In the crystal structure of the title compound, C26H36N2O4, the tripodal molecule adopts a conformation in which the substituents attached to the central benzene ring are arranged in an alternating order above and below the ring plane.
Keywords: crystal structure, tripodal molecule, hydrogen bonding, C—H⋯π interactions
Abstract
In the crystal structure of the title compound, C26H36N2O4, the tripodal molecule exists in a conformation in which the substituents attached to the central arene ring are arranged in an alternating order above and below the ring plane. The heterocyclic unit is inclined at an angle of 79.6 (1)° with respect to the plane of the benzene ring. In the crystal, the molecules are connected via N—H⋯O bonds, forming infinite supramolecular strands. Interstrand association involves weak C—H⋯O and C—H⋯π interactions, with the pyridine ring acting as an acceptor in the latter case.
1. Chemical context
Recognition units based on 2-aminopyridine have proved to be valuable building blocks for the construction of artificial carbohydrate receptors that act via non-covalent interactions (Mazik et al., 2004 ▸, 2005 ▸; Mazik, 2009 ▸, 2012 ▸; Lippe & Mazik, 2015 ▸; Seidel et al., 2023 ▸). Such units are able to participate in the formation of hydrogen-bonding motifs similar to those observed in natural complexes for the primary amide groups (side chains of asparagine and glutamine). The latter are used by carbohydrate-binding proteins in combination with other functional groups such as hydroxy, carboxy, imidazolyl and isopropyl groups (side chains of serine, aspartic acid, histidine and valine, respectively). The use of a combination of different functional groups enables not only the formation of neutral and charge-reinforced hydrogen bonds, but also of C—H⋯π interactions and numerous van der Waals contacts, and is responsible for the observed binding selectivities and efficiencies of the proteins (Quiocho, 1989 ▸; Sharon & Lis, 2007 ▸; Gabius, 2009 ▸; Gabius et al., 2011 ▸). Our studies with various acyclic and macrocyclic artificial receptors have also shown that selective and effective binding is favourably influenced by the involvement of different functional groups in the binding process. Among the acyclic receptor molecules, 1,3,5-substituted 2,4,6-trialkylbenzene derivatives have been studied particularly intensively (Lippe et al., 2015 ▸; Kaiser et al., 2019 ▸; Stapf et al., 2020a ▸; Köhler et al., 2020 ▸, 2021 ▸, 2024 ▸), and different binding properties have been observed depending on the nature of the receptor building blocks. In this article, we describe the crystal structure of 1,3-bis(acetoxymethyl)-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene, which is a precursor for the synthesis of a triethylbenzene derivative bearing a 2-aminopyridine-based recognition moiety and two hydroxymethyl groups.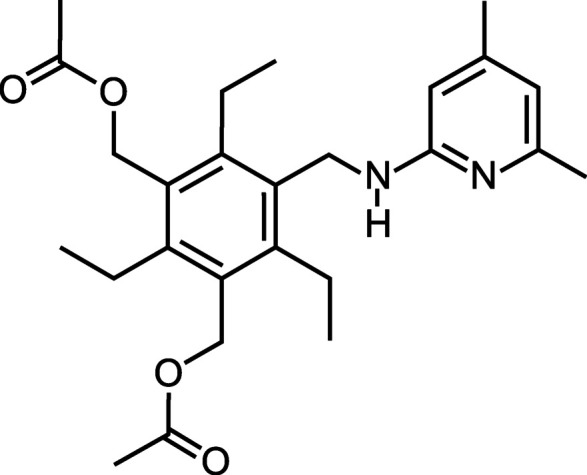
2. Structural commentary
The crystal structure of the title compound, C26H36N2O4, was solved in the monoclinic space group P21/n with the asymmetric unit containing one molecule. As shown in Fig. 1 ▸, the molecule adopts a conformation in which the pyridinylamino moiety and the two acetoxy groups are located on one side of the central benzene ring, whereas the ethyl substituents are directed to the opposite side of the ring plane (ababab arrangement; Das & Barbour, 2008a ▸,b ▸, 2009 ▸; Koch et al., 2017 ▸; Schulze et al., 2017 ▸). The molecule exists in a strongly distorted conformation with torsion angles of −166.6 (1) (anti) and −121.3 (1)° (eclipsed) along the Caryl—C—O—C sequences and an interplanar angle of 79.6 (1)° between the aromatic rings. The conformation appears to be stabilized by an intramolecular C—H⋯N hydrogen bond [d(H⋯N) = 2.65 Å] and a C—H⋯O bond [d(H⋯O) = 2.52 Å] involving the ethyl hydrogen atoms H25A, H25B and the acceptor positions N1 and O3 (Table 1 ▸).
Figure 1.
Perspective view of the title molecule including atom labelling. Anisotropic displacement ellipsoids are drawn at the 50% probability level.
Table 1. Hydrogen-bond geometry (Å, °).
Cg represents the centroid of the C8–C12/N2 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯O4i | 0.88 (1) | 2.10 (1) | 2.9776 (16) | 173 (1) |
| C19—H19C⋯O2ii | 0.98 | 2.61 | 3.1785 (18) | 117 |
| C14—H14B⋯Cgiii | 0.98 | 2.73 | 3.5955 (17) | 147 |
| C25—H25A⋯N1 | 0.99 | 2.65 | 3.3598 (17) | 128 |
| C25—H25B⋯O3 | 0.99 | 2.52 | 3.2250 (15) | 128 |
Symmetry codes: (i) 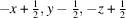 ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
3. Supramolecular features
The crystal structure is composed of zigzag-like strands of N-H⋯O=C bonded molecules [N1—H1⋯O4, 2.10 (1) Å, 173 (1)°], that extend parallel to the crystallographic b axis (Fig. 2 ▸). Interstrand association is confined to only one C—H⋯π contact (Nishio et al., 2009 ▸, 2012 ▸) per molecule with the pyridine ring acting as an acceptor [C14—H14B⋯Cg, 2.73 Å, 147°] as well as a weak C—H⋯O bond (Desiraju & Steiner, 1999 ▸) involving the oxygen atom O2 [C19—H19C⋯O2, 2.61 Å, 117°].
Figure 2.
Packing diagram of the title compound. The N—H⋯O hydrogen bonds are shown as dashed lines.
4. Database survey
Our previous studies have shown that representatives of 1,3,5-substituted 2,4,6-trialkylbenzenes with side arms bearing different functional groups have a better ability to discriminate between various carbohydrate substrates than compounds possessing identical functionalized side arms. In this context, the combination of 2-aminopyridine-based building blocks with other functional groups was shown to provide compounds capable of acting as effective and selective carbohydrate receptors. The search in the Cambridge Structural Database (CSD, Version 5.45, update June 2024; Groom et al., 2016 ▸) for such molecules with one or two pyridin-2-yl-aminomethyl unit(s) yielded thirteen hits. All crystal structures of the triethylbenzene derivatives listed below have in common that the tripodal molecules adopt a conformation with an alternating arrangement of the substituents above and below the plane of the central benzene ring. The crystal structures of the monohydrate and the methanol solvate of {1-[(3,5-bis{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzyl)amino]cyclopentyl}methanol (CADTAG, CADTEK; Stapf et al., 2020b ▸) as well as that of the methanol solvate of 1-{[N,N′-bis(tert-butoxycarbonyl)guanidino]methyl}-3,5-bis{[(6-methylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (HEXVAI; Mazik & Cavga, 2007 ▸) are composed of inversion-symmetric molecular dimers in which the water or methanol molecules are enclosed. Thus, the dimers are held together by solvent-mediated hydrogen bonds. In a similar way, the solvent-free crystal structures of 1,3-bis{[N,N-bis(2-hydroxyethyl)amino]methyl}-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (BEFGAY; Stapf et al., 2022 ▸) and 1-{[N,N-bis(ethoxycarbonylmethyl)amino]methyl}-3,5-bis{[(6-methylpyridin-2-yl)amino]methyl}-2,4,6-trimethylbenzene (KEGWID; Mazik & Cavga, 2006 ▸) also consist of centrosymmetric dimers as the smallest supramolecular entity. In the crystal structure of 1,3-bis{[N-(1,10-phenanthrolin-2-ylcarbonyl)amino]methyl-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (TUGVEX; Mazik et al., 2009 ▸), two water molecules and one ethanol molecule are accommodated in the binding pocket created by the heterocyclic units (one pyridinyl and two phenanthrolinyl groups) of the host molecule. The related compound 1-{[N-(1,10-phenanthrolin-2-ylcarbonyl)amino]methyl}-3,5-bis{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (ROKJEH, ROKJEH01; Mazik & Hartmann, 2008 ▸; Mazik et al., 2009 ▸), possessing one phenanthrolinyl and two pyridinyl groups, encloses three water molecules in the binding pocket. Both host–water/ethanol aggregates are stabilized by O—H⋯O, N—H⋯O and O—H⋯N hydrogen bonds. In the crystal structures of the formamide monosolvate and the n-propanol/H2O solvate of 1-{[2,6-bis(hydroxymethyl)-4-methylphenoxy]methyl}-3,5-bis{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (FIZDOL, FIZDUR; Stapf et al., 2023 ▸), the tripodal host molecules adopt similar conformations despite the different solvent molecules. 1-(Bromomethyl)-3,5-bis{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene was found to crystallize as a diethyl ether solvate (BIYTOT; Mazik & Kuschel, 2008 ▸), with the ether oxygen bound to one of the amino groups by hydrogen bonding. Finally, the crystal structures of triethylbenzene derivatives bearing one or two cationic moieties, namely 3-methylpyridinium group(s), in combination with 4,6-dimethylpyridin-2-yl unit(s) (hexafluorophosphate salts; ZITRAZ and ZITRON, respectively; Weisse et al., 2023 ▸) should be mentioned.
5. Synthesis and crystallization
A suspension of 1,3-bis(bromomethyl)-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene (0.30 g, 0.62 mmol) and anhydrous sodium acetate (0.42 g, 5.12 mmol) in acetic acid (3 mL) was stirred at 373 K for 12 h. The solvent was evaporated under reduced pressure. To the obtained white solid, water (3 mL) and CH2Cl2 (3 mL) were added. The aqueous phase was extracted twice with CH2Cl2 (5 mL). The combined organic extracts were treated with a saturated NaHCO3 solution (3 mL), washed with water (5 mL), dried (Na2SO4) and then concentrated. The pale yellow resin was recrystallized from methanol to give the title compound (0.17 g, 62%) as colourless crystals. Single crystals suitable for X-ray diffraction were obtained by slow evaporation from a solution of the title compound in N,N-dimethylacetamide.
Analytical data: m.p. 429–431 K; 1H NMR (500 MHz, CDCl3, ppm): δ = 1.18–1.22 (m, 9H, CH3), 2.09 (s, 6H, CH3), 2.24 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.76 (q, 6H, J = 7.6 Hz, CH2), 4.15 (br s, 1H, NH), 4.39 (d, 2H, J = 4.2 Hz, CH2), 5.21 (s, 4H, OCH2), 6.08 (s, 1H, aryl), 6.36 (s, 1H, aryl); 13C NMR (125 MHz, CDCl3, ppm): δ = 16.3, 16.5 (CH3), 21.0 (CH3), 21.1 (CH3), 22.8, 23.0 (CH2), 24.1 (CH3), 40.4 (NHCH2), 60.9 (OCH2), 103.5, 114.0, 130.0, 133.2, 145.4, 145.8, 148.9, 156.7, 158.1 (all aryl), 171.1 (C=O); MS (APCI): m/z calculated for C26H37N2O4: 441.3 [M + H]+, found 441.2. Elemental analysis for C26H36N2O4 (%): calculated C 70.88, H 8.24, N 6.36; found C 70.68, H 8.20, N 6.40. TLC: Rf = 0.41 [SiO2, toluene/ethyl acetate 3:1 (v/v)].
The educt, 1,3-bis(bromomethyl)-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylbenzene, was synthesized according to the reported procedure (Weisse et al., 2023 ▸).
6. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. The non-hydrogen atoms were refined anisotropically. All hydrogen atoms were positioned geometrically and refined isotropically using a riding model with C—H = 0.98–0.99 Å (alkyl), 0.95 Å (aryl); Uiso(H) = 1.2–1.5Ueq(C).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C26H36N2O4 |
| M r | 440.57 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 100 |
| a, b, c (Å) | 12.5184 (4), 9.1855 (2), 22.1339 (6) |
| β (°) | 105.1331 (15) |
| V (Å3) | 2456.87 (12) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.08 |
| Crystal size (mm) | 0.42 × 0.11 × 0.06 |
| Data collection | |
| Diffractometer | Bruker Kappa APEXII CCD area detector |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 23620, 5984, 4634 |
| R int | 0.031 |
| (sin θ/λ)max (Å−1) | 0.663 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.042, 0.120, 1.03 |
| No. of reflections | 5984 |
| No. of parameters | 300 |
| No. of restraints | 1 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.40, −0.24 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989024007515/ex2085sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024007515/ex2085Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024007515/ex2085Isup3.cml
CCDC reference: 2374683
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
Open Access Funding by the Publication Fund of the Technische Universität Bergakademie Freiberg is gratefully acknowledged.
supplementary crystallographic information
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Crystal data
| C26H36N2O4 | F(000) = 952 |
| Mr = 440.57 | Dx = 1.191 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| a = 12.5184 (4) Å | Cell parameters from 6078 reflections |
| b = 9.1855 (2) Å | θ = 2.4–28.3° |
| c = 22.1339 (6) Å | µ = 0.08 mm−1 |
| β = 105.1331 (15)° | T = 100 K |
| V = 2456.87 (12) Å3 | Needle, colourless |
| Z = 4 | 0.42 × 0.11 × 0.06 mm |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Data collection
| Bruker Kappa APEXII CCD area detector diffractometer | Rint = 0.031 |
| phi and ω scans | θmax = 28.1°, θmin = 2.8° |
| 23620 measured reflections | h = −15→16 |
| 5984 independent reflections | k = −12→11 |
| 4634 reflections with I > 2σ(I) | l = −29→29 |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Refinement
| Refinement on F2 | 1 restraint |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.042 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.120 | w = 1/[σ2(Fo2) + (0.0653P)2 + 0.589P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 5984 reflections | Δρmax = 0.40 e Å−3 |
| 300 parameters | Δρmin = −0.24 e Å−3 |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.57195 (8) | 0.63700 (10) | 0.27696 (5) | 0.0206 (2) | |
| O2 | 0.75502 (8) | 0.62688 (10) | 0.28844 (5) | 0.0251 (2) | |
| O3 | 0.11605 (8) | 0.87452 (10) | 0.19905 (4) | 0.0185 (2) | |
| O4 | 0.13164 (10) | 0.90318 (11) | 0.10076 (4) | 0.0284 (2) | |
| N1 | 0.27865 (10) | 0.66995 (12) | 0.44466 (5) | 0.0184 (2) | |
| N2 | 0.22044 (10) | 0.74686 (12) | 0.53067 (5) | 0.0187 (2) | |
| C1 | 0.35590 (11) | 0.83570 (12) | 0.38032 (5) | 0.0133 (2) | |
| C2 | 0.45669 (10) | 0.79830 (12) | 0.36834 (6) | 0.0136 (2) | |
| C3 | 0.47462 (10) | 0.83050 (12) | 0.30955 (6) | 0.0135 (2) | |
| C4 | 0.39092 (11) | 0.89452 (12) | 0.26199 (6) | 0.0129 (2) | |
| C5 | 0.28887 (10) | 0.92766 (12) | 0.27416 (5) | 0.0122 (2) | |
| C6 | 0.27068 (10) | 0.89825 (12) | 0.33303 (5) | 0.0125 (2) | |
| C7 | 0.33606 (11) | 0.80813 (13) | 0.44406 (6) | 0.0166 (3) | |
| H7A | 0.4078 | 0.8061 | 0.4762 | 0.020* | |
| H7B | 0.2911 | 0.8883 | 0.4545 | 0.020* | |
| C8 | 0.23040 (11) | 0.63821 (14) | 0.49228 (6) | 0.0166 (3) | |
| C9 | 0.16947 (12) | 0.71630 (15) | 0.57598 (6) | 0.0216 (3) | |
| C10 | 0.12987 (12) | 0.58025 (16) | 0.58463 (6) | 0.0234 (3) | |
| H10 | 0.0956 | 0.5637 | 0.6176 | 0.028* | |
| C11 | 0.14056 (11) | 0.46587 (15) | 0.54437 (6) | 0.0205 (3) | |
| C12 | 0.19076 (11) | 0.49616 (14) | 0.49736 (6) | 0.0186 (3) | |
| H12 | 0.1986 | 0.4224 | 0.4687 | 0.022* | |
| C13 | 0.15911 (15) | 0.84358 (17) | 0.61719 (7) | 0.0320 (4) | |
| H13A | 0.2330 | 0.8761 | 0.6403 | 0.048* | |
| H13B | 0.1172 | 0.8137 | 0.6468 | 0.048* | |
| H13C | 0.1204 | 0.9236 | 0.5912 | 0.048* | |
| C14 | 0.09729 (13) | 0.31615 (16) | 0.55260 (7) | 0.0262 (3) | |
| H14A | 0.1176 | 0.2486 | 0.5232 | 0.039* | |
| H14B | 0.0165 | 0.3197 | 0.5445 | 0.039* | |
| H14C | 0.1296 | 0.2827 | 0.5955 | 0.039* | |
| C15 | 0.54724 (11) | 0.72755 (14) | 0.41921 (6) | 0.0186 (3) | |
| H15A | 0.5922 | 0.6634 | 0.3996 | 0.022* | |
| H15B | 0.5130 | 0.6664 | 0.4459 | 0.022* | |
| C16 | 0.62274 (12) | 0.84108 (16) | 0.46005 (7) | 0.0260 (3) | |
| H16A | 0.6584 | 0.9000 | 0.4340 | 0.039* | |
| H16B | 0.6795 | 0.7916 | 0.4925 | 0.039* | |
| H16C | 0.5786 | 0.9043 | 0.4798 | 0.039* | |
| C17 | 0.58334 (11) | 0.78884 (13) | 0.29743 (6) | 0.0176 (3) | |
| H17A | 0.5994 | 0.8517 | 0.2645 | 0.021* | |
| H17B | 0.6442 | 0.7992 | 0.3360 | 0.021* | |
| C18 | 0.66651 (11) | 0.56814 (14) | 0.27606 (6) | 0.0182 (3) | |
| C19 | 0.64560 (13) | 0.41217 (15) | 0.25739 (8) | 0.0301 (3) | |
| H19A | 0.6259 | 0.4045 | 0.2116 | 0.045* | |
| H19B | 0.5847 | 0.3748 | 0.2732 | 0.045* | |
| H19C | 0.7125 | 0.3550 | 0.2751 | 0.045* | |
| C20 | 0.41205 (11) | 0.93293 (13) | 0.19939 (6) | 0.0161 (3) | |
| H20A | 0.3421 | 0.9246 | 0.1661 | 0.019* | |
| H20B | 0.4656 | 0.8629 | 0.1899 | 0.019* | |
| C21 | 0.45783 (12) | 1.08797 (14) | 0.19947 (7) | 0.0217 (3) | |
| H21A | 0.4083 | 1.1567 | 0.2125 | 0.033* | |
| H21B | 0.4629 | 1.1129 | 0.1573 | 0.033* | |
| H21C | 0.5315 | 1.0932 | 0.2287 | 0.033* | |
| C22 | 0.19501 (11) | 0.99175 (13) | 0.22362 (6) | 0.0154 (3) | |
| H22A | 0.1582 | 1.0705 | 0.2412 | 0.018* | |
| H22B | 0.2238 | 1.0332 | 0.1897 | 0.018* | |
| C23 | 0.09262 (11) | 0.84169 (14) | 0.13812 (6) | 0.0171 (3) | |
| C24 | 0.01268 (13) | 0.71748 (16) | 0.12222 (6) | 0.0248 (3) | |
| H24A | −0.0613 | 0.7550 | 0.1019 | 0.037* | |
| H24B | 0.0107 | 0.6656 | 0.1606 | 0.037* | |
| H24C | 0.0363 | 0.6506 | 0.0937 | 0.037* | |
| C25 | 0.16236 (11) | 0.93941 (13) | 0.34683 (6) | 0.0155 (2) | |
| H25A | 0.1467 | 0.8708 | 0.3780 | 0.019* | |
| H25B | 0.1016 | 0.9312 | 0.3080 | 0.019* | |
| C26 | 0.16604 (12) | 1.09505 (14) | 0.37219 (7) | 0.0229 (3) | |
| H26A | 0.2244 | 1.1025 | 0.4114 | 0.034* | |
| H26B | 0.0945 | 1.1191 | 0.3800 | 0.034* | |
| H26C | 0.1815 | 1.1631 | 0.3415 | 0.034* | |
| H1 | 0.3047 (13) | 0.5952 (13) | 0.4283 (7) | 0.022 (4)* |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0143 (5) | 0.0159 (5) | 0.0321 (5) | 0.0015 (3) | 0.0069 (4) | −0.0065 (4) |
| O2 | 0.0159 (5) | 0.0221 (5) | 0.0394 (6) | 0.0014 (4) | 0.0107 (4) | 0.0042 (4) |
| O3 | 0.0164 (5) | 0.0236 (5) | 0.0143 (4) | −0.0058 (4) | 0.0019 (3) | 0.0013 (3) |
| O4 | 0.0416 (7) | 0.0271 (5) | 0.0184 (5) | −0.0097 (5) | 0.0111 (4) | 0.0003 (4) |
| N1 | 0.0270 (7) | 0.0129 (5) | 0.0186 (5) | −0.0006 (4) | 0.0117 (5) | −0.0002 (4) |
| N2 | 0.0195 (6) | 0.0203 (5) | 0.0172 (5) | 0.0008 (4) | 0.0065 (4) | −0.0007 (4) |
| C1 | 0.0170 (6) | 0.0103 (5) | 0.0127 (5) | −0.0019 (4) | 0.0038 (4) | −0.0007 (4) |
| C2 | 0.0149 (6) | 0.0094 (5) | 0.0152 (6) | −0.0004 (4) | 0.0014 (5) | −0.0012 (4) |
| C3 | 0.0131 (6) | 0.0096 (5) | 0.0181 (6) | −0.0014 (4) | 0.0046 (5) | −0.0025 (4) |
| C4 | 0.0159 (6) | 0.0087 (5) | 0.0150 (6) | −0.0016 (4) | 0.0052 (5) | −0.0013 (4) |
| C5 | 0.0129 (6) | 0.0093 (5) | 0.0138 (5) | −0.0008 (4) | 0.0024 (4) | −0.0013 (4) |
| C6 | 0.0138 (6) | 0.0093 (5) | 0.0149 (6) | −0.0009 (4) | 0.0047 (4) | −0.0016 (4) |
| C7 | 0.0219 (7) | 0.0151 (6) | 0.0130 (6) | −0.0013 (5) | 0.0046 (5) | 0.0006 (4) |
| C8 | 0.0163 (7) | 0.0191 (6) | 0.0141 (6) | 0.0024 (5) | 0.0037 (5) | 0.0032 (5) |
| C9 | 0.0211 (7) | 0.0271 (7) | 0.0184 (6) | 0.0025 (5) | 0.0085 (5) | −0.0010 (5) |
| C10 | 0.0239 (8) | 0.0300 (7) | 0.0193 (6) | −0.0005 (6) | 0.0110 (5) | 0.0028 (5) |
| C11 | 0.0168 (7) | 0.0226 (7) | 0.0218 (6) | 0.0015 (5) | 0.0046 (5) | 0.0057 (5) |
| C12 | 0.0202 (7) | 0.0181 (6) | 0.0183 (6) | 0.0024 (5) | 0.0065 (5) | 0.0022 (5) |
| C13 | 0.0421 (10) | 0.0322 (8) | 0.0286 (8) | −0.0012 (7) | 0.0214 (7) | −0.0069 (6) |
| C14 | 0.0265 (8) | 0.0247 (7) | 0.0291 (7) | −0.0021 (6) | 0.0107 (6) | 0.0070 (6) |
| C15 | 0.0179 (7) | 0.0164 (6) | 0.0188 (6) | 0.0042 (5) | −0.0002 (5) | 0.0010 (5) |
| C16 | 0.0218 (8) | 0.0269 (7) | 0.0234 (7) | 0.0033 (6) | −0.0046 (5) | −0.0039 (6) |
| C17 | 0.0145 (6) | 0.0149 (6) | 0.0241 (6) | −0.0002 (5) | 0.0061 (5) | −0.0034 (5) |
| C18 | 0.0164 (7) | 0.0194 (6) | 0.0207 (6) | 0.0044 (5) | 0.0079 (5) | 0.0036 (5) |
| C19 | 0.0254 (8) | 0.0191 (7) | 0.0486 (9) | 0.0040 (6) | 0.0144 (7) | −0.0057 (6) |
| C20 | 0.0188 (7) | 0.0154 (6) | 0.0162 (6) | 0.0001 (5) | 0.0081 (5) | −0.0001 (5) |
| C21 | 0.0279 (8) | 0.0163 (6) | 0.0257 (7) | −0.0013 (5) | 0.0157 (6) | 0.0026 (5) |
| C22 | 0.0155 (6) | 0.0141 (6) | 0.0157 (6) | 0.0001 (5) | 0.0024 (5) | 0.0011 (4) |
| C23 | 0.0168 (7) | 0.0174 (6) | 0.0156 (6) | 0.0028 (5) | 0.0018 (5) | 0.0027 (5) |
| C24 | 0.0233 (8) | 0.0270 (7) | 0.0201 (6) | −0.0070 (6) | −0.0015 (5) | 0.0009 (5) |
| C25 | 0.0127 (6) | 0.0173 (6) | 0.0173 (6) | 0.0004 (5) | 0.0053 (5) | −0.0015 (5) |
| C26 | 0.0248 (8) | 0.0190 (6) | 0.0260 (7) | 0.0052 (5) | 0.0085 (6) | −0.0033 (5) |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Geometric parameters (Å, º)
| O1—C18 | 1.3469 (16) | C13—H13C | 0.9800 |
| O1—C17 | 1.4619 (15) | C14—H14A | 0.9800 |
| O2—C18 | 1.1980 (17) | C14—H14B | 0.9800 |
| O3—C23 | 1.3377 (15) | C14—H14C | 0.9800 |
| O3—C22 | 1.4669 (15) | C15—C16 | 1.5336 (19) |
| O4—C23 | 1.2051 (16) | C15—H15A | 0.9900 |
| N1—C8 | 1.3761 (16) | C15—H15B | 0.9900 |
| N1—C7 | 1.4603 (16) | C16—H16A | 0.9800 |
| N1—H1 | 0.878 (9) | C16—H16B | 0.9800 |
| N2—C8 | 1.3375 (17) | C16—H16C | 0.9800 |
| N2—C9 | 1.3511 (17) | C17—H17A | 0.9900 |
| C1—C2 | 1.3982 (18) | C17—H17B | 0.9900 |
| C1—C6 | 1.4073 (17) | C18—C19 | 1.4952 (19) |
| C1—C7 | 1.5164 (16) | C19—H19A | 0.9800 |
| C2—C3 | 1.4083 (17) | C19—H19B | 0.9800 |
| C2—C15 | 1.5194 (17) | C19—H19C | 0.9800 |
| C3—C4 | 1.4057 (17) | C20—C21 | 1.5349 (17) |
| C3—C17 | 1.5038 (18) | C20—H20A | 0.9900 |
| C4—C5 | 1.4060 (17) | C20—H20B | 0.9900 |
| C4—C20 | 1.5185 (16) | C21—H21A | 0.9800 |
| C5—C6 | 1.4063 (16) | C21—H21B | 0.9800 |
| C5—C22 | 1.5140 (16) | C21—H21C | 0.9800 |
| C6—C25 | 1.5137 (17) | C22—H22A | 0.9900 |
| C7—H7A | 0.9900 | C22—H22B | 0.9900 |
| C7—H7B | 0.9900 | C23—C24 | 1.4977 (19) |
| C8—C12 | 1.4108 (18) | C24—H24A | 0.9800 |
| C9—C10 | 1.376 (2) | C24—H24B | 0.9800 |
| C9—C13 | 1.5092 (19) | C24—H24C | 0.9800 |
| C10—C11 | 1.406 (2) | C25—C26 | 1.5322 (17) |
| C10—H10 | 0.9500 | C25—H25A | 0.9900 |
| C11—C12 | 1.3770 (18) | C25—H25B | 0.9900 |
| C11—C14 | 1.5064 (19) | C26—H26A | 0.9800 |
| C12—H12 | 0.9500 | C26—H26B | 0.9800 |
| C13—H13A | 0.9800 | C26—H26C | 0.9800 |
| C13—H13B | 0.9800 | ||
| C18—O1—C17 | 115.95 (10) | H15A—C15—H15B | 107.9 |
| C23—O3—C22 | 119.24 (10) | C15—C16—H16A | 109.5 |
| C8—N1—C7 | 120.44 (10) | C15—C16—H16B | 109.5 |
| C8—N1—H1 | 115.8 (11) | H16A—C16—H16B | 109.5 |
| C7—N1—H1 | 116.1 (11) | C15—C16—H16C | 109.5 |
| C8—N2—C9 | 117.18 (12) | H16A—C16—H16C | 109.5 |
| C2—C1—C6 | 120.38 (11) | H16B—C16—H16C | 109.5 |
| C2—C1—C7 | 120.69 (11) | O1—C17—C3 | 106.17 (10) |
| C6—C1—C7 | 118.92 (11) | O1—C17—H17A | 110.5 |
| C1—C2—C3 | 119.53 (11) | C3—C17—H17A | 110.5 |
| C1—C2—C15 | 120.01 (11) | O1—C17—H17B | 110.5 |
| C3—C2—C15 | 120.43 (11) | C3—C17—H17B | 110.5 |
| C4—C3—C2 | 120.82 (11) | H17A—C17—H17B | 108.7 |
| C4—C3—C17 | 120.35 (11) | O2—C18—O1 | 123.31 (12) |
| C2—C3—C17 | 118.78 (11) | O2—C18—C19 | 125.41 (13) |
| C3—C4—C5 | 118.95 (11) | O1—C18—C19 | 111.28 (12) |
| C3—C4—C20 | 120.47 (11) | C18—C19—H19A | 109.5 |
| C5—C4—C20 | 120.53 (11) | C18—C19—H19B | 109.5 |
| C4—C5—C6 | 120.72 (11) | H19A—C19—H19B | 109.5 |
| C4—C5—C22 | 120.75 (10) | C18—C19—H19C | 109.5 |
| C6—C5—C22 | 118.50 (11) | H19A—C19—H19C | 109.5 |
| C5—C6—C1 | 119.53 (11) | H19B—C19—H19C | 109.5 |
| C5—C6—C25 | 120.62 (11) | C4—C20—C21 | 111.62 (10) |
| C1—C6—C25 | 119.80 (11) | C4—C20—H20A | 109.3 |
| N1—C7—C1 | 110.74 (10) | C21—C20—H20A | 109.3 |
| N1—C7—H7A | 109.5 | C4—C20—H20B | 109.3 |
| C1—C7—H7A | 109.5 | C21—C20—H20B | 109.3 |
| N1—C7—H7B | 109.5 | H20A—C20—H20B | 108.0 |
| C1—C7—H7B | 109.5 | C20—C21—H21A | 109.5 |
| H7A—C7—H7B | 108.1 | C20—C21—H21B | 109.5 |
| N2—C8—N1 | 117.44 (11) | H21A—C21—H21B | 109.5 |
| N2—C8—C12 | 123.12 (12) | C20—C21—H21C | 109.5 |
| N1—C8—C12 | 119.40 (11) | H21A—C21—H21C | 109.5 |
| N2—C9—C10 | 123.30 (12) | H21B—C21—H21C | 109.5 |
| N2—C9—C13 | 114.79 (12) | O3—C22—C5 | 107.84 (9) |
| C10—C9—C13 | 121.90 (13) | O3—C22—H22A | 110.1 |
| C9—C10—C11 | 119.53 (12) | C5—C22—H22A | 110.1 |
| C9—C10—H10 | 120.2 | O3—C22—H22B | 110.1 |
| C11—C10—H10 | 120.2 | C5—C22—H22B | 110.1 |
| C12—C11—C10 | 117.74 (13) | H22A—C22—H22B | 108.5 |
| C12—C11—C14 | 121.68 (13) | O4—C23—O3 | 124.38 (12) |
| C10—C11—C14 | 120.58 (12) | O4—C23—C24 | 124.16 (12) |
| C11—C12—C8 | 119.11 (12) | O3—C23—C24 | 111.45 (11) |
| C11—C12—H12 | 120.4 | C23—C24—H24A | 109.5 |
| C8—C12—H12 | 120.4 | C23—C24—H24B | 109.5 |
| C9—C13—H13A | 109.5 | H24A—C24—H24B | 109.5 |
| C9—C13—H13B | 109.5 | C23—C24—H24C | 109.5 |
| H13A—C13—H13B | 109.5 | H24A—C24—H24C | 109.5 |
| C9—C13—H13C | 109.5 | H24B—C24—H24C | 109.5 |
| H13A—C13—H13C | 109.5 | C6—C25—C26 | 111.35 (11) |
| H13B—C13—H13C | 109.5 | C6—C25—H25A | 109.4 |
| C11—C14—H14A | 109.5 | C26—C25—H25A | 109.4 |
| C11—C14—H14B | 109.5 | C6—C25—H25B | 109.4 |
| H14A—C14—H14B | 109.5 | C26—C25—H25B | 109.4 |
| C11—C14—H14C | 109.5 | H25A—C25—H25B | 108.0 |
| H14A—C14—H14C | 109.5 | C25—C26—H26A | 109.5 |
| H14B—C14—H14C | 109.5 | C25—C26—H26B | 109.5 |
| C2—C15—C16 | 111.82 (10) | H26A—C26—H26B | 109.5 |
| C2—C15—H15A | 109.3 | C25—C26—H26C | 109.5 |
| C16—C15—H15A | 109.3 | H26A—C26—H26C | 109.5 |
| C2—C15—H15B | 109.3 | H26B—C26—H26C | 109.5 |
| C16—C15—H15B | 109.3 | ||
| C6—C1—C2—C3 | 3.27 (17) | C7—N1—C8—N2 | 11.94 (18) |
| C7—C1—C2—C3 | −177.42 (11) | C7—N1—C8—C12 | −170.12 (12) |
| C6—C1—C2—C15 | −178.56 (11) | C8—N2—C9—C10 | 1.1 (2) |
| C7—C1—C2—C15 | 0.75 (17) | C8—N2—C9—C13 | −179.11 (13) |
| C1—C2—C3—C4 | −2.52 (17) | N2—C9—C10—C11 | −0.9 (2) |
| C15—C2—C3—C4 | 179.31 (11) | C13—C9—C10—C11 | 179.24 (14) |
| C1—C2—C3—C17 | −179.72 (11) | C9—C10—C11—C12 | −0.1 (2) |
| C15—C2—C3—C17 | 2.11 (17) | C9—C10—C11—C14 | −179.63 (13) |
| C2—C3—C4—C5 | 0.69 (17) | C10—C11—C12—C8 | 1.0 (2) |
| C17—C3—C4—C5 | 177.86 (10) | C14—C11—C12—C8 | −179.52 (12) |
| C2—C3—C4—C20 | 178.20 (10) | N2—C8—C12—C11 | −0.9 (2) |
| C17—C3—C4—C20 | −4.63 (17) | N1—C8—C12—C11 | −178.70 (12) |
| C3—C4—C5—C6 | 0.39 (17) | C1—C2—C15—C16 | −88.79 (15) |
| C20—C4—C5—C6 | −177.12 (10) | C3—C2—C15—C16 | 89.37 (14) |
| C3—C4—C5—C22 | −177.83 (10) | C18—O1—C17—C3 | −166.61 (11) |
| C20—C4—C5—C22 | 4.67 (17) | C4—C3—C17—O1 | −92.28 (13) |
| C4—C5—C6—C1 | 0.36 (17) | C2—C3—C17—O1 | 84.94 (13) |
| C22—C5—C6—C1 | 178.61 (10) | C17—O1—C18—O2 | −3.15 (18) |
| C4—C5—C6—C25 | 177.60 (11) | C17—O1—C18—C19 | 177.29 (11) |
| C22—C5—C6—C25 | −4.15 (16) | C3—C4—C20—C21 | −89.67 (14) |
| C2—C1—C6—C5 | −2.21 (17) | C5—C4—C20—C21 | 87.81 (14) |
| C7—C1—C6—C5 | 178.47 (10) | C23—O3—C22—C5 | −121.28 (12) |
| C2—C1—C6—C25 | −179.47 (11) | C4—C5—C22—O3 | 102.10 (12) |
| C7—C1—C6—C25 | 1.21 (16) | C6—C5—C22—O3 | −76.15 (13) |
| C8—N1—C7—C1 | −165.33 (11) | C22—O3—C23—O4 | −0.62 (19) |
| C2—C1—C7—N1 | −95.56 (14) | C22—O3—C23—C24 | 178.70 (11) |
| C6—C1—C7—N1 | 83.76 (14) | C5—C6—C25—C26 | −88.44 (13) |
| C9—N2—C8—N1 | 177.71 (12) | C1—C6—C25—C26 | 88.80 (14) |
| C9—N2—C8—C12 | −0.15 (19) |
{3-[(Acetyloxy)methyl]-5-{[(4,6-dimethylpyridin-2-yl)amino]methyl}-2,4,6-triethylphenyl}methyl acetate. Hydrogen-bond geometry (Å, º)
Cg represents the centroid of the C8–C12/N2 ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···O4i | 0.88 (1) | 2.10 (1) | 2.9776 (16) | 173 (1) |
| C19—H19C···O2ii | 0.98 | 2.61 | 3.1785 (18) | 117 |
| C14—H14B···Cgiii | 0.98 | 2.73 | 3.5955 (17) | 147 |
| C25—H25A···N1 | 0.99 | 2.65 | 3.3598 (17) | 128 |
| C25—H25B···O3 | 0.99 | 2.52 | 3.2250 (15) | 128 |
Symmetry codes: (i) −x+1/2, y−1/2, −z+1/2; (ii) −x+3/2, y−1/2, −z+1/2; (iii) −x, −y+1, −z+1.
References
- Bruker (2014). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Das, D. & Barbour, L. J. (2008a). J. Am. Chem. Soc.130, 14032–14033. [DOI] [PubMed]
- Das, D. & Barbour, L. J. (2008b). Chem. Commun. pp. 5110–5112. [DOI] [PubMed]
- Das, D. & Barbour, L. J. (2009). Cryst. Growth Des.9, 1599–1604.
- Desiraju, G. R. & Steiner, T. (1999). In The Weak Hydrogen Bond. Oxford University Press.
- Farrugia, L. J. (2012). J. Appl. Cryst.45, 849–854.
- Gabius, H.-J. (2009). The Sugar Code – Fundamentals of Glycosciences. Weinheim: Wiley-VCH.
- Gabius, H.-J., André, S., Jiménez-Barbero, J., Romero, A. & Solís, D. (2011). Trends Biochem. Sci.36, 298–313. [DOI] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Kaiser, S., Geffert, C. & Mazik, M. (2019). Eur. J. Org. Chem. pp. 7555–7562.
- Koch, N., Seichter, W. & Mazik, M. (2017). CrystEngComm, 19, 3817–3833.
- Köhler, L., Hübler, C., Seichter, W. & Mazik, M. (2021). RSC Adv.11, 22221–22229. [DOI] [PMC free article] [PubMed]
- Köhler, L., Kaiser, S. & Mazik, M. (2024). Nat. Prod. Commun.19 (accepted). https://doi.org/10.1177/1934578X241258352
- Köhler, L., Seichter, W. & Mazik, M. (2020). Eur. J. Org. Chem. pp. 7023–7034.
- Lippe, J. & Mazik, M. (2015). J. Org. Chem.80, 1427–1439. [DOI] [PubMed]
- Lippe, J., Seichter, W. & Mazik, M. (2015). Org. Biomol. Chem.13, 11622–11632. [DOI] [PubMed]
- Mazik, M. (2009). Chem. Soc. Rev.38, 935–956. [DOI] [PubMed]
- Mazik, M. (2012). RSC Adv.2, 2630–2642.
- Mazik, M. & Cavga, H. (2006). J. Org. Chem.71, 2957–2963. [DOI] [PubMed]
- Mazik, M. & Cavga, H. (2007). J. Org. Chem.72, 831–838. [DOI] [PubMed]
- Mazik, M., Cavga, H. & Jones, P. G. (2005). J. Am. Chem. Soc.127, 9045–9052. [DOI] [PubMed]
- Mazik, M. & Hartmann, A. (2008). J. Org. Chem.73, 7444–7450. [DOI] [PubMed]
- Mazik, M., Hartmann, A. & Jones, P. G. (2009). Chem. Eur. J.15, 9147–9159. [DOI] [PubMed]
- Mazik, M. & Kuschel, M. (2008). Chem. Eur. J.14, 2405–2419. [DOI] [PubMed]
- Mazik, M., Radunz, W. & Boese, R. (2004). J. Org. Chem.69, 7448–7462. [DOI] [PubMed]
- Nishio, M., Umezawa, Y., Honda, K., Tsuboyama, S. & Suezawa, H. (2009). CrystEngComm, 11, 1757–1788.
- Nishio, M., Umezawa, Y., Suezawa, H. & Tsuboyama, S. (2012). In The Importance of Pi-Interactions in Crystal Engineering: Frontiers in Crystal Engineering, edited by E. R. T. Tiekink and J. Zukerman-Schpector, pp. 1–40. Chichester: Wiley.
- Quiocho, F. A. (1989). Pure Appl. Chem.61, 1293–1306.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Schulze, M. M., Schwarzer, A. & Mazik, M. (2017). CrystEngComm, 19, 4003–4016.
- Seidel, P., Seichter, W. & Mazik, M. (2023). ChemistryOpen, 12, e202300019. [DOI] [PMC free article] [PubMed]
- Sharon, N. & Lis, H. (2007). Lectins, 2nd ed. Dordrecht: Springer.
- Stapf, M., Schmidt, U., Seichter, W. & Mazik, M. (2022). Acta Cryst. E78, 825–828. [DOI] [PMC free article] [PubMed]
- Stapf, M., Schmidt, U., Seichter, W. & Mazik, M. (2023). Acta Cryst. E79, 1067–1071. [DOI] [PMC free article] [PubMed]
- Stapf, M., Seichter, W. & Mazik, M. (2020a). Eur. J. Org. Chem. pp. 4900–4915.
- Stapf, M., Seichter, W. & Mazik, M. (2020b). Acta Cryst. E76, 1679–1683. [DOI] [PMC free article] [PubMed]
- Weisse, A., Seichter, W. & Mazik, M. (2023). Molecules, 28, 6485–6503. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989024007515/ex2085sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989024007515/ex2085Isup2.hkl
Supporting information file. DOI: 10.1107/S2056989024007515/ex2085Isup3.cml
CCDC reference: 2374683
Additional supporting information: crystallographic information; 3D view; checkCIF report




