Abstract
The cardinal pathophysiology of Parkinson’s disease (PD) is considered to be the increase in the activities of basal ganglia (BG) output nuclei, which excessively inhibits the thalamus and superior colliculus (SC) and causes preferential impairment of internal over external movements. Here we recorded saccade performance in 66 patients with PD and 87 age-matched controls, and studied how the abnormality changed with disease progression. PD patients were impaired not only in memory guided saccades, but also in visually guided saccades, beginning in the relatively early stages of the disease. On the other hand, they were impaired in suppressing reflexive saccades (saccades to cue). All these changes deteriorated with disease progression. The frequency of reflexive saccades showed a negative correlation with the latency of visually guided saccades and Unified Parkinson’s Disease Rating Scale motor subscores reflecting dopaminergic function. We suggest that three major drives converging on SC determine the saccade abnormalities in PD. The impairment in visually and memory guided saccades may be caused by the excessive inhibition of the SC due to the increased BG output and the decreased activity of the frontal cortex-BG circuit. The impaired suppression of reflexive saccades may be explained if the excessive inhibition of SC is “leaky.” Changes in saccade parameters suggest that frontal cortex-BG circuit activity decreases with disease progression, whereas SC inhibition stays relatively mild in comparison throughout the course of the disease. Finally, SC disinhibition due to leaky suppression may represent functional compensation from neural structures outside BG, leading to hyper-reflexivity of saccades and milder clinical symptoms.
Keywords: Parkinson’s disease, Saccades, Superior colliculus, Inhibition, Basal ganglia
1. Introduction
Although most of the anatomical connections and physiology of the basal ganglia (BG) within the oculomotor network have been studied in primates (Hikosaka & Wurtz, 1989; Kato et al., 1995; Kori et al., 1995), a similar oculomotor system organization has been suggested in humans by means of lesion (see Pierrot-Deseilligny, Milea, & Muri, 2004 for review) and neuroimaging studies (Brown et al., 2004; O’Driscoll et al., 1995; Sweeney et al., 1996). Outputs from BG reach the superior colliculus (SC) via the substantia nigra pars reticulata (SNr), where the SC serves as the common terminal for controlling visually and memory guided saccades, with converging commands arriving through the BG–SC pathway and cortex-SC pathways (Hikosaka & Wurtz, 1989). Since eye movement reflects the output of BG relatively directly, saccade recordings can provide insights into the pathophysiology underlying neurological disorders at the systems level, especially for BG disorders such as Parkinson’s disease (PD) (Hikosaka & Wurtz, 1989). The SC is spared until the later stages of the disease, even when other neural structures, including the cerebral cortex, become affected (see Jellinger, 2001 for review). Cortical mechanisms could also explain the predominant impairment of voluntary saccades, such as functional changes in the frontal eye field and prefrontal cortices, especially in the later stages of the disease. We thus considered it important to examine the pathophysiology of PD using saccade performance as an indicator of BG function.
Although a number of human and primate studies have been conducted in this area, some aspects of the pathophysiology of PD remain unclear. The diagram presented by Alexander and Crutcher (1990) provides relatively clear explanations for Parkinsonian symptoms. In PD, GABAergic inhibitory projection neurons of the BG output nuclei, the globus pallidus interna (GPi), and SNr exhibit overactive tonic firing rates and produce excessive suppression of the thalamus and the SC (Albin, Young, & Penney, 1995). This may prevent the thalamus from producing quick movements of appropriate size, resulting in bradykinesia/akinesia. As for oculomotor control, the SC is excessively inhibited by the overactive SNr and all types of saccades are thus suppressed. Indeed, saccades are hypometric and require a multistep sequence to reach the target in PD. Although highly simplified in some respects, the general framework of this classical model provides a good starting point and conceptual guide to understand the pathophysiology of PD.
Excessive SC inhibition, however, does not explain all aspects of saccade abnormalities in PD. Most studies have found marked impairment of memory guided saccades (MGS), saccades made to remembered target locations in the absence of a visual target, whereas visually guided saccades, which are made to targets appearing at unpredictable locations, are relatively spared (e.g., Briand, Strallow, Hening, Poizner, & Sereno, 1999; Bronstein & Kennard, 1985; Vidailhet et al., 1994). The involvement of the BG in PD appears to provide a good explanation for this dissociation, since different neural structures subserve both types of saccades. For the visually guided saccades, the parietal eye field, including the posterior parietal cortex, mainly integrates visuospatial information to generate a motor signal that is sent to the SC via the parietal lobe-SC pathway (Gaymard, Lynch, Ploner, Condy, & Rivaud-Péchoux, 2003). In contrast, the processing for MGS, a voluntary saccade, mainly takes place in the frontal lobe, where the motor signal is emitted directly or via the BG to the SC (the direct pathway of the BG circuit). A phasic reduction from the high resting rates of the SNr temporarily releases the saccade cells in the recipient SC, resulting in the generation of voluntary saccades (Hikosaka & Wurtz, 1985a, 1985b, 1989).
However, it is not only the voluntary saccades such as MGS that are affected but also visually guided saccades. Other saccade abnormalities in PD do not fit in with the aforementioned views. Along with the impairment of memory guided saccades, some studies have found a mild but definite abnormality of visually guided saccades (Rascol et al., 1989; Shibasaki, Tsuji, & Kuroiwa, 1979). PD patients also have difficulty in suppressing unwanted saccades to the novel appearance of visual targets (Chan, Armstrong, Pari, Riopelle, & Munoz, 2005; Joti, Kulashekhar, Behari, & Murthy, 2007; Kitagawa, Fukushima, & Tashiro, 1994; van Koningsbruggen, Pender, Machado, & Rafal, 2009; van Stockum, MacAskill, Anderson, & Dalrymple-Alford, 2008), which is also an important function of the BG.
The drive to suppress the excitability of the SC and impair saccade initiation and that leading to its “hyper-reflexivity” are in apparent contradiction if they were to converge in the SC. Excessive inhibition of SC would tend to prolong latencies of visually guided saccades. Conversely, if “hyper-reflexivity” predominates, we would expect that the latency of visually guided saccades would be shortened. Indeed, there is no agreement among many of the above studies as to whether the latency of visually guided saccades, including VGS and gap saccades (see below), is shortened or prolonged. A recent meta-analytic review across 47 studies (Chambers & Prescott, 2010) suggested that PD patients initiate saccades faster than controls at small target eccentricities, while they respond more slowly for large eccentricities. However, the target eccentricity effect has not been formally ascertained in a single study, and how this trend happens and also how it relates to the pathophysiology of PD remains entirely unknown.
Altogether, a comprehensive explanation that can integrate all aspects of saccade abnormalities is still lacking. Although anatomical studies have shown that the FEF (Stanton, Deng, Goldberg, & McMullen, 1989) and SEF (Shook, Schlag-Rey, & Schlag, 1990) innervate the brainstem saccade generator directly, direct fronto-reticular projections from frontal eye field do not appear to be sufficient to evoke saccades (Hanes & Wurtz, 2001) and thus the majority of cortical saccade-related brain areas influence saccade generation by projections to SC neurons, which then project to the brainstem saccade generators (Johnston & Everling, 2008). The present study aimed to clarify the pathophysiology of PD through saccades from the perspective of the SC. For this purpose, unlike previous studies, we considered it important to study saccades toward targets of different eccentricities (5–30°), since saccades of large and small amplitudes may be generated by a different mechanism and the extent to which inhibition is involved may also differ (van Donkelaar, Saavedra, & Woollacott, 2007). We studied saccade parameters in PD patients at various stages to investigate how the initiation and inhibitory control of saccades varied with the advance of the disease. To measure the inhibitory control of saccades, we studied the frequency of saccades to cue, that is, inadvertent saccades made to a visual cue presented during an MGS task, instead of directional errors in the antisaccade task. Insights into the pathophysiology of PD would support the usefulness of saccadic parameters as a biomarker for gauging the progression of the disease (Blekher et al., 2009) and would also help in optimizing the treatment. This study has been previously presented in abstract form (Terao et al., 2007).
2. Methods
2.1. Subjects
Sixty-six PD patients (36 men, 30 women, age: 66.7rf ± 10.6, Hoehn and Yahr [H–Y] stages 1–4.5 (on medication), disease duration: 6.5 ± 5.3, Table 1) were studied, with a mean Unified Parkinson’s Disease Rating Scale (UPDRS) motor score (on medication) of 26.8 ± 11.3 (range: 7–56.5). Patients with mini-mental state examination scores of less than 25 and those with psychiatric and affective disorders were excluded. We also collected control data from 89 age-matched normal subjects (42 men, 47 women, age: 65.6 ± 4.3).
Table 1.
Patient characteristics.
| Subject group | Subject | Male | Female | Age (yr) | Onset age (yr) | Duration (yrs) | UPDRS motor score | Dopa equivalent dose (mg) | RT (ms) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Normal | 89 | 42 | 47 | 65.6 ± 4.3 | – | – | – | – | 417.6 ± 123.7 |
| H–Y stage 1 | 11 | 9 | 2 | 61.4 ± 10.9 | 58.0 ± 13.0 | 4.2 ± 3.5 | 12.1 ± 4.0 | 152.6 ± 194.2 | 429.3 ± 97.7 |
| H–Y stage 2 | 19 | 10 | 9 | 67.8 ± 9.8 | 62.3 ± 12.2 | 5.5 ± 3.8 | 21.4 ± 6.7 | 233.7 ± 203.6 | 573.4 ± 151.0 |
| H–Y stage 3 | 29 | 14 | 15 | 66.5 ± 9.7 | 59.6 ± 12.7 | 6.8 ± 5.2 | 31.0 ± 7.0 | 259.1 ± 229.2 | 642.6 ± 215.2 |
| H–Y stage 4 | 7 | 3 | 4 | 72.0 ± 12.0 | 60.2 ± 18.7 | 11.8 ± 7.3 | 45.1 ± 6.3 | 439.0 ± 184.7 | 783.1 ± 168.9 |
Experiments were conducted as part of clinical assessment according to the guidelines of the local ethical committee after obtaining informed consent. Both for ethical and practical reasons, patients had to continue taking their regular doses of medication. This was because many of the studied patients were outpatients who had to commute to visit our hospital to take the tests. In addition, some advanced patients could not do without dopaminergic medication to carry on with their daily life, and were not able to properly perform the task trials, which were all started by a button press and terminated by a button release (see below).
PD medications can influence parameters of saccadic eye movement (Michell et al., 2006). To cope with this problem, we studied the saccade performance in 10 denovo PD patients in preliminary experiments, who took their first dose of l-dopa (100–200 mg). We followed up the saccade performance of VGS and MGS for 4 h (Yugeta, Terao, Fukuda, & Ugawa, 2008). There was a small effect both on latency and amplitude of visually and memory guided saccades, which effect was maximal at around 1–2 h but faded away by 3 h of intake. Thus, the subjects’ performance was measured 4 h after the final intake of l-dopa in the morning.
For statistical assessment, patients with H–Y stages 1–1.5 were assigned a disease stage of 1 (11 patients); those with H–Y stages of 2–2.5 stage 2 (19 patients); those with H–Y stages of 3–3.5 stage 3 (29 patients); and those with higher disease stages were assigned a stage of 4 (7 patients).
2.2. Experimental setup
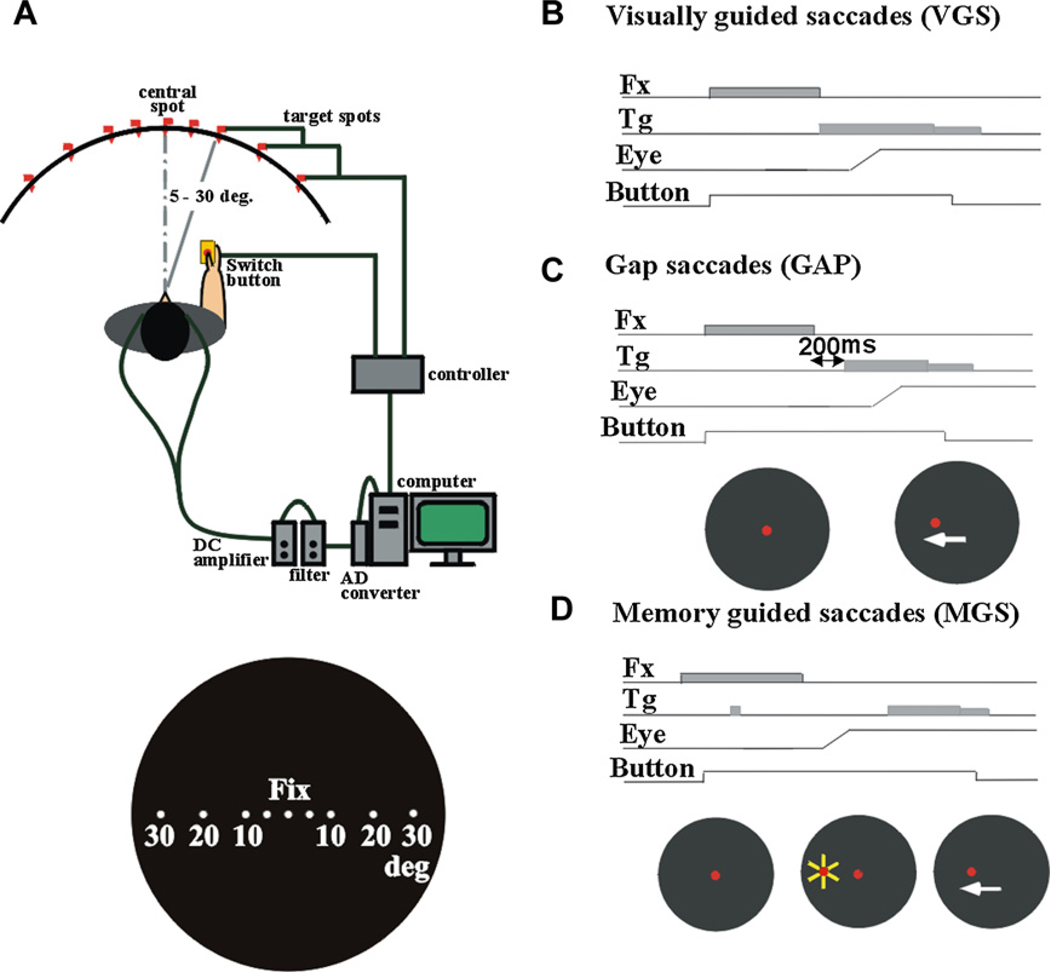
Subjects were seated in front of a black, concave dome-shaped screen of 90-cm diameter that contained light-emitting diodes which served as the fixation points and saccade targets. Their heads were placed on a chin rest to restrict head movements (Kato et al., 1995; Terao et al., 1998; Fig. 1A). They faced the center of the screen at a viewing distance of ~66 cm. The subjects held a microswitch button which was connected to the microcomputer and allowed them to initiate a trial by pressing the button with their thumb.
Fig. 1.
Experimental setup (A) and oculomotor tasks (B: VGS, C: GAP, D: MGS).
Horizontal electro-oculographic (EOG) recordings were made using two Ag–AgCl gel electrodes placed at the bilateral outer canthi. Vertical EOG recordings were made using electrodes placed above and below the right eye. The signals were fed to a DC amplifier (AN-601G, Nihon Kohden, Tokyo, Japan) that was low-pass filtered at 20 Hz and then digitized (500 Hz). The impedance was monitored and maintained below 5 kΩ throughout the measurements. Eye movement calibration was administered before each test session. A target appeared 20° to the left and right of the fixation point. While the subjects fixated on this spot, we adjusted the EOG gain so that the eye position displayed on the computer monitor matched the target position displayed on the screen.
2.3. Behavioral paradigms
We used two types of visually guided saccades, VGS and gap saccade (GAP), as well as the MGS task. In VGS (Fig. 1B), a central spot of light on which subjects were required to fixate was turned on shortly after they pressed a button. After a random period of time (1.2–2.0 s), the fixation spot was extinguished, and a target stimulus simultaneously appeared randomly at various eccentricities, 5, 10, 20, or 30 degrees to the left or right of it. We instructed the subjects to foveate the target as quickly as possible.
The GAP task (Fig. 1C) was identical to the VGS task, except that the target spot was turned on 200 ms after the fixation point was extinguished.
In the MGS task (Fig. 1D), while the subject fixated on the central spot, a peripheral stimulus (“cue”) appeared for a brief period of 50 ms. The subjects were required to maintain visual fixation until the fixation point was turned off (delay period), when the subjects had to make a saccade based on their memory to the spatial location where the cue had appeared. The target spot turned on again 600 ms after the offset of the fixation point. Saccades unintentionally made to the cue during the delay period were termed saccades to cue.
Additionally, a hand reaction time (RT) task was implemented. A central spot of light came on shortly after the subject pressed a button and it stayed on throughout each trial. The subjects were instructed to fixate on it continuously. After a random period (2–6.5 s), another spot came on at one of the various eccentricities and the subjects released the button as soon as possible while fixating on the central cross point. Inadvertent saccades made to the target were termed saccades to target.
The subjects performed 50 trials each of VGS, GAP, and MGS tasks and 40 trials of the hand RT task (20 trials for each hand), for a total of 190 trials. The total duration of examination ranged from 30 to 40 min. In the following, we use the term visually guided saccades to indicate both VGS and GAP because these are tasks in which saccades are guided by the visual signals. Abbreviations such as VGS and GAP will be used to denote the individual tasks.
2.4. Data analysis and statistical assessment
Four parameters were determined off-line for each saccade: onset latency, amplitude, duration, and peak velocity. The onset of an eye movement was defined as the time when velocity and acceleration exceeded predetermined values (28°/s and 90°/s2, respectively). Eye movement was accepted as a saccade based on its velocity and duration. After the onset, the velocity had to exceed 88°/s, and this suprathreshold velocity had to be maintained for at least 10 ms. The end of an eye movement was considered to have occurred when the velocity decreased below 40°/s. The total duration had to exceed 30 ms. Records contaminated by noise and those with onset latency < 100 ms were excluded from the analysis. Hand RT was measured from the time of target presentation to that of button release. Accuracy of the first saccade amplitude was expressed as a ratio in percentages to the target eccentricity.
Statistical analyses were conducted using SPSS 10 software (SPSS Japan, Inc., Tokyo). First, saccade parameters (latencies and accuracies of VGS, GAP, and MGS as well as the frequencies of saccades to cue and saccades to target) and hand RT were entered into repeated-measures analysis of variance (ANOVA), with group (PD patients and normal controls) as a between-subject factor and target eccentricity as a within-subject factor. Where necessary, we corrected for sphericity by the Greenhouse and Geisser correction. The significance criterion was set at p < 0.05. The effect size was assessed by partial eta squared (ηp2). For each ANOVA, post hoc analysis by Bonferroni’s method was performed to determine what differences contributed to the significance detected. Second, saccade parameters were subjected to ANOVA with disease stage as a between-subject and target eccentricity as a within-subject factor to determine how they changed with disease progression. Since the number of subjects differed for different disease stages, the equality of error variance was assessed using the Levene’s test. Third, we looked at the correlation of the saccade latencies in various tasks with the frequency of saccades to cue using the Pearson’s product-moment correlation test. The p-value for correlation was calculated by means of Fisher’s z-transformation applied to the sample correlation coefficient r. We also correlated the frequency of saccades to cue with subscores of UPDRS part III. Similar analyses were performed after splitting the subjects into patients with disease stages 1–2 and stages 3–4.
Saccade parameters are known to be influenced by age (Munoz et al., 1998). To take account of the possible effect of age, we used the analysis of covariance (ANCOVA) with age as a covariate to see whether this factor showed any interaction with other factors of interest. We analyzed this effect by taking age as a covariate for ANCOVA. Age did not interact significantly with disease stage for VGS latency [age × disease stage: F(4,616) = 0.209, p = 0.933], MGS latency [F(4,612) = 0.666, p = 0.617], VGS accuracy [F(4,616) = 1.623, p = 0.172], MGS accuracy [F(4,612) = 0.506, p = 0.732], MGS success rate: F(4,544) = 1.018, p = 0.401], or for frequencies of saccades to cue [F(4,612) = 1.459, p = 0.218] and saccades to target [F(4,612) = 0.860, p = 0.490]. However, age showed a slight interaction with disease stage only for GAP accuracy [F(4,520) = 2.918, p = 0.020]. Therefore, overall, age and disease stage were considered not to be significantly related in this sample for these parameters. In the following analysis, we consider the effect of disease stage as largely independent of age.
3. Results
3.1. Impaired initiation and inhibitory control of saccades in PD
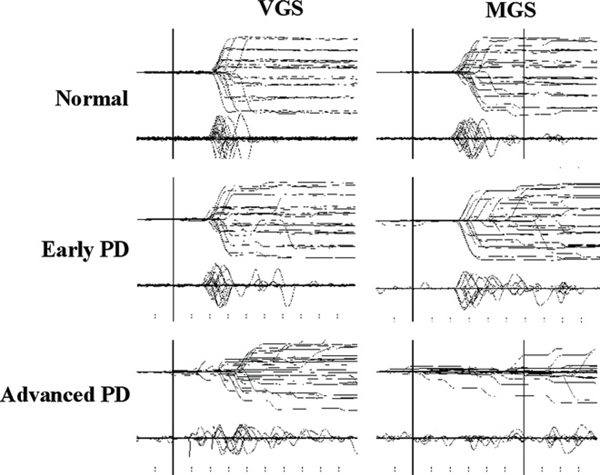
Fig. 2 presents typical examples of MGS and VGS traces recorded in a normal subject and two PD patients at different stages. In a patient with early PD patient (middle row), the onset of MGS was delayed and more variable compared to that of normal subjects at eccentricities of 5–10 degrees (Fig. 2), but not at 30 degrees. In a patient with more advanced PD (bottom row), the onset of MGS became markedly delayed for all eccentricities. Within the preset time limit of 600 ms, the patient in the figure succeeded in initiating MGS in 25% of trials (6/24 trials); many of these were saccades of very small amplitude. In contrast, the normal subject could successfully initiate MGS in almost every trial.
Fig. 2.
Traces of VGS and MGS. Traces of VGS and MGS in a normal subject (top row) and patients with early and advanced PD (middle and bottom rows). Twenty to thirty trials of eye movements (upper traces) and velocity curves (lower traces) are superimposed, time-locked to the presentation of the target (VGS) or to the offset of the central fixation point (MGS). The abscissa is the time axis and the ordinate gives the angle.
VGS was also affected, but to a lesser degree. Saccades toward targets at 5–10 degrees from the central fixation point were initiated slightly faster compared to the normal subject, whereas the latency toward targets at 20–30 degrees was similar. As a result, the traces for smaller saccades appeared more prominent than and to protrude out from the others.
Both VGS and MGS in PD patients toward larger eccentricities (20–30°) often consisted of an initial hypometric saccade followed by multiple corrective saccades. While normal subjects tended to undershoot target eccentricities of 20–30 degrees by 10–15%, PD patients tended to undershoot by approximately 10–20% in VGS and 40–50% in MGS. In contrast, smaller eccentricities of 5–10 degrees were usually covered by a single saccade both in normal subjects and PD patients.
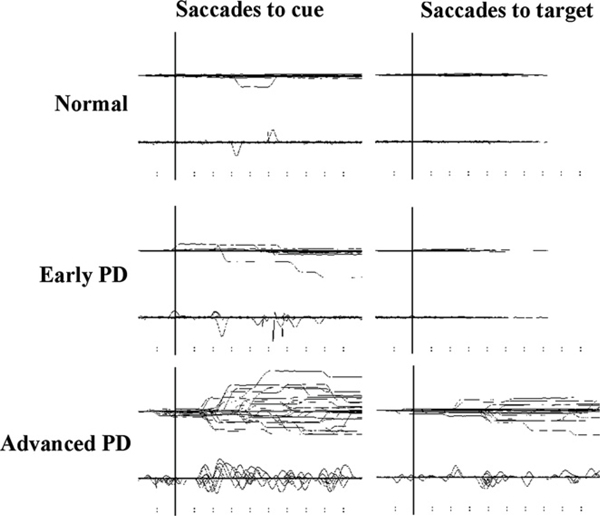
PD patients not only had difficulty in initiating saccades, but also in suppressing unwanted saccades. During the delay period in MGS and the hand RT task, PD patients often made inadvertent saccades toward the briefly presented cue, especially at smaller eccentricities (Fig. 3). These occurred despite repeated instructions and were therefore not because the subjects failed to understand the task. The unsuppressed saccades tended to increase with disease stage.
Fig. 3.
Traces of saccades to cue and saccades to target. Inadvertent eye movements made during the delay period of MGS (saccades to cues) and during the hand RT task (saccades to target) in a normal subject (top row) and patients with early and advanced PD (middle and bottom rows).
3.2. Abnormalities of saccade parameters in PD
Table 2 compares the saccade parameters of normal controls and PD patients. The latencies of VGS and GAP were significantly longer (VGS: F[1,153] = 38.535, p < 0.0001, ηp2 = 0.201; GAP: F[1,129] = 5.167, p < 0.025, ηp2 = 0.039) and their accuracy significantly reduced (VGS: F[1,153] = 45.922, p < 0.0001, ηp2 = 0.211, GAP: F[1,129] = 57.538, p < 0.0001, ηp2 = 0.249) compared to control subjects. The latency of MGS was greatly prolonged (F[1,152] = 37.768, p < 0.0001, ηp2 = 0.199) and accuracy more reduced (F[1,138] = 101.344, p < 0.0001, ηp2 = 0.450). The MGS success rate within 600 ms was lower than control subjects (F[1,152] = 24.134, p < 0.0001, ηp2 = 0.137).
Table 2.
Saccade parameters of normal controls and PD patients.
| Parameter | Normal | PD | |
|---|---|---|---|
|
| |||
| VGS | Latency (ms) | 232.9±35.4 | 282.2±62.6 |
| Accuracy (%) | 96.6 ± 5.1 | 85.6 ± 15.1 | |
| GAP | Latency (ms) | 202.9 ± 55.0 | 224.9 ± 54.5 |
| Accuracy (%) | 95.9 ± 4.7 | 83.3 ± 13.3 | |
| MGS | Latency (ms) | 415.8 ± 166.0 | 581.9 ± 165.3 |
| Accuracy (%) | 86.6 ± 13.6 | 57.4 ± 20.1 | |
| Success rate of MGS (%) | 88.7 ± 10.1 | 69.5 ± 21.6 | |
| Frequency of saccades to cue (%) | 24.2 ± 17.3 | 46.2 ± 23.9 | |
| Frequency of saccades to target (%) | 13.7 ± 22.0 | 32.0 ± 28.7 (mean ± SD) |
|
The frequencies of saccades to cue and saccades to target were higher in patients than in controls (saccades to cue F[1,152] = 53.970, p < 0.0001; saccades to target F[1,140] = 22.312, p < 0.0001, ηp2 = 0.137). Hand RT was significantly prolonged in PD (F[1,135] = 67.467, p < 0.0001, ηp2 = 0.282; Table 2).
3.3. The influence of target eccentricity on saccade parameters
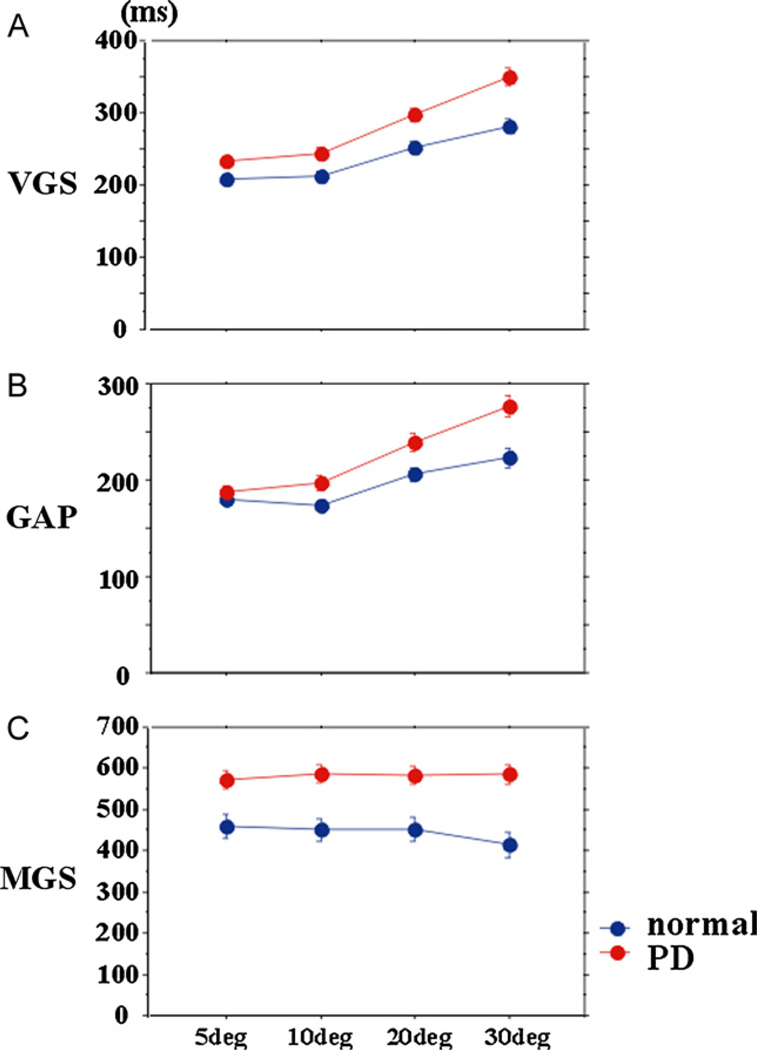
The latency of visually guided saccades was influenced by target eccentricity. VGS latency in PD patients was within the normal range at smaller eccentricities (5–10°), but was prolonged at larger eccentricities (20–30°) (Fig. 4A; effect of eccentricity: F[1.859,284.434] = 198.520, p < 0.0001, ηp2 = 0.565; eccentricity × group: F[1.859,284.434] = 18.547, p < 0.0001, ηp2 = 0.108). A similar trend was noted for GAP (Fig. 4B; effect of group: effect of eccentricity: F[2.391,308.4] = 153.779, p < 0.0001, ηp2 = 0.418; group × eccentricity: F[2.391, 308.4] = 27.559, p < 0.0001, ηp2 = 0.107).
Fig. 4.
Saccade latencies at different eccentricities. The latency of VGS, GAP, and MGS plotted as a function of target eccentricity (abscissa). The red curve represents PD patients and the blue curve represents normal subjects. The parameters were averaged for each subject, and the mean and standard errors for each parameter was computed for each parameter. Error bars indicate standard errors in this and the following figures.
In contrast, MGS latency was less dependent on target eccentricity. MGS latency in PD patients was similarly prolonged across all eccentricities (Fig. 4C; MGS latency: effect of eccentricity: F[2.669,405.709] = 2.060, p = 0.112, ηp2 = 0.013, group × eccentricity: F[2.669,405.709] = 0.778, p = 0.501, ηp2 = 0.005). Furthermore, the success rate of MGS decreased in PD patients (MGS success rate: effect of eccentricity: F[2.755,418.76] = 7.491, p < 0.0001, ηp2 = 0.047, group × eccentricity: F[2.755,418.76] = 0.439, p = 0.709, ηp2 = 0.003).
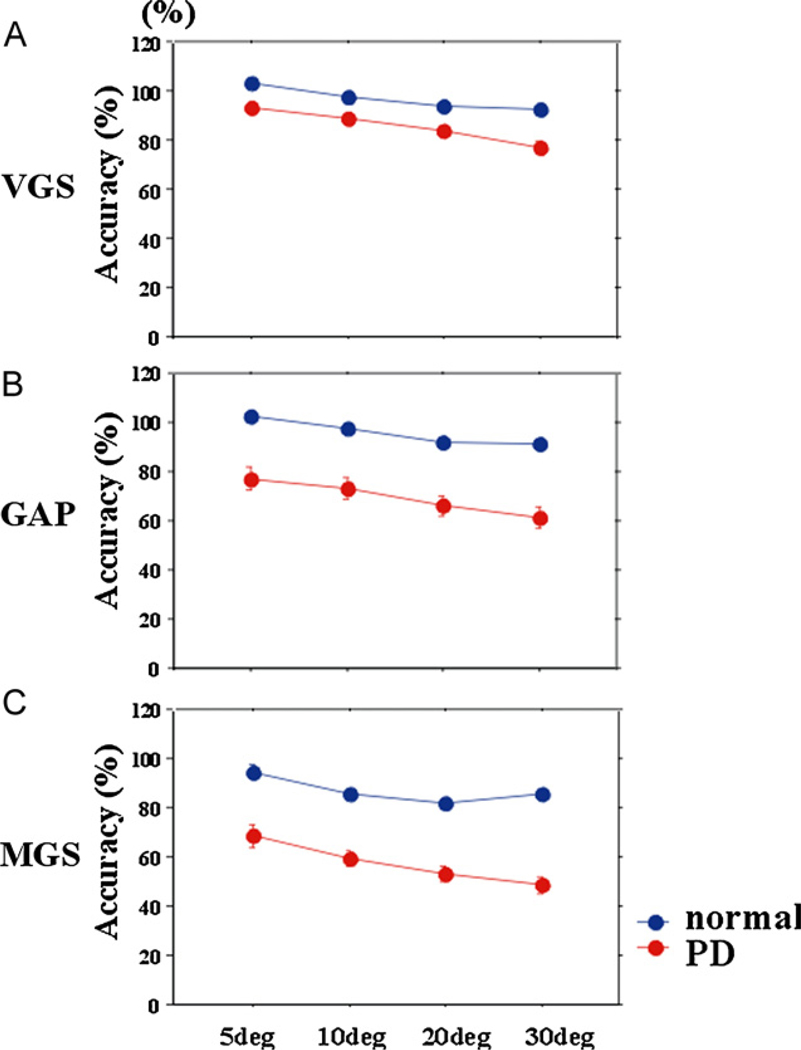
The accuracy of both visually and memory guided saccades was significantly or showed a trend to be reduced, especially at larger target eccentricities (Fig. 5; VGS: effect of eccentricity: F[2.195,335.870] = 86.927, p < 0.0001, ηp2 = 0.362,eccentricity × group F[2.195,335.870] = 5.911, p = 0.002, ηp2 = 0.037; GAP: effect of eccentricity: F[1.822,255.116] = 64.382, p < 0.0001, ηp2 = 0.314, eccentricity × group F[1.822,255.116] = 2.974, p = 0.058, ηp2 = 0.20; MGS: effect of eccentricity: F[2.054,262.912] = 15.647, p < 0.0001, ηp2 = 0.095, group × eccentricity F[2.054, 262.912] = 2.265, p = 0.104, ηp2 = 0.015).
Fig. 5.
Saccade accuracies at different eccentricities. Accuracy of the first saccade amplitude was expressed as a ratio in percentages of the target eccentricity.
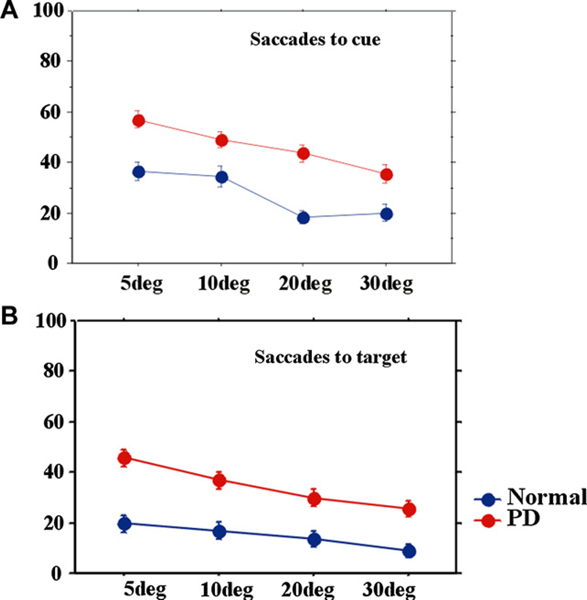
The frequencies of saccades to cue and saccades to target were also influenced by target eccentricity (Fig. 6). They were larger in PD at all eccentricities, but more prominently for smaller eccentricities (effect of eccentricity; saccades to cue: F[2.721,413.556] = 66.299, p < 0.0001, ηp2 = 0.304; saccades to target: F[2.425,339.434] = 37.649, p < 0.0001, ηp2 = 0.212; eccentricity × group; saccades to cue: F[2.721,413.556] = 3.253, p = 0.026, ηp2 = 0.021; saccades to target: F[2.425,339.434] = 3.499, p = 0.023, ηp2 = 0.024).
Fig. 6.
Frequency of saccades to cue and saccades to target at different eccentricities. The frequency of saccades to cue (A) and saccades to target (B) plotted as a function of target eccentricity.
3.4. Changes in saccade parameters and hand RT with disease stage
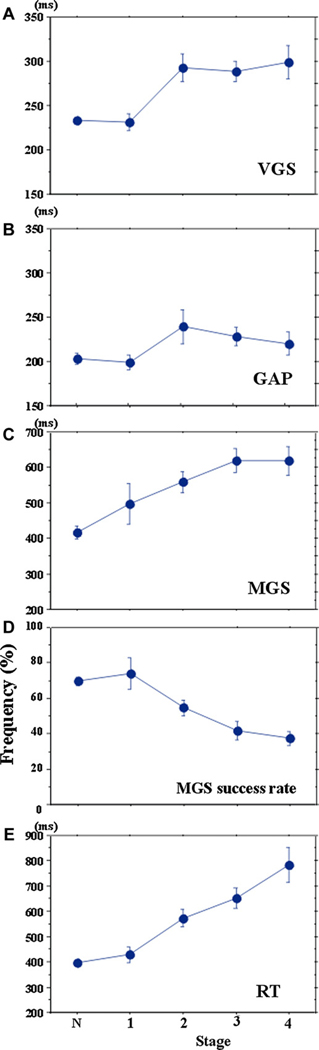
VGS latency increased from disease stages 1 to 2 and saturated thereafter (Fig. 7A; effect of disease stage: F[4,616] = 13.004, p < 0.0001, ηp2 = 0.269, difference between disease stages before 1 and those after stage 2 [p < 0.0044]). GAP latency increased up to stage 2, but decreased slightly at later disease stages (Fig. 7B; F[4,520] = 1.980, p = 0.102, ηp2 = 0.064; difference between disease stages 2–3 and other stages [p < 0.005]). At disease stage 1, the latencies of VGS and GAP were slightly shorter than but not significantly different from those of age-matched controls.
Fig. 7.
Changes in saccade latencies of VGS (A), GAP (B), and MGS (C) and success rate of MGS (D) and RT (E) with disease stage. The latencies of VGS, GAP, and MGS and the success rate of MGS and RT plotted as a function of disease stage. N, 1, 2, 3, and 4 on the abscissa represent normal subjects and patients at H–Y stages 1–4, respectively.
MGS latency increased more robustly and progressively with advancement of the disease (Fig. 7C; F[4,612] = 10.031, p < 0.0001, ηp2 = 0.224). At stages 3–4, it approached 600 ms, that is, patients were frequently unable to initiate MGS within the preset time limit. Accordingly, the MGS success rate declined progressively with advancing disease stages (Fig. 6D; effect of disease stage: F[4,432] = 8.635, p < 0.0001, ηp2 = 0.206), and correlated negatively with disease stage (r = 0.496, p < 0.0001).
To analyze how saccade parameters varied with disease progression, we assessed the correlation between saccade parameters and disease stage. The latencies of VGS, GAP, and MGS all showed significant positive correlations with stage (VGS: r = 0.443, p < 0.0001, GAP: r = 0.292, p = 0.0082; MGS: r = 0.468, p < 0.0001). When the latencies for each task were normalized to the respective median values of age-matched controls, VGS and GAP latencies showed a prolongation of 8.3% and 4.6% per disease stage, whereas MGS latency showed a larger prolongation of 13.6% per stage (effect of task: F[1.829,91.436] = 9.208, p = 0.0002, ηp2 = 0.227, task × disease stage: F[5.486,274.308] = 0.385, p = 0.8730, ηp2 = 0.034).
Similarly, hand RT increased progressively and showed a significant correlation with disease stage (Fig. 7E; effect of disease stage: F[4,616] = 18.313, p < 0.0001, ηp2 = 0.432; r = 0.807, p < 0.0001).
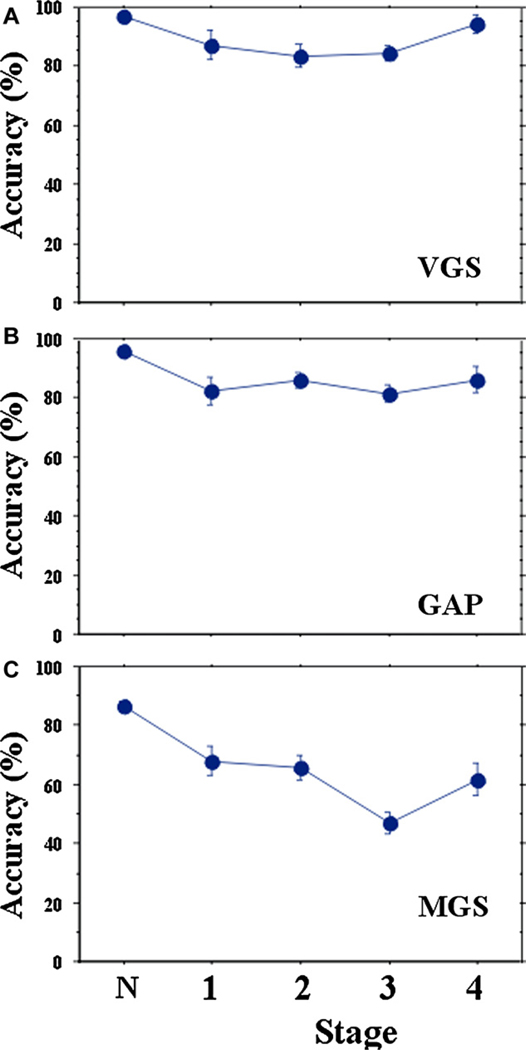
The accuracies of VGS and GAP decreased at disease stage 1 but leveled off thereafter (Fig. 8A and B; VGS: F[4,600] = 10.453, p < 0.0001, ηp2 = 0.243; GAP: F[4,520] = 15.766, p < 0.0001, ηp2 = 0.329). MGS accuracy decreased more progressively with disease stage (Fig. 8C; F[4,612] = 32.996, p < 0.0001, ηp2 = 0.520).
Fig. 8.
Changes in saccade amplitudes (accuracy) of VGS (A), GAP (B), and MGS (C) with disease stage.
The accuracy of MGS showed weak but significant negative correlations with disease stage (MGS: r = −0.303, p = 0.0138), but the accuracies of VGS and GAP did not [VGS: R = −0.0744, p = 0.5575, GAP: r = −0.013, p = 0.9263]. The accuracies of VGS and GAP declined by 3.3% and 4.2% per disease stage, respectively, whereas that of MGS showed a larger reduction of 8.1% (effect of task: F[1.289,68.315] = 65.508, p < 0.0001, ηp2 = 0.567; task × disease stage: F[1.289,68.315] = 1.855, p = 0.0960, ηp2 = 0.100).
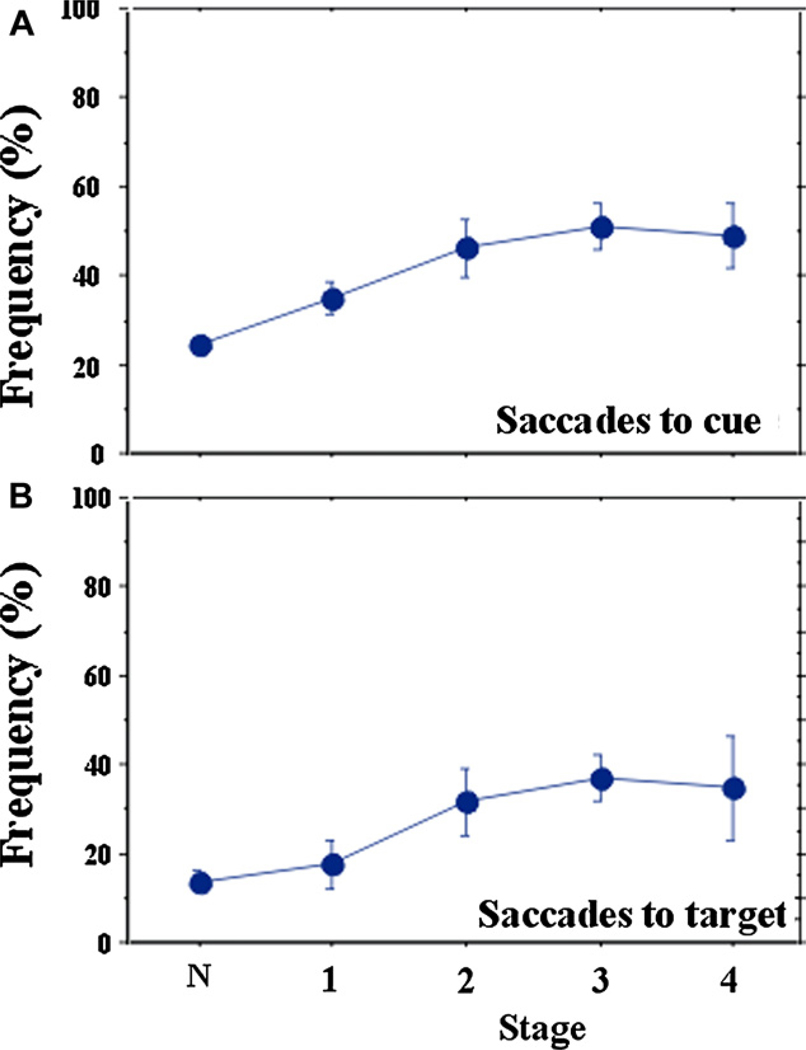
Both saccades to cue and saccades to target increased progressively up to stage 4 (Fig. 9) and showed a significant correlation with disease stage (saccade to cue: r = 0.488, p < 0.0001; saccade to target r = 0.381, p < 0.0001).
Fig. 9.
Changes in inhibitory control of saccades (A: saccades to cue, B: saccades to target) with disease stage.
3.5. Latency distribution of visually and memory guided saccades
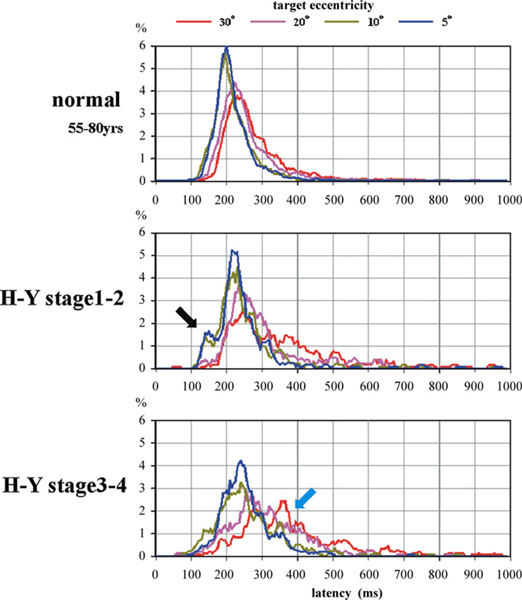
Across subjects, we pooled the VGS latency in single trials, separately for the four different target eccentricities, and then constructed histograms of latency distributions. All four curves for different eccentricities began to rise at approximately 100 ms for both controls and PD patients (Fig. 10). Patients with early PD showed a prominent peak in the early range (110–160 ms) for 5- to 10-degree targets (black arrow). At later stages, this “early” peak diminished, while VGS latencies toward 20- to 30-degree targets became markedly prolonged (light blue arrow). Early saccades comprised 5.2% in normal subjects, 8.3% in early PD patients, and 3.7% in advanced PD patients. This difference in proportion between normal and early PD subjects reached significance (χ-square test: p < 0.0001).
Fig. 10.
Frequency distribution of VGS latency. Frequency distribution of VGS latency in normal subjects (top), PD patients at H–Y stages 1–2 (middle) and 3–4 (bottom). Curves of different colors in each figure represent different target eccentricities. Please refer to the panel on top of the figures.
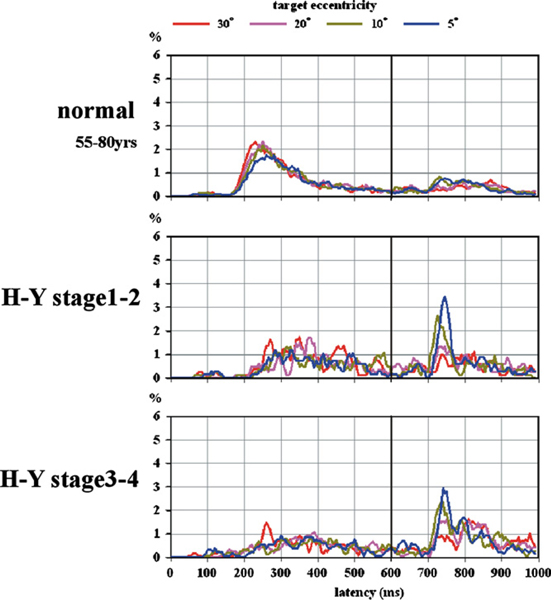
Fig. 11 presents a latency histogram for the MGS task. For both normal subjects and PD patients, the four curves for the different eccentricities almost overlapped. The latency was prolonged and variable across individual PD patients. The success rate of MGS decreased with disease stage. When the target location was lighted for the second time (black vertical line), however, PD patients frequently made a visually guided saccade toward this location. These visually guided saccades mostly occurred at latencies of 100–180 ms after the target presentation, for which the four curves diverged from each other, with the highest peak for the 5-degree target and the lowest peak for the 30-degree target.
Fig. 11.
Frequency distribution of MGS latency.
3.6. Correlation between various saccade parameters and that of inhibitory control
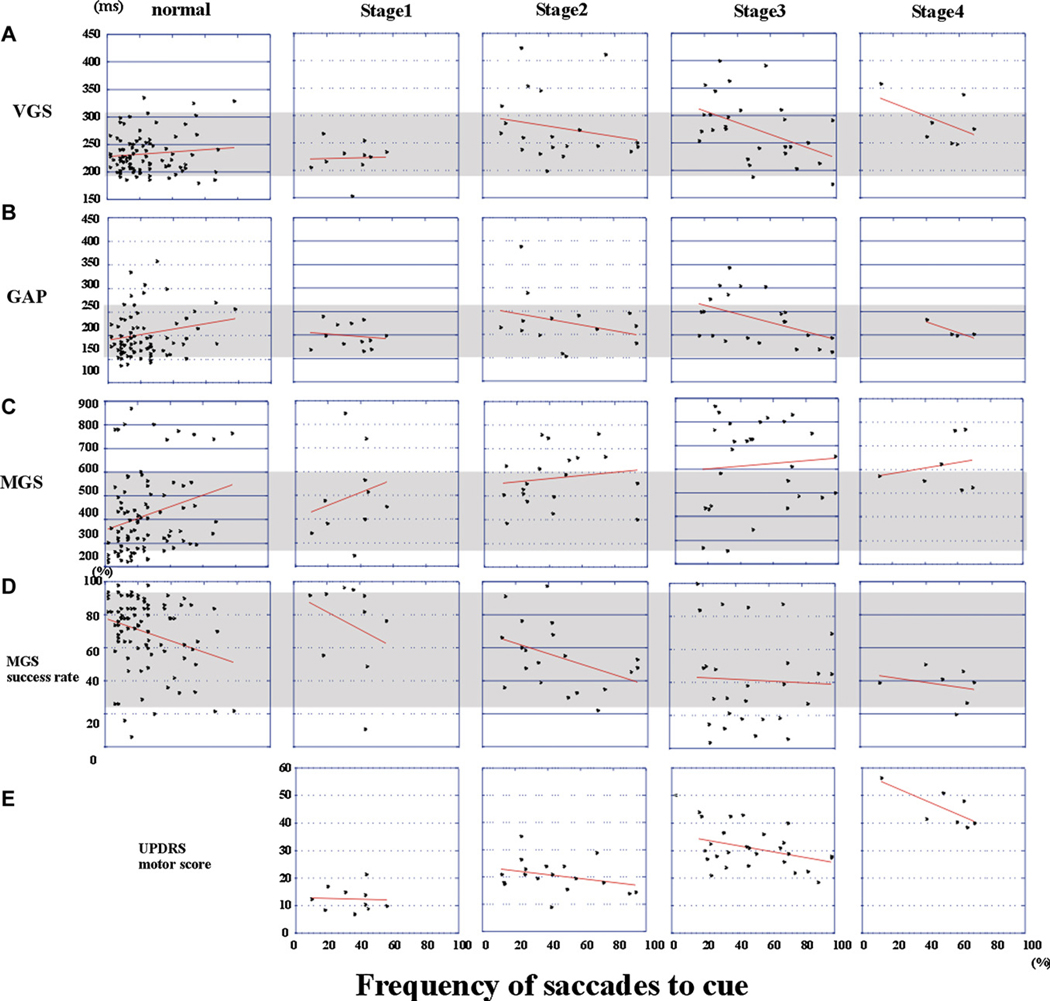
If the frequency of saccades to cue reflects the excitability of the SC, it would also show a negative correlation with the latency of visually guided saccades (see Section 4). In normal controls, the frequency of saccades to cue did not correlate significantly with VGS (r = 0.087, p = 0.5833) or GAP latency (r = 0.080, p = 0.6990) (Fig. 12A and B, normal), consistent with a previous report (Gowen & Abadi, 2005). In PD patients, however, the correlation between latencies of visually guided saccades and the frequency of saccades to cue reached significance (VGS: r = −0.320, p = 0.0353; GAP: r = −0.388, p = 0.0117). When the correlation was studied separately for different disease stages (Fig. 12A and B), we found a significant negative correlation at stage 3 (VGS: r = −0.462, p = 0.0123; GAP: r = −0.466, p = 0.0276) and stage 4 (VGS: r = −0.822, p = 0.0442; GAP: r = −0.919, p = 0.0250), although the correlation was not significant at stage 1 (VGS: r = −0.242, p = 0.5803; GAP: r = −0.152, p = 0.6467) or stage 2 (VGS: r = −0.204, p = 0.3934; GAP: r = −0.296, p = 0.3109). Correlation was not found for MGS latency and its success rate (Fig. 12C and D).
Fig. 12.
Correlations between the frequency of saccades to cue (abscissa) and saccade parameters and UPDRS score (ordinate) at disease stages. (A) VGS latency, (B) GAP latency, (C) MGS latency, (D) Success rate of MGS, (E) total UPDRS motor score. Plots are made separately for normal subjects (the leftmost column), and patients at H–Y stages 1–4 (the four columns on the right). Each dot represents the data of individual subjects. Regression lines are shown in red. Shaded areas represent the 95% confidence ranges of each parameter in normal subjects.
3.7. Behavioral correlates of the frequency of saccades to cue
To study the possible clinical correlates of the frequency of saccades to cue, we correlated the frequency of saccades to cue with the scores of UPDRS part III (Fig. 12E). Pooled across all disease stages, the frequency did not correlate significantly with the total or any of the motor subscores of UPDRS (r = 0.002, p = 0.9860). However, when we split patients into stages 1–2 and 3–4, the frequency of saccades to cue showed a significant negative correlation with the total UPDRS motor score at stages 3–4 (r = −0.407, p = 0.0221), but not at stages 1–2 (r = 0.121, p = 0.4520). Subscores for resting tremor and rigidity showed a significant negative correlation with the frequency for patients at stages 3–4 (resting tremor: r = −0.397, p = 0.0416; rigidity: r = −0.378, p = 0.0353), but not at stages 1–2 (resting tremor: r = 0.044, p = 0.8259; rigidity: r = 0.039, p = 0.8449). The subscore for rapid alternating movements of the hand showed a trend for significant negative correlation (r = −0.378, p = 0.0702), whereas those for leg agility and bradykinesia approached a trend level of statistical significance (leg agility: r = −0.284, p = 0.1223, bradykinesia: r = −0.293, p = 0.1105). All subscores showing correlation were classified as subscore A of the UPDRS motor score, indicative of dopaminergic deficiency (Levy et al., 2000). In contrast, subscores reflecting nondopaminergic function failed to show any correlation (rising from a chair: r = 0.089, p = 0.5016, posture: r = 0.032, p = 0.8079, postural stability: r = 0.035, p = 0.7908, gait: r = 0.035, p = 0.7908; speech: r = 0.134, p = 0.3048).
4. Discussion
In this study, we examined the initiation and inhibitory control of saccades in PD. We demonstrated that PD patients are impaired not only in MGS, but also in VGS to a lesser degree, beginning in the relatively early stages of the disease. PD patients also exhibited impaired ability to suppress unwanted saccades (saccades to cue and to target). Both the initiation of memory-guided saccades and the inhibitory control of reflexive saccades (saccades to cue and saccades to target) deteriorated with the progress of the disease. The frequency of saccades to cue showed a negative correlation with some clinical scores, including UPDRS motor scores. We discuss these findings from the perspective of the SC, where inhibitory and excitatory drives for saccade generation converge.
4.1. Excessive SC inhibition and decreased pre-oculomotor drive in PD
It has been hypothesized that the initiation of VGS is mediated predominantly by structures that bypass the BG (Braun, Weber, Mergner, & Schulte-Monting, 1992; Heide & Kömpf, 1998; Pierrot-Deseilligny et al., 2004). However, we found that VGS was affected even in the relatively early stages of PD; both VGS and MGS were affected, with prolonged latencies and reduced amplitudes.
SC comprises part of the final common pathway where the descending commands for visually and memory-guided saccades converge. We propose that excessive inhibition of SC neurons by the BG (specifically the SNr) leads to prolonged latency and reduced velocity and amplitude for all types of saccades (Machado & Rafal, 2004). Indeed, electrical stimulation of the SNr reduces the amplitude of both VGS and MGS in primates (Basso & Liu, 2007). Moreover, deep brain stimulation of the subthalamic nucleus (DBS-STN), which is thought to reduce the level of SNr–SC inhibition, improved VGS and MGS in PD patients (Yugeta et al., 2010); hypometria of various visually and memory-guided saccades were improved, supporting the notion that the SC serves as the bottleneck region for all types of volitional saccades.
Saccades in PD showed hypometria and prolonged latency, reaching the target with a multistep sequence, especially for MGS. Kimmig, Haussmann, Mergner, and Lucking (2002) suggested that this phenomenon (“gaze shift segmentation”) occurs because the pre-oculomotor drive through the BG to SC becomes abnormally weak and/or because SC receives inadequately strong inhibition. We propose that both of these factors are involved in the oculomotor abnormalities in PD. Commands for initiating visually guided saccades such as VGS arise in the posterior parietal cortex that is directly connected to the SC (Pierrot-Deseilligny et al., 2004). The abnormality of VGS may therefore be explained mainly by the excessive inhibition of the “bottleneck” SC. In contrast, along with this excessive inhibition, MGS engages two serial inhibitory projections mediated by the frontal cortex (FC) and the BG, finally reaching the SC. DBS-STN normalized VGS latency and amplitude, but not MGS latency (Yugeta et al., 2010); DBS-STN may act on the SNr–SC pathway and normalize the function of the “bottleneck” SC, but it may not act directly on the FC–BG pathway.
4.2. Changes in the pre-oculomotor drive and inhibition of the SC change with disease stage
VGS amplitude initially decreased progressively with disease stage, although to a much smaller degree than MGS. Therefore, SC suppression as reflected in VGS may comprise only a part of PD pathophysiology. Although VGS latency and amplitude tended to worsen with disease stage, especially from H–Y stage 1 to 2, it subsequently tended to saturate. This saturation suggests that a mechanism other than SC suppression is at work for determining VGS latency (see below).
In contrast, MGS latency and amplitude progressively increased and the MGS success rate declined with disease progression. This suggests that the function of the FC–BG circuit, the pre-oculomotor drive, deteriorated progressively with disease stage. The success rate of MGS appears to reach a minimum level at H–Y stages 3–4. This suggests that dopaminergic denervation due to neuronal death in the SNr may have already reached a near-exhausted level at these stages. Hypometria in MGS is more pronounced than that of VGS, probably because MGS is affected by both the excessive inhibition of the SC and the deteriorated pre-oculomotor drive through the FC–BG circuit.
4.3. Impaired inhibitory control of saccades in PD
Apart from the excessive inhibition from BG and decreased frontal cortex-BG activity, the inhibitory control of saccades was also impaired in PD. This is difficult to explain by excessive SC suppression, which is expected to prevent saccade initiation in response to cue stimulus. Furthermore, although PD patients had difficulty in initiating memory-guided saccades because of the decrease in frontal cortex-BG activity, they had no difficulty in making them in response to the target that was presented at the end of the delay period (Fig. 11). It was evident that although PD patients have difficulty in initiating voluntary saccades in the absence of a visual target, they show a tendency to make saccades to it reflexively once it is presented.
There are several possible explanations for the emergence of “hyper-reflexivity.” Visually guided saccades can occur readily if the suppression is “leaky,” which can occur due to fluctuation in the level of the BG-induced SC inhibition. Studies have shown that in PD, pathological firing patterns, both oscillatory and nonoscillatory, develop in the BG circuit, which can lead to the phasic inhibition and disinhibition of SC saccade cells and the release of unwanted saccades (Bergman et al., 1998; Brown, 2003). Wichmann et al. (1999) demonstrated that neuronal discharge in the SNr is affected in Parkinsonism in the same way as the GPi, exhibiting an increased proportion of cells with oscillatory burst patterns at 3–8 Hz. Because SC receives inhibitory input from the SNr, this fluctuation in firing activity can lead to the phasic disinhibition of SC. In contrast, a group of neurons in the monkey STN enable switching from an automatic saccade to a voluntary saccade by inhibiting the automatic saccade (Isoda & Hikosaka, 2008). If this control is compromised by abnormal oscillatory activities both within and outside the STN, a saccade will be initiated when a novel visual stimulus is presented. Shaikh, Xu-Wilson, Grill, and Zee (2010) found “staircase” square-wave jerks in patients with early PD; they ascribed them to the oscillations in the STN being transferred to SNr and subsequently to SC to intermittently inhibit the ongoing saccades. Conversely, a “leakage” in suppression may lead to the disinhibition of SC and the generation of unwanted saccades.
The “leaky” suppression may also be phasic. The phasic disinhibition of SC may occur in response to the appearance of the visual target. In primate studies, the high-frequency activity of SNr has been demonstrated to decrease phasically in response to suddenly appearing visual signals (Hikosaka & Wurtz, 1983). Because SNr sends inhibitory inputs to SC, the decrease in discharge of SNr leads to the disinhibition of SC. We noted that the saccades to cue occur mostly around 200 ms and around 400–500 ms after the cue is presented in MGS (data not shown); with similar intervals, the discharge of SNr neurons decreased in response to visual stimuli. In PD, there may be an apparent general hyperexcitability of BG neurons to phasic sensory inputs (e.g., Bergman, Wichmann, Karmon, & Delong, 1994), so this phasic disinhibition may also be enhanced.
However, it should be noted that neither the tonic or phasic disinhibition noted above can explain why the timing of periods of low inhibition happen to coincide with the cue-target interval where saccades to cue may take place involuntarily, but not with the target onset-saccade interval when VGS are being initiated later; a phasic disinhibition can occur in response to either the visual target in VGS or to the cue in MGS. Our data suggested that VGS are delayed in latency, whereas saccades to cue are increased in frequency.
Thus, although the precise mechanism remains unknown, we may have to take the task-related modulation of oscillation within the BG into consideration. There is growing evidence that PD is associated with pathological synchronous oscillatory activity in the BG, primarily in the β range (11–30 Hz), which may play a key role in BG dysfunction in PD (Bergman et al., 1994; Nini, Feingold, Slovin, & Bergman, 1995; Raz, Feingold, Zelanskaya, Vaadia, & Bergman, 1996; Wichmann, Bergman, & DeLong, 1994). In normal primates, the local field potential oscillations have been proposed to serve the function of filtering striatal input–output transmission (Hutchison et al., 2004) or acting as a dynamic filter to sharpen the action-selection network activity in the striatum (Courtemanche, Fujii, & Graybiel, 2003). β band oscillations are also reduced before and during self- and externally paced voluntary movements (Cassidy et al., 2002; Doyle et al., 2005; Kempf et al., 2007; Kuhn et al., 2004; Williams et al., 2003) and are considered to be involved in movement inhibition (Kuhn et al., 2004).
Here, saccade generation was required in VGS whereas in the delay period of MGS, it was required to be inhibited. Evidence is limited as to how oscillatory activity in BG is modulated in PD. In PD, these oscillations may entrain more neurons to give rise to pathological oscillatory spike activity. Therefore, BG may be resistant to normal modulation, compromising its function to filter out the execution of saccades other than the ones to be performed and allowing unnecessary saccades to be issued (Weinberger, Hutchison, & Dostrovsky, 2009).
4.4. Interaction between inhibition and disinhibition within SC
Because the hyper-reflexivity and the decreased drive for saccade initiation is apparently contradictory, it is important to consider how the drives for hyper-reflexivity and the impairment of saccade initiation converge on SC to produce the oculomotor abnormalities in PD. As yet, there is no direct evidence to explain how SC inhibition and disinhibition interacts and we can therefore only speculate about it. According to the model proposed by Brown, Bullock, and Grossberg (1999), visual inputs to SC dominate “reflexive” movements by default and allow fast visually guided saccades to take place toward a suddenly appearing target. When a planned movement is required instead, frontal activity can suppress visually guided saccades and allow time for processing a planned response to a different target. Although not supported by neuronal recording, it has been suggested that the purpose of the descending input from the cortex is to delay saccade initiation so that accurately planned responses are elicited (Carpenter, 2004; Neggers, Raemaekers, Lampmann, Postma, & Ramsey, 2005; Reddi & Carpenter, 2000; van Donkelaar et al., 2007). Consistent with this notion, van Donkelaar et al. (2007) demonstrated that when a peripheral object is foveated by a sequence of multiple saccades, the initial saccade in the sequence is initiated markedly faster than a single accurate saccade to the same object. It is possible that the execution of a visually guided saccade is delayed until a saccade of precise direction and amplitude is programmed. Therefore, visually guided saccades such as VGS and GS in this study are not “reflexive” in the sense that they are solely triggered by visual signals.
In PD, the voluntary drive mediated by the FC–BG circuit declines, leading to delayed latency of MGS. The BG-induced suppression of SC increases, leading to delayed latency of visually guided saccades as well. Meanwhile, hyper-reflexivity also develops. The dual pattern of decreased voluntary drive and increased hyper-reflexivity suggests that functional balance in PD patients gradually approaches the default “reflexive” mode as the disease progresses.
Although excessive inhibition of SC cells may lead to the prolongation of latency for all types of saccades, hyper-reflexivity of saccades can also work to shorten the latency of saccades. The overall negative correlation between VGS latency and the frequency of saccades to cue suggests that the disinhibition of SC underlies both of these phenomena. Indeed, the disinhibition of SC by injections of bicuculline into the SC in primates facilitates the initiation of saccades including VGS and MGS and increases irrepressible saccades toward the center of the movement field of the SC cells at the injection site (Hikosaka & Wurtz, 1985a).
4.5. Preferential disinhibition of small amplitude saccades in PD
Not only was the latency of visually guided saccades shortened by disinhibition of SC, there was also a preferential facilitation of small amplitude saccades. VGS latency was preserved for saccades made toward smaller target eccentricities (5–10°) until later disease stages, whereas those made toward large target eccentricities were markedly delayed. The same held for saccades made toward the target when it was presented for the second time in the MGS task (Fig. 11). These phenomena can also be explained by the disinhibition of the SC. Although visually guided saccades may not be directly triggered by visual inputs, the predominance of smaller saccades may be related to the broader representation within the SC of small amplitude saccades as well as the large input it receives from cortical and subcortical areas (Sparks, Rohrer, & Zhang, 2000). Even if SC were homogenously inhibited by the excessive output from the SNr, smaller saccades would overcome this inhibition because of their large representation, whereas larger saccades would be greatly suppressed.
The difference in processing for large and small amplitude saccades may also contribute to the preferential facilitation of smaller saccades. As stated above, because of the leaky inhibition, saccades that were initially programmed as large amplitude saccades may be prematurely released with small amplitude. Consequently, the latency of small amplitude saccades in VGS and GS tasks would be relatively normal or even shortened compared to normal subjects, whereas large amplitude VGS or GS may be slowed in latency.
In summary, the prolongation in latency of visually guided saccades may be caused by the heightened level of the SNr–SC inhibition. In addition to SC inhibition, the pre-oculomotor drive decreases as the disease progresses, which increases the latency of MGS more prominently than that of visually guided saccades. The facilitation of saccades to cue as well as the preferential facilitation of smaller saccades may be caused by temporary removal (or leakage) of the SNr–SC inhibition. This SC disinhibition causes VGS latencies to decrease slightly.
4.6. SC disinhibition may reflect compensatory mechanisms in PD
We found a significant correlation between the symptoms of PD that are indicative of dopaminergic deficiency and the frequency of saccades to cue; this was especially prominent in H–Y stages 3–4 (Fig. 12E).
Motor functions may be better for PD patients in which compensatory mechanisms outside the BG work better (Bezard, Gross, & Brotchie, 2003), even if the dopaminergic neurons have degenerated equally. It is known that movements that are driven by external stimuli employ different cortical routes than those driven by an internal trigger. Behaviorally, PD patients in the advanced stage compensate for their motor deficits by using sensory guidance (Brown & Marsden, 1988). Patients are poor at suppressing prepotent behavior in response to sensory stimuli (Praamstra, Stegeman, Cools, & Horstink, 1998) because information from sensory stimuli relevant to the generation of a response can have rapid access to motor structures to facilitate prepotent movements. Structures outside BG may compensate for the progressive loss of dopamine in PD, for example, through overactivity of the parietal cortex. Neuroimaging studies also demonstrate that PD patients exhibit a strategic compensatory shift of the use from striato-mesial frontal to parietal–lateral premotor circuits to recruit parallel motor circuits and to overcome the functional BG deficit and facilitate the performance of complex limb movements (Praamstra, Stegeman, Cools, Meyer, & Horstink, 1998; Sabatini et al., 2000).
Consistent with this notion, Shaikh et al. (2010) ascribed the abnormally large square-wave jerks in PD to a compensatory increase in FEF activity secondary to the increased inhibition upon SC. SC disinhibition, as indicated by the increased frequency of saccades to cue, may reflect such inputs to SC from structures outside BG that become active to compensate for the PD-associated changes in BG.
In addition, abnormal firing patterns, especially synchronous oscillations, developing within the BG circuit, or exaggerated response of BG (e.g., SNr) in response to sensory stimuli (Bergman et al., 1994) may phasically remove the excessive gating by the BG and make the downstream motor structures more responsive to sensory inputs; they may also make the intended movement easier to perform. In the dopamine-depleted state, there is increased coupling between cortex and STN activities (Magill, Bolam, & Bevan, 2001) perhaps due to a greater efficacy of the excitatory synaptic inputs (Bevan, Hallworth, & Baufreton, 2007). The abnormal firing pattern that allows saccades to cue to occur may also enhance this coupling in a way that facilitates voluntary movements.
4.7. Pathophysiology of PD studied through saccade performance; clinical relevance and treatment implications
Various therapies can alter the aforementioned pathophysiological changes in PD. For example, l-DOPA and dopamine agonists improve the function of premotor drive by altering the balance between direct and indirect pathways. They shorten the latency of MGS, prolong the latency of visually guided saccades, and improve the performance of antisaccades by suppressing the default mode (Hood et al., 2007; Michell et al., 2006); they also alleviate the motor symptoms.
In contrast, DBS-STN reverses the excessive inhibition of SC by targeting the overactive STN-SNr circuit, which is further downstream than the aforementioned circuit. It thereby improves the amplitudes of all types of saccades (Yugeta et al., 2010) and can also change small multistep movements into a single large movement (Kumru, Summerfield, Valldeoriola, & Valls-Solé, 2004; Sauleau et al., 2008). DBS-STN can also occlude the oscillatory activity and normalize the excitability of SC, decreasing the occurrence of saccades to cue (Yugeta et al., 2010).
With further clarification of associated pathophysiology, treatment could be optimized for each patient, using saccade parameters as an indicator of current pathological status.
Abbreviations:
- PD
Parkinson’s disease
- BG
Basal ganglia
- SC
superior colliculus
- VGS
visually guided saccade
- MGS
memory guided saccade
- SNr
substantia nigra pars reticulata
- GPi
globus pallidus interna
- GABA
gamma aminobutyric acid
- GAP
gap saccade
- RT
reaction time
References
- Albin RL, Young AB, & Penney JB (1995). The functional anatomy of disorders of the basal ganglia. Trends in Neurosciences, 18, 63–64, 1995. [PubMed] [Google Scholar]
- Alexander GE, & Crutcher MD (1990). Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neurosciences, 13, 266–271. [DOI] [PubMed] [Google Scholar]
- Basso MA, & Liu P. (2007). Context-dependent effects of substantia nigra stimulation on eye movements. Journal of Neurophysiology, 97, 4129–4142. [DOI] [PubMed] [Google Scholar]
- Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, et al. (1998). Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends in Neurosciences, 21, 32–38. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, & Delong MR (1994). The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. Journal of Neurophysiology, 72, 507–520. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Hallworth NE, & Baufreton J. (2007). GABAergic control of the subthalamic nucleus. Progress in Brain Research, 160, 173–188. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, & Brotchie JM (2003). Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends in Neurosciences, 26, 215–221. [DOI] [PubMed] [Google Scholar]
- Blekher T, Weaver M, Rupp J, Nichols WC, Hui SL, Gray J, et al. (2009). Multiple step pattern as a biomarker in Parkinson disease. Parkinsonism & Related Disorders, 15, 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D, Weber H, Mergner T, & Schulte-Monting J. (1992). Saccadic reaction times in patients with frontal and parietal lesions. Brain, 115, 1359–1386. [DOI] [PubMed] [Google Scholar]
- Briand KA, Strallow D, Hening W, Poizner H, & Sereno AB (1999). Control of voluntary and reflexive saccades in Parkinson’s disease. Experimental Brain Research, 129, 38–48. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, & Kennard C. (1985). Predictive ocular motor control in Parkinson’s disease. Brain, 108, 925–940. [DOI] [PubMed] [Google Scholar]
- Brown MR, DeSouza JF, Goltz HC, Ford K, Menon RS, Goodale MA, et al. (2004). Comparison of memory and visually guided saccades using event-related fMRI. Journal of Neurophysiology, 91, 873–889. [DOI] [PubMed] [Google Scholar]
- Brown J, Bullock D, & Grossberg S. (1999). How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. Journal of Neuroscience, 19, 10502–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. (2003). Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Movement Disorders, 18, 357–363. [DOI] [PubMed] [Google Scholar]
- Brown RG, & Marsden CD (1988). Internal versus external cues and the control of attention in Parkinson’s disease. Brain, 111, 323–345. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS (2004). Contrast, probability, and saccadic latency; evidence for independence of detection and decision. Current Biology, 14, 1576–1580. [DOI] [PubMed] [Google Scholar]
- Cassidy M, Mazzone P, Oliviero A, Insola A, Tonali P, Di Lazzaro V, et al. (2002). Movement-related changes in synchronization in the human basal ganglia. Brain, 125, 1235–1246. [DOI] [PubMed] [Google Scholar]
- Chambers JM, & Prescott TJ (2010). Response times for visually guided saccades in persons with Parkinson’s disease: A meta-analytic review. Neuropsychologia, 48, 887–899. [DOI] [PubMed] [Google Scholar]
- Chan F, Armstrong IT, Pari G, Riopelle RJ, & Munoz DP (2005). Deficits in saccadic eye-movement control in Parkinson’s disease. Neuropsychologia, 43, 784–796. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, & Graybiel AM (2003). Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. Journal of Neuroscience, 23, 11741–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LM, Kuhn AA, Hariz M, Kupsch A, Schneider GH, & Brown P. (2005). Levodopa-induced modulation of subthalamic beta oscillations during self-paced movements in patients with Parkinson’s disease. European Journal of Neuroscience, 21, 1403–1412. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Lynch J, Ploner CJ, Condy C, & Rivaud-Péchoux S. (2003). The parieto-collicular pathway: Anatomical location and contribution to saccade generation. European Journal of Neuroscience, 17, 1518–1526. [DOI] [PubMed] [Google Scholar]
- Gowen E, & Abadi RV (2005). Saccadic instabilities and voluntary saccadic behaviour. Experimental Brain Research, 164, 29–40. [DOI] [PubMed] [Google Scholar]
- Hanes DP, & Wurtz RH (2001). Interaction of the frontal eye field and superior colliculus for saccade generation. Journal of Neurophysiology, 85, 804–815. [DOI] [PubMed] [Google Scholar]
- Heide W, & Kömpf D. (1998). Combined deficits of saccades and visuo-spatial orientation after cortical lesions. Experimental Brain Research, 123, 164–171. [DOI] [PubMed] [Google Scholar]
- Hood AJ, Amador SC, Cain AE, Briand KA, Al-Refai AH, Schiess MC, et al. (2007). Levodopa slows prosaccades and improves antisaccades: An eye movement study in Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry, 78, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, & Wurtz RH (1983). Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. Journal of Neurophysiology, 49, 1230–1253. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, & Wurtz RH (1985a). Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. Journal of Neurophysiology, 53, 266–291. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, & Wurtz RH (1985b). Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. Journal of Neurophysiology, 53, 292–308. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, & Wurtz RH (1989). The basal ganglia. Reviews of Oculomotor Research, 3, 257–281. [PubMed] [Google Scholar]
- Hutchison WD, Dostrovsky JO, Walters JR, Courtemanche R, Boraud T, Goldberg J, et al. (2004). Neuronal oscillations in the basal ganglia and movement disorders: Evidence from whole animal and human recordings. Journal of Neuroscience, 24, 9240–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M, & Hikosaka O. (2008). Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. Journal of Neuroscience, 28, 7209–7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA (2001). The pathology of Parkinson’s disease. Advances in Neurology, 86, 55–72. [PubMed] [Google Scholar]
- Johnston K, & Everling S. (2008). Neurophysiology and neuroanatomy of reflexive and voluntary saccades in non-human primates. Brain and Cognition, 68, 271–283. [DOI] [PubMed] [Google Scholar]
- Joti P, Kulashekhar S, Behari M, & Murthy A. (2007). Impaired inhibitory oculomotor control in patients with Parkinson’s disease. Experimental Brain Research, 177, 447–457. [DOI] [PubMed] [Google Scholar]
- Kato M, Miyashita N, Hikosaka O, Matsumura M, Usui S, & Kori A. (1995). Eye movements in monkeys with local dopamine depletion in the caudate nucleus. I. Deficits in spontaneous saccades. Journal of Neuroscience, 15, 912–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig H, Haussmann K, Mergner T, & Lucking CH (2002). What is pathological with gaze shift fragmentation in Parkinson’s disease? Journal of Neurology, 249, 683–692. [DOI] [PubMed] [Google Scholar]
- Kempf F, Kuhn AA, Kupsch A, Brucke C, Weise L, Schneider GH, et al. (2007). Premovement activities in the subthalamic area of patients with Parkinson’s disease and their dependence on task. European Journal of Neuroscience, 25, 3137–3145. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Fukushima J, & Tashiro K. (1994). Relationship between antisaccades and the clinical symptoms in Parkinson’s disease. Neurology, 44, 2285–2289. [DOI] [PubMed] [Google Scholar]
- Kori A, Miyashita N, Kato M, Hikosaka O, Usui S, & Matsumura M. (1995). Eye movements in monkeys with local dopamine depletion in the caudate nucleus. II. Deficits in voluntary saccades. Journal of Neuroscience, 15, 928–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn A, Williams D, Kupsch A, Limousin P, Hariz M, Schneider GH, et al. (2004). Event related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain, 127, 735–746. [DOI] [PubMed] [Google Scholar]
- Kumru H, Summerfield C, Valldeoriola F, & Valls-Solé J. (2004). Effects of subthalamic nucleus stimulation on characteristics of EMG activity underlying reaction time in Parkinson’s disease. Movement Disorders, 19, 94–100. [DOI] [PubMed] [Google Scholar]
- Levy G, Tang MX, Cote LJ, Louis ED, Alfaro B, Mejia H, et al. (2000). Motor impairment in PD: Relationship to incident dementia and age. Neurology, 55, 539–544. [DOI] [PubMed] [Google Scholar]
- Machado L, & Rafal RD (2004). Control of fixation and saccades in humans with chronic lesions of oculomotor cortex. Neuropsychology, 18, 115–123. [DOI] [PubMed] [Google Scholar]
- Magill PJ, Bolam JP, & Bevan MD (2001). Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience, 106, 313–330. [DOI] [PubMed] [Google Scholar]
- Michell AW, Xu Z, Fritz D, Lewis SJ, Foltynie T, Williams-Gray CH, et al. (2006). Saccadic latency distributions in Parkinson’s disease and the effects of l-dopa. Experimental Brain Research, 174, 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neggers SF, Raemaekers MA, Lampmann EE, Postma A, & Ramsey NF (2005). Cortical and subcortical contributions to saccade latency in the human brain. European Journal of Neuroscience, 21, 2853–2863. [DOI] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, & Bergman H. (1995). Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of Parkinson. Journal of Neurophysiology, 74, 1800–1805. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Alpert NM, Matthysse SW, Levy DL, Rauch SL, & Holzman PS (1995). Functional neuroanatomy of antisaccade eye movements investigated with positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America, 92, 925–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Milea D, & Muri R. (2004). Eye movement control by the cerebral cortex. Current Opinion in Neurology, 17, 17–25. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, & Horstink MW (1998). Reliance on external cues for movement initiation in Parkinson’s disease. Evidence from movement-related potentials. Brain, 121, 167–177. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman DF, Cools AR, Meyer AS, & Horstink MW (1998). Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain, 121, 769–772. [DOI] [PubMed] [Google Scholar]
- Rascol O, Clanet M, Montastruc JL, Simonetta M, Soulier-Esteve MJ, Doyon B, et al. (1989). Abnormal ocular movements in Parkinson’s disease: Evidence for involvement of dopaminergic systems. Brain, 112, 1193–1214. [DOI] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, & Bergman H. (1996). Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. Journal of Neurophysiology, 76, 2083–2088. [DOI] [PubMed] [Google Scholar]
- Reddi BA, & Carpenter RH (2000). The influence of urgency on decision time. Nature Neuroscience, 3, 827–830. [DOI] [PubMed] [Google Scholar]
- Sauleau P, Pollak P, Krack P, Courjon JH, Vighetto A, Benabid AL, et al. (2008). Subthalamic stimulation improves orienting gaze movements in Parkinson’s disease. Clinical Neurophysiology, 119, 1857–1863. [DOI] [PubMed] [Google Scholar]
- Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. (2000). Cortical motor reorganization in akinetic patients with Parkinson’s disease: A functional MRI study. Brain, 123, 394–403. [DOI] [PubMed] [Google Scholar]
- Shaikh AG, Xu-Wilson M, Grill S, & Zee DS (2010). ‘Staircase’ square-wave jerks in early Parkinson’s disease. British Journal of Ophthalmolology [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Shibasaki H, Tsuji S, & Kuroiwa Y. (1979). Oculomotor abnormalities in Parkinson’s disease. Archives of Neurology, 36, 360–364. [DOI] [PubMed] [Google Scholar]
- Shook BL, Schlag-Rey M, & Schlag J. (1990). Primate supplementary eye field. I. Comparative aspects of mesencephalic and pontine connections. Journal of Comparative Neurology, 301, 618–642. [DOI] [PubMed] [Google Scholar]
- Sparks D, Rohrer WH, & Zhang Y. (2000). The role of the superior colliculus in saccade initiation: A study of express saccades and the gap effect. Vision Research, 40, 2763–2777. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Deng SY, Goldberg ME, & McMullen NT (1989). Cytoarchitectural characteristic of the frontal eye fields in macaque monkeys. Journal of Comparative Neurology, 282, 415–427. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, et al. (1996). Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology, 75, 454–468. [DOI] [PubMed] [Google Scholar]
- Terao Y, Fukuda H, Ugawa Y, Hikosaka O, Furubayashi T, Hanajima R, et al. (1998). Visualization of the information through human oculomotor cortical regions by transcranial magnetic stimulation. Journal of Neurophysiology, 80, 936–946. [DOI] [PubMed] [Google Scholar]
- Terao Y, Fukuda H, Ugawa Y, Tsuji S, Nomura Y, & Segawa M. (2007). Saccade abnormalities in Parkinson’s disease. Clinical Neurophysiology, 118, e205 [abstract]. [Google Scholar]
- van Donkelaar P, Saavedra S, & Woollacott M. (2007). Multiple saccades are more automatic than single saccades. Journal of Neurophysiology, 97, 3148–3151. [DOI] [PubMed] [Google Scholar]
- van Stockum S, MacAskill M, Anderson T, & Dalrymple-Alford J. (2008). Don’t look now or look away: Two sources of saccadic disinhibition in Parkinson’s disease? Neuropsychologia, 46, 3108–3115. [DOI] [PubMed] [Google Scholar]
- van Koningsbruggen MG, Pender T, Machado L, & Rafal RD (2009). Impaired control of the oculomotor reflexes in Parkinson’s disease. Neuropsychologia, 47, 2909–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Rivaud S, Gouder-Khouja N, Pillon B, Bonnet AM, Gaymard B, et al. (1994). Eye movements in parkinsonian syndrome. Annals of Neurology, 35, 420–426. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Hutchison WD, & Dostrovsky JO (2009). Pathological subthalamic nucleus oscillations in PD: Can they be the cause of bradykinesia and akinesia? Experimental Neurology, 219, 58–61. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, & DeLong MR (1994). The primate subthalamic nucleus. I. Functional properties in intact animals. Journal of Neurophysiology, 72, 494–506. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, & DeLong MR (1999). Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Experimental Brain Research, 125, 397–409. [DOI] [PubMed] [Google Scholar]
- Williams D, Kuhn A, Kupsch A, Tijssen M, van Bruggen G, Speelman H, et al. (2003). Behavioural cues are associated with modulations of synchronous oscillations in the human subthalamic nucleus. Brain, 126, 1975–1985. [DOI] [PubMed] [Google Scholar]
- Yugeta A, Terao Y, Fukuda H, Okiyama R, Yokochi F, Taniguchi M, et al. (2010). Effects of STN stimulation on the initiation and inhibition of saccade in Parkinson disease. Neurology, 74, 743–748. [DOI] [PubMed] [Google Scholar]
- Yugeta A, Terao Y, Fukuda H, & Ugawa Y. (2008). Effects of levodopa on saccade performance in Parkinson’s disease. Movement Disorders, 23(Suppl. 1), S296. [Google Scholar]