Abstract
Background
This evaluation aims to provide a reference for clinical decision-making regarding the use of SPN in intensive care unit (ICU) patients. The objective of this study is to evaluate the quality of clinical practice guidelines for the use of supplementary parenteral nutrition (SPN) in ICU patients, both domestically and internationally.
Patients and Methods
The quality of clinical practice recommendations for SPN use in ICU patients was assessed using a systematic approach.
Results
Five nutrition recommendations in total were included for analysis. The average standardized scores for the recommendations across the six domains of the Appraisal of Guidelines for Research and Evaluation II (AGREE II) are as follows: Scope and purpose scored 87.96%, stakeholder Involvement scored 68.52%, rigour of development scored 73.40%, clarity of presentation scored 84.80%, applicability scored 64.72%, and editorial independence scored 91.10%. In the comprehensive evaluation, two guidelines were rated as grade A recommendations, and three were rated as grade B recommendations. Most guidelines recommended against early use of SPN when energy and protein requirements could not be met solely through enteral nutrition (EN) in ICU patients. The guidelines emphasized achieving target energy levels and discontinuing parenteral nutrition (PN) as soon as the energy requirements were met to prevent overfeeding.
Conclusion
This study utilized the AGREE II scale to assess the quality of five nutrition guidelines. All 5 guidelines were deemed acceptable Recommendations include focusing on participants, rigour, and applicability to enhance guideline quality. Clinicians should exercise professional judgment when applying guidelines as they complement training and judgment, rather than replacing them.
Keywords: ICU, patients, supplementary parenteral nutrition, clinical practice guidelines, guideline evaluation
Introduction
Disease-related malnutrition (DRM) is a form of malnutrition that is caused by disease rather than poverty. It was first described in the 1970s. 1 DRM is caused by insufficient nutritional intake during the disease progression and treatment. DRM is associated with various adverse clinical outcomes, including an increased risk of complications, impaired wound healing, prolonged hospital stays, and higher mortality rates. These outcomes are also associated with increased healthcare costs.2–7 Critically ill patients in the (ICU) often find themselves in a hypercatabolic state due to the influence of trauma, stress, and other factors.8 According to a recent meta-analysis of 20 studies involving 1168 patients, the malnutrition rate among ICU patients is 38–78%. This highlights the need for optimal and personalized medical nutrition therapy in the ICU.9 Additionally, varying degrees of restricted anabolism make ICU patients more susceptible to malnutrition. Malnutrition rates in ICU patients have been reported to be as high as 40%.10
Therefore, the problem of insufficient nutritional intake in ICU patients has garnered significant attention, and the use of Supplementary Parenteral Nutrition (SPN) in ICU patients at the early stage has been shown to be harmful. There are no randomized controlled trials investigating SPN initiated after the acute phase. Clinical Practice Guidelines (CPGs) are systematically developed statements aimed at assisting clinicians and patients in making appropriate healthcare decisions in specific clinical situations. 11 Their purpose is to improve clinical efficacy, ensure patient safety, control medical costs, and serve other objectives.12 In recent years, there has been a continuous introduction and updating of recommended guidelines for the use of SPN in ICU patients. To better understand the status and quality of these guidelines, this study adopts the Appraisal of Guidelines for Research and Evaluation II (AGREE II),13 which is an internationally recognized gold standard for guideline evaluation.
The AGREE II tool is a widely-used instrument designed to assess the methodological rigour and transparency of guideline development. It has been tested for its validity and reliability. The tool consists of 23 items organized within six domains, followed by two global rating items for an overall assessment. Each domain captures a specific aspect of guideline development quality.14,15
This study aims to systematically evaluate existing guidelines on Supplementary Parenteral Nutrition (SPN) use in ICU patients, providing a comprehensive summary of treatment recommendations. The findings will serve as valuable references for clinicians, aiding in informed clinical decision-making and enhancing medical practices.
Data and Methods
Research Data
The research data retrieval system involved the following components: Major electronic databases: PubMed, Embase, CNKI (China National Knowledge Infrastructure), WanFang Data, VIP, China Biomedical Abstracted Service System. Websites of professional associations: European Society for Parenteral and Enteral Nutrition (ESPEN), ASPEN (American Society for Parenteral and Enteral Nutrition), Chinese Society for Parenteral Nutrition (CSPEN). Inclusion of relevant guidelines from website resources such as the National Guideline Clearinghouse (NGC), the National Institute for Health and Care Excellence (NICE), the Guidelines International Network (GIN), the New Zealand Guidelines Collaboration Group (NZGG), the Ontario Association of Registered Nurses (RNAO), the China Clinical Guidelines Library (CGC), Jimaitong, and others.
The search terms used in Chinese included “enteral nutrition”, “parenteral nutrition”, “nutrition”, “critically ill patients”, and “guidelines”. The corresponding English search terms were “enteral nutrition”, “parenteral nutrition”, “nutrition”, “Intensive Care Unit”, and “guideline”. The search period covered the entire database records up until December 2022. The search strategy used in all database is as follows:
#1: Search for articles with the MeSH term “Enteral Nutrition”.
#2: Search for articles with titles or abstracts containing keywords related to enteral nutrition, such as “Nutrition, Enteral”, “Enteral Feeding”, “Feeding, Enteral”, and various variations of “Force Feeding” and “Tube Feeding”.
#3: Combine the results of #1 and #2.
#4: Search for articles with the MeSH term “Parenteral Nutrition”.
#5: Search for articles with titles or abstracts containing keywords related to parenteral nutrition, such as “Nutrition, Parenteral”, “Parenteral Feeding”, “Feeding, Parenteral”, and variations of “Intravenous Feeding” and “Intravenous Feedings”.
#6: Combine the results of #4 and #5.
#7: Combine the results of #3 and #6.
#8: Search for articles with titles or abstracts containing keywords related to guidelines, such as “practice guideline”, “guideline”, and “guide”.
#9: Search for articles with MeSH terms and keywords related to critical care and intensive care, including “Critical Care”, “Care, Critical”, “Intensive Care”, “Care, Intensive”, “Intensive Care Unit”, “Unit, Intensive Care”, and “ICU Intensive Care Units”.
#10: Combine the results of #7, #8, and #9 to retrieve articles that satisfy all the search criteria related to enteral nutrition, parenteral nutrition, guidelines, and critical care or intensive care units.
A thorough literature search was performed across multiple domestic and international databases, resulting in the retrieval of 593 articles: 177 from PubMed, 164 from Embase, 86 from CNKI, 64 from Wanfang, 34 from VIP, and 68 from SinoMed. To enhance the search results, relevant literature from other guideline websites was included, adding 68 more articles. In total, 661 articles were incorporated into the initial review.
Inclusion Criteria
The clinical practice guidelines considered for this analysis must specifically address the use of SPN and be directed towards adult ICU patients suffering from severe diseases. The guidelines should provide clear indications of evidence and recommended grades, and only the most recent editions will be included. Furthermore, guidelines must be available in either Chinese or English.
Exclusion Criteria
Guidelines that fall under expert consensus or norms that do not meet the established criteria for clinical practice guidelines will be excluded from the analysis. Translations of foreign guidelines will not be considered, except for excerpts. Additionally, interpretations, reviews, and other non-original guidelines are excluded. Incomplete versions, such as executive summaries, technical reports, and simplified documents, along with any older versions from the same organization or body, will also be omitted.
Data Screening and Extraction
Two researchers conducted statistical analyses of the included guidelines, considering criteria such as publication/update year, publishing institution, country, page count, number of references, financial support, and evidence quality grading.
Guide Quality Evaluation
Two impartial evaluators used the AGREE II scale to rate the included guidelines’ transparency and methodological quality independently during the evaluation procedure. Each researcher was blinded for the score of the other researcher while scoring A third researcher was brought in to make a decision if the two assessors’ conclusions differed. The 23 elements that make up the AGREE II scale are divided into six categories that measure the quality of the work: “scope and purpose”, “stakeholder involvement”, “rigour of development”, “clarity of presentation”, “applicability”, and “editorial independence”. The nutritional experts who have received speaker fees/honoraria or grants by companies marketing parenteral nutrition solutions were excluded from the researcher group(author group).
Each item on the AGREE II scale is graded on a seven-point scale, with 1 being the strongest disagreement and 7 being the strongest agreement.16 If an object does not satisfy all the requirements, it may receive between 2 and 6 points, depending on the situation. The scores provided by the two evaluators for each field’s elements are added up to create an independent score for each field. The average standardized score for each field is then calculated by dividing the actual score by the lowest score that may be awarded, using the formula: (actual score - minimum possible score) / (maximum possible score - minimum possible score) 100%.14,15 The greatest possible score, 7 points, is determined by multiplying the number of items by the number of evaluators. Similarly, the smallest possible score, 1 point, is calculated by multiplying the number of items by the number of evaluators.14–16
Overall Guideline Assessment
Based on the final scores for the six fields of the guideline and the evaluators’ overall judgment, the guidelines are categorized into three recommendation levels:
Recommended for Use Level (Graded as A): Guidelines with scores ≥60% in all six fields can be directly recommended without modification.
Recommended for Use with Modification Level (Graded as B): Guidelines with scores ≥30% in at least three fields but <60% can be recommended after modification.
Not Recommended for Use Level (Graded as C): If the number of fields with a final score <30% is ≥3, the guideline is not recommended temporarily due to poor formulation methods or low-quality evidence.14–17 These recommendation levels provide guidance on the suitability of the guidelines and assist in determining their potential for clinical decision-making.
Evaluators’ Judgment on the Consistency of Evaluation Results
To assess the consistency of the guideline results between the two evaluators, and it was done using the Intraclass Correlation Coefficient (ICC). The ICC is a statistical indicator that rates the consistency or agreement among numerous raters or assessors. The SPSSAU online analysis tool (https://spssau.com/indexs.html) was used in this instance to calculate the ICC. The range of the ICC value is 0 to 1, with values closer to 1 indicating greater consistency among the evaluators. A 95% Confidence Interval (CI) was used to calculate the ICC’s statistical significance, and a significance level of P <0.05 was regarded as statistically significant. ICC values can be interpreted as follows: ICC ≥ 0.75 indicates good inter-rater reliability, while values between 0.4 and 0.75 suggest average consistency among the raters. A low ICC of 0.4 indicates inconsistent evaluator performance.
By using the ICC, the researchers can ensure that each entry or item in the guidelines is consistently understood and evaluated by the evaluators. The ICC analysis helps establish the reliability and agreement between the evaluators, providing valuable insights into the consistency of the guideline results. The review and validation of data was proceeded by a statistician in a blind method.
Results
Literature Search Results
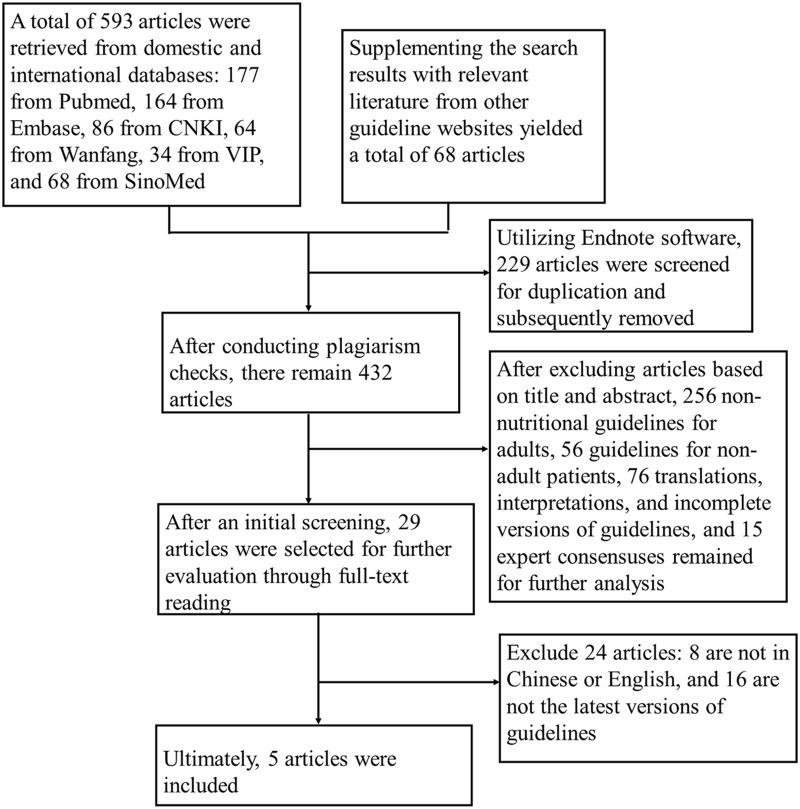
During the research process, a total of 661 literature sources were initially collected. To remove duplicates, the researchers utilized Endnote software. Following this, a screening process was conducted, which included both primary and secondary evaluations. Ultimately, 5 nutritional guidelines were selected for the study—1 in Chinese and 4 in English. A comprehensive overview of the literature screening process and its results can be seen in Figure 1.
Figure 1.
Flowchart of guidelines screening.
Overview of Guidelines for Analysis
Following the systematic retrieval process, a total of 5 guidelines were ultimately included for analysis.18–22 Among these, one guideline was in Chinese, while the other four were in English. The publication dates of the guidelines ranged from 2008 to 2019, with all four English guidelines being developed and published within the past decade. The essential characteristics of the included guidelines can be found in Table 1, providing a comprehensive overview of their key details. Supplementary Table 1 lists the abbreviations and terms used in clinical nutrition and guidelines.
Table 1.
Basic Features of the Included Guidelines
| Guideline Title (References) | Published Date | Developer | Country | Number of Pages | Number of References | Funding Situation | Grading of Evidence Quality |
|---|---|---|---|---|---|---|---|
| Clinical Diagnosis and Treatment Guide-Parenteral Enteral Nutrition Volume (Association., 2008)18 | 2008* | CSPEN | China | 7 | 43 | N | OCEBM |
| The Canadian Critical Care Nutrition Guidelines in 2013 Update on Current Recommendations and Implementation Strategies (Dhaliwal et al, 2014)19 | 2013* | CCPGC | Canada | 15 | 105 | N | GRADE |
|
Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) (McClave et al, 2016)21 |
2016* | SCCM/ASPEN | American | 53 | 480 | N | GRADE |
| ESPEN guideline on clinical nutrition in the intensive care unit (Singer et al, 2019)22 | 2019* | ESPEN | European Union | 32 | 358 | N | GRADE |
| Clinical Nutrition in Critical Care Medicine e Guideline of the German Society for Nutritional Medicine (DGEM) (Elke et al, 2019)20 | 2019* | DGEM | Germany | 56 | 502 | N | S2K |
Notes: * stands for Guideline Update Time; N: No.
Abbreviations: CSPEN, Chinese Society for Parenteral Enteral Nutrition; CCPGC, The Canadian Critical Care Practice Guidelines Committee; SCCM, Society for Critical Care Medicine; ASPEN, American Society for Parenteral Enteral Nutrition; ESPEN, European Society for Parenteral Enteral Nutrition; DGEM, German Society for Nutritional Medicine; OCEBM, Oxford Classification; GRADE, Grading, Development and Evaluation of Evidence Recommendations.
Guideline Quality Evaluation Results
Consistency of the Evaluators
Two researchers independently evaluated the included 5 nutrition guidelines using AGREE II. The ICC values for the evaluation results ranged from 0.864 to 0.957, with an average value exceeding 0.75. The F-test result indicated statistical significance (P < 0.05), demonstrating good agreement between the two evaluators in terms of understanding the entries and evaluation results (Table 2).
Table 2.
ICC Value of Evaluation Results
| Guideline (Reference) | ICC | 95% CI | P |
|---|---|---|---|
| CSPEN (Association., 2008)18 | 0.949 | (0.900–0.976) | <0.001 |
| CCPGs(Dhaliwal et al, 2014)19 | 0.864 | (0.812–0.941) | <0.001 |
| SCCM/APEN(McClave et al, 2016)21 | 0.883 | (0.818–0.954) | <0.001 |
| ESPEN(Singer et al, 2019)22 | 0.940 | (0.885–0.972) | <0.001 |
| DGEN(Elke et al, 2019)20 | 0.957 | (0.916–0.980) | <0.001 |
Abbreviations: CSPEN, Chinese Society for Parenteral Enteral Nutrition; CCPGs, The Canadian Critical Care Practice Guidelines; SCCM, Society for Critical Care Medicine; ASPEN, American Society for Parenteral Enteral Nutrition; ESPEN, European Society for Parenteral Enteral Nutrition; DGEM, German Society for Nutritional Medicine; ICC, Intraclass Correlation Coefficient; CI, confidence interval.
Scores of Each Domain in AGREE II and Overall Evaluation of Guideline Quality
The overall scores of the guidelines assessed using AGREE II were satisfactory. The discussion of each domain is as follows:
Scope and Purpose: This domain had an average standardized score of 87.96%, with all five guidelines scoring above 80%. The ESPEN guide22 achieved the highest score, providing detailed descriptions for all three items. The high scores indicate that the guidelines clearly defined their objectives, health questions, and target populations.
Clarity of Presentation: This domain received an average standardized score of 84.8%, with each guideline scoring above 80%. The guidelines elaborated on the formulated methodology, statistics, and supplementary materials, enhancing the clarity of presentation. They focused on the level of evidence, recommendation strength, and specific recommendations.
Editorial Independence: This domain had the highest average standardized score of 91.1%. All five guidelines explicitly stated that external funding did not influence the production of the guideline and disclosed the absence of conflicts of interest among members. High scores in this domain suggest strong adherence to maintaining editorial independence.
Stakeholder Involvement: This domain had a lower average standardized score of 68.52%. Low scores were primarily due to a lack of consultation with the target population (patients, the public, etc). The guidelines of CSPEN,18 DGEM,20 and ESPEN22 received deductions for this. The Canadian Critical Care Practice Guidelines (CCPGs)19 scored high by including 70 ICU patients in the research and conducting comprehensive assessments. The lower scores indicate a need for better involvement of stakeholders in the guideline development process.
Rigour of Development: This domain had an average standardized score of 73.4%. Except for the DGEM guide,20 the guidelines had similar scores, providing clear descriptions of evidence retrieval, screening, analysis, and guideline updates. They considered health benefits, side effects, and risks associated with the recommendations. Most points were deducted for not conducting external reviews before the release of the guidelines. The scores suggest a generally rigorous development process, but with room for improvement in external reviews.
Application: This domain had the lowest average standardized score of 64.72%. The DGEM Guide20 and CSPEN Guide18 had the highest scores in this area. The low scores indicate challenges in the practical application of the guidelines, suggesting the need for better implementation strategies. Based on the comprehensive evaluation of the field scores of the five guidelines, CCPGs19 and ACCM/APEN21 were recommended as Grade A, and three guidelines, CSPEN,18 DGEM,20 and ESPEN22 were recommended as Grade B. For the scores in various fields included in the guidelines, please refer to Table 3.
Table 3.
Evaluation Result of the Included Guidelines
| Guideline (Reference) | Standardized Scores in all Areas (%) | Score ≥60% field Number | Score≤ 30% Field Number | Recommendation Level | |||||
|---|---|---|---|---|---|---|---|---|---|
| Scope and Purpose | Stakeholder Involvement | Rigour of Development | Clarity of Presentation | Applicability | Editorial Independence | ||||
| CSPEN (Association., 2008)18 | 80.6 | 57.4 | 73.3 | 85.2 | 47.2 | 91.7 | 4 | 0 | B |
| CCPGs(Dhaliwal et al, 2014)19 | 92.6 | 90.7 | 73.6 | 85.2 | 77.8 | 88.9 | 6 | 0 | A |
| SCCM/APEN(McClave et al, 2016)21 | 85.2 | 81.5 | 79.9 | 83.3 | 62.5 | 94.4 | 6 | 0 | A |
| ESPEN(Singer et al, 2019)22 | 94.4 | 53.7 | 79.1 | 85.2 | 56.9 | 97.2 | 4 | 0 | B |
| DGEN(Elke et al, 2019)20 | 87.0 | 59.3 | 61.1 | 85.1 | 79.2 | 83.3 | 5 | 0 | B |
| Average score | 87.96 | 68.52 | 73.40 | 84.80 | 64.72 | 91.1 | – | – | – |
Abbreviations: CSPEN, Chinese Society for Parenteral Enteral Nutrition; CCPGs, The Canadian Critical Care Practice Guidelines; SCCM, Society for Critical Care Medicine; ASPEN, American Society for Parenteral Enteral Nutrition; ESPEN, European Society for Parenteral Enteral Nutrition; DGEM, German Society for Nutritional Medicine.
Recommendations on the Use of SPN Included in the Guidelines
As per the guidelines, if ICU patients with severe illness cannot meet their target energy and protein requirements through gastrointestinal intake, PN supplementation should be administered alongside therapy to treat malnutrition. However, there is still controversy regarding the timing of SPN supplementation. The guidelines recommend that PN should be supplemented until the target energy requirements are met. Once the SPN feeding reaches this target energy value, it is advised to discontinue PN immediately to avoid overfeeding. Please refer to Table 4 for detailed recommendations regarding SPN supplementation and target energy requirements as suggested by the guidelines.
Table 4.
Recommendations for Using SPN of Guideline
| Guideline (Reference) | Recommendations for the Use of SPN | AGREE II Evaluate and Recommend the Opinion |
|---|---|---|
| CSPEN (Association., 2008)18 | PN can be added from 3 to 5 days after early EN fails to improve malnutrition. The nutritional caloric target in the acute stress period is 20–25 kcal/ (kg·d), and the energy supply can be appropriately increased to 25–30 kcal/ (kg·d) after the stress and metabolic state are stabilized. | B |
| CCPGs (Dhaliwal et al, 2014)19 | Early supplemental PN and high IV glucose not be used in unselected critically ill patients (ie, low risk patients with short stay in ICU). In the patient who is not tolerating adequate EN, there are insufficient data to put forward a recommendation about when PN should be initiated. | A |
| SCCM/APEN(McClave et al, 2016)21 | In patients at either low or high nutrition risk, use of supplemental PN be considered after 7–10 days if unable to meet >60% of energy and protein requirements by the enteral route alone. | A |
| ESPEN(Singer et al, 2019)22 | In patients who do not tolerate full dose EN during the first week in the ICU, the safety and benefits of initiating PN should be weighed on a case-by-case basis.PN should not be started until all strategies to maximize EN tolerance have been attempted. The caloric target of nutritional support is 20–25 kcal/(kg · d). | B |
| DGEN(Elke et al, 2019)20 | Parenteral nutrients should be added to enteral nutrients, when exclusive enteral nutrition cannot deliver calories/proteins to the patient at a rate specified by disease phase and individual metabolic tolerance. | B |
Abbreviations: CSPEN, Chinese Society for Parenteral Enteral Nutrition; CCPGs, The Canadian Critical Care Practice Guidelines; SCCM, Society for Critical Care Medicine; ASPEN, American Society for Parenteral Enteral Nutrition; ESPEN, European Society for Parenteral Enteral Nutrition; DGEM, German Society for Nutritional Medicine.
Discussion
Based on the AGREE II ratings, the overall quality of the five guidelines is acceptable The standardized scores for the domains of “Scope and Purpose”, “Clarity and presentation”, and “Editorial independence” all exceed 80%, while those for “Stakeholder Involvement”, “Rigour of development” and “Applicability” are slightly lower, all falling below 80%. Clinical practice guidelines are crucial tools in clinical decision-making, with their quality increasingly recognized by healthcare professionals. The quality of guidelines directly impacts clinical outcomes, thus, for the guidelines recommended for use in critically ill patients, improvements are needed in stakeholder involvement, rigour, and applicability to enhance overall quality.
Scope and Purpose
The “scope and purpose” domain represents an overall assessment of the purpose of guideline development, target population, and health issues covered. In this study, the inclusion of five guidelines clearly delineates the objectives of guideline development, the intended beneficiaries of the guidelines, and provides definitions, utilizing PICO to describe the health issues addressed.
Clarity of Presentation
In this domain, the evaluation pertains to the language, structure, and presentation of guidelines, assessing the clarity of recommendations, inclusion of various options or health issues, and ease of identifying key recommendations. The overall average score in this domain is 84.80%, indicating a high level of adherence to guideline development standards in terms of language, structure, and presentation. The CSPEN guidelines, characterized by their concise and clear language, demonstrate a unique advantage in identifying key recommendations. During the development of most English guidelines, the guideline development organizations or institutions elaborate on the methodology, statistical aspects, and other supplementary materials in detail on their websites and in appendices. The content of the guidelines focuses on evidence levels, strength of recommendations, and specific recommendations to enhance clarity.
Editorial Independence
In the domain of editorial independence, the generation of recommendations in guidelines is intended to remain uninfluenced by competing interests. This domain boasts the highest overall mean score of 91.1%. It is widely recognized that the formulation of guidelines necessitates substantial financial support, with funding from governmental and commercial entities being indispensable. Consequently, conflicts of interest are inevitable In order to mitigate potential bias and enhance the credibility of guidelines, it is imperative for the organizing bodies involved in guideline development to provide explicit declarations regarding conflicts of interest. Notably, all five guidelines explicitly state that sponsors did not influence the guideline development process, indicating a high level of credibility in the guidelines.
Stakeholder Involvement
This domain is essential for guidelines development to involve professionals from relevant fields and the intended target audience, while also specifying the guidelines’ users. A notable reason for scoring lower in this domain is related to item 5: the collection of viewpoints and preferences from the target population (patients, the public, etc). The ASPEN guidelines21 explicitly affirm that patients and their families should never feel neglected, however, they do not elaborate on specific implementation plans. Among the guidelines reviewed, only the CCPGs19 clearly explain how to gather patient opinions, with the rest of the guidelines failing to address this aspect.
Rigour of Development
The “rigour of development” domain encompasses various aspects of guideline formulation, including the formation of evidence, development of recommended opinions, external review, and guideline updates. Rigour is a comprehensive evaluation of the methodology used in guideline formulation and is a crucial factor influencing the overall quality of the guidelines.23 In the “rigour” domain, the average score is 73.4%. The ASPEN Guidelines received the highest score in this domain, with descriptions provided for eight items. However, the details provided in these guidelines were not comprehensive. On the other hand, the DGEM’s guidance20 mentioned the use of S2k guideline classification, indicating a lack of evidence from systematic reviews. It is also unclear whether external input was sought during the guideline development process. Additionally, the guidelines lacked sufficient detail regarding health benefits, side effects, and risks, resulting in a lower score in this domain.
To enhance the credibility and persuasiveness of guidelines, it is crucial to follow standardized, rigorous, and transparent formulation processes.24 This suggests the need for greater emphasis on the rigour of the formulation methodology during the guideline development process, ultimately improving the overall quality of the guidelines.
Applicability
In the “applicability” domain, which assesses the conditions and factors affecting the implementation of guidelines, as well as related resources and improvement strategies, the average score is 64.72%. This domain received the lowest score among all domains. The application of guidelines is essential for evidence-based clinical practice and is the ultimate goal of guideline formulation. Effective applicability can truly assist clinical medical staff and patients in making appropriate recommendations and improve patient outcomes. Therefore, it is recommended that guideline developers pay more attention to the applicability of guidelines and create authoritative standardized documents that address this aspect in greater detail.20
Enhancing the Quality and Applicability of Clinical Practice Guidelines
Clinical practice guidelines play a crucial role in guiding clinical decision-making and treatment outcomes. Therefore, the quality of these guidelines is of utmost importance and has gained increased attention from clinical practitioners. The evaluation results highlight certain areas for improvement. To enhance the quality of guidelines, it is recommended that guideline developers prioritize the views and opinions of the target population. This can be achieved through actively seeking input from patients, their families, and other relevant stakeholders. Additionally, increasing the transparency and openness of the evidence and recommendation formulation process is essential. Clear descriptions of the methodology used, including systematic review procedures, will enhance the credibility of the guidelines. Furthermore, guidelines should clearly state both favorable conditions and potential obstacles related to guideline implementation. Providing strategies for overcoming these obstacles and improving implementation will help bridge the gap between guideline recommendations and actual clinical practice. Emphasizing the applicability of guidelines and considering the resources and context of the healthcare system will contribute to their effective implementation and impact on patient care. In addition, it is recommended that national guideline formulation organizations take into account specific national conditions when developing nutrition clinical practice guidelines. Factors such as disease burden, healthcare resource availability, and insurance reimbursement policies should be considered. This approach will ensure that guidelines are tailored to the local context, facilitating the standardization of diagnosis and treatment pathways and guiding clinical practice more effectively. By implementing these suggestions, the overall quality of clinical practice guidelines can be improved, leading to better clinical decision-making and improved patient outcomes.13
Guideline Development and Nutritional Transition in Patient Care
In light of the assessment of guidelines, it is advised that forthcoming guideline developers emphasize the viewpoints and choices of the target population, increase the transparency of the formation process of evidence and recommendations, explicitly outline the favorable conditions, obstacles, and improvement strategies during guideline implementation to enhance the overall quality of guidelines. Furthermore, it is recommended that guideline development organizations in different countries formulate nutrition clinical practice guidelines that align with their respective national disease burdens, healthcare resource conditions, and medical insurance reimbursement policies to steer clinical diagnosis and treatment activities and regulate treatment protocols.
The guidelines emphasize the importance of avoiding both overfeeding and malnutrition, and recommend that SPN should be conducted based on the target energy requirements of a normal individual. Once the target energy demand is reached, PN feeding should be promptly discontinued, and a cautious transition to EN feeding should be initiated to prevent overfeeding, which can be detrimental to patients. Abruptly discontinuing PN and starting EN can disrupt metabolic balance and gastrointestinal tolerance. A gradual transition allows the body to adjust and avoids overwhelming the digestive system. It also helps manage fluid balance and allows the intestines to adapt to the change in nutrient source.25 By closely monitoring patients’ tolerance and adjusting nutrient delivery, healthcare professionals can promote patient safety and optimize nutrition during the transition.26
Nutritional Screening Tools and Guidelines for Critically Ill Patients
A number of nutritional screening tools have been approved for use in acute care settings to help clinicians, including the Mini-Nutritional Assessment (MNA), Malnutrition Universal Screening Tool (MUST), Nutrition Risk Screening (NRS) tool, Subjective Global Assessment (SGA), Patient-Generated SGA (PG-SGA), and NUTRIC (Nutrition Risk in the Critically Ill) score.27–30 A more recent evaluation instrument being tested for use in acute care settings is the Global Leadership Initiative on Malnutrition (GLIM) Criteria.31
Regarding the study itself, the literature retrieval was restricted to Chinese and English guidelines, which may result in incomplete retrieval and selective bias since nutrition guidelines are released in different languages by various countries and regions. It is important to note that the AGREE II evaluation tool used in this study is an international consensus tool primarily designed to assess the methodological quality of guidelines, rather than the quality of guideline content. As well, the guidelines included in the evaluation targeted all critically ill patients, including both comprehensive ICU patients and specialized ICU patients. The guidelines did not specifically subdivide the critically ill patients when forming the evidence groups. Therefore, in clinical practice, clinicians should develop individualized treatment plans based on the specific conditions and needs of each patient, taking into account their unique circumstances and requirements.
Furthermore, a more in-depth comparison and analysis can be conducted to explain the reasons behind the differing viewpoints of different guidelines, citing relevant research or evidence to support each viewpoint. Providing explanations and background information on the evaluation methodology will also enhance reader understanding. Finally, recommendations should be accompanied by more specific reasons and supporting evidence to make them more persuasive.
Conclusion
This study employed the AGREE II scale to assess the quality of five nutrition guidelines that were included in the analysis. The findings of the study indicated that all five guidelines were deemed acceptable in terms of their quality. However, it is recommended that the creators of these guidelines focus on certain areas, such as participants, rigour, and applicability, in order to enhance their overall quality. It is important to note that the guidelines may not fully address the wide range of clinical situations that clinicians encounter. Therefore, it is advised that healthcare professionals exercise their professional judgment when applying these guidelines.
Data Sharing Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.
Consent for Publication
Not applicable.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jensen GL, Mirtallo J, Compher C, et al. Adult starvation and disease-related malnutrition: a proposal for etiology-based diagnosis in the clinical practice setting from the International Consensus Guideline Committee. Clin Nutr. 2010;29(2):151–153. doi: 10.1016/j.clnu.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 2.Bernstein LH, Shaw-Stiffel TA, Schorow M, Brouillette R. Financial implications of malnutrition. Clin Lab Med. 1993;13(2):491–507. [PubMed] [Google Scholar]
- 3.Braunschweig C, Gomez S, Sheean PM. Impact of declines in nutritional status on outcomes in adult patients hospitalized for more than 7 days. J Am Diet Assoc. 2000;100(11):1316–1322;quiz1323–1314. [DOI] [PubMed] [Google Scholar]
- 4.Schneider SM, Veyres P, Pivot X, et al. Malnutrition is an independent factor associated with nosocomial infections. Br J Nutr. 2004;92(1):105–111. doi: 10.1079/BJN20041152 [DOI] [PubMed] [Google Scholar]
- 5.Somanchi M, Tao X, Mullin GE. The facilitated early enteral and dietary management effectiveness trial in hospitalized patients with malnutrition. JPEN J Parenter Enteral Nutr. 2011;35(2):209–216. doi: 10.1177/0148607110392234 [DOI] [PubMed] [Google Scholar]
- 6.Sullivan DH. Risk factors for early hospital readmission in a select population of geriatric rehabilitation patients: the significance of nutritional status. J Am Geriatr Soc. 1992;40(8):792–798. doi: 10.1111/j.1532-5415.1992.tb01851.x [DOI] [PubMed] [Google Scholar]
- 7.Sullivan DH, Bopp MM, Roberson PK. Protein-energy undernutrition and life-threatening complications among the hospitalized elderly. J Gen Intern Med. 2002;17(12):923–932. doi: 10.1046/j.1525-1497.2002.10930.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagve M, Gjessing P, Ytrebø LM, Ø I. Nutritional support for critically ill patients in the intensive care unit. Tidsskr nor Laegeforen. 2020;140(2):426. [DOI] [PubMed] [Google Scholar]
- 9.Hill A, Elke G, Weimann A. Nutrition in the Intensive Care Unit-A narrative review. Nutrients. 2021;13(8):2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seron-Arbeloa C, Zamora-Elson M, Labarta-Monzon L, Mallor-Bonet T. Enteral nutrition in critical care. J Clin Med Res. 2013;5(1):1–11. doi: 10.4021/jocmr1210w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quality AfHa. Clinical Guidelines and Recommendations. https://www.ahrq.gov/prevention/guidelines/index.html. Accessed August 21, 2024.
- 12.Institute of Medicine (US) Committee on Standards for Developing Trustworthy Clinical Practice Guidelines.Graham RMM, Miller Wolman D editors Clinical Practice Guidelines We Can Trust.Washington (DC):National Academies Press (US);2011. doi: 10.17226/13058 [DOI] [Google Scholar]
- 13.Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Cmaj. 2010;182(18):E839–842. doi: 10.1503/cmaj.090449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.APPRAISAL OF GUIDELINES FOR RESEARCH & EVALUATION lI.Appraisal Of Guidelines For Research & Evaluation Li; 2017. https://www.agreetrust.org/wp-content/uploads/2017/12/AGREE-II-Users-Manual-and-23-item-Instrument-2009-Update-2017.pdf. Accessed August 21, 2024.
- 15.Hoffmann-Eßer W, Siering U, Neugebauer EA, et al. Guideline appraisal with AGREE II: systematic review of the current evidence on how users handle the 2 overall assessments. PLoS One. 2017;12(3):e0174831. doi: 10.1371/journal.pone.0174831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.AGREE II-Global Rating Scale (AGREE II-GRS) Instrument. AGREE II-Global Rating Scale (AGREE II-GRS) Instrument. https://www.agreetrust.org/wp-content/uploads/2013/12/AGREE-II-GRS-Instument.pdf. Accessed August 21, 2024.
- 17.Chen Y, Hu SL, Li YP. A systematic review of global guidelines for drug intervention in the treatment of simple hypertension. Chin Evid-Based Med. 2012;12(101):1180–1194. [Google Scholar]
- 18.Association CM. Clinical Diagnosis and Treatment Guide-Parenteral Enteral Nutrition Volume (2008. edition) ed. Beijing: People’s Medical Publishing House; 2008:1. [Google Scholar]
- 19.Dhaliwal R, Cahill N, Lemieux M, Heyland DK. The Canadian critical care nutrition guidelines in 2013: an update on current recommendations and implementation strategies. Nutr Clin Pract. 2014;29(1):29–43. doi: 10.1177/0884533613510948 [DOI] [PubMed] [Google Scholar]
- 20.Elke G, Hartl WH, Kreymann KG, et al. Clinical nutrition in critical care medicine - guideline of the German society for nutritional medicine (DGEM). Clin Nutr ESPEN. 2019;33:220–275.5. doi: 10.1016/j.clnesp.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 21.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically III patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863 [DOI] [PubMed] [Google Scholar]
- 22.Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Li Z, Shi DL, et al. Evaluation of guidelines for prevention of central venous catheter-related hematoinfection from 2004 to 2014. J Nurs. 2017;32(2):98–103. [Google Scholar]
- 24.Yan MQ, Li QL, Lu ZQ. Establishment of clinical practice guidelines and AGREE evaluation for pre-catheterization evaluation of PICC. J Nurs. 2013;28(14):1–5. [Google Scholar]
- 25.Oshima T, Heidegger CP, Pichard C. Supplemental parenteral nutrition is the key to prevent energy deficits in critically Ill patients. Nutr Clin Pract. 2016;31(4):432–437. doi: 10.1177/0884533616651754 [DOI] [PubMed] [Google Scholar]
- 26.Zaczek Z, Jurczak-Kobus P, Panczyk M, et al. Changes in parenteral nutrition requirements and BMI in patients with parenteral nutrition-dependent short bowel syndrome after stopping teduglutide-9 years of follow-up. Nutrients. 2022;14(8):1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35(2):247–307. doi: 10.1016/j.clnu.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 28.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15(6):R268. doi: 10.1186/cc10546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11(1):8–13. doi: 10.1177/014860718701100108 [DOI] [PubMed] [Google Scholar]
- 30.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–785. doi: 10.1038/sj.ejcn.1601412 [DOI] [PubMed] [Google Scholar]
- 31.Jensen GL, Cederholm T, Correia MI, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report From the global clinical nutrition community. JPEN J Parenter Enteral Nutr. 2019;43(1):32–40. doi: 10.1002/jpen.1440 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study available from the corresponding author on reasonable request.