Abstract
The spectrin-based membrane skeleton, a multi-protein scaffold attached to diverse cellular membranes, is presumed to be involved in the stabilization of membranes, the establishment of membrane domains as well as in vesicle trafficking and nuclear functions. Spectrin tetramers made of α- and β-subunits are linked to actin microfilaments, forming a network that binds a multitude of proteins. The most prevalent α-spectrin subunit in non-erythroid cells, αII-spectrin, contains two particular spectrin repeats in its central region, α9 and α10, which host an Src homology 3 domain, a tissue-specific spliced sequence of 20 residues, a calmodulin-binding site and major cleavage sites for caspases and calpains. Using yeast two-hybrid screening of kidney libraries, we identified two partners of the α9-α10 repeats: the potential tumour suppressor Tes, an actin-binding protein mainly located at focal adhesions; and EVL (Ena/vasodilator-stimulated phosphoprotein-like protein), another actin-binding protein, equally recruited at focal adhesions. Interactions between spectrin and overexpressed Tes and EVL were confirmed by co-immunoprecipitation. In vitro studies showed that the interaction between Tes and spectrin is mediated by a LIM (Lin-11, Isl-1 and Mec3) domain of Tes and by the α10 repeat of αII-spectrin whereas EVL interacts with the Src homology 3 domain located within the α9 repeat. Moreover, we describe an in vitro interaction between Tes and EVL, and a co-localization of these two proteins at focal adhesions. These interactions between αII-spectrin, Tes and EVL indicate new functions for spectrin in actin dynamics and focal adhesions.
Keywords: Ena/vasodilator-stimulated phosphoprotein-like protein (EVL), focal adhesion, LIM domain membrane, skeleton, spectrin repeat, Tes
Abbreviations: DO-WLH, medium lacking tryptophan, leucine and histidine; EVL, Ena/vasodilator-stimulated phosphoprotein-like protein; GST, glutathione S-transferase; LIM, Lin-11, Isl-1 and Mec3; mAb, monoclonal antibody; PET, prickle espinas, testin; SH3, Src homology 3; VASP, vasodilator-stimulated phosphoprotein
INTRODUCTION
The spectrin-based skeleton, first identified at the inner surface of the erythrocyte plasma membrane, is considered as an essential scaffold underlying different lipid bilayers in all animal cells [1]. Spectrins are giant extended flexible molecules composed of two subunits (α and β), which intertwine to form αβ-heterodimers, a pair of αβ-heterodimers forms the spectrin tetramer by head-to-head self-association. These tetramers constitute the filaments of this network, the nodes of which are cross-linked by short actin filaments in a complex including protein 4.1 and other proteins. As defined for the red blood cells, the attachment of the spectrin-based skeleton to biological membranes is mediated by interactions with membrane proteins and phospholipids [1,2]. The best understood linkages involve the adapter protein ankyrin, which connects spectrin to a variety of transmembrane proteins such as ionic channels (anion-exchanger channel, voltage-gated channel), ionic pumps (Na+/K+-ATPase, H+/K+-ATPase) and cell adhesion molecules of the immunoglobulin superfamily such as neuroglian, neurofascin or neuronal-cell adhesion molecule.
Two genes for the α-chains (αI and αII) and five for β-chains (βI–βV) as well as different spliced isoforms give rise to a great diversity of αβ-heterodimers. In most, if not all, vertebrate-nucleated cells, spectrin heterodimers contain the αII-chain, which can form a heterodimer with different β-chains. All spectrin chains are made up of a succession of triple-helical repeat units, which probably confer the flexible properties to spectrins. Each repeat unit is about 106 residues long and folds into a coiled-coil structure made of three helices (A, B and C). Certain repeat units host additional sequences that represent specific domains. These domains as well as certain repeat units mediate numerous protein interactions, defining the multiple physiological functions of spectrins. Functions of the spectrin-based skeleton are well-defined in red blood cells where it is required for shape maintenance, for resistance to shear stress and for deformability. However, the functions of the more complex spectrin-based skeleton in non-erythroid cells is less understood. Suggested functions of spectrin and of the spectrin-based skeleton in non-erythroid cells include the establishment and maintenance of specialized membrane subdomains, vesicle trafficking and targeting as well as exo- and endocytosis [1]. Spectrin was suggested to participate in the formation and maintenance of specialized membrane subdomains by accumulating and conferring resiliency and durability on integral membrane proteins [3–6].
One prominent role of the spectrin-based membrane skeleton in non-erythroid cells was suggested to be the stabilization of cell–cell contacts [4]. Spectrin has been demonstrated to be present in a complex with Zonula occludens-1, a protein of tight junctions [7]. The spectrin β-chain binds also directly α-catenin, a protein of the adherens junction complexes [8].
We focused our work on αII-spectrin, highly expressed in vertebrate nucleated cells. αII-spectrin contains in its central region an SH3 (Src homology 3) domain included in the α9 repeat. The α9 repeat also hosts a 20-residue loop, whose coding region is spliced out in about 50% of the epithelial cell mRNAs (G. Nicolas, unpublished work), resulting in two isoforms αIIΣ1 (including the 20-residue loop) and αIIΣ2 (lacking the loop). In addition, the following adjacent α10 repeat of αII-spectrin is characterized by a 36-residue insert that contains a hypersensitive domain to proteases (caspases-2, -3 and -7; m- and μ-calpains) and a binding site for calmodulin [9,10]. Cleavage at these protease sites is of major importance for the stability of the spectrin skeleton [11] and is implicated in apoptosis, necrosis and a number of pathologies associated with increased spectrin-breakdown [12].
In yeast two-hybrid screens of kidney libraries, we identified EVL (Ena/vasodilator-stimulated phosphoprotein-like protein) (O. Bournier, Y. Kroviarski, B. Rotter, G. Nicolas, M.C. Lecomte and D. Dhermy, unpublished work) and Tes as two new interacting proteins of the central region of αII-spectrin. Both proteins are actin-binding proteins present in cell adhesion complexes [13–16]. Tes, also called Testin, is different from the rat protease of the same name. Tes was identified as a candidate tumour suppressor [17,18]. It contains a PET (prickle espinas, testin) domain in its central region, followed by three C-terminal LIM (Lin-11, Isl-1 and Mec3) domains. In the present study, we demonstrate that Tes interacts in vitro through a LIM domain with the α10 repeat of αII-spectrin, whereas EVL interacts with the SH3 domain of the α9 repeat. EVL belongs to a protein family that includes Drosophila Ena (Enabled), Mena (mammalian Ena) and VASP (vasodilator-stimulated phosphoprotein) involved in actin-based motility and localized at focal adhesions [14,19,20]. Tes has been recently described to interact with Mena and VASP [15,16]. We show, in the present study that Tes could also interact with EVL. These results reveal that αII-spectrin could interact with different proteins at cell-matrix contacts and might indicate a function for spectrin in focal adhesions and actin-cytoskeleton dynamics.
MATERIALS AND METHODS
Yeast two-hybrid screening
Two-hybrid screening was performed as described in [10], using the αII-spectrin sequence (accession number U83867) from Asp885 to Leu1229 as baits. This sequence corresponding to repeat units α9-α10, including or not including the 20-residue insert, named αIIΣ1 and αIIΣ2 respectively, was cloned into the pLEX12 vector (a modified version of pBMT116 vector carrying the tetracycline resistance gene) in-frame with the C-terminus of the LEXA DNA-binding domain. The yeast strain L40 established with the αII-Sp-baits was transformed with 100 μg of plasmid cDNA originating from either a rat kidney library made in the laboratory [11] or a human kidney library (ClonTech, Basingstoke, U.K.). The baits did not induce any background and His+ clones were selected on DO-WLH (medium lacking tryptophan, leucine and histidine) after 3–4 days of growth at 30 °C. β-Galactosidase activity was analysed on a filter using X-gal as substrate. Recombinant pGAD and pAct2 plasmids were recovered from His+lacZ+ phenotype yeasts and selected after transformation of DH5α Escherichia coli grown on ampicillin plates. Sequences were obtained from PCR-amplified products and were subsequently submitted to the BLAST search (http://www.ncbi.NLM.NIH.gov/BLAST).
Analysis of interaction specificity using the yeast two-hybrid system
Interactions were analysed by mating of the L40 and AMR70 strains. Two additional αII-spectrin constructs spanning from Asp885 to Leu1229, bearing the Pro1017→Leu mutation within the SH3 domain were subcloned into the pLEX12 vector; they are referred to as αIIΣ1SH3mt and αIIΣ2SH3mt.
Expression and purification of recombinant peptides in E. coli
The sequences of αII-spectrin corresponding to α9 repeat (Asp 885 to Lys1089), α9-α10 repeats (Asp885 to Leu1229) and α8-α11 repeats (Lys778 to Gly 1336) were cloned into pGEX-6P2 (Amersham Biosciences, Europe, Sarclay, France) and pQE 80 (Qiagen, Courtaboeuf, France) vectors. Full-length EVL-1 isoform was subcloned in pQE 80. GST (glutathione S-transferase)- and His6-tagged peptides were expressed in BL21 Gold E. coli strain after induction with 0.5 mM isopropyl β-D-thiogalactoside and purified according to the manufacturer's instructions.
In vitro protein–protein interactions
In vitro interactions were performed overnight at 4 °C with 30–40 μg of either recombinant GST or His6 peptides immobilized on glutathione-Sepharose 4B or nickel beads (Amersham Biosciences) respectively and S35-labelled Tes peptides obtained by transcription and translation in vitro (TNT® T7 Quick kit for PCR DNA, Promega, Charbonnières, France) in 20 mM PBS, 50 μM ZnCl2 and 1 μM 2-mercaptoethanol. After six washes in PBS/Tween 20 (0.1%), bound proteins were eluted by either GSH (10 mM) or 300 mM imidazol. Amounts of eluted, radiolabelled proteins were evaluated after SDS/PAGE using ‘Instant Imager’ apparatus (Packard, Packard Instrument S.A., 45 Rue d'Arcueil Zl Silic Rungis, Cedex France). The binding activity (as evaluated by the counted radioactivity of the eluted Tes peptides) was adjusted according to the number of methionines in each peptide.
Cell culture, transfections and immunoprecipitation assays
The monkey kidney COS-7 cell line (A.T.C.C. CRL-1651) and the fibroblastic Rat2 cell line (A.T.C.C. CRL-1764) were grown at 37 °C in 5% CO2 in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) heat-inactivated foetal calf serum, sodium pyruvate and penicillin/streptomycin.
Human Tes cDNA was subcloned into the pEF-DEST51 Gateway™ vector (Invitrogen) from the pENTR vector containing the Tes-cDNA, according to the manufacturer's instructions. The resulting construct (pEF-DEST51-Tes) allows the production of Tes with the His6 and V5 epitope tags at its C-terminus. The rat EVL cDNA was subcloned in pDEST 12.2 Gateway™ vector (Invitrogen) from the pDONR™ 201 vector in which the EVL cDNA was subcloned as a PCR product, according to the manufacturer's instructions. The resulting construct (DEST-EVL) allows the production of EVL without tag. The central region of human αII-spectrin corresponding to repeats α8-α11 (Lys779 to Gly1336) fused to the FLAG epitope was subcloned in pCEP4 vector (Invitrogen).
COS-7 cells were seeded on to 100 mm plate dishes, grown for one day, and transiently transfected using Lipofectamine Plus™ Reagent (Invitrogen) according to the manufacturer's instructions. Transfected COS-7 cells were then scraped and lysed for 30 min on ice in lysis buffer (50 mM Tris/HCl, pH7.5, 150 mM NaCl, 0.5% Nonidet P 40, 1 mM O-vanadate, 10 μM ZnCl2, 0.1 mM 2-mercaptoethanol and anti-protease cocktail (Sigma). The detergent-soluble proteins were incubated overnight at 4 °C with ANTI-FLAG® M2-agarose affinity gel (Sigma). After extensive washing using the lysis buffer, immunoprecipitated proteins were eluted either with glycine (pH 2.2), then equilibrated with Tris/HCl buffer (pH 9), or with SDS (Laemmli sample buffer). Samples were analysed by SDS/PAGE (10% polyacrylamide gel) and transferred on to nitrocellulose membrane (Optitran®, Schleicher and Schuell, Elquevilly, France) using a Tris/glycine buffer containing 10% (v/v) methanol. After saturation in 5% (v/v) non-fat-milk, 0.05% Tween 20 and PBS buffer (pH 7.5), blots were probed with either immunopurified IgG directed against EVL (prepared in the laboratory after immunization of rabbit with recombinant EVL) and anti-rabbit IgG conjugated with horseradish peroxidase (Nordic Immunological Laboratories, Tilburg, the Netherlands) or anti-V5 antibodies conjugated with horseradish peroxidase (Invitrogen). Immunocomplexes were detected using the Supersignal West Pico chemiluminescence's substrate (Pierce, Tattenhall, Cheshire, U.K.).
Immunofluorescence studies of transfected cells
Immunofluorescence studies were performed 48 h after transient transfection with Lipofectamine Plus™ of COS-7 and Rat2 cells seeded on to coverslips (coated with collagen for Rat2 cells). The cells were fixed with 4% (w/v) paraformaldehyde and permeabilized with 0.5% Triton X-100. Tes was detected by anti-V5 mAb (monoclonal antibody; 1/200 dilution) and EVL by immunopurified anti-EVL polyclonal antibodies (1/200 dilution). F-actin was labelled with Alexa Fluor™ 568 phalloidin (Molecular Probes). All dilutions were made in buffered saline solution containing 0.1% BSA. Secondary labelled anti-IgG antibodies [Alexa Fluor™ 488 goat anti-rabbit (highly cross-absorbed) and Alexa Fluor™ 546 goat anti-mouse (highly cross-absorbed)] were purchased from Molecular Probes. Nuclei were counterstained with 0.4 μM TO-PRO 3 (Molecular Probes) after treatment with RNAse for 30 min. The stained cells were then mounted in ProLong™ Antifade solution (Molecular Probes). The fluorescence was observed by confocal microscopy (Zeiss LSM 510; Carl Zeiss, Cologne, Germany) using the multitracking-scanning procedure to avoid cross-talk between channels.
RESULTS
The central region of αII-spectrin recruits Tes and EVL
To identify αII-spectrin-interacting proteins, yeast two-hybrid screenings were performed using the two isoforms of the central region of the αII-spectrin as baits, referred to as αIIΣ1 and αIIΣ2 peptides [10]. The baits consisted of Asp885 to Leu1229 corresponding to α9 and α10 repeats of αII-spectrin. The α9 repeat includes an SH3 domain and an alternatively spliced 20-residue insert; α10 repeat contains a 36-residue loop that hosts a calmodulin binding site, and two cleavage sites recognized by calpains and caspases (termed here as CCC-loop, for calmodulin, calpain, caspase).
The screening of the rat kidney library with both baits resulted in the identification of nine clones expressing a part of the Tes protein (accession number gi:475209), starting at Glu144. Tes was first identified as a ubiquitously expressed protein of 421 amino acids [21]. This protein is characterized by the presence of a PET domain of unknown function followed by three C-terminal LIM domains (from the N- to the C-terminus). All the selected clones contain the three LIM domains, since they start at Glu144, located in the PET domain. Tes is a probable tumour suppressor [17,18,21] and has been described recently to interact with proteins of focal adhesion such as zyxin, vinculin and talin [15,16]. Among the clones selected by baits from the human library, we also identified three clones coding for the full-length sequence of EVL, a protein belonging to the Mena/VASP family of proteins, described as multifunctional organizers of the actin cytoskeleton [13,14].
Tes and EVL interact with the central region of αII-spectrin in a cellular context
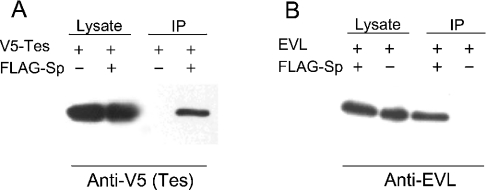
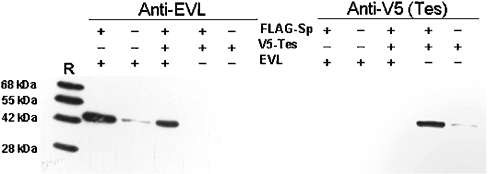
The lysates of COS-7 expressing either Tes as V5-tagged protein or both V5–Tes and spectrin α8-α11 peptide bearing the FLAG epitope were submitted to immunoprecipitation by anti-FLAG antibodies. Western blots of the immunocomplexes probed with anti-V5 antibodies revealed the presence of Tes only in COS cells expressing both peptides, indicating that interaction between Tes and spectrin occurs in cells (Figure 1A). Similar experiments performed in COS-7 expressing both EVL and the FLAG-tagged α8-α11 peptide confirmed the interaction between EVL and spectrin (Figure 1B).
Figure 1. Co-immunoprecipitation of spectrin peptide with Tes (A) and EVL (B).
(A) Lysates of COS-7 cells expressing V5-tagged Tes and FLAG-tagged spectrin α8-α11 peptide were immunoprecipitated with anti-FLAG M2 beads. The complexes eluted at pH 2 were analysed by Western blotting using antibodies directed against the V5-epitope. (B) Lysates of COS-7 cells expressing EVL and FLAG-tagged spectrin α8-α11 peptide were immunoprecipitated with anti-FLAG M2 beads. The complexes eluted at pH 2 were analysed by Western blotting using antibodies directed against EVL.
The spectrin α10 repeat is required for interaction with Tes
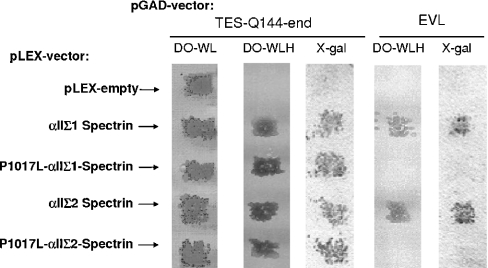
The spectrin baits used in the two-hybrid screenings contained several putative interacting domains: an SH3 domain, two spectrin repeat units, an alternatively spliced 20-residue insert and the CCC-loop. As both the αIIΣ1 peptide and αIIΣ2 peptide recruited Tes, the 20-residue insert is not essential for the interaction. To identify better the spectrin site required for the interaction with Tes, several different versions of the bait-constructs were tested in the yeast two-hybrid system and in GST-pull down assays. Involvement of the SH3 domain was tested with a construct carrying the Pro1017→Leu mutation within the SH3 domain (αIIΣ1SH3mt peptide). This mutation is associated with a loss of SH3-dependent interaction [11]. The presence of the Pro1017→Leu mutation within the SH3 domain did not affect the interaction with Tes as revealed by the yeast growth on the DO-WLH selective medium, and the β-galactosidase activity (Figure 2). In contrast, the Pro1017→Leu mutation totally abolished the interaction with EVL, as revealed by the absence of yeast growth on DO-WLH medium and of β-galactosidase activity. These results demonstrate that the interaction between spectrin and Tes does not require the SH3 domain, whereas the interaction with EVL is SH3-dependent.
Figure 2. Analysis of the interaction between spectrin constructs and Tes and EVL using yeast-two-hybrid assays.
L40 yeast cells transformed with pLEX plasmids containing different spectrin constructs (wild-type and P1017L mutant constructs) were mated with AMR70 yeast cells transformed with pGAD plasmids containing either Tes (from Glu144) or full-length EVL sequence. Yeasts containing both pLEX and pGAD plasmids are capable of growing on the double selective medium DO-WL. As revealed by yeast growth on triple-selective medium, DO-WLH and β-galactosidase activity (X-gal-lanes), all spectrin constructs interacted with Tes. In contrast, the presence of the Pro1017→Leu mutation (P1017L) abolished the interaction with EVL, indicating the dependence of the SH3 domain in this interaction.
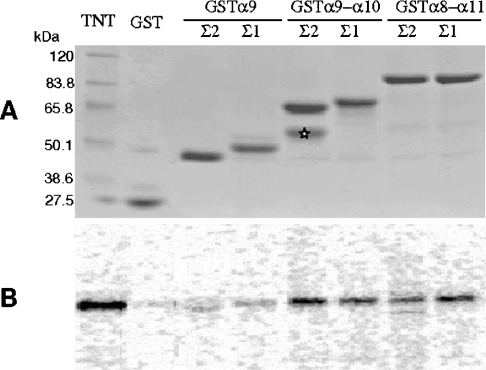
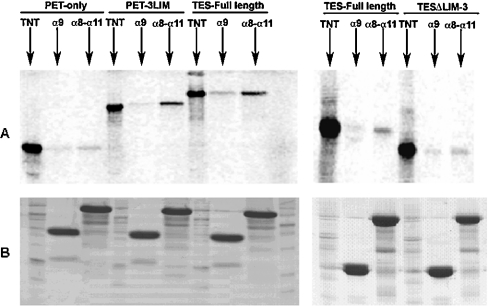
We further investigated the spectrin–Tes interaction using human full-length S35-labelled Tes and several spectrin constructs as GST fusion peptides. These spectrin peptides corresponded to the human sequences of αII-spectrin from the α9, the α9-α10 or the α8-α11 repeat units. Neither GST alone, nor the GST–α9 peptide containing the SH3 domain could retain full-length Tes isoforms as shown in Figure 3. These results confirm that the SH3 domain is not involved in the interaction with Tes. Tes was only retained by the GST–α9-α10 and the GST–α8-α11 peptides, suggesting involvement of the α10 repeat in the interaction. The amounts of Tes (as evaluated by S35-counting) bound to either GST–α9-α10 or GST–α8-α11 peptides were in a similar range, indicating that the α10 repeat represents the major binding site. The similar amount of Tes bound to αIIΣ1 and αIIΣ2 peptides confirmed that the 20-residue insert has no effect on the interaction.
Figure 3. In vitro interaction between different αII-spectrin peptides and human Tes.
S35-labelled Tes was incubated with GST and several GST-spectrin peptides bound to glutathione-Sepharose 4B beads. After extensive washes, the complexes were eluted and analysed by SDS/PAGE. (A) Coomassie Blue-stained gel showing different eluted GST peptides. The Σ2 version of GST–α9-α10 (*) is partly degraded. The first lane contained molecular mass standards as well as the TNT product. (B) Gel analysed by ‘Instant Imager’ and reveals the presence of S35-labelled Tes retained by the different spectrin constructs shown in (A). The first lane contained a sample of the labelled Tes. Tes strongly bound to spectrin peptides when they contained the α10 spectrin repeat.
Tes interacts with spectrin via a LIM domain
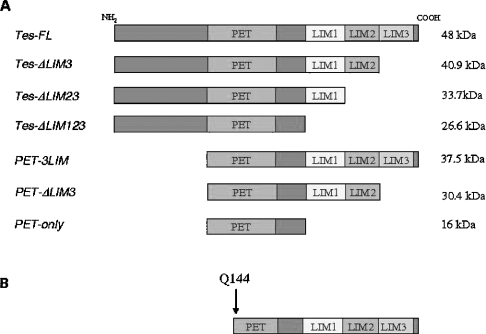
To identify the Tes-domains involved in the interaction with the α10 spectrin repeat, different S35-labelled Tes peptides (depicted in Figure 4) were tested for interaction with the GST-constructs. The latter consisted either of the spectrin α8-α11 repeats (as interacting partner) or the α9 repeat (for evaluation of non-specific binding). Binding of the different S35-labelled Tes constructs was regarded as specific when the amounts of Tes peptides bound to spectrin α8-α11 repeats (as evaluated by radioactivity counts) was more than twice the amount retained by the α9 repeat.
Figure 4. Presentation of the different Tes peptides obtained by in vitro TNT (A) and identified in the yeast-two-hybrid screening (B).
As shown in Figure 5, both the PET-3LIM Tes peptide (with a full PET domain and the three LIM domains, resembling the peptide identified in the two-hybrid screening) and the full-length Tes bound specifically to the spectrin α8-α11 repeats. In contrast, deletion of the LIM3 domain (TesΔLIM3) abolished specific binding to the spectrin α8-α11 repeats, as shown on Figure 5. No specific binding was observed for the PET domain (PET only; Figure 5).
Figure 5. In vitro interaction between different human Tes peptides and αII-spectrin peptides.
Different S35-labelled Tes peptides obtained by in vitro transcription and translation (Lanes named TNT) were incubated with GST–α9 and GST–α8-α11 spectrin peptides bound to glutathione-Sepharose 4B beads. After extensive washings, the complexes were eluted and analysed by SDS/PAGE. (A) The gel is analysed by the Instant Imager and reveals the presence of S35-labelled Tes, which could be retained by the GST-spectrin peptides. (B) The Coomassie Blue-stained gel showing the different eluted GST peptides. While full-length Tes and the peptide deleted of the N-terminal end (containing the PET domain with the three LIM domains) strongly bound spectrin α8-α9 peptides, deletion of the C-terminal end (ΔLIM3 domain) reduced considerably the specific binding.
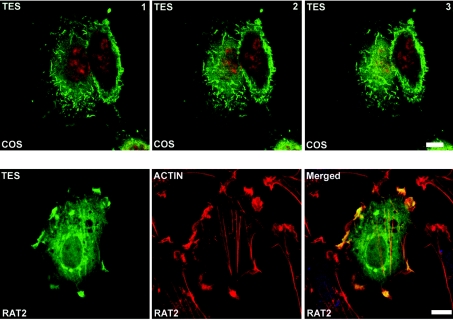
Tes localizes to plasma membrane and actin-rich structures
Immunofluorescence staining of Tes overexpressed in COS-7 and Rat2 cells appeared diffuse throughout the cytoplasm. However, in several COS-7 cells, overexpressed Tes was accumulated in ruffles covering the plasma membrane surface (Figure 6, upper panel). In fibroblastic Rat2 cells cultured on collagen, Tes staining was reinforced in actin-rich structures like stress fibres and filopodia where it co-localized with F-actin (Figure 6, lower panel).
Figure 6. Immunofluorescence studies of Tes overexpressed in COS-7 and Rat2 cells.
COS-7 cells (upper panel) and Rat2 cells (lower panel) were transiently transfected with the plasmid pEF-DEST51-Tes. The upper panel corresponds to three acquisitions of the same COS-7 cells from the basal surface (image 1) to the middle part and the top of the cells (images 2 and 3, respectively; Z scaling: 0.39 μm). Tes (pseudo-coloured in green) was stained with anti-V5 mAb and Alexa Fluor™ 546 goat anti-mouse. Nuclei (pseudo-coloured in red) were counterstained with TO-PRO 3. In the lower panel (Rat2 cells), Tes was stained with anti-V5 antibody and Alexa Fluor™ 488 goat anti-mouse. Tes (pseudo-coloured in green) co-localizes with F-actin, labelled with Alexa Fluor™ 568 phalloidin and pseudo-coloured in red (see yellow staining in merged image). Scale bar=10 μm.
Tes interacts with EVL in vitro
Tes has been reported to interact with Mena and VASP [15,16]. Both proteins belong to the Ena/VASP family of proteins, described as multifunctional organizers of the actin cytoskeleton [13]. We have identified an interaction between αII-spectrin and EVL, another member of this protein family (O. Bournier, Y. Kroviarski, B. Rotter, G. Nicolas, M. C. Lecomte and D. Dhermy, unpublished work). This interaction requires the SH3 domain of αII-spectrin, located in the α9 repeat. As Tes interacts with the adjacent repeat (α10), we tested if Tes interacts equally with EVL.
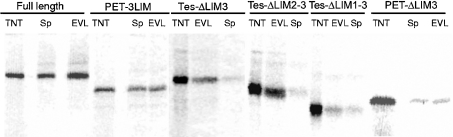
We first compared the ability of Tes to interact with EVL or with spectrin α8-α11 repeats in vitro. Results in Figure 7 show that a larger amount of full-length S35-labelled Tes is retained on His6-tagged EVL bound on nickel beads compared with His6-tagged spectrin peptide in the same conditions.
Figure 7. In vitro interaction between different human Tes peptides and EVL.
Different S35-labelled Tes peptides obtained by in vitro transcription and translation (lanes TNT) were incubated with EVL (lanes EVL) and spectrin α8-α11 peptides (lanes Sp) as His6-tagged peptides bound to nickel beads. After extensive washes, the complexes were eluted and analysed by SDS/PAGE. As revealed by ‘Instant Imager’, full-length Tes and the PET-3LIM peptide interact with EVL as well as with spectrin. The peptide made of the PET-domain with the two domains LIM1 and LIM2 was not retained, neither by spectrin nor by EVL. The peptides lacking the domain LIM3 were retained by EVL but not by spectrin.
We further defined the Tes domains involved in the interaction with EVL, again using the above-described S35-labelled Tes peptides (Figure 4). All peptides containing either the N-terminus or the LIM3 domain were retained by EVL. The peptide lacking both these domains (construct PET-ΔLIM3) was not retained by EVL. Therefore EVL appears to interact with the N-terminus as well as with the C-terminal LIM domain of Tes.
Tes co-localizes with EVL at sites of focal adhesion
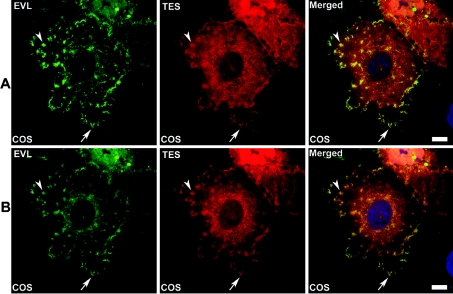
The in vitro characterization of an interaction between Tes and EVL prompted us to analyse such interactions in a cellular context. For this purpose, COS-7 cells were co-transfected with plasmids coding respectively for EVL (without tag) and a Tes-fusion protein bearing the V5 epitope. As previously observed ([14] and unpublished work), the overexpression of EVL modified the cell morphology, promoting the formation of filopodia and focal adhesions. The cellular distribution of EVL in co-transfected cells (Figure 8) was similar to those observed in cells expressing EVL alone: EVL was detected in filopodial tips (see arrow in Figure 8), at the leading edge of lamellipodia and focal adhesions (see arrow-head in Figure 8). In co-transfected cells expressing similar levels of EVL and Tes, Tes staining was reinforced in focal patches, and was co-localized with EVL in these structures (yellow staining in merged images). Co-localization between EVL and Tes was also observed in filopodial tips (see arrow in merged image – Figure 8). EVL staining in focal adhesions was the highest in optical sections acquired at the level of substratum contact (Figure 8A); in upper optical sections, EVL-staining in focal adhesion decreased when Tes-labelling appeared more concentrated (Figure 8B).
Figure 8. Immunofluorescence studies of EVL and Tes in COS-7 cells.
COS-7 cells transiently co-transfected with the plasmid DEST-EVL and the plasmid pEF-DEST51-Tes were stained using respectively anti-EVL polyclonal antibodies and Alexa Fluor™ 488 goat anti-rabbit (EVL pseudo-coloured in green) and anti-V5 mAb and Alexa Fluor™ 546 goat anti-mouse (Tes pseudo-coloured in red). Nuclei (pseudo-coloured in blue) were counterstained with TO-PRO 3. Filopodia and focal adhesions are pointed out by arrow and arrowhead, respectively. In (A), image acquisition was performed at the plane of coverslip contact and (B) corresponds to the upper optical section of the same cells (Z scaling: 0.32 μm). Scale bar=10 μm.
EVL prevents the interaction between spectrin and Tes
According to the interactions we have described between Tes and EVL, EVL and spectrin, as well as between spectrin and Tes, it was tempting to hypothesize the existence of a ternary complex between these three proteins. To test this hypothesis, COS-7 were transfected with the spectrin FLAG-tagged α8-α11 vector in combination with either V5-tagged Tes, or native EVL, or both vectors. Lysates of transfected cells were immunoprecipitated with anti-FLAG antibodies, and immunocomplexes were analysed by Western blotting using antibodies directed against EVL or V5 epitope. As shown in Figure 9, co-expression of the three partners prevents the interaction between Tes and the spectrin peptide, whereas the interaction between EVL and spectrin is maintained.
Figure 9. Co-immunoprecipitation of spectrin peptide, Tes and EVL.
Lysates of COS-7 cells overexpressing FLAG-tagged spectrin α8-α11 peptide, V5-tagged Tes and EVL were immunoprecipitated with anti-FLAG M2 beads. The complexes eluted by SDS were analysed by Western blotting using antibodies directed against EVL (left panel); after stripping, the blot was probed with antibodies directed against the V5-epitope to reveal Tes (right panel). When expressed separately, EVL and Tes each immunoprecipitates with the spectrin peptide. When EVL and Tes are co-expressed, only EVL is immunoprecipitated by the spectrin peptide.
DISCUSSION
Yeast two-hybrid assays, co-immunoprecipitation experiments, and in vitro analysis using recombinant peptides demonstrated an interaction between α10 repeat of αII-spectrin and human and rat Tes. The interaction probably involves the LIM3 domain of Tes. LIM domains (named after the Caenorhabditis elegans transcription factors LIM, [22,23]) are cysteine- and histidine-rich protein modules of 55 amino acids in length that form double zinc-finger domains. LIM-domains are present in many scaffolding proteins and act as modular protein-binding interfaces, mediating protein–protein interactions. Several LIM-proteins have been shown to be involved in the organization of actin filaments [24]. No single binding motif as common target of LIM domains is known. An interaction between a spectrin repeat unit and a LIM domain has been reported for repeat β7 of βI-spectrin and the LIM2 domain of the muscle LIM protein in heart [25]. Muscle LIM protein is a striated muscle-specific factor with a critical role in maintaining the structural integrity of the contractile apparatus. The interaction is proposed to stabilize the association of the contractile apparatus with the sarcolemma, by linking the β-spectrin network to the α-actinin cross-linked actin filaments of the myofibril. A protein BLAST search (at NCBI) with the peptide sequence of the β7 repeat of βI-spectrin revealed 47% resemblance to a stretch of 19 amino acids of α10 repeat of αII-spectrin. These resembling 19 amino acids, located at the very end of α10 repeat in helix C outside of the CCC-loop, could represent the binding site for the LIM3 domain of Tes. Hence Tes might represent a second protein that interacts with a spectrin repeat unit by a LIM domain.
Tes has been reported to interact with Mena and VASP, two members of the Ena/VASP protein family. Ena/VASP-proteins are concentrated at sites of actin dynamics such as lamellipodia, focal adhesions and adherens junctions [14] and have been associated with actin remodelling and actin-dynamics-dependent processes such as fibroblast migration, axon guidance, T-cell polarization and intracellular motility of Listeria [13]. Mena and VASP interact with the LIM3 domain of Tes [15]. We demonstrate, in the present study that Tes could also interact with EVL, another member of the Ena/VASP family. This interaction seems to involve not only the LIM3 domain but also the N-terminus of Tes. It has been suggested that Tes is present in two conformations [15], one ‘open’ and another one ‘closed’ resulting from an interaction between the N- and the C-terminus. As EVL interacts with both termini of Tes, it could interact with Tes in its closed conformation.
Tes has been described as an actin-binding protein, which can be located at focal adhesions, stress fibres and cell–cell contacts [15,16]. Tes interacts with Zyxin and paxillin and co-localizes with these proteins at focal adhesion sites. In our experiments (in COS-7 and Rat2 cells), we showed a localization of Tes in actin-rich structures (in membrane ruffles and stress fibres, Figures 5 and 7). When expressed in the presence of EVL, Tes shows a different cellular distribution: it is co-localized with EVL in focal patches, suggesting that EVL participates in the recruitment of Tes in these structures.
EVL has been also recruited by αII-spectrin baits in the yeast two-hybrid screenings. This interaction, which implicates the αII-spectrin SH3 domain, has been confirmed in a cellular context by co-immunoprecipitation. As the binding sites of EVL and Tes on spectrin are very close (located within the α9 repeat and α10 repeat respectively), a trimeric complex may be formed. However, no co-immunoprecipitation of spectrin and Tes could be detected in the presence of overexpressed EVL (Figure 9). EVL could inhibit the interaction between spectrin and Tes, probably as a result of the competition for the LIM3 binding site involved in both interactions.
The interactions of αII-spectrin with both Tes and EVL might represent a link of αII-spectrin to actin-dynamic processes and focal adhesions. Support for a potential role of αII-spectrin and especially of its central region in actin-dynamic processes was provided by previous observations [26,27], which revealed a localization of the αII-spectrin and its SH3 domain at sites of strong actin dynamics. In human lymphoblast cells, spectrin co-localizes with actin in regions of Fcγ receptor cap-assembly. Spectrin was suggested to influence the actin-skeleton rearrangements necessary for capping of the Fcγ receptor; antibodies directed against αII-spectrin resulted in decreased Fcγ receptor capping [28].
The spectrin-based membrane skeleton is regarded as an important accumulator of proteins required to strengthen cell–cell adhesions [1]. Spectrin isoforms are present at cell–cell contacts already at the two-cell state in C. elegans [4]. The spectrin-based membrane skeleton interacts (either directly or indirectly) with important members of cell–cell adhesion complexes, like Zonula occludens-1 [7], Zonula occludens-2 and protein 4.1 [29], α-catenin, [8] and L1-family of proteins [30]. The interaction of αII-spectrin with Tes and EVL, two focal adhesion proteins, could indicate resembling functions of spectrin in cell-matrix adhesion complexes.
Acknowledgments
This work was supported by a grant from the European Union: HPRNT-CT-2000-00096.
References
- 1.Bennett V., Baines A. J. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol. Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 2.An X., Guo X., Sum H., Morrow J., Gratzer W., Mohandas N. Phosphatidylserine binding sites in erythroid spectrin: location and implications for membrane stability. Biochemistry. 2004;43:310–315. doi: 10.1021/bi035653h. [DOI] [PubMed] [Google Scholar]
- 3.Dubreuil R. R., Wang P., Dahl S., Lee J., Goldstein L. S. Drosophila beta spectrin functions independently of alpha spectrin to polarize the Na,K ATPase in epithelial cells. J. Cell Biol. 2000;149:647–656. doi: 10.1083/jcb.149.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moorthy S., Chen L., Bennett V. Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function. J. Cell Biol. 2000;149:915–930. doi: 10.1083/jcb.149.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammarlund M., Davis W. S., Jorgensen E. M. Mutations in beta-spectrin disrupt axon outgrowth and sarcomere structure. J. Cell Biol. 2000;149:931–942. doi: 10.1083/jcb.149.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinder J. C., Baines A. J. A protein accumulator. Nature (London) 2000;406:253–254. doi: 10.1038/35018679. [DOI] [PubMed] [Google Scholar]
- 7.Tsukamoto T., Nigam S. K. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J. Biol. Chem. 1997;272:16133–16139. doi: 10.1074/jbc.272.26.16133. [DOI] [PubMed] [Google Scholar]
- 8.Pradhan D., Lombardo C. R., Roe S., Rimm D. L., Morrow J. S. Alpha-catenin binds directly to spectrin and facilitates spectrin-membrane assembly in vivo. J. Biol. Chem. 2001;276:4175–4181. doi: 10.1074/jbc.M009259200. [DOI] [PubMed] [Google Scholar]
- 9.Harris A. S., Morrow J. S. Proteolytic processing of human brain alpha spectrin (fodrin): identification of a hypersensitive site. J. Neurosci. 1988;8:2640–2651. doi: 10.1523/JNEUROSCI.08-07-02640.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotter B., Kroviarski Y., Nicolas G., Dhermy D., Lecomte M. C. AlphaII-spectrin is an in vitro target for caspase-2, and its cleavage is regulated by calmodulin binding. Biochem. J. 2004;378:161–168. doi: 10.1042/BJ20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolas G., Fournier C. M., Galand C., Malbert-Colas L., Bournier O., Kroviarski Y., Bourgeois M., Camonis J. H., Dhermy D., Grandchamp B., et al. Tyrosine phosphorylation regulates alpha II spectrin cleavage by calpain. Mol. Cell. Biol. 2002;22:3527–3536. doi: 10.1128/MCB.22.10.3527-3536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderklish P. W., Krushel L. A., Holst B. H., Gally J. A., Crossin K. L., Edelman G. M. Marking synaptic activity in dendritic spines with a calpain substrate exhibiting fluorescence resonance energy transfer. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2253–2258. doi: 10.1073/pnas.040565597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krause M., Dent E. W., Bear J. E., Loureiro J. J., Gertler F. B. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 14.Lambrechts A., Kwiatkowski A. V., Lanier L. M., Bear J. E., Vandekerckhove J., Ampe C., Gertler F. B. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 2000;275:36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 15.Garvalov B. K., Higgins T. E., Sutherland J. D., Zettl M., Scaplehorn N., Kocher T., Piddini E., Griffiths G., Way M. The conformational state of Tes regulates its zyxin-dependent recruitment to focal adhesions. J. Cell Biol. 2003;161:33–39. doi: 10.1083/jcb.200211015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutts A. S., MacKenzie E., Griffith E., Black D. M. TES is a novel focal adhesion protein with a role in cell spreading. J. Cell Sci. 2003;116:897–906. doi: 10.1242/jcs.00278. [DOI] [PubMed] [Google Scholar]
- 17.Tatarelli C., Linnenbach A., Mimori K., Croce C. M. Characterization of the human TESTIN gene localized in the FRA7G region at 7q31.2. Genomics. 2000;68:1–12. doi: 10.1006/geno.2000.6272. [DOI] [PubMed] [Google Scholar]
- 18.Tobias E. S., Hurlstone A. F., MacKenzie E., McFarlane R., Black D. M. The TES gene at 7q31.1 is methylated in tumours and encodes a novel growth-suppressing LIM domain protein. Oncogene. 2001;20:2844–2853. doi: 10.1038/sj.onc.1204433. [DOI] [PubMed] [Google Scholar]
- 19.Vasioukhin V., Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 20.Gertler F. B., Niebuhr K., Reinhard M., Wehland J., Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell (Cambridge, Mass.) 1996;87:227–239. doi: 10.1016/s0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 21.Divecha N., Charleston B. Cloning and characterisation of two new cDNAs encoding murine triple LIM domains. Gene. 1995;156:283–286. doi: 10.1016/0378-1119(95)00088-n. [DOI] [PubMed] [Google Scholar]
- 22.Bach I. The LIM domain: regulation by association. Mech. Dev. 2000;91:5–17. doi: 10.1016/s0925-4773(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 23.Dawid I. B., Breen J. J., Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- 24.Khurana T., Khurana B., Noegel A. A. LIM proteins: association with the actin cytoskeleton. Protoplasma. 2002;219:1–12. doi: 10.1007/s007090200000. [DOI] [PubMed] [Google Scholar]
- 25.Flick M. J., Konieczny S. F. The muscle regulatory and structural protein MLP is a cytoskeletal binding partner of betaI-spectrin. J. Cell Sci. 2000;113:1553–1564. doi: 10.1242/jcs.113.9.1553. [DOI] [PubMed] [Google Scholar]
- 26.Merilainen J., Palovuori R., Sormunen R., Wasenius V. M., Lehto V. P. Binding of the alpha-fodrin SH3 domain to the leading lamellae of locomoting chicken fibroblasts. J. Cell Sci. 1993;105:647–654. doi: 10.1242/jcs.105.3.647. [DOI] [PubMed] [Google Scholar]
- 27.Sormunen R., Lehto V. P. Spectrin in the leading lamella of cultured chicken fibroblasts. Eur. J. Cell Biol. 1995;68:387–397. [PubMed] [Google Scholar]
- 28.Kwiatkowska K., Sobota A. Engagement of spectrin and actin in capping of FcgammaRII revealed by studies on permeabilized U937 cells. Biochem. Biophys. Res. Commun. 1999;259:287–293. doi: 10.1006/bbrc.1999.0769. [DOI] [PubMed] [Google Scholar]
- 29.Mattagajasingh S. N., Huang S. C., Hartenstein J. S., Benz E. J., Jr Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J. Biol. Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura K., Yoshihara F., Tojima T., Ooashi N., Yoon W., Mikoshiba K., Bennett V., Kamiguchi H. L1-dependent neuritogenesis involves ankyrinB that mediates L1-CAM coupling with retrograde actin flow. J. Cell Biol. 2003;163:1077–1088. doi: 10.1083/jcb.200303060. [DOI] [PMC free article] [PubMed] [Google Scholar]