Abstract
HGF (hepatocyte growth factor), a heterodimeric glycoprotein composed of α- and β-chains, exerts biological activities through the c-Met receptor tyrosine kinase. The α-chain has three glycosylation sites, while the β-chain has two; however, the role of sugar chains on HGF is still unknown. To address the significance of glycosylation of HGF, three different types of glycosylation-deficient HGFs, i.e. non-glycosylated in the α-chain, the β-chain, and in both the α- and β-chains, were respectively expressed in COS-7 cells and then purified from culture supernatants. Unexpectedly, glycosylation-deficient HGFs induced tyrosine phosphorylation of the c-Met receptor and subsequent phosphorylation of ERK (extracellular-signal-regulated kinase) and Akt in rat hepatocytes with the same potency as glycosylated HGF. Consistent with this, glycosylation-deficient HGFs strongly stimulated DNA synthesis of hepatocytes equal to glycosylated HGF. Likewise, glycosylation-deficient HGFs induced cell scattering and branching tubulogenesis in MDCK (Madin–Darby canine kidney) cells, and thus were indistinguishable from glycosylated HGF in biological activities. Glycosylation also did not affect stability, protease sensitivity and tissue distribution, although the plasma clearance of HGF was slightly prolonged by glycosylation deficiency. Glycosylation deficiency resulted in a decrease in post-transcriptional biosynthesis of HGF in the cells, whereas extracellularly secreted HGFs were efficiently activated to a two-chain form. These results indicate that glycosylation influences post-transcriptional biosynthesis of HGF, whereas biological activities and basic physicochemical characteristics are retained, even in completely non-glycosylated HGF. Hence, non-glycosylated HGF is promising as an alternative for glycosylated HGF in clinical applications.
Keywords: glycosylation, hepatocyte growth factor (HGF), c-Met, mitogen, morphogen, motogen
Abbreviations: CHO, Chinese-hamster ovary; DIG, digoxigenin; DMEM, Dulbecco's modified Eagle's medium; ERK, extracellular-signal-regulated kinase; FCS, foetal calf serum; HGF, hepatocyte growth factor; HGF-αNG, HGF non-glycosylated in the α-chain; HGF-βNG, HGF non-glycosylated in the β-chain; HGF-αβNG, HGF non-glycosylated in the α- and β-chains; MDCK, Madin–Darby canine kidney; PI3K, phosphoinositide 3-kinase; WT, wild-type
INTRODUCTION
HGF (hepatocyte growth factor) was first identified and cloned as a potent mitogen for fully differentiated hepatocytes [1,2]; however, it is currently known that HGF has multiple biological activities on a wide variety of cells, including mitogenic, motogenic and morphogenic activities (reviewed in [3,4]). Typical biological activities of HGF are related to construction, remodelling and protection of tissues during development and regeneration. HGF exerts its biological activities through binding to the c-met protooncogene product of receptor tyrosine kinase [5]. Binding of HGF to the c-Met/HGF receptor induces activation of the tyrosine kinase domain of the receptor, the result being subsequent phosphorylation of C-terminally clustered tyrosine residues and recruitment of intracellular signalling molecules.
HGF is a heterodimeric molecule composed of a 69 kDa α-chain and a 34 kDa β-chain. The α-chain contains the N-terminal hairpin domain and four subsequent kringle domains, and the β-chain contains a serine-protease-like domain [2]. HGF is initially biosynthesized and secreted in a biologically inactive single-chain form, and is subsequently activated to the two-chain form by specific serine proteases, including HGF activator and urokinase [6,7]. On the other hand, before extracellular secretion, the singlechain HGF undergoes post-translational modification by glycosylation. HGF contains five glycosylation sites [2,8–10]. The α-chain of HGF has two N-glycosylation sites and one O-glycosylation site, while the β-chain has two N-glycosylation sites, and the structures of sugar chains at these sites have been well-defined [9,10]. Although glycosylation plays important roles in defining the properties of proteins, including biological functions and physicochemical properties [11–13], the roles of sugar chains of HGF remained to be determined.
To address the involvement of glycosylation in biological activities and physicochemical properties of HGF, we generated glycosylation-deficient HGF mutants, and analysed their characteristics. In the present study, we demonstrate that non-glycosylated HGF retains mitogenic, motogenic and morphogenic activities equivalent to glycosylated HGF.
EXPERIMENTAL
Expression of recombinant HGF
Recombinant human HGF was expressed in CHO (Chinese-hamster ovary) cells or COS-7 cells. HGF used in the present study was the 5-amino-acid-deleted type [14]. CHO-derived wild-type HGF (designated CHO-HGF-WT) was purified from conditioned medium of CHO cells transfected with human HGF cDNA [14] and was used as a standard glycosylated HGF. The purity of CHO-HGF-WT exceeded 98%, as determined by SDS/PAGE and protein staining. In order to generate glycosylation-deficient HGF mutants, oligonucleotide primers of 38–41 bases were designed to replace asparagine in N-glycosylation sites and threonine at the O-glycosylation site with glutamine and glycine respectively. In the case of the 5-amino-acid-deleted-type human HGF, glycosylation sites are located at Asn-289, Asn-397 and Thr-471 in the α-chain, and Asn-561 and Asn-648 in the β-chain (Figure 1). Mutagenesis was carried out using QuikChange Multi Site-Directed Mutagenesis kits (Stratagene, La Jolla, CA, U.S.A.). Constructions were confirmed by DNA sequencing. Non-mutated and mutated HGF cDNAs were respectively inserted into the unique XhoI site of the pCAGGS vector (a gift from Dr Jun-ichi Miyazaki, Division of Stem Cell Regulation Research, Osaka University, Japan) [15], in which a foreign gene is driven by the CAG promoter. The constructed vectors were designated pCAGGS-HGF for non-mutated HGF, pCAGGS-HGF-αNG for HGF mutated at three glycosylation sites in the α-chain, pCAGGS-HGF-βNG for HGF mutated at two glycosylation sites in the β-chain, and pCAGGS-HGF-αβNG for HGF mutated at all five glycosylation sites. Each of the expression vectors was introduced into 90–95% confluent COS-7 cells, using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, U.S.A.), according to the manufacturer's protocol. After these cells had been cultured for 6 h, the medium was changed to DMEM (Dulbecco's modified Eagle's medium) containing 1% (v/v) FCS (foetal calf serum) and 1 μg/ml heparin, then the cells were cultured for a further 3 days. As a control of transfection efficiency, cells were co-transfected with 2 μg of pCAGGS-lacZ (a gift from Dr Jun-ichi Miyazaki). The intracellular β-galactosidase activity was measured using a β-Galactosidase Enzyme Assay System (Promega, Madison, WI, U.S.A.).
Figure 1. Schematic description of wild-type and glycosylation-deficient mutants of HGF expressed in COS-7 cells.
N-linked and O-linked sugar chains are indicated by diamonds and circles respectively. Amino acids are numbered from the N-terminal methionine of the secretion signal sequence.
Purification of recombinant HGF
Culture supernatants of COS-7 cells transiently transfected with the expression vector were loaded on to a Hi-Trap heparin column (Amersham Biosciences, Piscataway, NJ, U.S.A.) equilibrated with 50 mM Tris/HCl buffer (pH 7.5) containing 0.3 M NaCl and 0.01% Tween 80. After washing with 50 mM Tris/HCl buffer (pH 7.5) containing 0.7 M NaCl and 0.01% Tween 80, adsorbed proteins were eluted with a linear gradient of 0.7–2.0 M NaCl in 50 mM Tris/HCl buffer (pH 7.5) containing 0.01% Tween 80. HGF in each fraction was determined using an ELISA, as described in [16]. Fractions containing HGF were applied further on to a Mini S column (Amersham Biosciences) equilibrated with 50 mM Tris/HCl buffer (pH 7.5) containing 0.3 M NaCl and 0.01% Tween 80. After washing with the same buffer, proteins were eluted with a linear gradient of 0.3–1.0 M NaCl in 50 mM Tris/HCl buffer (pH 7.5) containing 0.01% Tween 80. To analyse the purity of HGF, SDS/PAGE was performed using a 4–20% gradient gel under reducing conditions, and proteins were visualized by staining with Coomassie Brilliant Blue.
Enzymatic deglycosylation of HGF
To remove the O-linked sugar chain from HGF, CHO-HGF-WT (2 μg) was incubated with 5 m-units of neuraminidase (Nacalai Tesque, Kyoto, Japan) and 1 m-unit of O-glycanase (Prozyme, San Leandro, CA, U.S.A.) in 10 μl of 50 mM sodium phosphate buffer (pH 6.0) at 37 °C for 6 h. N-linked sugar chains were subsequently removed by treating with glycopeptidase F under native or denatured conditions. For treatment under the former, 10 μl of 100 mM Tris/HCl (pH 8.0) was added to the above reaction mixture, and the mixture was incubated with 2.5 m-units of glycopeptidase F (Takara, Otsu, Japan) at 37 °C for 16 h. For treatment under the latter, 10 μl of 1 M Tris/HCl (pH 8.6) containing 0.2 M 2-mercaptoethanol was added to the above reaction mixture, and the mixture was boiled for 3 min. Then, 10 μl of 10% Nonidet P-40 was added, and the preparation was incubated with 1 m-unit of glycopeptidase F at 37 °C for 16 h.
Western blot analysis
To analyse intracellular HGF, COS-7 cells transfected with expression vectors were lysed with 50 mM Tris/HCl (pH 7.5) containing 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 5 μg/ml pepstatin A. After rotation for 30 min at 4 °C, the cell lysate was centrifuged at 15000 g for 20 min. The supernatant was incubated with heparin–Sepharose (Amersham Biosciences) at 4 °C for 6 h. Precipitated materials were washed with 50 mM Tris/HCl (pH 7.5) containing 0.5 M NaCl and solubilized with the sample buffer for SDS/PAGE. The precipitates were separated by SDS/PAGE, electroblotted on to a PVDF membrane (Bio-Rad, Hercules, CA, U.S.A.), and probed with a biotinylated polyclonal antibody against human HGF. Immunoreactive proteins were detected with horseradish-peroxidase-conjugated streptavidin (Amersham Biosciences) and ECL® (enhanced chemiluminescence) reagents (Amersham Biosciences).
Northern blot analysis
Total RNA was purified from cultured cells using the acid/guanidium thiocyanate/phenol/chloroform method and the TRIzol® reagent (Invitrogen). A 1 μg sample of total RNA was electrophoresed, blotted on to a nylon membrane, and hybridized with a specific probe. Single-stranded antisense RNA for the XhoI/KpnI fragment of HGF cDNA encoding a part of HGF β-chain was labelled with DIG (digoxigenin) and used as a probe. The DIG-labelled RNA probe was prepared by in vitro transcription of a template DNA designed to generate the corresponding RNA sequence under the T7 promoter, using DIG RNA Labeling kits (Roche Diagnostics, Mannheim, Germany). The hybridized probe was immunodetected according to the manufacturer's protocol. The β-actin mRNA was also analysed as an internal control using DIG-labelled antisense RNA for human β-actin.
Assay of biological activities of HGF
MDCK (Madin–Darby canine kidney) (clone 3B) renal epithelial cells, a gift from Dr Roberto Montesano (Department of Morphology, University of Geneva Medical School, Switzerland), were cultured in DMEM containing 10% FCS. Cell scattering and tubulogenesis assays using MDCK cells were performed as described in [17]. Mitogenic activity of HGF was measured using adult rat hepatocytes in primary culture, as described in [17]. Briefly, HGF was added to cultures of hepatocytes, the culture was maintained for 20 h, then pulse-labelled with 2.5 μCi/ml of [3H]thymidine for 6 h. The cells were washed twice with PBS and once with trichloroacetic acid, then solubilized with 1 M NaOH. After neutralizing the pH, radioactivity of [3H]thymidine incorporated into nuclei was measured using a β-counter.
Detection of c-Met receptor tyrosine phosphorylation, ERK (extracellular-signal-regulated kinase) phosphorylation, and Akt phosphorylation
Subconfluent adult rat hepatocytes in primary culture were treated with HGF for 10 min, washed with ice-cold PBS, and lysed with lysis buffer. For immunoprecipitation of the c-Met receptor, the cells were lysed with 50 mM Tris/HCl (pH 7.5) containing 150 mM NaCl, 1% Triton X-100, 5 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 5 μg/ml pepstatin A. After rotation for 30 min at 4 °C, the cell lysate was centrifuged at 15000 g for 20 min. The supernatant was incubated with anti-c-Met antibody (B-2) (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) and Protein G-Sepharose (Amersham Biosciences). Immunoprecipitated materials were washed with lysis buffer and solubilized with the sample buffer for SDS/PAGE. The immunoprecipitates were separated by SDS/PAGE, electroblotted on to a PVDF membrane (Bio-Rad), and probed with an anti-phosphotyrosine monoclonal antibody (PY99) (Santa Cruz Biotechnology) or an anti-c-Met monoclonal antibody (B-2). For detection of phosphorylation of ERK and Akt, cells were lysed with 50 mM Tris/HCl (pH 7.5) containing 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 25 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 5 μg/ml aprotinin, 5 μg/ml leupeptin and 5 μg/ml pepstatin A. After centrifugation (15000 g for 20 min), supernatants containing 40 μg of protein were separated by SDS/PAGE, electroblotted on to a PVDF membrane, and probed with an anti-phospho-ERK (Thr-202/Tyr-204) monoclonal antibody (E10) (Cell Signaling Technology, Beverly, MA, U.S.A.), an anti-ERK polyclonal antibody (Upstate, Charlottesville, VA, U.S.A.), an anti-phospho-Akt (Ser-473) polyclonal antibody (Cell Signaling Technology) or an anti-Akt polyclonal antibody (Cell Signaling Technology). Proteins reacting with these antibodies were detected with horseradish-peroxidase-conjugated secondary antibodies against mouse or rabbit immunoglobulin (DakoCytomation, Tokyo, Japan). The resulting signals were detected using ECL®.
Stability and protease sensitivity of HGF
For the measurement of stability, 50 μg/ml HGF was incubated in 50 mM Tris/HCl buffer (pH 7.5) containing 0.3 M NaCl and 0.01% Tween 80 at 37 °C for various periods and biological activity to stimulate DNA synthesis of hepatocytes was measured, as described above. Susceptibility of HGF to trypsin digestion was examined by incubating 100 μg/ml HGF with 2 μg/ml trypsin at 37 °C for various periods. The digestion was stopped by the addition of SDS/PAGE sample buffer and boiling for 5 min. The extent of digestion was analysed by SDS/PAGE and subsequent protein staining.
Changes in plasma level and tissue distribution of HGF
HGF was radio-iodinated using IODO-BEADS Iodination Reagent (Pierce, Rockford, IL, U.S.A.). One bead of the IODO-BEADS was added to a solution of 50 μCi of Na125I in 80 μl of 0.1 M Tris/HCl buffer (pH 7.5) containing 0.3 M NaCl and 0.01% Tween 80, and the mixture was incubated at 25 °C for 5 min. A 5 μg sample of HGF in the same buffer was added to the reaction mixture and allowed to react for 5 min. The reaction was stopped by removing the beads from the reaction mixture. Radio-iodinated HGF was purified by gel filtration using a Sephadex G-25 (Amersham Biosciences) column. The 125I-labelled HGF solution was diluted with PBS containing 0.1% BSA and injected intravenously into male ICR mice (30–35 g of body mass) via a lateral tail vein. Changes in radioactivities in plasma and tissues were measured using a γ-counter.
RESULTS
Expression and purification of glycosylation-deficient HGFs
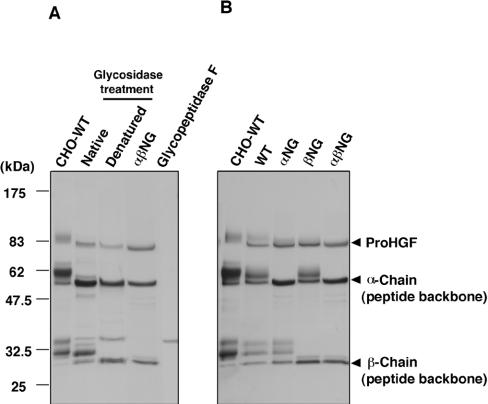
We first tried enzymatic deglycosylation of glycosylated HGF. CHO-derived glycosylated HGF (CHO-HGF-WT) was digested with a combination of neuraminidase, O-glycanase, and glycopeptidase F (Figure 2A). Under denatured conditions, sugar chains were almost completely removed. However, under native conditions, sugar chains on the α-chain were largely removed, whereas elimination of sugar chains from the β-chain was incomplete, indicating that glycosylation sites in the β-chain with native conformation are inaccessible to glycosidases. Therefore we used recombinant glycosylation-deficient HGFs in the following experiments.
Figure 2. SDS/PAGE of purified glycosylation-deficient HGFs.
(A) SDS/PAGE of enzymatically deglycosylated HGF. Purified glycosylated HGF (CHO-HGF-WT) was digested with a combination of glycosidases under native or denatured conditions, and subjected to SDS/PAGE (4–20% gradient gel) under reducing conditions. The gel was stained with Coomassie Brilliant Blue. Purified preparations of HGF-αβNG and glycopeptidase F were loaded for comparison. Neuraminidase and O-glycanase added to the reaction mixture were undetectable at concentrations used here (not shown). (B) SDS/PAGE of purified HGF-WT and glycosylation-deficient HGF mutants. Each preparation (1 μg of each) was separated by SDS/PAGE (4–20% gradient gel) under reducing conditions and stained with Coomassie Brilliant Blue.
To generate three different types of glycosylation-deficient HGFs, asparagine residues in N-glycosylation sites and threonine residues in O-glycosylation site, were respectively replaced with glutamine and glycine by site-directed mutagenesis (Figure 1). HGF-αNG, HGF-βNG and HGF-αβNG represent HGFs non-glycosylated in the α-chain, β-chain, and both α- and β-chains respectively. Expression vectors for these glycosylation-deficient HGFs, as well as the vector for HGF-WT (wild-type HGF), were transfected into COS-7 cells, and glycosylated and non-glycosylated HGFs were purified from the conditioned media by heparin-affinity and cation-exchange chromatography.
When the purified proteins were analysed using SDS/PAGE, all preparations had over 98% purity (Figure 2B). In CHO-HGF-WT, the main protein bands showed three distinct bands under reducing conditions: the upper band corresponds to single-chain proHGF, while the middle and lower bands correspond to the α-chain and the β-chain respectively. Protein bands of α-chain and β-chain were not single, showing that both α- and β-chains were glycosylated heterogeneously. The extent of glycosylation in HGF-WT derived from COS-7 cells seemed to be slightly lower than CHO-HGF-WT, because the band pattern of HGF-WT shifted down slightly compared with CHO-HGF-WT. In HGF-αNG, the bands of the α-chain converged to the position corresponding to the peptide backbone of the α-chain with a molecular mass of 53 kDa, while the band pattern of the β-chain was unchanged. On the other hand, in HGF-βNG, the bands of the β-chain converged to the position corresponding to the peptide backbone of the β-chain with a molecular mass of 26 kDa, while the band pattern of α-chain was unchanged. In HGF-αβNG, the bands of both the α-chain and the β-chain converged to positions corresponding to the peptide backbone of the α-chain and the β-chain respectively. These results indicated that glycosylation-deficient mutant HGFs were prepared as intended.
In the course of heparin-affinity chromatography, elution profiles of glycosylated and non-glycosylated HGFs were substantially equivalent (results not shown), indicating that affinity of HGF for heparin is not influenced by glycosylation. This result seems reasonable, because the N-terminal domain and the second kringle domain of HGF are responsible for the binding of HGF to heparin [18–20], while no glycosylation sites reside in these domains.
Influence of glycosylation on expression of HGF
Glycosylation deficiency often decreases secretion of glycoproteins, including erythropoietin, interferons and acetylcholinesterase [21–23]. We therefore analysed the influence of glycosylation deficiency on expression of HGF in COS-7 cells (Figure 3). The amount of HGF secreted into the culture medium indicated that the concentrations of glycosylation-deficient HGFs (HGF-αNG, HGF-βNG and HGF-αβNG) were lower than that of glycosylated HGF-WT (Figure 3A). When the production levels of HGF were normalized, using β-galactosidase activity obtained from co-transfected lacZ gene as an internal standard, the levels of HGF-αNG and HGF-βNG in the culture medium 72 h after transfection were 67.7% and 70.0% of HGF-WT respectively. Production level of completely non-glycosylated HGF (HGF-αβNG) decreased to 51.7% of HGF-WT. When the glycosylation-deficient HGFs in the culture medium were analysed using Western blotting, HGFs were processed to an active form and the ratio of active form to pro-form was equivalent among the glycosylation-deficient HGFs and HGF-WT (results not shown). Therefore activation of HGF did not seem to be affected by glycosylation deficiency.
Figure 3. Influence of glycosylation deficiency on expression of HGF in COS-7 cells.
(A) Change in extracellular levels of glycosylated and glycosylation-deficient HGFs. The concentration of HGF secreted into the culture medium was measured using ELISA. (B) Northern blot analysis of HGF mRNA expression. Total RNA was prepared from COS-7 cells 2 days after transfection of each expression vector and was electrophoresed. RNA was blotted on to a nylon membrane and hybridized with a specific probe for human HGF mRNA. (C) Change in intracellular levels of glycosylated and glycosylation-deficient HGFs. Cell lysates were prepared from COS-7 cells, and the concentration of HGF was measured by ELISA. (D) Western blot analysis of intracellular HGF. Lysates of COS-7 cells were prepared 24 h after transfection of each expression vector. Intracellular HGF was precipitated with heparin–Sepharose from the lysates, and was subjected to SDS/PAGE under reducing conditions. Proteins were electroblotted on to a PVDF membrane, and HGF was detected using an anti-human HGF polyclonal antibody.
To investigate the reason for decreases in the extracellular level of glycosylation-deficient HGFs, Northern blot analysis was performed for COS-7 cells transfected with non-mutated and mutated vectors (Figure 3B). The result showed that all mutant cDNAs were transcribed at levels similar to that of HGF-WT, indicating that decreases in the amount of HGF in the culture medium in glycosylation-deficient HGFs could not be ascribed to decreases in transcriptional efficiency. Rather, post-transcriptional processes might be involved in the decreased production in glycosylation-deficient HGFs.
Therefore we analysed intracellular HGF levels using ELISA for COS-7 cells transfected with expression vectors. Intracellular HGF levels reached a peak at 24 h after transfection and thereafter decreased gradually (Figure 3C). The intracellular level of HGF-WT was higher than that in glycosylation-deficient HGFs, and the level of HGF-αβNG was lowest. Intracellular HGF was also analysed by Western blotting under reducing conditions using cell lysates of 24 h after transfection (Figure 3D). Although some non-specific bands were detected in cell lysates of mock-transfectant, a specific major band that coincides with the mobility of single-chain proHGF was detected in lysates of cells expressing HGFs, indicating that the abundance of intracellular HGF exists in a pro-form. The densitometric analysis of proHGF bands indicated that the amounts of HGF-αNG, HGF-βNG and HGF-αβNG were 83.0%, 66.9% and 47.7% of HGF-WT respectively. Thus decreases in the extracellular levels of glycosylation-deficient HGFs were associated with decreases in intracellular levels, indicating that glycosylation deficiency may affect post-transcriptional processes that are involved in translation or peptide folding. Because sugar chains are known to be involved in quality control of newly synthesized proteins in the endoplasmic reticulum (reviewed in [24,25]), it is most likely that the efficiency of proper folding of newly synthesized HGF polypeptides was reduced owing to the lack of sugar chains.
Biological activities
Biological activities of glycosylation-deficient HGFs were assessed in distinct assay systems. Mitogenic activity was assayed by measuring DNA synthesis of rat hepatocytes in primary culture (Figure 4). Glycosylated HGFs (HGF-WT and CHO-HGF-WT) showed stimulatory effects on DNA synthesis as low as 0.5 ng/ml, and the maximal stimulatory effect was seen at 20 ng/ml. Likewise, glycosylation-deficient HGFs (HGF-αNG, HGF-βNG and HGF-αβNG) exhibited potent stimulatory effects on DNA synthesis of hepatocytes, and their activity was substantially the same as that of HGF-WT and CHO-HGF-WT, including the dose-dependency and the maximal activity. The result indicated that the mitogenic activity of HGF is retained even when glycosylation is deficient. When motogenic activity of HGF to stimulate motility of cells was assayed by measuring scattering of MDCK renal epithelial cells (see supplementary Figure S1 at http://www.BiochemJ.org/bj/388/bj3880555add.htm), the extent of scattering of MDCK cells was equivalent among glycosylated and glycosylation-deficient HGFs.
Figure 4. Mitogenic activity of glycosylated and glycosylation-deficient HGFs.
Mitogenic activity of glycosylated and glycosylation-deficient HGFs was determined by measuring DNA synthesis of rat hepatocytes in primary culture. Cells were stimulated with HGF for 18 h and pulse-labelled with [3H]thymidine for 6 h.
We also assessed the influence of glycosylation deficiency on the unique biological activity of HGF to induce branching tubulogenesis in MDCK cells [26] (Figure 5). When MDCK cells were placed in a collagen gel without HGF, they grew and had a spherical cystic structure. On the other hand, when MDCK cells were grown in the presence of HGFs, morphogenic change was seen at 1 ng/ml HGF, and branching tubulogenesis occurred with 5 and 10 ng/ml HGF. The potency to induce branching tubulogenesis was equivalent among glycosylated and glycosylation-deficient HGFs. Taken together, these results indicated that glycosylation-deficient HGFs, even in completely non-glycosylated HGF, retain mitogenic, motogenic and morphogenic activities with the same efficiency as glycosylated HGF.
Figure 5. Morphogenic activity of glycosylated and glycosylation-deficient HGFs.
Morphogenic activity of glycosylated and glycosylation-deficient HGFs was measured by evaluating the formation of branching tubes in MDCK cells. The cells were cultured in a collagen gel for 5 days in the absence or presence of HGF. Scale bar, 200 μm.
Tyrosine phosphorylation of the c-Met receptor and subsequent activation of ERK and Akt
Since biological activities of HGF depend on tyrosine phosphorylation of the c-Met receptor upon HGF binding, tyrosine phosphorylation of the c-Met receptor was analysed using rat hepatocytes in primary culture (Figure 6A). To detect tyrosine phosphorylation of the c-Met receptor, the c-Met receptor was immunoprecipitated from cell lysates, and immunoprecipitated materials were subjected to Western blotting. Tyrosine phosphorylation of the c-Met receptor was undetectable in non-stimulated cells, while addition of HGF induced tyrosine phosphorylation of the c-Met receptor. Tyrosine phosphorylation levels were submaximal at 5 ng/ml HGF, and were somewhat higher at 10 and 20 ng/ml HGF than those seen at 5 ng/ml HGF. Importantly, the extent of phosphorylation of the c-Met receptor was mostly equivalent among all HGF preparations at the same concentration, thereby supporting the notion that glycosylation deficiency does not affect the biological activities.
Figure 6. Activation of c-Met receptor, ERK and Akt by glycosylated and glycosylation-deficient HGFs.
(A) Tyrosine-phosphorylation of the c-Met receptor induced by glycosylated and glycosylation-deficient HGFs. HGF was added to subconfluent cultures of adult rat hepatocytes, and cells were lysed with lysis buffer 10 min later. The c-Met receptor was immunoprecipitated and subjected to SDS/PAGE under reducing conditions. Proteins were electroblotted on to a PVDF membrane, and phosphorylated tyrosine (pY) and total c-Met were detected with anti-phosphotyrosine antibody and anti-c-Met antibody, respectively. (B, C) Phosphorylation of ERK (B) and Akt (C). HGF was added to subconfluent cultures of adult rat hepatocytes, and cells were lysed with lysis buffer 10 min later. Total lysates (40 μg) were separated by SDS/PAGE under reducing conditions, and were electroblotted on to a PVDF membrane. Phosphorylated ERK, total ERK, phosphorylated Akt and total Akt were detected with anti-phospho-ERK (Thr-202/Tyr-204) antibody, anti-ERK antibody, anti-phospho-Akt (Ser-473) antibody and anti-Akt antibody respectively.
The ERK and PI3K (phosphoinositide 3-kinase) are activated downstream of the c-Met receptor, and are involved in HGF-induced multiple activities, including cellular proliferation, scattering and tubulogenesis [27–30]. The PI3K–Akt cascade is also a key component of survival signalling in a variety of cells [31,32]. Therefore HGF-induced phosphorylation of ERK and Akt was analysed using cell lysates from rat hepatocytes (Figures 6B and 6C). Phosphorylation of ERK and Akt was undetectable in non-stimulated cells, while addition of HGF induced their phosphorylation. The phosphorylation level of ERK and Akt together reached a maximum at 5 ng/ml HGF, and differences were not seen between any HGF preparations at the same concentration. Thus activation of signal molecules downstream of the c-Met receptor was also unaffected by glycosylation deficiency of HGF. Hence, the lack of influence of glycosylation deficiency of HGF on biological activities was also confirmed from the intracellular signalling.
Stability of HGF
To assess the effect of glycosylation on the stability of HGFs, glycosylated and glycosylation-deficient HGFs were incubated at 37 °C for various periods and change in biological activity was analysed (see supplemental Figure S2 at http://www.BiochemJ.org/bj/388/bj3880555add.htm). The result showed that glycosylation does not affect the stability of HGF. Next, sensitivity of glycosylated and glycosylation-deficient HGFs to protease digestion was tested. HGFs were incubated with trypsin, and change in the time-dependent digestion pattern was compared using SDS/PAGE (see supplemental Figure S3 at http://www.BiochemJ.org/bj/388/bj3880555add.htm). Glycosylation of HGF did not affect the trypsin resistance of HGF.
Plasma clearance and tissue distribution of 125I-labelled HGF
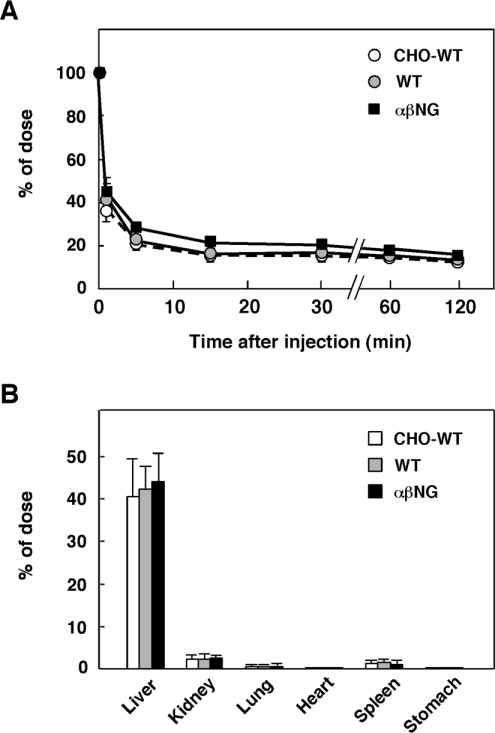
To examine the influence of glycosylation on the plasma clearance of HGF, 125I-labelled HGFs (CHO-HGF-WT, HGF-WT and HGF-αβNG) were administrated intravenously to mice, and changes in plasma levels of 125I-labelled HGFs were measured (Figure 7A). Details and pharmacokinetical analysis of changes in plasma HGF have been noted [33–35]. Similar to these previous reports, glycosylated 125I-labelled HGFs (CHO-HGF-WT and HGF-WT) disappeared rapidly from the blood circulation. Even 1 min after intravenous injection, <50% of the dose remained in the plasma, and the plasma HGF level at 5 min after injection was <30% of the dose. If anything, longevity of the non-glycosylated HGF (HGF-αβNG) was slightly prolonged compared with glycosylated HGFs (CHO-HGF-WT and HGF-WT). However, the time-dependent profiles did not differ significantly between glycosylated and non-glycosylated HGFs, indicating that glycosylation deficiency did not significantly affect the plasma clearance of HGF.
Figure 7. Changes in plasma levels and organ distribution of glycosylated and non-glycosylated HGFs in mice.
(A) Changes in plasma levels of 125I-labelled HGFs. Glycosylated (CHO-HGF-WT and HGF-WT) and non-glycosylated HGF (HGF-αβNG) were radiolabelled with 125I, and intravenously injected into mice. Radioactivities in plasma samples are expressed as a percentage of the dose. Each value represents the means±S.D. of measurements using three mice. (B) Organ distribution of 125I-labelled HGFs. Radioactivity per whole organ was measured 15 min after intravenous injection of 125I-labelled HGFs and expressed as a percentage of each dose. Each value represents the means±S.D. of measurements using three mice.
We also analysed tissue distribution of glycosylated and non-glycosylated HGFs (Figure 7B). At 15 min after intravenous injection of glycosylated 125I-labelled HGFs, the abundance of 125I-labelled HGFs accumulated in the liver, while much lower amounts were distributed in other organs, indicating that the liver is the main organ involved in the plasma clearance of HGF, as described in [33,34]. Substantially, non-glycosylated HGF showed the same tissue distribution as glycosylated HGFs, hence glycosylation deficiency did not affect the tissue distribution of HGF.
DISCUSSION
Hofmann et al. [36] reported that glycosylation is not required for the cell-scattering activity of HGF. However, to eliminate sugar chains from HGF, they used tunicamycin treatment of HGF-producing cells or glycosidase treatment of HGF. Since tunicamycin is an inhibitor of N-glycosylation, but not of O-glycosylation, elimination of sugar chains from HGF was restricted or was partial. Likewise, they described that glycosidase-treated HGF still adsorbed to concanavalin A columns, indicating that enzymatic deglycosylation of HGF was incomplete. Thus influences of glycosylation-deficiency of HGF remained to be addressed further. Moreover, functional involvement of sugar chains in distinct biological activities of HGF and in regulation of physicochemical characteristics, plasma clearance, and tissue distribution of HGF remained unknown, even though 20 years have passed since HGF was discovered.
To investigate the significance of sugar chains on HGF, we generated completely non-glycosylated HGF (HGF-αβNG), as well as partially glycosylation-deficient HGFs (HGF-αNG and HGF-βNG) and analysed their characteristics using distinct assay systems. Unexpectedly, all glycosylation-deficient HGFs (HGF-αNG, HGF-βNG and HGF-αβNG) showed activities almost equivalent to glycosylated HGFs (HGF-WT and CHO-HGF-WT) in mitogenic, motogenic and morphogenic activities, although glycosylation deficiency decreased secretion of HGF from cells. Thus we found that glycosylation is not essential for HGF to exert multiple biological activities, at least in vitro. The finding was also supported by the fact that tyrosine residues of the c-Met receptor were phosphorylated in response to HGF-αNG, HGF-βNG and HGF-αβNG at the same level as HGF-WT and CHO-HGF-WT. Likewise, intracellular signalling in response to the c-Met activation, including ERK and Akt activation, was unaffected by glycosylation deficiency of HGF. Considering that HGF-WT carries as many as five sugar chains, the present findings were beyond prediction.
Furthermore, completely non-glycosylated HGF retained the same activity as the fully and partially glycosylated HGFs when they were kept at 37 °C for 7 days. In addition, sensitivity of HGFs to trypsin was almost equivalent among glycosylation-deficient HGFs and glycosylated HGF-WT. Glycosylation is known to shield the peptide backbone of glycoproteins from proteases, and a lack of sugar chains often results in a decrease in the resistance to proteases. However, this was not the case with HGF. Sugar chains might not occupy surface areas of the HGF molecule to the extent that the molecule is shielded from trypsin digestion.
Non-glycosylated forms of glycoproteins are more readily cleared from blood circulation than are glycosylated forms in several cases, including interferons [37,38], granulocyte/macrophage colony-stimulating factor [39], and follicle-stimulating hormone [40]. On the other hand, non-glycosylated HGF (HGF-αβNG) showed almost the same longevity in plasma, and, if anything, the clearance of non-glycosylated HGF was slightly delayed compared with glycosylated HGF. Shortening of plasma half-life due to a lack of sugar chains seems to be significant in the case of proteins with molecular masses smaller than 20–30 kDa. Small proteins are extensively filtered through glomeruli in the kidney [41,42], while sugar chains serve to increase the apparent molecular size of small proteins, thereby shielding them from glomerular filtration. Since the molecular mass of the HGF polypeptide is as large as 80 kDa even when sugar chains are eliminated, filtration by the kidney will not be significant in the case of non-glycosylated HGF. Putative binding of glycosylated HGF to lectins expressed in various tissues may possibly be involved in a slightly earlier decay of glycosylated HGF in plasma levels compared with non-glycosylated HGF. On the other hand, HGF has an affinity for glycosaminoglycans such as heparan sulphate, and adsorption of HGF to glycosaminoglycans in tissues such as the liver predominantly participates in its plasma clearance [33,43]. Because the N-terminal and the second kringle domains with no glycosylation sites are responsible for binding of HGF to heparin/heparan sulphate and non-glycosylated HGF retains affinity to heparin equivalent to glycosylated HGF, non-glycosylated HGF may well show characteristics similar to glycosylated HGF in plasma clearance and tissue distribution.
HGF plays important roles in tissue regeneration and protection following injuries, and administration and gene expression of HGF in a variety of experimental models elucidated the therapeutic potential of HGF for treatment of subjects with clinical diseases (reviewed in [3,44,45]). In this context, elucidation of specific functions of sugar chains of HGF in physiological processes in vivo such as tissue regeneration remains to be addressed. However, similarity in characteristics between non-glycosylated HGF and native HGF seems to afford non-glycosylated HGF an advantage in potential therapeutic approaches. Since it seems feasible to mass-produce non-glycosylated HGF in non-mammalian host cells, such as yeasts or insect cells, non-glycosylated HGF may serve as an alternative for glycosylated HGF. On the other hand, sugar chains of proteins regulate profiles of their circulating levels and tissue distribution through specific interactions between sugar chains and their binding molecules. We speculate that creation of engineered HGF with unique characteristics, e.g. targeted delivery to specific tissues, may possibly be feasible by modification of sugar chains on HGF.
Multimedia adjunct
Acknowledgments
We thank Professor Junichi Miyazaki (Osaka University) for providing the pCAGGS vector, and M. Ohara (Fukuoka, Japan) for language assistance. This work was supported by grants from the Ministry of Education, Science, Technology, Sports and Culture of Japan (Nos. 12215082 and 14207005 to T.N., and the 21st Century COE programme to T.N.).
References
- 1.Nakamura T., Nawa K., Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem. Biophys. Res. Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A., Tashiro K., Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature (London) 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto K., Nakamura T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem. Biophys. Res. Commun. 1997;239:639–644. doi: 10.1006/bbrc.1997.7517. [DOI] [PubMed] [Google Scholar]
- 4.Birchmeier C., Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 5.Bottaro D. P., Rubin J. S., Faletto D. L., Chan A. M., Kmiecik T. E., Vande Woude G. F., Aaronson S. A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 6.Miyazawa K., Shimomura T., Kitamura A., Kondo J., Morimoto Y., Kitamura N. Molecular cloning and sequence analysis of the cDNA for a human serine protease responsible for activation of hepatocyte growth factor: structural similarity of the protease precursor to blood coagulation factor XII. J. Biol. Chem. 1993;268:10024–10028. [PubMed] [Google Scholar]
- 7.Naldini L., Vigna E., Bardelli A., Follenzi A., Galimi F., Comoglio P. M. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J. Biol. Chem. 1995;270:603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa K., Tsubouchi H., Naka D., Takahashi K., Okigaki M., Arakaki N., Nakayama H., Hirono S., Sakiyama O., Takahashi K., et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem. Biophys. Res. Commun. 1989;163:967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- 9.Hara H., Nakae Y., Sogabe T., Ihara I., Ueno S., Sakai H., Inoue H., Shimizu S., Nakamura T., Shimizu N. Structural study of the N-linked oligosaccharides of hepatocyte growth factor by two-dimensional sugar mapping. J. Biochem. (Tokyo) 1993;114:76–82. doi: 10.1093/oxfordjournals.jbchem.a124143. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu N., Hara H., Sogabe T., Sakai H., Ihara I., Inoue H., Nakamura T., Shimizu S. Hepatocyte growth factor is linked by O-glycosylated oligosaccharide on the α chain. Biochem. Biophys. Res. Commun. 1992;189:1329–1335. doi: 10.1016/0006-291x(92)90219-b. [DOI] [PubMed] [Google Scholar]
- 11.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- 13.Rudd P. M., Elliott T., Cresswell P., Wilson I. A., Dwek R. A. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 14.Seki T., Ihara I., Sugimura A., Shimonishi M., Nishizawa T., Asami O., Hagiya M., Nakamura T., Shimizu S. Isolation and expression of cDNA for different forms of hepatocyte growth factor from human leukocyte. Biochem. Biophys. Res. Commun. 1990;172:321–327. doi: 10.1016/s0006-291x(05)80212-8. [DOI] [PubMed] [Google Scholar]
- 15.Niwa H., Yamamura K., Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto K., Tajima H., Hamanoue M., Kohno S., Kinoshita T., Nakamura T. Identification and characterization of “injurin”, an inducer of expression of the gene for hepatocyte growth factor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:3800–3804. doi: 10.1073/pnas.89.9.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto K., Kataoka H., Date K., Nakamura T. Cooperative interaction between α- and β-chains of hepatocyte growth factor on c-Met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J. Biol. Chem. 1998;273:22913–22920. doi: 10.1074/jbc.273.36.22913. [DOI] [PubMed] [Google Scholar]
- 18.Mizuno K., Inoue H., Hagiya M., Shimizu S., Nose T., Shimohigashi Y., Nakamura T. Hairpin loop and second kringle domain are essential sites for heparin binding and biological activity of hepatocyte growth factor. J. Biol. Chem. 1994;269:1131–1136. [PubMed] [Google Scholar]
- 19.Zhou H., Casas-Finet J. R., Heath Coats R., Kaufman J. D., Stahl S. J., Wingfield P. T., Rubin J. S., Bottaro D. P., Byrd R. A. Identification and dynamics of a heparin-binding site in hepatocyte growth factor. Biochemistry. 1999;38:14793–14802. doi: 10.1021/bi9908641. [DOI] [PubMed] [Google Scholar]
- 20.Lietha D., Chirgadze D. Y., Mulloy B., Blundell T. L., Gherardi E. Crystal structures of NK1-heparin complexes reveal the basis for NK1 activity and enable engineering of potent agonists of the MET receptor. EMBO J. 2001;20:5543–5555. doi: 10.1093/emboj/20.20.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamaguchi K., Akai K., Kawanishi G., Ueda M., Masuda S., Sasaki R. Effects of site-directed removal of N-glycosylation sites in human erythropoietin on its production and biological properties. J. Biol. Chem. 1991;266:20434–20439. [PubMed] [Google Scholar]
- 22.Sareneva T., Pirhonen J., Cantell K., Kalkkinen N., Julkunen I. Role of N-glycosylation in the synthesis, dimerization and secretion of human interferon-γ. Biochem. J. 1994;303:831–840. doi: 10.1042/bj3030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velan B., Kronman C., Ordentlich A., Flashner Y., Leitner M., Cohen S., Shafferman A. N-glycosylation of human acetylcholinesterase: effects on activity, stability and biosynthesis. Biochem. J. 1993;296:649–656. doi: 10.1042/bj2960649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehrman M. A. Oligosaccharide-based information in endoplasmic reticulum quality control and other biological systems. J. Biol. Chem. 2001;276:8623–8626. doi: 10.1074/jbc.R100002200. [DOI] [PubMed] [Google Scholar]
- 25.Spiro R. G. Glucose residues as key determinants in the biosynthesis and quality control of glycoproteins with N-linked oligosaccharides. J. Biol. Chem. 2000;275:35657–35660. doi: 10.1074/jbc.R000022200. [DOI] [PubMed] [Google Scholar]
- 26.Montesano R., Matsumoto K., Nakamura T., Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 27.Nakagami H., Morishita R., Yamamoto K., Taniyama Y., Aoki M., Matsumoto K., Nakamura T., Kaneda Y., Horiuchi M., Ogihara T. Mitogenic and antiapoptotic actions of hepatocyte growth factor through ERK, STAT3, and AKT in endothelial cells. Hypertension. 2001;37:581–586. doi: 10.1161/01.hyp.37.2.581. [DOI] [PubMed] [Google Scholar]
- 28.Tanimura S., Chatani Y., Hoshino R., Sato M., Watanabe S., Kataoka T., Nakamura T., Kohno M. Activation of the 41/43 kDa mitogen-activated protein kinase signaling pathway is required for hepatocyte growth factor-induced cell scattering. Oncogene. 1998;17:57–65. doi: 10.1038/sj.onc.1201905. [DOI] [PubMed] [Google Scholar]
- 29.Paumelle R., Tulasne D., Leroy C., Coll J., Vandenbunder B., Fafeur V. Sequential activation of ERK and repression of JNK by scatter factor/hepatocyte growth factor in Madin–Darby canine kidney epithelial cells. Mol. Biol. Cell. 2000;11:3751–3763. doi: 10.1091/mbc.11.11.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khwaja A., Lehmann K., Marte B. M., Downward J. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J. Biol. Chem. 1998;273:18793–18801. doi: 10.1074/jbc.273.30.18793. [DOI] [PubMed] [Google Scholar]
- 31.Hong F., Nguyen V. A., Shen X., Kunos G., Gao B. Rapid activation of protein kinase B/Akt has a key role in antiapoptotic signaling during liver regeneration. Biochem. Biophys. Res. Commun. 2000;279:974–979. doi: 10.1006/bbrc.2000.4044. [DOI] [PubMed] [Google Scholar]
- 32.Kandel E. S., Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 33.Liu K. X., Kato Y., Narukawa M., Kim D. C., Hanano M., Higuchi O., Nakamura T., Sugiyama Y. Importance of the liver in plasma clearance of hepatocyte growth factors in rats. Am. J. Physiol. 1992;263:G642–G649. doi: 10.1152/ajpgi.1992.263.5.G642. [DOI] [PubMed] [Google Scholar]
- 34.Appasamy R., Tanabe M., Murase N., Zarnegar R., Venkataramanan R., Van Thiel D. H., Michalopoulos G. K. Hepatocyte growth factor, blood clearance, organ uptake, and biliary excretion in normal and partially hepatectomized rats. Lab. Invest. 1993;68:270–276. [PubMed] [Google Scholar]
- 35.Zioncheck T. F., Richardson L., DeGuzman G. G., Modi N. B., Hansen S. E., Godowski P. J. The pharmacokinetics, tissue localization, and metabolic processing of recombinant human hepatocyte growth factor after intravenous administration in rats. Endocrinology. 1994;134:1879–1887. doi: 10.1210/endo.134.4.8137756. [DOI] [PubMed] [Google Scholar]
- 36.Hofmann R., Joseph A., Bhargava M. M., Rosen E. M., Goldberg I. Scatter factor is a glycoprotein but glycosylation is not required for its activity. Biochim. Biophys. Acta. 1992;1120:343–350. doi: 10.1016/0167-4838(92)90258-f. [DOI] [PubMed] [Google Scholar]
- 37.Sareneva T., Cantell K., Pyhala L., Pirhonen J., Julkunen I. Effect of carbohydrates on the pharmacokinetics of human interferon-γ. J. Interferon Res. 1993;13:267–269. doi: 10.1089/jir.1993.13.267. [DOI] [PubMed] [Google Scholar]
- 38.Takamatsu S., Fukuta K., Asanagi M., Abe R., Yokomatsu T., Fujibayashi Y., Makino T. Monitoring biodistribution of glycoproteins with modified sugar chains. Biochim. Biophys. Acta. 2003;1622:179–191. doi: 10.1016/s0304-4165(03)00141-7. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto M., Nakai M., Nakayama C., Yanagi H., Matsui H., Noguchi H., Namiki M., Sakai J., Kadota K., Fukui M., Hara H. Purification and characterization of three forms of differently glycosylated recombinant human granulocyte-macrophage colony-stimulating factor. Arch. Biochem. Biophys. 1991;286:562–568. doi: 10.1016/0003-9861(91)90080-3. [DOI] [PubMed] [Google Scholar]
- 40.Sebok K., Meloche S., Sairam M. R. Pharmacokinetic analysis of the plasma disappearance of ovine follitropin and analogues in the male rat. Life Sci. 1990;46:927–934. doi: 10.1016/0024-3205(90)90094-8. [DOI] [PubMed] [Google Scholar]
- 41.Maack T., Johnson V., Kau S. T., Figueiredo J., Sigulem D. Renal filtration, transport, and metabolism of low-molecular-weight proteins: a review. Kidney Int. 1979;16:251–270. doi: 10.1038/ki.1979.128. [DOI] [PubMed] [Google Scholar]
- 42.Mihara K., Hojo T., Fujikawa M., Takakura Y., Sezaki H., Hashida M. Disposition characteristics of protein drugs in the perfused rat kidney. Pharm. Res. 1993;10:823–827. doi: 10.1023/a:1018996808153. [DOI] [PubMed] [Google Scholar]
- 43.Kato Y., Liu K. X., Nakamura T., Sugiyama Y. Heparin-hepatocyte growth factor complex with low plasma clearance and retained hepatocyte proliferating activity. Hepatology. 1994;20:417–424. [PubMed] [Google Scholar]
- 44.Matsumoto K., Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- 45.Funakoshi H., Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clin. Chim. Acta. 2003;327:1–23. doi: 10.1016/s0009-8981(02)00302-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.