic mice did not develop glomerular hypertrophy [32]. These observations suggest that the increased p21Waf1/Cip1 expression might also be involved in the mechanisms leading to hypertrophy of RMC in response to GlcN. Since the effects of GlcN on RMC are similar to those of high ambient glucose concentrations, one might speculate that the effects of high glucose concentrations on RMC growth and hypertrophy are, at least partly, mediated by a HP-induced p21Waf1/Cip1 pathway.
On the other hand, gangliosides are glycosphingolipids that have been involved in the regulation of proliferation of various cell types. Furthermore, it has been shown that when GlcN is added to cells, even at low concentrations, a marked increase of UDP-GlcNAc levels is observed [7,29]. This glycosidic precursor can be incorporated into ganglioside structures such as GM2 and GM1, after isomerization to UDP-GalNAc. Therefore we investigated whether the ganglioside pattern was affected in RMC submitted to GlcN treatment. Analyses revealed a modification of the ganglioside pattern with a massive increase in the levels of gangliosides GM2 and GM1. These results suggest that GlcN increases GM2 and GM1 levels through mass action by providing high amounts of precursors such as UDP-GalNAc. Radiolabelling experiments with [14C]GlcN also supported a connection between the HP and ganglioside biosynthesis pathways. However, other mechanisms regulating ganglioside biosynthesis in response to GlcN should not be excluded. For example, GM2 synthase presents putative binding sites for Sp1, a transcription factor whose activity is up-regulated by GlcN [33].
Next, we assessed whether GM2 and GM1 were involved in the hypertrophy and cell-cycle arrest of RMC in response to GlcN. Exogenous application of GM2 and GM1 resulted in an increase in the protein/cell number ratio. Furthermore, cell-cycle analysis of RMC treated with GM2 and GM1 showed an accumulation of cells at the G0/G1 phase and it was associated with an increase in the p21Waf1/Cip1 expression. Thus use of exogenous GM2 or GM1 reproduced the effects of 0.5 mM GlcN on RMC hypertrophy and cell-cycle arrest. Few studies implicated gangliosides in cell-cycle arrest. Nakatsuji and Miller [34] showed that ganglioside GM3 induces cell-cycle arrest and apoptosis of astrocytes partly through the CDKI p27Kip1. Our results further suggest that gangliosides might also contribute to mechanisms leading to cell hypertrophy. However, since gangliosides showed similar effects on p27Kip1 expression, results of the present study cannot rule out a role of p27Kip1 decrease in cell-cycle arrest induced by GlcN and gangliosides.
In conclusion, our results show that 0.5 mM GlcN induces cell-cycle arrest at the G0/G1 phase and hypertrophy of RMC. Moreover, our results suggest that gangliosides GM2 and GM1 are involved in mediating GlcN effects in RMC. Observations of the present study raise a new hypothesis on the potential involvement of the HP in the development of DN and add new mechanisms of action of the HP such as modulation of ganglioside levels in cells. Interestingly, gangliosides have recently been implicated in insulin resistance [35,36] and, as discussed earlier, HP has been shown to induce insulin resistance in vitro and in vivo. It would be of interest to investigate whether gangliosides are involved in the HP-induced insulin resistance.
References
- 1.Trevisan R., Barnes D. J., Viberti G. Pathogenesis of diabetic nephropathy. In: Pickup J., Williams G., editors. Textbook of Diabetes. Oxford, U.K.: Blackwell Science; 1997. pp. 52.1–52.21. [Google Scholar]
- 2.Wolf G. Cell cycle regulation in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S59–S66. doi: 10.1046/j.1523-1755.2000.07710.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuan C. J., al Douahji M., Shankland S. J. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J. Am. Soc. Nephrol. 1998;9:986–993. doi: 10.1681/ASN.V96986. [DOI] [PubMed] [Google Scholar]
- 4.Wolf G., Schroeder R., Ziyadeh F. N., Thaiss F., Zahner G., Stahl R. A. High glucose stimulates expression of p27Kip1 in cultured mouse mesangial cells: relationship to hypertrophy. Am. J. Physiol. 1997;273:F348–F356. doi: 10.1152/ajprenal.1997.273.3.F348. [DOI] [PubMed] [Google Scholar]
- 5.Wolf G., Schroeder R., Thaiss F., Ziyadeh F. N., Helmchen U., Stahl R. A. Glomerular expression of p27Kip1 in diabetic db/db mouse: role of hyperglycemia. Kidney Int. 1998;53:869–879. doi: 10.1111/j.1523-1755.1998.00829.x. [DOI] [PubMed] [Google Scholar]
- 6.Marshall S., Bacote V., Traxinger R. R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 7.Marshall S. The hexosamine signaling pathway: a new road to drug discovery. Curr. Opin. Endocrinol. Diabetes. 2002;9:160–167. [Google Scholar]
- 8.Vosseller K., Sakabe K., Wells L., Hart G. W. Diverse regulation of protein function by O-GlcNAc: a nuclear and cytoplasmic carbohydrate post-translational modification. Curr. Opin. Chem. Biol. 2002;6:851–857. doi: 10.1016/s1367-5931(02)00384-8. [DOI] [PubMed] [Google Scholar]
- 9.Rossetti L., Hawkins M., Chen W., Gindi J., Barzilai N. In vivo glucosamine infusion induces insulin resistance in normoglycemic but not in hyperglycemic conscious rats. J. Clin. Invest. 1995;96:132–140. doi: 10.1172/JCI118013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert L. F., Jr, Daniels M. C., Zhou J., Crook E. D., Turner R. L., Simmons S. T., Neidigh J. L., Zhu J. S., Baron A. D., McClain D. A. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J. Clin. Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J., Neidigh J. L., Cooksey R. C., McClain D. A. Transgenic mice with increased hexosamine flux specifically targeted to beta-cells exhibit hyperinsulinemia and peripheral insulin resistance. Diabetes. 2000;49:1492–1499. doi: 10.2337/diabetes.49.9.1492. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg H. J., Scholey J., Fantus I. G. Glucosamine activates the plasminogen activator inhibitor 1 gene promoter through Sp1 DNA binding sites in glomerular mesangial cells. Diabetes. 2000;49:863–871. doi: 10.2337/diabetes.49.5.863. [DOI] [PubMed] [Google Scholar]
- 13.James L. R., Fantus I. G., Goldberg H., Ly H., Scholey J. W. Overexpression of GFAT activates PAI-1 promoter in mesangial cells. Am. J. Physiol. Renal Physiol. 2000;279:F718–F727. doi: 10.1152/ajprenal.2000.279.4.F718. [DOI] [PubMed] [Google Scholar]
- 14.Kolm-Litty V., Sauer U., Nerlich A., Lehmann R., Schleicher E. D. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J. Clin. Invest. 1998;101:160–169. doi: 10.1172/JCI119875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh L. P., Crook E. D. Hexosamine regulation of glucose-mediated laminin synthesis in mesangial cells involves protein kinases A and C. Am. J. Physiol. Renal Physiol. 2000;279:F646–F654. doi: 10.1152/ajprenal.2000.279.4.F646. [DOI] [PubMed] [Google Scholar]
- 16.Singh L. P., Alexander M., Greene K., Crook E. D. Overexpression of the complementary DNA for human glutamine:fructose-6-phosphate amidotransferase in mesangial cells enhances glucose-induced fibronectin synthesis and transcription factor cyclic adenosine monophosphate-responsive element binding phosphorylation. J. Investig. Med. 2003;51:32–41. doi: 10.2310/6650.2003.33536. [DOI] [PubMed] [Google Scholar]
- 17.Weigert C., Friess U., Brodbeck K., Haring H. U., Schleicher E. D. Glutamine:fructose-6-phosphate aminotransferase enzyme activity is necessary for the induction of TGF-beta1 and fibronectin expression in mesangial cells. Diabetologia. 2003;46:852–855. doi: 10.1007/s00125-003-1122-8. [DOI] [PubMed] [Google Scholar]
- 18.van Echten G., Sandhoff K. Ganglioside metabolism. Enzymology, topology, and regulation. J. Biol. Chem. 1993;268:5341–5344. [PubMed] [Google Scholar]
- 19.Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- 20.Bremer E. G., Schlessinger J., Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J. Biol. Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- 21.Mirkin B. L., Clark S. H., Zhang C. Inhibition of human neuroblastoma cell proliferation and EGF receptor phosphorylation by gangliosides GM1, GM3, GD1A and GT1B. Cell Prolif. 2002;35:105–115. doi: 10.1046/j.1365-2184.2002.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachinidis A., Kraus R., Seul C., Meyer zu Brickwedde M. K., Schulte K., Ko Y., Hoppe J., Vetter H. Gangliosides GM1, GM2 and GM3 inhibit the platelet-derived growth factor-induced signalling transduction pathway in vascular smooth muscle cells by different mechanisms. Eur. J. Cell Biol. 1996;71:79–88. [PubMed] [Google Scholar]
- 23.Van Brocklyn J., Bremer E. G., Yates A. J. Gangliosides inhibit platelet-derived growth factor-stimulated receptor dimerization in human glioma U-1242MG and Swiss 3T3 cells. J. Neurochem. 1993;61:371–374. doi: 10.1111/j.1471-4159.1993.tb03581.x. [DOI] [PubMed] [Google Scholar]
- 24.Geoffroy K., Wiernsperger N., Lagarde M., El Bawab S. Bimodal effect of advanced glycation end products on mesangial cell proliferation is mediated by neutral ceramidase regulation and endogenous sphingolipids. J. Biol. Chem. 2004;279:34343–34352. doi: 10.1074/jbc.M403273200. [DOI] [PubMed] [Google Scholar]
- 25.Schleicher E. D., Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000;77(Suppl.):S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 26.Roos M. D., Han I. O., Paterson A. J., Kudlow J. E. Role of glucosamine synthesis in the stimulation of TGF-alpha gene transcription by glucose and EGF. Am. J. Physiol. 1996;270:C803–C811. doi: 10.1152/ajpcell.1996.270.3.C803. [DOI] [PubMed] [Google Scholar]
- 27.Weigert C., Brodbeck K., Lehmann R., Haring H. U., Schleicher E. D. Overexpression of glutamine:fructose-6-phosphate-amidotransferase induces transforming growth factor-beta1 synthesis in NIH-3T3 fibroblasts. FEBS Lett. 2001;488:95–99. doi: 10.1016/s0014-5793(00)02395-4. [DOI] [PubMed] [Google Scholar]
- 28.Ziyadeh F. N., Sharma K., Ericksen M., Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J. Clin. Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall S., Nadeau O., Yamasaki K. Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes: differential effects on glucosamine 6-phosphate, UDP-N-acetylglucosamine, and ATP levels. J. Biol. Chem. 2004;279:35313–35319. doi: 10.1074/jbc.M404133200. [DOI] [PubMed] [Google Scholar]
- 30.Griffin S. V., Pichler R., Wada T., Vaughan M., Durvasula R., Shankland S. J. The role of cell cycle proteins in glomerular disease. Semin. Nephrol. 2003;23:569–582. doi: 10.1053/s0270-9295(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 31.Fan Y. P., Weiss R. H. Exogenous attenuation of p21(Waf1/Cip1) decreases mesangial cell hypertrophy as a result of hyperglycemia and IGF-1. J. Am. Soc. Nephrol. 2004;15:575–584. doi: 10.1097/01.asn.0000114557.75244.5f. [DOI] [PubMed] [Google Scholar]
- 32.al Douahji M., Brugarolas J., Brown P. A., Stehman-Breen C. O., Alpers C. E., Shankland S. J. The cyclin kinase inhibitor p21WAF1/CIP1 is required for glomerular hypertrophy in experimental diabetic nephropathy. Kidney Int. 1999;56:1691–1699. doi: 10.1046/j.1523-1755.1999.00728.x. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg H. J., Whiteside C. I., Fantus I. G. The hexosamine pathway regulates the plasminogen activator inhibitor-1 gene promoter and Sp1 transcriptional activation through protein kinase C-beta I and -delta. J. Biol. Chem. 2002;277:33833–33841. doi: 10.1074/jbc.M112331200. [DOI] [PubMed] [Google Scholar]
- 34.Nakatsuji Y., Miller R. H. Selective cell-cycle arrest and induction of apoptosis in proliferating neural cells by ganglioside GM3. Exp. Neurol. 2001;168:290–299. doi: 10.1006/exnr.2000.7602. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki A., Hata K., Suzuki S., Sawada M., Wada T., Yamaguchi K., Obinata M., Tateno H., Suzuki H., Miyagi T. Overexpression of plasma membrane-associated sialidase attenuates insulin signaling in transgenic mice. J. Biol. Chem. 2003;278:27896–27902. doi: 10.1074/jbc.M212200200. [DOI] [PubMed] [Google Scholar]
- 36.Tagami S., Inokuchi J. J., Kabayama K., Yoshimura H., Kitamura F., Uemura S., Ogawa C., Ishii A., Saito M., Ohtsuka Y., et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]