Abstract
Background
We have previously studied the physiological and mechanical responses of the eye to blunt trauma in various situations using finite element analysis (FEA). In this study, we evaluated the volume kinetics of an airbag impact on the eye using FEA to sequentially determine the volume change rates of intraocular segments at various airbag deployment velocities.
Methods
The human eye model we created was used in simulations with the FEA program PAM-GENERISTM (Nihon ESI, Tokyo, Japan). Different airbag deployment velocities, 30, 40, 50, 60 and 70 m/s, were applied in the forward direction. The volume of the deformed eye impacted by the airbag was calculated as the integrated value of all meshes in each segment, and the decrease rate was calculated as the ratio of the decreased volume of each segment at particular timepoints to the value before the airbag impact.
Results
The minimum volume of the anterior chamber was 63%, 69% and 50% at 50, 60 and 70 m/s airbag impact velocity, respectively, showing a curve with a sharp decline followed by gradual recovery. In contrast to the anterior chamber, the volume of the lens recovered promptly, reaching 80–90% at the end of observation, except for the case of 60 m/s. Following the decrease, the volume increased to more than that of baseline at 60 m/s. The rate of volume change of the vitreous was distributed in a narrow range, 99.2–100.4%.
Conclusion
In this study, we found a large, prolonged decrease of volume in the anterior chamber, a similar large decrease followed by prompt recovery of volume in the lens, and a time-lag in the volume decrease between these tissues. These novel findings may provide an important insight into the pathophysiological mechanism of airbag ocular injuries through this further evaluation, employing a refined FEA model representing cuboidal deformation, to develop a more safe airbag system.
Keywords: airbag, ocular trauma, computer simulation, lens, anterior chamber, vitreous body, finite element analysis, volume
Introduction
Serious and visually devastating ocular injuries caused by airbags have been reported if the occupant comes into contact with the airbag during the inflation phase, since airbags are deployed at velocities of up to 200 mph.1–23 Minor ocular injuries due to airbag impact include corneal abrasion, hyphema, vitreous hemorrhage, retinal tear, maculopathy, and localized photoreceptor damage.5,13–18 Moderate-to-serious ocular injuries include corneoscleral laceration, bullous keratopathy, lens dislocation, lens capsule rupture, choroidal rupture, retinal detachment, and open globe rupture.3,4,6,9,12,19–23 It has been thought that, even at low velocities, there may be a delay before the sensors transmit the firing signal, causing the airbag to strike the occupant while it continues to expand, resulting in moderate-to-severe ocular injury in relatively low-velocity collisions.24 The mechanisms inducing intraocular damage and how they influence the severity of airbag ocular injuries have been reported in a limited number of studies using finite element analysis (FEA).25,26 Power et al reported that FEA of the eye impacted by an airbag proved that placing a protective lens in front of the eye reduced the stress to the eye but increased the force experienced by the surrounding orbital bones.27
We have previously developed a simulation model resembling a human eye and applied 3-dimensional FEA to determine the physical and mechanical conditions of an intraocular foreign body injury.28 This model human eye has also been used in our studies on airbag impact in various situations.29–36 From these studies on eye–airbag injury, we have obtained important information on the sequential physical and mechanical responses of intraocular segments at various airbag deployment velocities.
While deformation of the eye occurs in three dimensions with an airbag impact, the intraocular segment deformation rate was analyzed in two dimensions, in only the axial plane.36 Thus, it remains to be determined how each ocular segment undergoes deformation during the airbag impact process in three dimensions. This is closely related to the pathophysiological mechanism that induces severe ocular trauma resulting in a poor visual prognosis. Therefore, we planned to study the kinetic phenomenon of airbag impact on eyes with different axial lengths, using an FEA method to determine the sequential response of volume in each segment during airbag impact in this study. This might enable us to more precisely understand what happens inside the eye and the pathophysiological mechanism of ocular damage due to airbag impact.
Materials and Methods
We used a model human eye in simulations with a computer using an FEA program, PAM-GENERISTM (Nihon ESI, Tokyo, Japan).28 The model of the human eye is composed of three layers as reported previously in Uchio et al.28 In the biomechanical head of a dummy, it was assumed that everything excluding the eye was a solid element, to reduce the calculation time. An eye was inserted in the Hybrid III biomechanical model of the head.37
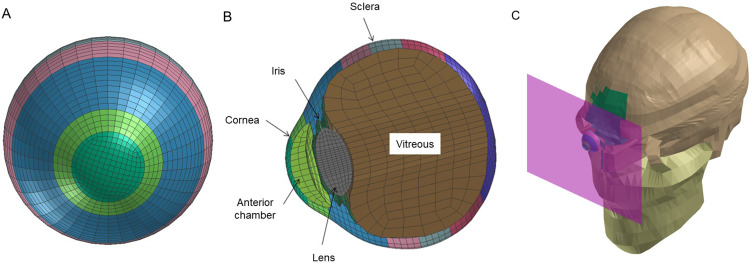
The meshing principles of the model eye are shown in Figure 1A and 1B according to previous reports.28,29 Corneal thickness was assigned as 0.45 mm. The depth of the anterior chamber was assumed to be 2.95 mm. The lens was assumed to be biconvex with a length of 3.80 mm. A vitreous model was assigned having hydrostatic pressure of 20 mmHg (2.7 kPa)33,34 and its length was assumed to be 16.65 mm, and the posterior curvature of the retina was assumed to be 12.0 mm.28 The elastic properties, meshing principles of the model normal human eye and mass densities of ocular tissues were similar to those in previous reports.29,36 Poisson ratio of the cornea, 0.420, and sclera, 0.470, were used to determine the standard stress strain curves for cornea and sclera.38,39 A normal eye with axial length of 23.85 mm was used.34–36 Deformation of the eyeball in the axial view was displayed sequentially.
Figure 1.
Simulation view of model eye and image of airbag impacting model eye. Frontal (A) and sagittal (B) view of model eye and eyeball and impacting airbag location (C) are displayed.
According to the data previously reported,40,41 different airbag deployment velocities, 30, 40, 50, 60 and 70 m/s, were used in this study (Figure 1C). The eye segments were divided into three segments: anterior chamber, lens, and vitreous. We calculated the volume change of the whole eyeball and each segment from 0.2 to 2.0 ms.36 Volume of the deformed eye impacted by the airbag was calculated in brief as the integrated value of all meshes in each segment, and the decrease rate was calculated as the ratio of the decreased volume of each segment at a particular timepoint to the value before the airbag impact. In our human eye FEA model, time-step size was 0.0002 ms on average. The mean volume in the five simulation situations was considered the volume of each segment at each timepoint. This article does not contain any studies with human or animal participants and informed consent is not required.
Results
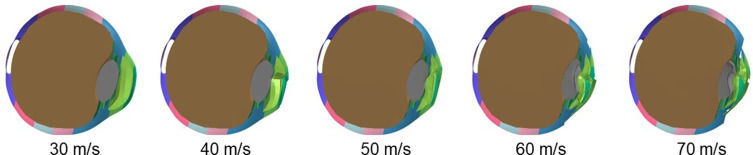
Sagittal section views of an airbag-impacted eye at 0.5 ms after the impact at impact velocities of 30, 40, 50, 60 and 70 m/s, respectively, are displayed as an example of the sequential deformation in Figure 2. Deformation was evident in the anterior part of the eye, such as the cornea, anterior chamber, and lens. Deformation rate of the anterior chamber and lens increased as impact velocity increased, and lens deformation rate seemed less than that of the anterior chamber. Deformation of vitreous was less than that of the anterior segments but it showed horizontal expansion of the equatorial zone after lens decompression, especially in high velocity impacts (60 and 70 m/s) (Figure 2).
Figure 2.
Deformation of eye upon airbag impact at five different velocities at 0.5 ms after impact. Sagittal view of eye at impact velocities of 30, 40, 50, 60 and 70 m/s is shown. Deformation of the anterior part of the eye is evident compared with posterior segments.
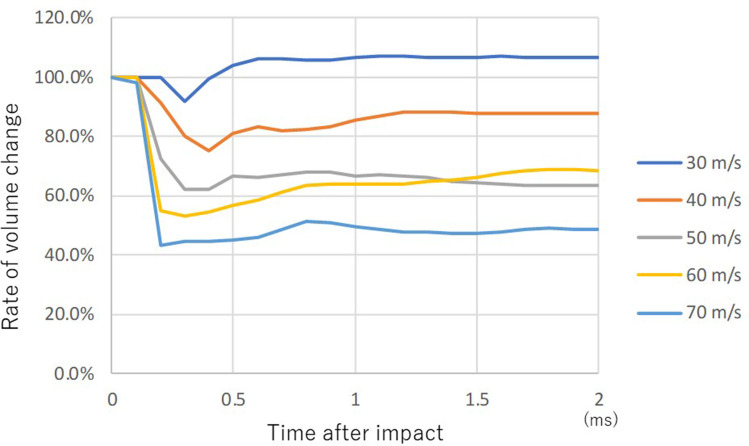
Curves of rate of volume change (RVC) in the anterior chamber showed a similar pattern at all impact velocities except for the case of 30 m/s (Figure 3). The volume of the anterior chamber decreased slightly after impact at 30 m/s airbag impact velocity, and it increased 0.4 ms after the impact. The final RVC of the anterior chamber was 108%. The rate of volume change at other higher impact velocities was similar, showing a sharp decline followed by gradual recovery, but it did not return to the baseline volume at all impact velocities. The volume of the anterior chamber at 2.0 ms after the impact was 63%, 69% and 50% at 50, 60 and 70 m/s airbag impact velocity, respectively (Figure 3).
Figure 3.
Sequential volume change rate of anterior chamber upon airbag impact at five different velocities. Cases of impact velocities of 30 (blue), 40 (brown), 50 (gray), 60 (yellow) and 70 (light blue) m/s are shown sequentially.
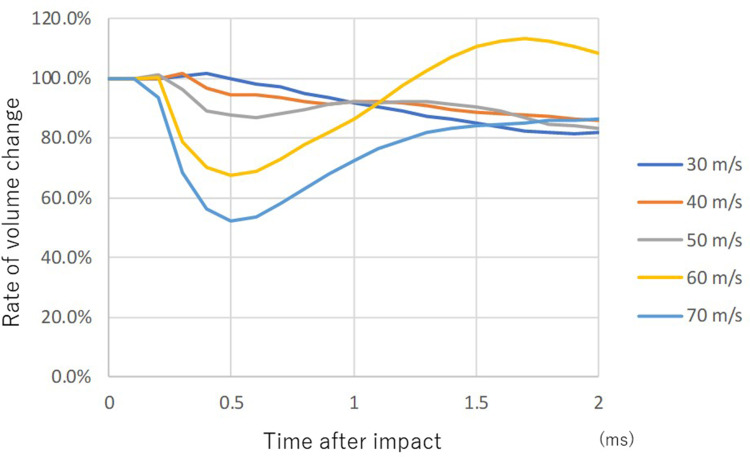
The start timepoint of lens volume change differed according to the impact velocity, and it was earliest at 70 m/s (0.1 ms after the impact) (Figure 4). The higher the impact velocity, the smaller the lowest value of the volume, and RVC at 70 m/s after 0.5 ms was 52%. However, in contrast to the anterior chamber, RVC of the lens recovered promptly, reaching 80–90% at 2.0 ms after the impact except with 60 m/s impact. Following the decrease, volume increased to more than that of baseline only in the case of 60 m/s impact velocity (Figure 4).
Figure 4.
Sequential volume change rate of lens upon airbag impact at five different velocities. Legends pertaining to the line drawings are the same as in Figure 3.
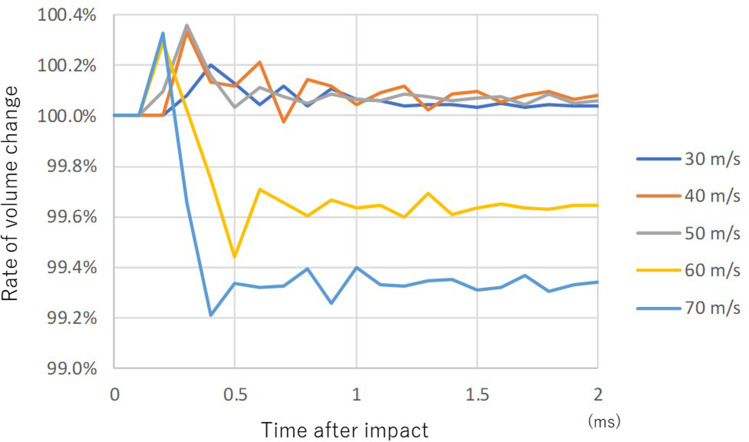
The rate of volume change of the vitreous was distributed in a very narrow range (99.2–100.4%) but two different patterns were observed (Figure 5). The volume was almost constant except for the early phase after the impact at lower impact velocities (30, 40 and 50 m/s). The volume of vitreous decreased rapidly after the impact, but it did not return to baseline volume in the later phase at higher impact velocities (60 and 70 m/s) (Figure 5).
Figure 5.
Sequential volume change rate of vitreous upon airbag impact at five different velocities. Legends pertaining to the line drawings are the same as in Figure 3.
Discussion
In our previous simulation studies of eyes impacted by an airbag or air soft gun, we mainly reported mechanical damage of the ocular surface, such as laceration, perforation, etc., and deformation of the whole eyeball was evaluated, but intraocular deformation, which is closely related to angle recession, lens rupture, vitreous traction, etc., was not evaluated.30–35 We recently reported the sequential changes in the length of each intraocular segment, anterior chamber, lens and vitreous, in an axial direction at various airbag deployment velocities using FEA.36 The eyeball is a small but very complicated and minute organ composed of various segments, and deformation of the intraocular segments and the relationship of regions of attachment among the segments have a critical role in the mechanism of ocular injury due to blunt trauma, as shown by Watson et al in a simulation study of blast insult ocular trauma.42 Because the airbag impacted perpendicular to the center of the cornea in the simulation, the deformation rate was highest in the axial plane of the intraocular segments in two dimensions and, therefore, we analyzed morphological change of intraocular segments from a three-dimensional standpoint using the volume of each segment as a candidate parameter of intraocular deformation in this study.
It is evident that RVC of the anterior chamber was higher than that of other segments, and it decreased to a trough value of 93–44% as impact velocity increased (Figure 3). Pearlman et al reported that damage to the anterior structures is most common with blunt ocular trauma, and posterior segment trauma is less common.43 It is also reasonable that the deformation rate of the anterior segment was higher than that of the posterior segment, because the anterior chamber is located in the anatomically most forward position, adjacent to the cornea, supporting our present results. It is also interesting that RVC of the anterior chamber at each impact velocity remained at a similar level even at the end of observation (2.0 ms) (Figure 3). This phenomenon was specific to the anterior chamber, and this might indicate that the prolonged deformation of the anterior chamber caused serious damage to the iris, angle and cornea, considering the long duration taken for recovery. Mohamed and Banerjee reported that self-limited injuries occurred at low velocity (10–30 mph).24 It was thought that, at low velocities, there may be a delay before the sensors transmit the firing signal, causing the airbag to strike the occupant while it continues to expand, resulting in moderate-to-severe ocular injury in relatively low-velocity collisions in which the airbag was fully deployed.24 This could be the reason for the peculiar RVC curve at a low impact velocity of 30 m/s. However, we should consider that the low velocity mentioned in the article of Mohamed et al24 meant the speed of the car in the crash, but we analyzed airbag ocular injury by changing airbag impact velocity without considering the car’s velocity and the vehicle’s inertial force on the passenger in our study. It should also be noted that the increase in volume of the lens after 1.2 ms at 60 m/s impact velocity coincided with the continual recovery curve observed in the anterior chamber at 60 m/s impact velocity, indicating a close relationship in the kinetics between the anterior chamber and lens.
Several cases of lens injury by airbag ocular trauma, such as opacification of the anterior capsule and cortex, lens dislocation and lens capsule rupture, have been reported.20,21,44 In this study, the lens was simulated as a shell element considering its linear elastic material property, different from the anterior chamber and vitreous (solid elements), and its mass density, Young’s modulus and Poisson’s ratio were assumed to be 1.078 (kg/m3), 0.00056 GPa and 0.42, respectively, according to past reports.26,28 In contrast to the irregular RVC curve of the anterior chamber and vitreous, the characteristic smooth RVC curve of the lens might be due to the element feature. The value of the highest deformation rate of the lens, ranging from 30% to 40%,36 was similar to the peak volume decrease rate in this study. It should also be noted that the time of greatest decrease in volume of the lens was 0.5 ms; in contrast, the peak decrease in volume of the anterior chamber was observed 0.2–0.4 ms after the impact. This time-lag in the deformation rate of the anterior chamber and lens in the axial direction was also observed in our previous study.36 This indicates that the decrease in volume of the anterior chamber did not directly induce the decrease in volume of the lens, but the lens decreased in volume after an interval from the peak volume decrease of the anterior chamber. Stein et al reported that the impact between the airbag and the eye in severe blunt trauma results in indentation of the cornea, a reduction in anterior–posterior diameter of the globe, and horizontal expansion of the equatorial zone after lens decompression.44 This could explain the time-lag in the volume decrease between the anterior chamber and lens. The volume of the lens had returned to more than 80% of baseline volume at the end of the simulation period (Figure 4). The reason for this recovery is unclear, but it might be derived from the kinetic behavior of vitreous behind the lens, as discussed later.
The rate of volume change of the vitreous was very low, as little as 0.8% at 70 m/s impact velocity (Figure 5). Considering the wide variety of vitreoretinal injury reported due to airbag impact,6,12,15,17,18,45,46 it is difficult to comment on the results of the present study in relation to the biomechanisms in vitreoretinal complications, but the vitreous might act as a shock absorber against the deformation caused by the anterior segment, due to its large volume.36 Traction at the vitreous base by expansion of the equatorial diameter of the eyeball by an airbag impact is the main factor in vitreoretinal complications of airbag ocular injury, as proposed by Stein et al.44 In contrast to the constant volume of vitreous after an impact at lower impact velocities, 30, 40 and 50 m/s, the volume of vitreous decreased rapidly after the impact and a persistent decrease was observed at higher impact velocities, 60 and 70 m/s (Figure 5). The phenomenon observed at the higher impact velocities might be supported by the hypothesis of Stein et al.44 However, it should be noted that they analyzed the velocity of motor vehicles, and did not consider airbag impact velocity in their study.44
Despite our careful calculations using the simulation model, there are still limitations to our study. First, there is a basic question of whether intraocular segments really change in volume as shown in this study. From the physical standpoint, a liquid might decrease in volume only a little on applying high pressure. The impact from a deploying airbag can exert extraordinarily high pressure on the eyeball. Gray et al reported in numerical modeling of paintball impact ocular trauma that the pressure rise and deformation rate experienced in dynamic impact-type events are several orders of magnitude greater than those experienced in hydrostatic-like intraocular events.47 Cirovic et al reported that the maximal pressure in the anterior chamber was close to 300 mm Hg as a direct consequence of compression of the globe in a blunt trauma FEA modeling study.48 The methods of modeling intraocular segments also had an influence on the results of this study, due to differences from the clinical situation. We modeled the anterior chamber and vitreous as solid elements in this study. Shirzadi et al employed shock equations of state (EOS) material for the aqueous humor,26 and Stitzel et al used Eulerian brick elements to comprise the aqueous humor,49 while Watson et al simulated the aqueous humor as compositionally similar to a saline solution, which does not exhibit complex rheological behavior in an FEA study;42 however, all of them modeled the lens with an elastic model, similarly to us. The lens was simulated as having linear elastic material property with elastic modulus of 0.00056 GPa and Poisson’s ratio of 0.42, as shown above. Shirzadi et al reported that the lens showed high stress with airbag impact, 8 MPa, in an FEA of airbag impact.26 Collectively, in brief, aqueous humor and vitreous were modeled as mesh elements having an elastic modulus in this study; therefore, they showed strain due to stress, resulting in volume change. Therefore, the volume change rates of the anterior chamber and vitreous were amplified more than those in clinical situations. Further verification is necessary for the volume kinetics of intraocular segments against high energy impact, and refinement of the modeling properties for intraocular segments will be considered in our future research.
Second, we analyzed sequential RVC in intraocular segments by airbag impact in this study, following our recent study on the deformation rate of intraocular segments in an axial direction by airbag impact. Refinement of the parameters in the FEA study in two dimensions, length and depth, to three dimensions, volume, produced novel information in this study. However, if some parameter representing cuboidal deformation could be introduced in 3D topology in the future, more precise evaluation of intraocular segment kinetics would be possible, leading to more useful information on the pathophysiological mechanisms of blunt ocular trauma.
In conclusion, we found a large, prolonged decrease of volume in the anterior chamber, a similar large decrease followed by prompt recovery of volume in the lens, and a time-lag in the volume decrease between the anterior chamber and lens. Although further evaluation, such as 3D topological analysis in an FEA airbag-impact ocular injury model, is required, these findings might provide an important insight into the pathophysiological mechanism of airbag ocular injuries observed in various ocular tissues, and might also play a role in developing a safer airbag system for the human body and eyeball in the near future.
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement of Scientists (24K12755) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Dr W. Gray for editing this manuscript.
Funding Statement
The authors have no relevant financial or non-financial interests to disclose for this study.
Disclosure
The authors report no conflict of interest in this work.
References
- 1.O’Halloran HS, Draud K, Stevens JL. Primary enucleation as a consequence of airbag injury. J Trauma. 1998;44(6):1090. doi: 10.1097/00005373-199806000-00025 [DOI] [PubMed] [Google Scholar]
- 2.de Vries S, Geerards AJ. Long-term sequelae of isolated chemical ”airbag” keratitis. Cornea. 2007;26(8):998–999. doi: 10.1097/ICO.0b013e3180ca9a35 [DOI] [PubMed] [Google Scholar]
- 3.Baker RS, Flowers CW, Singh P, Smith A, Casey R. Corneoscleral laceration caused by airbag trauma. Am J Ophthalmol. 1996;121(6):709–711. [DOI] [PubMed] [Google Scholar]
- 4.Scott IU, Greenfield DS, Parrish RK. Airbag-associated injury producing cyclodialysis cleft and ocular hypotony. Ophthalmic Surg Lasers. 1996;27(11):955–957. [PubMed] [Google Scholar]
- 5.Fukagawa K, Tsubota K, Kimura C, et al. Corneal endothelial cell loss induced by airbags. Ophthalmology. 1993;100(12):1819–1823. doi: 10.1016/S0161-6420(13)31394-3 [DOI] [PubMed] [Google Scholar]
- 6.Han DP. Retinal detachment caused by airbag injury. Arch Ophthalmol. 1993;111(10):1317–1318. doi: 10.1001/archopht.1993.01090100023010 [DOI] [PubMed] [Google Scholar]
- 7.Scott IU, John GR, Stark WJ. Airbag-associated ocular injury and periorbital fractures. Arch Ophthalmol. 1993;111(1):25. doi: 10.1001/archopht.1993.01090010027016 [DOI] [PubMed] [Google Scholar]
- 8.Vichnin MC, Jaeger EA, Gault JA, Jeffers JB. Ocular injuries related to airbag inflation. Ophthalmic Surg Lasers. 1995;26(6):542–548. [PubMed] [Google Scholar]
- 9.Onwuzuruigbo CJ, Fulda GJ, Larned D, Hailstone D. Traumatic blindness after airbag deployment: bilateral lenticular dislocation. J Trauma. 1996;40(2):314–316. doi: 10.1097/00005373-199602000-00029 [DOI] [PubMed] [Google Scholar]
- 10.Ghafouri A, Burgess SK, Hrdlicka ZK, Zagelbaum BM. Airbag-related ocular trauma. Am J Emerg Med. 1997;15(4):389–392. doi: 10.1016/S0735-6757(97)90135-2 [DOI] [PubMed] [Google Scholar]
- 11.Koisaari T, Leivo T, Sahraravand A, Haavisto A-H, Sulander P, Tervo TMT. Airbag deployment-related eye injuries. Traffic Inj Prev. 2017;18(5):493–499. doi: 10.1080/15389588.2016.1271945 [DOI] [PubMed] [Google Scholar]
- 12.Wang SH, Lim CC, Teng YT. Airbag-associated severe blunt eye injury causes choroidal rupture and retinal hemorrhage: a case report. Case Rep Ophthalmol. 2017;8(1):13–20. doi: 10.1159/000452652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vohra V, Chawla H. Corneal endothelial decompensation due to airbag injury. Int Ophthalmol. 2018;38(5):2171–2174. doi: 10.1007/s10792-018-0999-7 [DOI] [PubMed] [Google Scholar]
- 14.Lesher MP, Durrie DS, Stiles MC. Corneal edema, hyphema, and angle recession after air bag inflation. Arch Ophthalmol. 1993;111(10):1320–1322. doi: 10.1001/archopht.1993.01090100026014 [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Moreno JM. Air bag-associated retinal tear. Eur J Ophthalmol. 1998;8(1):52–53. doi: 10.1177/112067219800800112 [DOI] [PubMed] [Google Scholar]
- 16.Kaizu Y, Nakao S, Yamaguchi M, Murakami Y, Salehi-Had H, Ishibashi T. Detection of airbag impact-induced cone photoreceptor damage by adaptive optics scanning laser ophthalmoscopy: a case report. BMC Ophthalmol. 2016;16(1):99. doi: 10.1186/s12886-016-0275-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savastano A, Donati MC, Rizzo S. Retinal tear related to air bag deployment. JAMA Ophthalmol. 2016;34(4):e155021. doi: 10.1001/jamaophthalmol.2015.5021 [DOI] [PubMed] [Google Scholar]
- 18.Kung J, Leung LS, Leng T, Liao YJ. Traumatic airbag maculopathy. JAMA Ophthalmol. 2013;131(5):685–687. doi: 10.1001/jamaophthalmol.2013.883 [DOI] [PubMed] [Google Scholar]
- 19.Geggel HS, Griggs PB, Freeman MI. Irreversible bullous keratopathy after air bag trauma. Case Reports CLAO J. 1996;22(2):148–150. [PubMed] [Google Scholar]
- 20.Zabriskie NA, Hwang IP, Ramsey JF, Crandall AS. Anterior lens capsule rupture caused by air bag trauma. Am J Ophthalmol. 1997;123(6):832–833. doi: 10.1016/S0002-9394(14)71133-X [DOI] [PubMed] [Google Scholar]
- 21.Blackmon SM, Fekrat S, Setlik DE, Afshari NA. Posterior dislocation of a crystalline lens associated with airbag deployment. J Cataract Refract Surg. 2005;31(12):2431–2432. doi: 10.1016/j.jcrs.2005.08.050 [DOI] [PubMed] [Google Scholar]
- 22.Salam T, Stavrakas P, Wickham L, Bainbridge J. Airbag injury and bilateral globe rupture. Am J Emerg Med. 2010;28(8):982.e5–982.e6. [DOI] [PubMed] [Google Scholar]
- 23.Eliott D, Hauch A, Kim RW, Fawzi A. Retinal dialysis and detachment in a child after airbag deployment. J AAPOS. 2011;15(2):203–204. doi: 10.1016/j.jaapos.2010.11.021 [DOI] [PubMed] [Google Scholar]
- 24.Mohamed AA, Banerjee A. Patterns of injury associated with automobile airbag use. Postgrad Med J. 1998;74(874):455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duma SM, Crandall JR. Eye injuries from airbags with seamless module covers. J Trauma. 2000;48(4):786–789. doi: 10.1097/00005373-200004000-00036 [DOI] [PubMed] [Google Scholar]
- 26.Shirzadi H, Zohoor H, Naserkhaki S. Biomechanical simulation of eye-airbag impacts during vehicle accidents. Proc Inst Mech Eng H. 2018;232(7):699–707. doi: 10.1177/0954411918778063 [DOI] [PubMed] [Google Scholar]
- 27.Power ED, Duma SM, Stitzel JD, et al. Computer modeling of airbag-induced ocular injury in pilots wearing night vision goggles. Aviat Space Environ Med. 2002;73(10):1000–1006. [PubMed] [Google Scholar]
- 28.Uchio E, Ohno S, Kudoh J, Aoki K, Kisielewicz LT. Simulation model of an eyeball based on finite element analysis on a supercomputer. Br J Ophthalmol. 1999;83(10):1106–1111. doi: 10.1136/bjo.83.10.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchio E, Kadonosono K, Matsuoka Y, Goto S. Simulation of airbag impact on an eye with transsclerally fixated posterior chamber intraocular lens using finite element analysis. J Cataract Refract Surg. 2004;30(2):483–490. doi: 10.1016/S0886-3350(03)00520-0 [DOI] [PubMed] [Google Scholar]
- 30.Uchio E, Ohno S, Kudoh K, Kadonosono K, Andoh K, Kisielewicz LT. Simulation of airbag impact on post-radial keratotomy eye using finite element analysis. J Cataract Refract Surg. 2001;27(11):1847–1853. doi: 10.1016/S0886-3350(01)00966-X [DOI] [PubMed] [Google Scholar]
- 31.Uchio E, Watanabe Y, Kadonosono K, Matsuoka Y, Goto S. Simulation of airbag impact on eyes after photorefractive keratectomy by finite element analysis method. Graefes Arch Clin Exp Ophthalmol. 2003;241(6):497–504. doi: 10.1007/s00417-003-0679-8 [DOI] [PubMed] [Google Scholar]
- 32.Huang J, Uchio E, Goto S. Simulation of airbag impact on eyes with different axial lengths after transsclerally fixated posterior chamber intraocular lens by using finite element analysis. Clin Ophthalmol. 2015;9:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamura K, Shimokawa A, Takahashi R, Saeki Y, Ozaki H, Uchio E. Finite element analysis of air gun impact on post-keratoplasty eye. Clin Ophthalmol. 2020;14:179–186. doi: 10.2147/OPTH.S236825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi R, Okamura K, Tsukahara-Kawamura T, et al. Finite element analysis of changes in tensile strain by airsoft gun impact on eye and deformation rate in eyes of various axial lengths. Clin Ophthalmol. 2020;14:1445–1450. doi: 10.2147/OPTH.S249483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi A, Izaki R, Fujita H, et al. Finite element analysis of changes in tensile strain and deformation by airbag impact in eyes of various axial lengths. Int Ophthalmol. 2022;43(7):2143–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno T, Fujita H, Harada K, Kawamura-Tsukahara T, Ozaki H, Uchio E. Finite element analysis of changes in deformation of intraocular tissues by airbag impact in eyes of various axial lengths. Clin Ophthalmol. 2024;18:699–712. doi: 10.2147/OPTH.S445253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruan JS, Pradad P. Coupling of a finite element human head model with a lumped parameter hybrid III dummy model: preliminary results. J Neurotrauma. 1995;42(4):725–734. doi: 10.1089/neu.1995.12.725 [DOI] [PubMed] [Google Scholar]
- 38.Hoeltzel DA, Altman P, Buzard K, Choe K. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng. 1992;114(2):202–215. [DOI] [PubMed] [Google Scholar]
- 39.Reichel E, Miller D, Blanco E, Mastanduno R. The elastic modulus of central and perilimbal bovine cornea. Ann Ophthalmol. 1989;21(6):205–208. [PubMed] [Google Scholar]
- 40.Kim JM, Kim KO, Kim YD, Choi GJ. A case of air-bag associated severe ocular injury. Korean J Ophthalmol. 2004;18(1):84–88. doi: 10.3341/kjo.2004.18.1.84 [DOI] [PubMed] [Google Scholar]
- 41.Schreck RM, Rouhana SW, Santrock J, et al. Physical and chemical characterization of airbag effluents. J Trauma. 1995;38(4):528–532. doi: 10.1097/00005373-199504000-00011 [DOI] [PubMed] [Google Scholar]
- 42.Watson R, Gray W, Sponsel WE, et al. Simulations of porcine eye exposure to primary blast insult. Transl Vis Sci Technol. 2015;4(4):8. doi: 10.1167/tvst.4.4.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearlman JA, Au Eong KG, Kuhn E, Pieramici DJ. Airbags and eye injuries: epidemiology, spectrum of injury, and analysis of risk factors. Surv Ophthalmol. 2001;46(3):234–242. doi: 10.1016/S0039-6257(01)00256-9 [DOI] [PubMed] [Google Scholar]
- 44.Stein JD, Jaeger EA, Jeffers JB. Air bags and ocular injuries. Trans Am Ophthalmol Soc. 1999;97:59–82. [PMC free article] [PubMed] [Google Scholar]
- 45.Yang CS, Chou TF, Liu JH, Hsu WM. Air bag associated posterior segment ocular trauma. J Chin Med Assoc. 2004;67(8):425–431. [PubMed] [Google Scholar]
- 46.Madreperla SA, Benetz BA. Formation and treatment of a traumatic macular hole. Arch Ophthalmol. 1997;115(9):1210–1211. doi: 10.1001/archopht.1997.01100160380026 [DOI] [PubMed] [Google Scholar]
- 47.Gray W, Sponsel WE, Scribbick FW, et al. Numerical modeling of paintball impact ocular trauma: identification of progressive injury mechanisms. Invest Ophthalmol Vis Sci. 2011;52(10):7506–7513. doi: 10.1167/iovs.11-7942 [DOI] [PubMed] [Google Scholar]
- 48.Cirovic S, Bhola RM, Hose DR, et al. Computer modelling study of the mechanism of optic nerve injury in blunt trauma. Br J Ophthalmol. 2006;90(6):778–783. doi: 10.1136/bjo.2005.086538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stitzel JD, Duma SM, Cormier JM, Herring IP. A nonlinear finite element model of the eye with experimental validation of the prediction of globe rupture. Stapp Car Crash J. 2002;46:81–102. [DOI] [PubMed] [Google Scholar]