Abstract
Purpose
To characterize the asymmetrical loss of bone mass and identify the association between scoliosis and osteopenia in patients with adolescent idiopathic scoliosis (AIS).
Methods
Demographic information, Cobb angle, and Hounsfield unit (HU) of the neutral vertebra (NV) and apical vertebra (apex) of the major curve were collected retrospectively in 54 AIS patients. For 84 control subjects, HU values were measured at T12 and L5. Propensity score matching was performed to balance the interference of age and BMI.
Results
In the AIS group, the concave and convex lateral HU of the NV and the convex lateral HU of the apex were negatively correlated with the Cobb angle. The AIS patients had lower bilateral HU. The mean HU and the apex-convex HU were also lower in the AIS group, while the apex-concave HU was slightly higher. After matching, the apex-convex HU of the AIS group remained lower, while the apex-concave HU was higher.
Conclusion
Patients with AIS exhibit osteopenia, particularly on the convex side. The severity of scoliosis was found to be directly proportional to the severity of bone loss and the degree of bilateral osteopenia asymmetry. Appropriate intervention for bone loss may be able to curb the progression of scoliosis.
Keywords: adolescent idiopathic scoliosis, osteopenia, osteoporosis, Hounsfield unit, propensity score matching
Introduction
Adolescent idiopathic scoliosis (AIS) is the most prevalent form of structural spinal deformity among adolescents, characterized by a Cobb angle of ≥10°. Typically emerging during early puberty, AIS affects an estimated 1–4% of adolescents, with a notable prevalence in young women.1 Our study hypothesizes a significant interplay between AIS, bone metabolism, and energy homeostasis, which may influence the condition’s progression and severity.1–3
While AIS has been traditionally linked with osteopenia and lower body mass index (BMI), recent findings underscore a potential association with reduced leptin levels as well.2 Osteopenia in AIS is not confined to the axial skeleton but extends peripherally, affecting the distal tibia and radius, and may serve as a prognostic indicator for curve progression.4–6
The gold standard for diagnosing osteopenia, Dual-energy X-ray absorptiometry (DXA), faces limitations due to its planar nature and reliance on cortical bone measurements.7 To address these limitations, Computed Tomography (CT) attenuation, quantified through Hounsfield units (HU), offers a normalized index of x-ray attenuation that correlates strongly with bone mineral density (BMD).8,9 Unlike DXA, HU measurements are not susceptible to errors from cortical bone or calcification and are particularly useful in severe scoliosis cases.8,10
Given the intricate patterns of osteopenia in AIS patients, where bone mineral loss and scoliosis progression mutually exacerbate each other, it is imperative to delineate bone mass alterations in AIS to better understand the etiology of scoliosis. To this end, we conducted a case-control study focusing on the asymmetry of bone loss in AIS patients and the correlation between scoliosis and vertebral HU values.
Materials and Methods
Subjects
We conducted a comprehensive review of all patients diagnosed with scoliosis at our hospital from January 2018 to December 2023. The inclusion criteria for the AIS patients were as follows: (1) age at first visit ranging from 10 to 16 years, (2) a confirmed diagnosis of AIS, and (3) the availability of HU values on CT scans. Patients were excluded based on the following criteria: (1) congenital scoliosis or other etiologies such as neuromuscular or neurofibromatosis type; (2) the presence of metabolic bone disease or the use of medications known to influence bone metabolism; (3) a history of lumbar surgery or low back trauma; and (4) any other diagnosis that would be inconsistent with AIS.
The control group was selected to ensure comparability with the AIS cohort. We reviewed the patients who visited our hospital during the same period. The control subjects were chosen based on these criteria: (1) age between 10 and 18 years, (2) absence of any AIS or other spinal scoliosis diagnosis, and (3) completion of thoracic/lumbar spine three-dimensional CT scans. The control group was selected through a systematic process that prioritized age and BMI similarity to the AIS group. Following initial age screening, participants were stratified and matched by BMI to the AIS group, ensuring they controlled for confounding factors that might affect the study results. Furthermore, to ensure a robust match between the AIS patients and controls, we employed a propensity score matching (PSM) technique. This statistical method allowed us to pair each AIS patient with a control subject who had similar age and BMI characteristics, thereby enhancing the validity of our comparative analyses.
Informed consents were obtained from all patients enrolled. This study was approved by the local ethics committee of the hospital where it was conducted.
Data Collection
Demographic data including age, sex, and body mass index (BMI), and radiological measures including Cobb angle, the neutral vertebra (NV) and apical vertebra (apex) of the major curve were recorded for all patients enrolled (Figure 1). The NV refers to the vertebra with the greatest symmetrical inclination of the bilateral pedicles immediately below the major curve, while the apex specifically indicates the vertebra that is farthest from the vertical line within the major curve.
Figure 1.
The Cobb angle and apical vertebra (apex) of the major curve of scoliosis.
The measurement of HU values was based on the methods proposed in the previous literature.9,11,12 The three-dimensional reconstructive CT used for the examinations was from GE discovery, with a tube voltage of 120kV and a slice thickness of 1.25 mm with a spacing of 0.625 mm. Subjects were scanned in the supine position, in a relaxed neutral position. Image data were analyzed using ROI Manager. The region of interest (ROI) was placed on the coronal images of the vertebral body. The largest possible elliptical ROIs were plotted on the whole, the concave side and the convex side of the cancellous bone of the apex (for the control group, measurements were taken on both sides of the vertebrae), excluding the cortical margins, hemangiomas, and segmental vessels (Figure 2). HU values of the superior and inferior vertebrae of each apex of the major curve were also measured and averaged. For subjects in the control group, T12 and L5 vertebrae were selected, and HU values were measured on bilateral vertebrae, according to the method described previously.
Figure 2.
The ROIs plotted on the whole, the concave side and the convex side of the cancellous bone of the apex. (A). The ROIs of the whole cancellous bone of the apex. (B). The ROIs of the concave and convex side of the cancellous bone of the apex.
The control group was selected based on the basis of an adolescent population, attempting to maintain similar levels of spinal degeneration and BMD values as the AIS group. However, there were still differences in age, and BMI levels between the two groups of subjects, limited by the different etiology of the subjects. To provide a rigorous baseline for consistency comparison, the propensity score matching (PSM) analyses was addressed in both the AIS and control groups, where the PSM matches were then subjected to statistical analysis once again. Two potential confounders (age and BMI), were matched, and matching was performed on a 1:1 ratio (nearest-matching algorithm), with caliper width=0.6 of the standard deviation of the logit of the propensity score. Statistical analysis was repeated after PSM matching.
Apex/NV selection, Cobb angle measurements, and HU value measurements were performed by two independent observers, and mean values were calculated. Disagreements were resolved by consultation with a third observer, a specialist with many years of experience in spinal surgery.
Statistical Analysis
The SPSS (Ver. 26.0, Chicago, IL) and R 4.3.2 (The R Foundation for Statistical Computing, Vienna, Austria) were used for the analysis. Continuous data were expressed as the mean±standard deviation. A t-test was used to analyze the difference in HU values between the concave and convex sides within the target vertebral level. The Pearson correlation coefficient was calculated to assess the correlation between the Cobb angle of the major curve and HU values of different vertebrae, as well as the concave-convex-lateral HU difference. Differences were considered statistically significant when P < 0.05.
Results
A total of 54 AIS patients and 84 controls were included in the study. The demographics of AIS and non-AIS patients are summarized in Table 1. The sex ratio of the AIS and non-AIS groups was 33:21 and 39:45, respectively (P=0.092). The age of the AIS group at the time of hospitalization was 13.87±3.17 years while the mean age of the control group was 17.62±2.73 years (P<0.01). The mean height, weight and BMI of the patients in the AIS group were lower than those in the control group (all P<0.01).
Table 1.
The Characteristics of All Participants
| AIS Group n = 54 | Control Group n = 84 | P value | |
|---|---|---|---|
| Sex (female: male) | 33: 21 | 39: 45 | 0.092 |
| Age (years) | 13.87 ± 3.17 | 17.62 ± 2.73 | <0.001 ** |
| Weight (kg) | 39.64 ± 13.03 | 81.74 ± 17.76 | <0.001 ** |
| Height (m) | 1.47 ± 0.23 | 1.77 ± 0.08 | <0.001 ** |
| BMI | 17.87 ± 3.07 | 26.06 ± 4.66 | <0.001 ** |
Note: **P < 0.01.
Characteristics of Cobb Angle and HU Value in AIS Patients
The mean Cobb angle of the primary curve for enrolled AIS patients was 62.97° with a standard deviation of 26.12°, ranging from 18.71° to 119.40°. As shown in Supplemental Table 1, there are no differences in Cobb angle and vertebral HU values, including both mean and concave/convex HU values in terms of gender in AIS group. The HU values of the apex and the vertebrae above and below the apex in the AIS group are shown in Supplemental Table 2. The bilateral difference in apex HU was statistically different (162.22±49.45 vs 230.70±56.63, P<0.001). However, there was no statistical difference in the bilateral HU difference of the NV (169.02±52.23 vs 167.87±52.71, P=0.251).
The Cobb angle was not correlated with either age or BMI (P=0.724 and 0.268, respectively). However, as shown in Table 2, the HU values of NV were significantly negatively correlated with Cobb angle for both concave and convex sides (r=−0.329 and −0.327; P=0.015 and 0.016, respectively). The Cobb angle was negatively correlated with the apex-convex HU (r=−0.305, P=0.025), but not with the mean HU of the apex-concave lateral (P=0.656). The convex-concave difference of the apex was significantly correlated with the Cobb angle (r=0.410, P=0.002).
Table 2.
The Bilateral and Overall HU of the NV and the Apex, and the Correlation with the Cobb Angle
| HU value | Correlation with Cobb angle | ||
|---|---|---|---|
| Pearson Correlation Coefficient (r) | P value | ||
| NV-concave | 167.87 ± 52.71 | −0.329 | 0.015 * |
| NV-convex | 169.02 ± 52.23 | −0.327 | 0.016 * |
| NV-overall | 168.44 ± 52.34 | −0.329 | 0.015 * |
| Apex-concave | 230.70 ± 56.63 | −0.062 | 0.656 |
| Apex-convex | 162.22 ± 49.45 | −0.305 | 0.025 * |
| Apex-overall | 199.44 ± 42.54 | −0.241 | 0.079 |
| Convex-concave difference of the apex | 72.46 ± 39.88 | 0.410 | 0.002 ** |
Notes: *P < 0.05. **P < 0.01.
Comparison of HU Values Between AIS and Controls
The differences in the ipsilateral HU values were not statistically significant for either T12 or L1 in the control group (P=0.581, 0.347, respectively). Therefore, the average HU value of the vertebrae (217.01±47.06) was used in the subsequent analysis. The HU values of the NV in the AIS group were lower (168.44±52.34, P<0.001) than the average HU value of the control subjects. The apex-concave lateral HU values were higher in the AIS group (235.67±52.78, P=0.032), but the apex-convex lateral and the mean apex HU values were lower (163.21±40.37, P<0.001; 199.44±42.54, P=0.028).
Comparison of HU Values Between AIS and PSM-Matched Controls
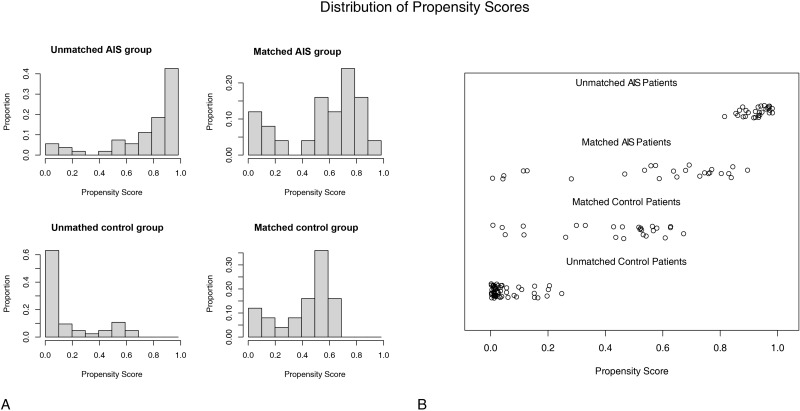
The PSM procedure adjusted for 2 potential confounders of age and BMI. Twenty-five patient pairs were matched and included in the next step of the research. The potential confounders were well balanced (Figure 3). As shown in Table 3, the ages of the AIS group and the PSM-matched control group were 14.84±2.34 years and 15.60±1.73 years, respectively. No significant differences in age and BMI were observed between the two groups (P=0.298 and 0.303, respectively). NV-HU values were lower in the AIS group (172.68±50.90) than in the control group (P=0.006). The apex-convex HU values in the AIS group remained significantly lower than in the control group (170.19±43.11, P=0.002), and the apex-concave HU values were higher than that in the control group (240.65±59.22, P=0.042). The mean apex HU value was indifferent from the control group (P=0.694).
Figure 3.
The potential confounders were well balanced after the propensity score matching. (A). The histograms of the propensity scores of the AIS and control group. (B). The jitter plots of the propensity scores of the AIS and control group.
Table 3.
The Comparison of the PSM-Matched Control Group and the AIS Group
| AIS | PSM-Matched Control | P value | |
|---|---|---|---|
| Age (years) | 14.84 ± 2.34 | 15.60 ± 1.73 | 0.198 |
| BMI | 20.25 ± 2.82 | 21.25 ± 3.90 | 0.303 |
| NV-concave HU | 171.40 ± 50.01 | 210.36 ± 41.83 | 0.004 ** |
| NV-convex HU | 173.96 ± 52.04 | 210.36 ± 41.83 | 0.009 ** |
| NV-overall HU | 172.68 ± 50.90 | 210.36 ± 41.83 | 0.006 ** |
| Apex-concave HU | 240.65 ± 59.22 | 210.36 ± 41.83 | 0.042 * |
| Apex-convex HU | 170.19 ± 43.11 | 210.36 ± 41.83 | 0.002 * |
| Apex-overall HU | 205.42 ± 46.40 | 210.36 ± 41.83 | 0.694 |
Notes: *P < 0.05. **P < 0.01.
Discussion
In this study, we collected and analyzed bilateral HU values for several interested vertebrae in AIS patients and compared them with unmatched/matched control subjects. We found that there was no difference in the bilateral NV-HU values for patients with AIS, while the overall NV-HU values were lower than normal; the concave lateral apex had mildly higher HU values than the control group, while the convex lateral had much lower HU values, and the concave-convex difference showed statistical difference. Meanwhile, correlation analysis showed that the more severe the scoliosis, the more significant the HU difference between convex and concave lateral. We noted that the HU values within concave side of the apex, although mildly increased, were not related to the severity of scoliosis, suggesting that it may be a secondary manifestation of asymmetric loading.
With scoliosis excluded in the control subjects of this study, we chose the T12 and L5 vertebrae as the reference based on the fact that the HU values of these two vertebrae in this study did not show a statistical difference for either bilateral or the overall HU values. The same conclusion is supported by several studies. In a study conducted by Schreiber et al on spinal trauma patients aged 10–90 years, there were no significant differences in HU values from L1 to L4, regardless of age or sex.9 The study conducted by Choi et al that included degenerative and non-degenerative patients also reached consistent conclusions.13 Their study also found that although the correlation was weaker in the degenerative patients, HU values were strongly positively correlated with BMD and T scores in the non-degenerative group.
The etiology of AIS remains largely unknown, but it’s generally attributed to the interplay of genetic and environmental factors that disrupt the normal regulation of bone growth, metabolism, and modeling. This disruption can lead to spinal deformities, systemic osteopenia, and skeletal abnormalities. Studies have consistently shown that reduced bone mineral density is prevalent even in early and mild AIS cases.14,15 Non-coding RNAs (ncRNAs) linked to AIS are thought to influence musculoskeletal development, including osteoblast differentiation and chondrogenesis.16 Additionally, histological findings in AIS patients indicate lower osteoblast counts and reduced trabecular bone activity, hinting at primary bone metabolic dysfunction rather than a consequence of the spinal deformity.17
Alterations in energy and metabolic homeostasis, as well as disturbances in oxidative stress levels, may link AIS and osteopenia in pathogenesis. Various hormones may act as mediators between them. Studies have investigated the relationship between AIS and hormones such as melatonin, leptin, estrogen, and growth hormone,18–21 which are closely linked to bone metabolism.22,23 Taking melatonin as an example, Li et al analyzed paraspinal muscle damage in patients with idiopathic scoliosis and found high levels of reactive oxygen species (ROS) in idiopathic scoliosis patients compared to controls, as well as upregulation of oxidative stress-related gene (PGC1A) expression.24 Previous studies have suggested that ROS-mediated oxidative stress can promote bone resorption by inducing RANKL to increase osteoclast differentiation.25 Melatonin and its signaling pathways are closely related to the protection against oxidative stress, which may explain the role in the pathogenesis of AIS.26 A randomized controlled trial has confirmed that melatonin supplementation at a dose of 6 mg/day can significantly reduce serum inflammatory indicators and increase antioxidant capacity in women.27 Therefore, exploring the alterations in energy and metabolic homeostasis in AIS patients may help to elucidate the pathogenesis of AIS and identify new therapeutic targets.
Decreased BMD is a significant risk factor for curve progression in AIS, with affected patients facing a higher likelihood of surgery, and early detection of bone loss in AIS patients is vital for disease management and minimizing the need for surgical treatment.28 It is noted that the NV-HU values of the AIS group were significantly lower than the mean HU values of the controls, but still higher than that of apex-convex, which suggests that although there is systematic osteopenia in AIS patients, it does not fully explain the severe bone loss on the apex-convex lateral. Scoliosis is likely initiated by skeletal growth and development, as AIS progresses most rapidly during puberty, when growth spurts occur. Skeletal development is associated with mechanical loading. A meta-study, which included 16 studies involving a total of 9627 subjects, found that appropriate physical exercise was negatively associated with the onset of AIS.29 However, the study also found that ballet or gymnastics may exacerbate the development of AIS. It can be reasonably inferred that ballet and gymnastics involve repetitive exercises that promote a wide range of spinal motion and place high loads on the spine. Performing these exercises intensively during growth could potentially affect spinal development.30 Ruff et al proposed that trabecular bone could be remodeled to accommodate mechanical loading, ie bone functional adaptation theory.31 The Hueter-Volkmann principle states that spinal curvature leads to asymmetric loading of the vertebrae, which leads to asymmetric growth, creating a vicious circle.32 However, the reduced bone density observed in individuals with AIS cannot be solely attributed to mechanical loading. GPR126 is a member of the adhesion G protein-coupled receptor family and has been identified as a susceptibility gene for AIS.33 Xu et al found a significant concave-convex lateral asymmetric expression of GPR126 in vertebrae in AIS patients. They also discovered that down-regulation of GPR126 promoted osteogenic differentiation of MSCs.34 The association between scoliosis and low bone mass, as well as imbalanced bone mass, has been demonstrated in several studies.11,35,36 However, further studies are needed to explore the causal link between them.
This study suggests the potential asymmetric osteopenia of the AIS curve. Attention to the low bone density condition in AIS patients is of significant clinical importance in certain situations, such as the selection of brace treatment. A review confirms that reduced BMD independently predicts curve progression in idiopathic scoliosis, with AIS patients having lower BMD facing a higher risk of surgery.28,37 Based on the results of this study, disease control personnel may consider including bone mineral density indicators in the AIS monitoring items. Appropriate intervention for bone loss may be able to curb the progression of scoliosis. We believe that monitoring changes in the HU values of key vertebrae in AIS patients is a cost-effective and clinically significant practice. Further large-scale studies are needed to establish a reasonable range and alert values for the HU values of the curve apex and NV in AIS patients, which can then be applied to the diagnosis and treatment of all AIS patients. For AIS patients suspected of having reduced bone density beyond the acceptable range, treatment plans should be dynamically adjusted according to individual conditions. This may include enhancing education for caregivers to improve brace compliance, closely monitoring curve progression, and adopting measures in line with guidelines for the management of osteoporosis in children and adolescents, such as promoting regular physical activity and optimizing nutritional status, while seeking assistance from pediatric osteoporosis clinics. At the same time, for orthopedic surgeons, the preoperative assessment of HU values of the vertebral bodies involved may serve as an additional guide for surgical planning. For patients with severe AIS, appropriate measures such as bone cement screw may be considered.
There are some limitations in this study. Propensity score matching conducted to balance baseline levels reduces the sample size and limits the robustness of the findings. Our study does not make a plausible theory for a causal association between AIS and osteopenia. The HU values were measured manually by researchers, with an unavoidable subjective bias. Large sample size cohort studies, case-control studies, and causal association analysis can help to further reveal the association between AIS and osteopenia.
Conclusion
The study found evidence of systemic low bone mass in AIS patients, which was more pronounced on the convex side of the spine. We advocate routine monitoring of HU values in key vertebrae for AIS patients as an economical and meaningful approach. Large-scale studies should define optimal HU value ranges and thresholds for curve apex and NV to guide AIS diagnosis and treatment.
Funding Statement
This work was supported by grants from the Beijing Natural Science Foundation [7232182], Research and Development Fund of Peking University People’s Hospital [RDL2022-52], and Clinical Medicine Plus X-Young Scholars Project Peking University, the Fundamental Research Funds for the Central Universities [PKU2023LCXQ042].
Data Sharing Statement
The data used in this study are available from the corresponding author upon request.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards, and this study was approved by the ethics committee of Peking People’s hospital (IRB approval: 2024PHB103-001).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Cheng JC, Castelein RM, Chu WC, et al. Adolescent idiopathic scoliosis. Nat Rev Dis Primers. 2015;1(1):1–21. doi: 10.1038/nrdp.2015.30 [DOI] [PubMed] [Google Scholar]
- 2.Clark EM, Taylor HJ, Harding I, et al. Association between components of body composition and scoliosis: a prospective cohort study reporting differences identifiable before the onset of scoliosis. J Bone Miner Res. 2014;29(8):1729–1736. doi: 10.1002/jbmr.2207 [DOI] [PubMed] [Google Scholar]
- 3.Azeddine B, Letellier K, Wang DS, Moldovan F, Moreau A. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res. 2007;462:45–52. doi: 10.1097/BLO.0b013e31811f39fa [DOI] [PubMed] [Google Scholar]
- 4.Hung VWY, Qin L, Cheung CSK, et al. Osteopenia: a new prognostic factor of curve progression in adolescent idiopathic scoliosis. J Bone Joint Surg Am. 2005;87(12):2709–2716. doi: 10.2106/JBJS.D.02782 [DOI] [PubMed] [Google Scholar]
- 5.Cheung CSK, Lee WTK, Tse YK, et al. Generalized osteopenia in adolescent idiopathic scoliosis--association with abnormal pubertal growth, bone turnover, and calcium intake? Spine. 2006;31(3):330–338. doi: 10.1097/01.brs.0000197410.92525.10 [DOI] [PubMed] [Google Scholar]
- 6.Cheng JC, Qin L, Cheung CS, et al. Generalized low areal and volumetric bone mineral density in adolescent idiopathic scoliosis. J Bone Miner Res. 2000;15(8):1587–1595. doi: 10.1359/jbmr.2000.15.8.1587 [DOI] [PubMed] [Google Scholar]
- 7.Morgan SL, Prater GL. Quality in dual-energy X-ray absorptiometry scans. Bone. 2017;104:13–28. doi: 10.1016/j.bone.2017.01.033 [DOI] [PubMed] [Google Scholar]
- 8.Zaidi Q, Danisa OA, Cheng W. Measurement techniques and utility of Hounsfield unit values for assessment of bone quality prior to spinal instrumentation: a review of current literature. Spine. 2019;44(4):E239. doi: 10.1097/BRS.0000000000002813 [DOI] [PubMed] [Google Scholar]
- 9.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93(11):1057–1063. doi: 10.2106/JBJS.J.00160 [DOI] [PubMed] [Google Scholar]
- 10.Mergler S, Rieken R, Tibboel D, Evenhuis HM, van Rijn RR, Penning C. Lumbar spine and total-body dual-energy X-ray absorptiometry in children with severe neurological impairment and intellectual disability: a pilot study of artefacts and disrupting factors. Pediatr Radiol. 2012;42(5):574–583. doi: 10.1007/s00247-011-2307-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zou D, Sun Z, Wang L, Ding W, Li W. Hounsfield unit for assessing vertebral bone quality and asymmetrical vertebral degeneration in degenerative lumbar scoliosis. Spine. 2020;45(22):1559. doi: 10.1097/BRS.0000000000003639 [DOI] [PubMed] [Google Scholar]
- 12.Pickhardt PJ, Pooler BD, Lauder T, Del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158(8):588–595. doi: 10.7326/0003-4819-158-8-201304160-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi MK, Kim SM, Lim JK. Diagnostic efficacy of Hounsfield units in spine ct for the assessment of real bone mineral density of degenerative spine: correlation study between t-scores determined by dexa scan and Hounsfield units from CT. Acta Neurochir. 2016;158(7):1421–1427. doi: 10.1007/s00701-016-2821-5 [DOI] [PubMed] [Google Scholar]
- 14.Thomas KA, Cook SD, Skalley TC, et al. Lumbar spine and femoral neck bone mineral density in idiopathic scoliosis: a follow-up study. J Pediatr Orthop. 1992;12(2):235–240. doi: 10.1097/01241398-199203000-00016 [DOI] [PubMed] [Google Scholar]
- 15.Li XF, Li H, Liu ZD, Dai LY. Low bone mineral status in adolescent idiopathic scoliosis. Eur Spine J. 2008;17(11):1431–1440. doi: 10.1007/s00586-008-0757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Li X, Shen J, Zhang L, Chan MTV, Wu WKK. Emerging roles of non-coding RNAs in scoliosis. Cell Prolif. 2020;53(2):e12736. doi: 10.1111/cpr.12736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng JC, Tang SP, Guo X, Chan CW, Qin L. Osteopenia in adolescent idiopathic scoliosis: a histomorphometric study. Spine. 2001;26(3):E19–23. doi: 10.1097/00007632-200102010-00002 [DOI] [PubMed] [Google Scholar]
- 18.Gargano G, Oliva F, Migliorini F, Maffulli N. Melatonin and adolescent idiopathic scoliosis: the present evidence. Surgeon. 2022;20(6):e315–e321. doi: 10.1016/j.surge.2021.07.008 [DOI] [PubMed] [Google Scholar]
- 19.Girardo M, Bettini N, Dema E, Cervellati S. The role of melatonin in the pathogenesis of adolescent idiopathic scoliosis (AIS). Eur Spine J. 2011;20(Suppl 1):S68–74. doi: 10.1007/s00586-011-1750-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goultidis TT, Papavasiliou KA, Petropoulos AS, Philippopoulos A, Kapetanos GA. Higher levels of melatonin in early stages of adolescent idiopathic scoliosis: toward a new scenario. J Pediatr Orthop. 2014;34(8):768. doi: 10.1097/BPO.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Wang SR, Qiu GX, Zhang JG, Zhuang QY. Research progress on the etiology and pathogenesis of adolescent idiopathic scoliosis. Chin Med J. 2020;133(4):483–493. doi: 10.1097/CM9.0000000000000652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao R, Tao L, Qiu S, et al. Melatonin rescues glucocorticoid-induced inhibition of osteoblast differentiation in MC3T3-E1 cells via the PI3K/AKT and BMP/Smad signalling pathways. Life Sci. 2020;257:118044. doi: 10.1016/j.lfs.2020.118044 [DOI] [PubMed] [Google Scholar]
- 23.Emch MJ, Wicik Z, Aspros KGM, et al. Estrogen-regulated miRs in bone enhance osteoblast differentiation and matrix mineralization. Mol Ther Nucleic Acids. 2023;33:28–41. doi: 10.1016/j.omtn.2023.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Tang M, Yang G, Wang L, Gao Q, Zhang H. Muscle injury associated elevated oxidative stress and abnormal myogenesis in patients with idiopathic scoliosis. Int J Biol Sci. 2019;15(12):2584–2595. doi: 10.7150/ijbs.33340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agidigbi TS, Kim C. Reactive OXYGEN SPECIES IN OSTEOCLAST DIFFERENTIATION AND POSSIBLE PHARMACEUTICAL TARGETS OF ROS-MEDIATED OSTEOCLAST DISEASEs. Int J Mol Sci. 2019;20(14):3576. doi: 10.3390/ijms20143576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galano A, Tan DX, Reiter RJ. Melatonin as a natural ally against oxidative stress: a physicochemical examination. J Pineal Res. 2011;51(1):1–16. doi: 10.1111/j.1600-079X.2011.00916.x [DOI] [PubMed] [Google Scholar]
- 27.Mesri Alamdari N, Mahdavi R, Roshanravan N, Lotfi Yaghin N, Ostadrahimi AR, Faramarzi E. A double-blind, placebo-controlled trial related to the effects of melatonin on oxidative stress and inflammatory parameters of obese women. Horm Metab Res. 2015;47(7):504–508. doi: 10.1055/s-0034-1384587 [DOI] [PubMed] [Google Scholar]
- 28.Hawary RE, Zaaroor-Regev D, Floman Y, Lonner BS, Alkhalife YI, Betz RR. Brace treatment in adolescent idiopathic scoliosis: risk factors for failure-a literature review. Spine J. 2019;19(12):1917–1925. doi: 10.1016/j.spinee.2019.07.008 [DOI] [PubMed] [Google Scholar]
- 29.Newman M, Hannink E, Barker KL. Associations between physical activity and adolescent idiopathic scoliosis: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2023;104(8):1314–1330. doi: 10.1016/j.apmr.2023.01.019 [DOI] [PubMed] [Google Scholar]
- 30.Marin-Puyalto J, Mäestu J, Gomez-Cabello A, et al. Vigorous physical activity patterns affect bone growth during early puberty in boys. Osteoporos Int. 2018;29(12):2693–2701. doi: 10.1007/s00198-018-4731-2 [DOI] [PubMed] [Google Scholar]
- 31.Ruff C, Holt B, Trinkaus E. Who’s afraid of the big bad Wolff?: “Wolff’s law” and bone functional adaptation. Am J Phys Anthropol. 2006;129(4):484–498. doi: 10.1002/ajpa.20371 [DOI] [PubMed] [Google Scholar]
- 32.Stokes IAF, Spence H, Aronsson DD, Kilmer N. Mechanical modulation of vertebral body growth: implications for scoliosis progression. Spine. 1996;21(10):1162. doi: 10.1097/00007632-199605150-00007 [DOI] [PubMed] [Google Scholar]
- 33.Kou I, Takahashi Y, Johnson TA, et al. Genetic variants in GPR126 are associated with adolescent idiopathic scoliosis. Nat Genet. 2013;45(6):676–679. doi: 10.1038/ng.2639 [DOI] [PubMed] [Google Scholar]
- 34.Xu E, Lin T, Jiang H, et al. Asymmetric expression of GPR126 in the convex/concave side of the spine is associated with spinal skeletal malformation in adolescent idiopathic scoliosis population. Eur Spine J. 2019;28(9):1977–1986. doi: 10.1007/s00586-019-06001-5 [DOI] [PubMed] [Google Scholar]
- 35.Jin LY, Su XJ, Xu S, Liu HY, Li XF. Reliability of Hounsfield unit for assessing asymmetrical vertebral bone mass in adult degenerative scoliosis. Int J Gen Med. 2022;15:5869–5877. doi: 10.2147/IJGM.S368718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Y, Yang H, Hai Y, Pan A, Zhang Y, Zhou L. Hounsfield unit for assessing asymmetrical loss of vertebral bone mineral density and its correlation with curve severity in adolescent idiopathic scoliosis. Front Surg. 2022;9:1000031. doi: 10.3389/fsurg.2022.1000031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bagheri A, Liu XC, Tassone C, et al. 13th international conference on conservative management of spinal deformities and first joint meeting of the international research society on spinal deformities and the society on scoliosis orthopaedic and rehabilitation treatment – SOSORT-IRSSD 2016 meeting. Scol Spinal Disord. 2017;12(1):17. doi: 10.1186/s13013-017-0124-0 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used in this study are available from the corresponding author upon request.