Abstract
MT-SP1 (membrane-type serine protease 1)/matriptase is an epithelial-derived integral membrane enzyme. The purpose of the present study was to examine whether the enzyme exists on the basolateral side of simple columnar epithelial cells, such as enterocytes, of normal adult animals. Using COS-1 monkey kidney cells transiently transfected with rat MT-SP1/matriptase expression plasmids, we found that the enzyme is post-translationally processed by the cleavage between Gly149 and Ser150, that a portion of the C-terminal part (Ser150–Val855) remains in the cells by association with the NTF (N-terminal fragment) (Met1–Gly149), while the other portions are released into the medium and that the release is increased on activation by co-expression with hepatocyte growth factor activator inhibitor type-1. Western-blot analysis of crude membranes prepared from rat jejunum demonstrated the presence of the NTF but negligible or no occurrence of the C-terminal part of the protein. Fractionation of the crude membranes by ultracentrifugation with Percoll followed by Western-blot analysis showed that the fractionation profile of the NTF correlated significantly with that of E-cadherin, an adhesion molecule on the lateral membrane. Immunostaining of the jejunum demonstrated the occurrence of the NTF on the lateral membranes but not on the apical membranes. These results suggest that considerable MT-SP1/matriptase molecules occur on the basolateral sides of normal epithelial cells and support our hypothesis that a possible physiological function of this enzyme is the control of epithelial-cell turnover by regulating cell–cell and/or cell–substratum adhesions.
Keywords: cell–cell and/or cell–substratum adhesion, enterocyte, MT-SP1/matriptase, N-terminal fragment, small-intestine epithelial cell, subcellular distribution
Abbreviations: CTF, C-terminal fragment; endo H, endoglycosidase H; HGF, hepatocyte growth factor; HAI-1, HGF-activator inhibitor type-1; HRP, horseradish peroxidase; MT-SP1, membrane-type serine protease 1; NTF, N-terminal fragment; PBST, PBS containing 0.1% (v/v) Tween 20; SEA, sea-urchin sperm protein, enterokinase and agrin
INTRODUCTION
Cell–cell and/or cell–substratum adhesions are complex and tightly regulated processes that play crucial roles in tissue homoeostasis [1]. This is particularly important for cells with short life cycles and high rates of proliferation, such as enterocytes [2]. Enterocytes, simple columnar polarized epithelial cells, are generated by stem cells at the crypt base and migrate towards the lumen within a few days [2]. At the villus tip, their short life cycle is terminated as the cells undergo apoptosis, lose cell–cell and/or cell–substratum adhesions and are shed into the lumen [2,3]. Although various proteolytic events may be involved in the regulation of the cell–cell and/or cell–substratum adhesions, details of the underlying mechanisms are largely unknown.
MT-SP1 (membrane-type serine protease 1)/matriptase is a member of the type II membrane serine proteases and shows proteolytic activity with trypsin-like specificity [4–6]. MT-SP1/matriptase has a multidomain structure consisting of a short cytosolic domain, a putative transmembrane domain, an SEA (sea-urchin sperm protein, enterokinase and agrin) domain, two CUB (complement protein subcomponents C1r/C1s, urchin embryonic growth factor and bone morphogenetic protein 1) domains, four LDLRA (low-density lipoprotein receptor A) modules and a C-terminal serine protease (catalytic) domain (Figure 1A). The enzyme activity was initially discovered in 1993 by Shi et al. [7] in a human breast cancer cell line (T-47D) and was considered to control breast cancer invasion and metastasis. Later, the protein was purified from human breast milk and named matriptase [8]. The purified matriptase was a soluble form of MT-SP1, which lacks the N-terminal transmembrane domain. MT-SP1/matriptase has been shown in vitro to cleave and activate single-chain urokinase-type plasminogen activator [5,9,10], to activate protease-activated receptor-2 [10], to cleave the precursor form of HGF (hepatocyte growth factor) to produce its active form [9] and to digest the extracellular matrix proteins directly [5,8]. The identification of these molecules as putative in vivo substrates suggests that MT-SP1/matriptase regulates the functions mediated by these molecules such as cell–cell and/or cell–substratum adhesion, as well as cancer invasion and metastasis [11,12].
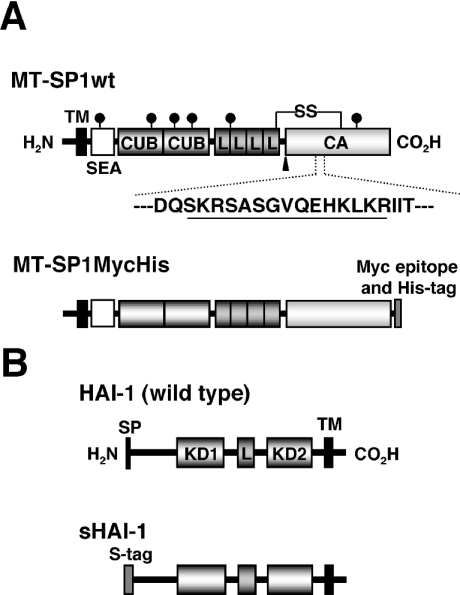
Figure 1. Domain structures of rat MT-SP1 and rat HAI-1 and diagrams of the expression constructs.
(A) Schematic representation of the structure of rat MT-SP1 and diagram of an expression construct. Wild-type MT-SP1/matriptase (MT-SP1wt) consists of 855 amino acids. The amino acid numbering starts from the putative N-terminus of the protein. The predicted disulphide linkages are shown as SS. The potential N-glycosylation sites are marked with a ‘lollipop’ symbol. The putative activation cleavage site is indicated by an arrowhead. The site of antibody production (Spr992) is indicated by appropriate underlining in the amino acid sequence in single-letter code. MT-SP1MycHis represents a recombinant form fused to the myc epitope (Myc) and His6 tag at the C-terminus. (B) Schematic representation of the structure of rat HAI-1 and diagram of an expression construct. Wild-type rat HAI-1 consists of 508 amino acids. The amino acid numbering starts from the putative N-terminus of the protein. sHAI-1 represents a recombinant form in which the N-terminal region containing the signal peptide (Met1–Pro35) is replaced by the human IgG κ-chain signal peptide and S tag. TM, transmembrane domain; SEA, SEA domain; CUB, CUB domain; L, LDLRA; CA, catalytic domain; SP, signal peptide; KD, Kunitz domain.
MT-SP1/matriptase is expressed by the epithelial elements of almost all the organs examined so far [5,13]. The pattern of expression in normal tissues suggests that the enzyme plays a ubiquitous role in the biology of surface-lining epithelial cells. Recently, MT-SP1/matriptase−/− knockout mice showed that this enzyme is essential for postnatal survival. The postnatal death of the MT-SP1/matriptase−/− mice resulted from a deficient epidermal barrier function in the skin of newborn mice [14]. However, its physiological role in normal adult animals and its localization in simple columnar epithelial cells such as enterocytes remain to be elucidated. We previously found that the mRNA for rat MT-SP1/matriptase is expressed most strongly in the small intestine of the normal tissues examined and that the signal is most prominent in the epithelium of the villus tip, where cell–cell and cell–substratum adhesions are loose and cells frequently undergo apoptosis [5]. These results led us to hypothesize that the enzyme participates in the control of epithelial cell–cell and/or cell–substratum adhesions, which are key processes in cell turnover.
The plasma membranes of simple columnar epithelial cells, including enterocytes, are characterized by two structurally and functionally different domains: the apical and basolateral domains [15]. If our hypothesis regarding the role of MT-SP1/matriptase is correct, this enzyme must exist on the basolateral side where cell–cell and/or cell–substratum adhesion occurs. However, the subcellular distribution of this enzyme in the enterocytes is controversial. We have previously showed that the precursor form of MT-SP1/matriptase localized predominantly on the basolateral surfaces of transfected Caco-2 cells, a human colonic cancer cell line [5]. However, Caco-2 cells have been shown apparently to lose their polarity and to mimic a pathological situation, and thus they do not reflect the normal physiological situation [16]. Kishi et al. [17] showed, by the immunostaining of normal adult rat duodenum with an antibody raised against the catalytic domain, that a membrane-bound arginine-specific serine proteinase, identical with MT-SP1/matriptase, localized to the brush border (apical) membranes of epithelial cells. They proposed that the enzyme participates in the processing or digestion of some specific proteins or peptides on the brush border membranes. Furthermore, the existence of a soluble form of MT-SP1/matriptase in human breast milk [8] suggests the apical sorting of the enzyme in normal epithelial cells.
The purpose of the present study was to determine the subcellular distribution of MT-SP1/matriptase in simple columnar epithelial cells, such as enterocytes, of normal adult animals. For this purpose, we characterized the post-translational processing of the enzyme and prepared an antibody that can detect the enzyme when associated with cells. In the present study, we provide evidence that a considerable number of MT-SP1/matriptase molecules occur on the basolateral sides of normal enterocytes. The results presented here support our hypothesis that a physiological role for this enzyme is in the control of epithelial cell–cell and/or cell–substratum adhesion.
EXPERIMENTAL
Materials
Male Wistar rats and male Dutch rabbits were purchased from the Japan Shizuoka Laboratory Center (Shizuoka, Japan). HRP (horseradish peroxidase)-conjugated S-protein was purchased from Novagen (Madison, WI, U.S.A.). Anti-myc-tagged antibody and synthetic oligonucleotides were purchased from Invitrogen (Carlsbad, CA, U.S.A.). Complete™, a protease inhibitor cocktail, neuraminidase (from Arthrobacter ureafaciens), endo H (endoglycosidase H; from Streptomyces plicatus) and O-glycosidase (from Diplococcus pneumoniae) were purchased from Roche Diagnostics (Mannheim, Germany). Glycopeptidase F (N-glycosidase F; from Flavobacterium meningosepticum) was purchased from Takara Bio (Otsu, Shiga, Japan), prestained protein markers (broad range) and restriction endonucleases were purchased from New England Biolabs (Beverly, MA, U.S.A.) and Kaleidoscope Polypeptide Standards from Bio-Rad Laboratories (Hercules, CA, U.S.A.). All other reagents used were of analytical grade.
Animal care
The animals were maintained in accordance with the guidelines of Kyoto University for the care and use of laboratory animals. This study was approved by the Kyoto University Animal Committee, Graduate School of Agriculture.
Antibody preparation
Polyclonal antibodies (Spr992 and Tmc172) were raised against rat MT-SP1/matriptase using the synthetic peptides SKRSASGVQEHKLKRC and GCGPYHKKSTVTAFSEG, corresponding to the catalytic domain of rat MT-SP1/matriptase (Figure 1A) and the C-terminus of the NTF (N-terminal fragment) (Met1–Gly149; Figure 3B) respectively, each of them conjugated with haemocyanin. Synthesis of the peptides and production of the peptide–haemocyanin conjugates were performed by the Peptide Institute (Osaka, Japan). Male Dutch rabbits were immunized with 1 mg of each conjugate/Freund's complete adjuvant (50:50) at five subcutaneous sites. Booster immunizations (0.5 mg of conjugate/Freund's incomplete adjuvant) were administered over a 3 month period. The antibodies were affinity-purified on a peptide-antigen-coupled Sepharose column (Amersham Biosciences, Piscataway, NJ, U.S.A.) following the manufacturer's instructions.
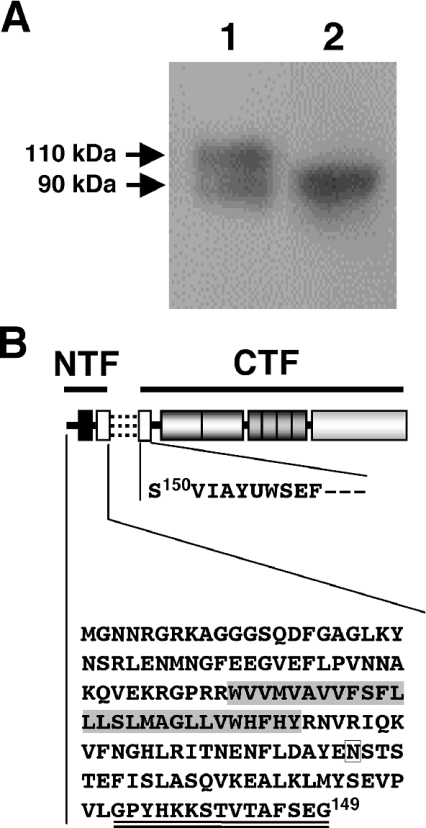
Figure 3. Post-translational cleavage of MT-SP1/matriptase at Gly149 and Ser150 within the SEA domain.
(A) Representative Western blot showing the expression of MT-SP1MycHis in COS-1 cells. Samples were separated by SDS/PAGE (8% polyacrylamide) under reducing conditions, and Western-blot analysis was performed using anti-myc-tagged antibody. Lane 1, crude membrane sample; lane 2, conditioned media. The molecular masses of the bands are indicated on the left. (B) Schematic representation of the post-translational cleavage between Gly149 and Ser150. The association between the NTF and CTF is illustrated with three broken lines. The predicted amino acid sequence of the NTF and the amino acid sequence of the N-terminus of the CTF, which was determined in the present study, are shown by their single-letter codes. The C-terminal glycine residue (Gly149) of the NTF and the N-terminal serine residue (Ser150) of the CTF are indicated. The highlighted amino acid sequence represents the putative transmembrane domain. A potential N-glycosylation site (Asn107) is boxed. The region corresponding to the antigenic peptide for the production of antibody (Tmc172) is double-underlined.
Construction of expression plasmids for MT-SP1/matriptase
Procedure for the construction of the vector pcMT-SP1MycHis has been described previously (Figure 1A) [5]. An expression plasmid for MT-SP1wt was constructed as follows. A DNA fragment containing the sequence of rat MT-SP1/matriptase (Met1–Val855) was amplified by PCR using synthetic oligonucleotides (5′-ATGGGGAACAATCGGGGC-3′ and 5′-CTATACCCCAGTTTGCTC-3′) as primers and rat small-intestinal cDNA (Nippon Gene, Tokyo, Japan) as the template. The PCR product was ligated into the PmeI-linearized pcDNA3.1(+) vector (Invitrogen). This expression plasmid was designated pcMT-SP1wt. Sequences of the expression plasmids constructed in the present study were determined in both directions by the dideoxynucleotide chain-termination method as described previously [5].
Cloning of rat HAI-1 (HGF activator inhibitor type-1) cDNA and construction of the expression plasmid
PCR amplification of HAI-1 cDNA from rat small-intestine cDNA (Nippon Gene) was performed with various synthetic oligonucleotides. One set of primers, 5′-CTGGGCAACAAGAACAACTA-3′ and 5′-CATCCTTCTTGGAGATGC-3′, yielded a fragment of approx. 400 bp. Using the PCR product as a probe, a rat jejunal cDNA library (Stratagene, La Jolla, CA, U.S.A.) was screened by plaque hybridization to obtain the full-length cDNA. The longest clone was subcloned into the pBluescript KS(–) vector (pBlueHAI-1) and its DNA sequence was determined. Construction of an expression plasmid for rat HAI-1 was performed as follows. A DNA fragment containing the sequence of rat HAI-1 (Pro35–Leu507; amino acid numbering starts from the putative N-terminal methionine residue of the protein) was amplified by PCR using the synthetic oligonucleotides 5′-CCCTGAGTTTTCCGGGGG-3′ and 5′-GCTCTAGAGCTCAGAGAGGCTGGGTTGT-3′ as primers and pBlueHAI-1 as the template. The PCR product was subcloned into the SmaI-linearized PT7Blue-2 vector (Novagen) (PT7HAI-1) and, then, PT7HAI-1 was double-digested with KpnI and XbaI. The restriction fragment was subcloned into SfiI- and XbaI-digested pSecTag/Hygro vector (Invitrogen). This construct was designated pSecHAI-1.
Expression and detection of recombinant rat MT-SP1/matriptases
The procedure for the transfection of constructs into COS-1 cells using Lipofectamine 2000™ (Invitrogen) has been described previously [5]. Cells were left undistributed for 24 h after transfection. COS-1 cells expressing MT-SP1wt, MT-SP1MycHis or sHAI-1 (Figure 1) were then washed three times with PBS (8 mM Na2HPO4, 1.5 mM KH2PO4, 136 mM NaCl and 2.7 mM KCl, pH 7.4), and then cultured for an additional 24 h in Dulbecco's modified Eagle's medium without foetal bovine serum. After the conditioned media were collected, the cells were washed three times with ice-cold PBS, mechanically scraped and collected by centrifugation at 3000 g for 5 min at 4 °C. The cells were resuspended in a hypo-osmotic buffer (50 mM Tris/HCl, pH 7.0, containing Complete™) and quickly lysed in liquid nitrogen. After thawing and centrifugation at 500 g for 5 min at 4 °C, the postnuclear supernatants were re-centrifuged at 48000 g for 30 min at 4 °C. The resulting pellets (crude membranes) were solubilized with Laemmli buffer [0.05 M Tris/HCl (pH 6.8), 10% (v/v) glycerol, 2% (w/v) SDS and 0.005% (w/v) Bromophenol Blue with dithiothreitol at a final concentration of 12 mM] [18] and stored at −20 °C until use. The conditioned media collected were concentrated by ultrafiltration (Ultrafree; Millipore, Bedford, MA, U.S.A.) and stored at −20 °C until use. Samples were heated to 95 °C for 5 min and subjected to SDS/PAGE under reducing conditions. After separation, the proteins were transferred by electroblotting on to a PVDF membrane (Fluorotrans W; Nihon Genetics, Tokyo, Japan) and then the blots were incubated with PBST [PBS containing 0.1% (v/v) Tween 20] and the primary antibody Spr992 (1:200 dilution) or Tmc172 (1:1000 dilution). Protein bands were visualized using HRP-conjugated anti-rabbit IgG secondary antibody (Dako Japan, Kyoto, Japan) and the ECL® detection system (Amersham Biosciences).
N-terminal sequencing of recombinant MT-SP1/matriptases
Procedures for the purification of a His-tagged recombinant protein (MT-SP1MycHis) with Ni2+-charged resin and for N-terminal sequencing have been described previously [19].
Glycosidase digestions
Samples (30 μl of crude membranes or concentrated conditioned media) were heated to 95 °C for 5 min in 20 mM sodium citrate phosphate (pH 5.5) containing 0.2% (w/v) SDS and 5% (v/v) 2-mercaptoethanol, and cooled to 22 °C. The detergent Nonidet P40 was added to a final concentration of 2% (v/v), and the samples were incubated at 37 °C for 18 h with or without 1 m-unit/μl endo H. Reactions with glycopeptidase F were performed similarly, except that the buffer was 20 mM sodium phosphate (pH 7.5) containing 50 mM EDTA, 5% 2-mercaptoethanol and 0.2% (v/v) SDS with 0.5 unit of enzyme. To analyse proteins for O-glycosylation, samples (30 μl) were digested with 0.5 m-unit/μl neuraminidase at 37 °C for 1 h in 100 mM sodium acetate (pH 5.0), then digested with 0.05 m-unit/μl O-glycanase in 100 mM sodium phosphate (pH 6.0) and 100 μg/ml BSA at 37 °C for 16 h. Digested samples were analysed by SDS/PAGE and Western blotting.
Cell-surface biotinylation of recombinant MT-SP1/matriptases expressed in COS-1 cells
The procedures for cell-surface biotinylation with sulpho-NHS-SS-biotin [sulphosuccinimidyl 2-(biotinamido)ethyl-1,3-dithiopropionate; Pierce, Rockford, IL, U.S.A.] and precipitation of the biotinylated proteins with streptavidin–agarose (Invitrogen) have been described previously [5]. The flow-through fractions were precipitated with the SDS/PAGE Clean-Up kit (Amersham Biosciences) and analysed by Western blotting.
Preparation of samples from rat jejunum
Male Wistar rats (200–300 g) were killed by cervical dislocation and the proximal quarter of their small intestines (jejunum) was excised. The excised intestines were washed thoroughly with ice-cold 0.155 M NaCl solution and then the mucosae were scraped off with a stainless-steel spatula and suspended in ice-cold buffer A (250 mM sucrose/10 mM triethanolamine, pH 7.6). The mucosae were suspended in buffer A at a concentration of 1 g of cells/3 ml of buffer and were homogenized using a Polytron homogenizer (setting 7; three 10 s bursts) to produce a crude homogenate, which was centrifuged at 2500 g for 10 min at 4 °C. The resulting supernatants were removed and re-centrifuged at 19500 g for 20 min at 4 °C. This spin resulted in a double pellet composed of a dark centre surrounded by a ‘fluffy’ white layer that represents the crude membranes [20]. The supernatant resulting from this spin was concentrated by ultrafiltration, subjected to gel filtration equilibrated with buffer B (20 mM Tris/HCl, pH 8.0, and 145 mM NaCl) and purified with benzamidine–Sepharose resin (Amersham Biosciences) as described below. Portions of the crude membranes were further separated with Percoll (described below). Other portions were washed once with buffer A and solubilized with 2% (v/v) Triton X-100 (1 ml/proximal quarter of the small intestine) as described above. Triton X-100 extracts were subjected to gel filtration equilibrated with buffer B and purified on a benzamidine column. Purification, using benzamidine, of MT-SP1/matriptase from the supernatants of initial homogenates or the Triton X-100 extracts was performed as follows. The benzamidine resin (30 μl) was added to 1 ml of each mixture, and the mixtures were incubated for 2 h at 22 °C. After precipitating the resin by a brief centrifugation, the resin was washed three times with buffer B and suspended in Laemmli buffer. After the resin was heated at 95 °C for 5 min, the eluted protein was subjected to SDS/PAGE (12% polyacrylamide) followed by probing of Western blots with Spr992. When purification with benzamidine resin was not performed, the Triton X-100 extracts were precipitated with the SDS/PAGE Clean-Up kit and analysed by SDS/PAGE (16% polyacrylamide), and Western blots were probed with Tmc172 (see Figure 6A).
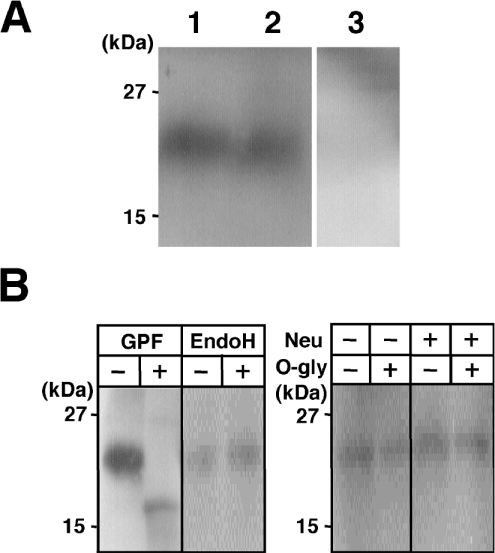
Figure 6. Western-blot analysis of the NTF of MT-SP1/matriptase expressed in jejunal enterocytes of normal adult rats.
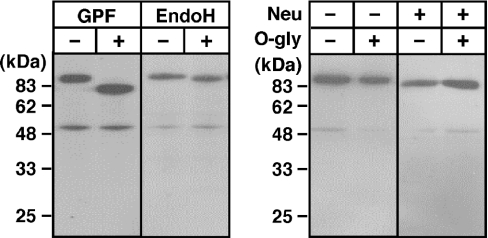
(A) Representative Western blot showing the presence of the 22 kDa band in the crude membranes from jejunal enterocytes. Triton X-100 extracts of crude membranes were separated by SDS/PAGE (16% polyacrylamide) under reducing conditions, and Western blots were probed with Tmc172. The 22 kDa band was detected in the crude membrane extracts (lane 1) but not when the antibody was preadsorbed with the antigenic peptide at a concentration of 1 mM (lane 3). For comparison, a sample from the crude membranes of COS-1 cells expressing MT-SP1wt is shown in lane 2. Molecular masses of the standards (Kaleidoscope Polypeptide Standards) are indicated on the left. (B) Glycosidase digestion of the NTF. The crude membranes from rat jejunal enterocytes were incubated with glycopeptidase F (GPF), endo H or O-glycosidase (O-gly) and neuraminidase (Neu). The digested samples were separated by SDS/PAGE (16% polyacrylamide), and Western-blot analysis was performed with Tmc172.
Fractionation of crude membranes by Percoll density gradient and detection of MT-SP1/matriptase
The procedure used to fractionate crude membranes was based on previously reported methods with some modifications [20,21]. Crude membranes were prepared as described above, except for the inclusion of Complete™ in all the solutions used. Crude membrane fractions suspended in buffer A were homogenized (15 strokes at setting 12) in a Teflon/glass Potter homogenizer. This crude membrane suspension was diluted with Percoll (Amersham Biosciences) to a final Percoll concentration of 15% (v/v). The mixture was rehomogenized and subsequently spun in a fixed-angle ultracentrifuge rotor at 48000 g for 1 h at 4 °C. The spontaneously formed gradient was divided, from top to bottom, into eight fractions of 2.5 ml each, which were removed with a syringe and an 18-gauge needle. These were diluted with buffer A and subsequently repelleted by centrifugation at 190000 g for 90 min at 4 °C. The pellets from this spin were then resuspended in small aliquots of buffer A. The protein content of each membrane fraction was measured using the BCA (bicinchoninic acid) protein assay kit (Pierce) with BSA as the standard. Portions of the prepared membrane fractions (30 μg for the NTF of MT-SP1/matriptase and 5 μg for other membrane proteins) were heated at 95 °C for 5 min, subjected to SDS/PAGE and Western blots were probed with Tmc172 or an anti-alkaline-phosphatase antibody (Research Diagnostic, Flanders, NJ, U.S.A.; 1:1000 dilution) or an anti-Na,K-ATPase antibody (Upstate Biotechnology, Lake Placid, NY, U.S.A.; 1:3000 dilution) or an anti-E-cadherin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.; 1:2000 dilution). Blots were digitally scanned with an Epson GT-5000 flatbed scanner (Seiko Epson, Nagano, Japan) and densitometric analysis of band intensities was performed with public domain NIH Image software. The fractionation profiles were compared by linear correlation analysis using Instat statistical software (GraphPAD Software for Science, San Diego, CA, U.S.A.).
Immunostaining of rat jejunum
Male Wistar rats of approx. 220 g body weight were anaesthetized with pentobarbital and perfused through the aorta with 0.155 M NaCl solution followed by 300 ml of periodate/lysine/paraformaldehyde mixture for a 5–10 min period. The jejunum was then removed and immersed in the same fixative for an additional 6 h. The tissue was incubated in 30% (w/v) sucrose solution overnight at 4 °C and frozen rapidly in liquid nitrogen. Immunostaining was performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA, U.S.A.) with minor modifications to the manufacturer's instructions. Frozen sections, approx. 5 μm in thickness, were hydrated with PBS for 10 min and then incubated for 20 min with PBST containing 5% (v/v) normal goat serum. After the sections were washed with PBST, they were incubated with Tmc172 (1:5000 dilution) in PBST containing 5% goat serum in a humid chamber for 2 h at 22 °C. After several washes with PBST, the sections were incubated with biotinylated goat anti-rabbit IgG (1:200 dilution) for 1 h at 22 °C. Endogenous peroxidase activity was blocked by incubating the sections for 45 min in 1% (v/v) H2O2 in water. The sections were then washed with PBST and incubated with streptavidin–HRP conjugate (1:300 dilution) for 10 min. The immunoperoxidase colour reaction was developed by incubating the sections in diaminobenzidene substrate chromogen solution. The sections were counterstained with haematoxylin and further processed according to routine procedures. To assess the specificity of Tmc172, control experiments were performed by incubating the sections with Tmc172 preadsorbed with the antigenic peptide at a final concentration of 1 mM. All other steps and conditions were the same as those used for experimental immunostaining.
RESULTS
Detection of MT-SP1/matriptase using Spr992 antibody
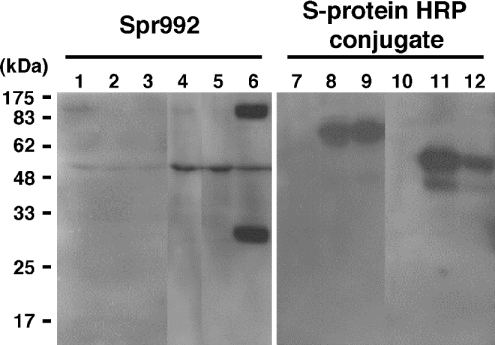
In the present study, MT-SP1wt (Figure 1A) was expressed in COS-1 cells with or without recombinant rat HAI-1 (sHAI-1, Figure 1B), cognate inhibitor of MT-SP1/matriptase, which paradoxically facilitates the activation of this protease [22]. Probing of the Western blots with HRP-conjugated S-protein revealed that recombinant HAI-1 was visualized with a mass of 66 kDa in the crude membrane fraction (Figure 2, lane 8 or 9) and with masses of 58 and 40 kDa in the conditioned medium (lane 11 or 12), confirming the expression of sHAI-1. When the blot was probed with Spr992 (Figure 1A), which was designed to recognize the catalytic domain of MT-SP1/matriptase (Figure 2), a 90 kDa band was visualized in the crude membranes of COS-1 cells transfected with pcMT-SP1wt plasmid (lane 1) but not in those of the cells transfected with pSecHAI-1 (lane 2) or the parent pcDNA3.1(+) vector alone (results not shown). The 90 kDa band was also detected in the conditioned medium from the cells transfected with pcMT-SP1wt (lane 4) but not that from cells transfected with pSecHAI-1 (lane 5) or the pcDNA3.1(+) vector alone (results not shown), indicating that a portion of the MT-SP1/matriptase is released. A 28 kDa band that shows the occurrence of activation cleavage between Arg614 and Val615 of this enzyme [5] was detected in the conditioned medium (lane 6) but not in the crude membranes (lane 3) of cells transfected with both pcMT-SP1wt and pSecHAI-1. This indicates that the activation cleavage of MT-SP1/matriptase also occurs in COS-1 cells when it is co-expressed with HAI-1 and that the activated enzyme is no longer present in the cells. Release of the 90 kDa non-activated enzyme was also greater in the co-expressing cells (lane 6) compared with the cells expressing MT-SP1wt alone (lane 4). On the other hand, the signal for the 90 kDa band was lower in the crude membranes of co-expressing cells (lane 3) compared with those of cells expressing MT-SP1wt alone (lane 1).
Figure 2. Representative Western blots showing the expression of MT-SP1wt or sHAI-1 expressed in COS-1 cells and the activation of MT-SP1wt by co-expression with sHAI-1.
Transfection was performed when cells cultured on 100 mm dishes reached approx. 90% confluence. Samples were prepared as described in the Experimental section. All samples were separated by SDS/PAGE (12% polyacrylamide) under reducing conditions; Western blots were probed with Spr992 (lanes 1–6) or HRP-conjugated S-protein (lanes 7–12). Lanes 1 and 7, crude membranes from cells transfected with pcMT-SP1wt (12.5 μg) and pSecTagHygro vector (12.5 μg); lanes 2 and 8, crude membranes from cells transfected with pSecHAI-1 (12.5 μg) and pcDNA3.1(+) vector (12.5 μg); lanes 3 and 9, crude membranes from cells transfected with pcMT-SP1wt (12.5 μg)+pSecHAI-1 (12.5 μg); lanes 4 and 10, conditioned media of cells transfected with pcMT-SP1wt (12.5 μg) and pSecTagHygro vector (12.5 μg); lanes 5 and 11, conditioned media of cells transfected with pSecHAI-1 (12.5 μg) and pcDNA3.1(+) vector (12.5 μg); lanes 6 and 12, conditioned media of cells transfected with pcMT-SP1wt (12.5 μg)+pSecHAI-1 (12.5 μg). The 28 kDa band, which represents the catalytic domain of MT-SP1/matriptase after activation cleavage, is apparent in lane 6 but not in lane 3. A protein with a mass of approx. 50 kDa visible in lanes 1–6 is a non-specific band representing an unknown protein. The molecular-mass standards are indicated on the left in kDa.
We probed Western blots of normal rat enterocytes using Spr992. However, neither the 90 kDa nor the 28 kDa band could be detected in the crude membranes from the tissues (results not shown). We then concentrated the MT-SP1/matriptase using benzamidine–Sepharose, which can be used to purify the active enzyme [16], either from the 2% (v/v) Triton X-100-soluble fraction of the crude membranes or from the supernatants of the initial jejunal homogenates. However, no MT-SP1/matriptase signal was detected in any sample (results not shown).
N-terminal sequencing of recombinant MT-SP1/matriptase expressed in COS-1 cells
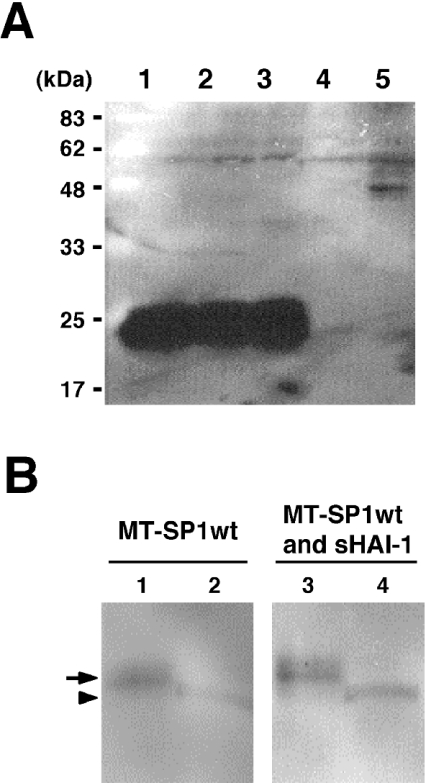
We purified MT-SP1MycHis from Triton X-100 extracts of the transfected COS-1 cells using Ni2+-charged resin. Partial N-terminal sequencing of the 90 kDa fragment (Figure 3A, lane 1) gave the sequence SVIAYYWSEF, demonstrating post-translational processing of the enzyme by cleavage between Gly149 and Ser150 within the SEA domain (Figure 3B) [23]. N-terminal sequencing of the MT-SP1MycHis released into the medium (Figure 3A, lane 2) gave the same result. The 110 kDa form (Figure 3A, lane 1) appeared to be the unprocessed enzyme, possibly persisting by evading N-terminal cleavage due to the high level of expression from exogenous cDNA under the control of strong promoters. The amino acid sequence could not be determined, probably because of modifications to the N-terminus of the protein.
Glycosidase digestion of the CTF (C-terminal fragment) of recombinant MT-SP1/matriptase
We analysed the oligosaccharides that were added to recombinant MT-SP1/matriptase (Figure 4). Digestion with glycopeptidase F of the MT-SP1wt released into the medium (90 kDa) decreased its mass to the calculated mass (78.2 kDa) of the C-terminal region (Ser150–Val855; CTF). Endo H digestion decreased the mass to approx. 86 kDa, indicating that one (or at most two) potential N-glycosylation site on the recombinant protein is modified with high-mannose-type oligosaccharides. Neuraminidase digestion slightly decreased the mass, whereas O-glycosidase digestion did not affect the mass, irrespective of pretreatment with neuraminidase or not.
Figure 4. Glycosidase digestion of recombinant MT-SP1/matriptase expressed in COS-1 cells.
Concentrated conditioned medium of COS-1 cells expressing MT-SP1wt was incubated with (+) or without (−) glycopeptidase F (GPF), endo H or O-glycosidase (O-gly) and neuraminidase (Neu). The digested samples were separated by SDS/PAGE (12% polyacrylamide), and Western-blot analysis was performed with Spr992. The molecular-mass standards are indicated on the left in kDa.
Analysis of the NTF of recombinant MT-SP1/matriptase
We prepared another antibody (Tmc172), using as the immunogen a synthetic peptide corresponding to the C-terminus of the NTF, consisting of the cytosolic and transmembrane domains and the N-terminal part of the SEA domain (Figure 3B). By probing Western blots with Tmc172 (Figure 5A), a 22 kDa band was visualized in the crude membranes from COS-1 cells expressing either MT-SP1MycHis (lane 1) or MT-SP1wt (lane 2) but not in those from cells transfected with pSecHAI-1 (lane 4) or the pcDNA3.1(+) vector (lane 5). The 22 kDa band was not detected when the antibody was preadsorbed with the antigenic peptide at a concentration of 1 mM (results not shown). The crude membranes of COS-1 cells expressing MT-SP1wt with HAI-1 contained the 22 kDa protein (lane 3) at almost the same level observed in cells expressing MT-SP1MycHis or MT-SP1wt alone. The 22 kDa band was not detected in the conditioned medium from these transfected COS-1 cells (results not shown).
Figure 5. Western-blot analysis of the NTF of a recombinant MT-SP1/matriptase expressed in COS-1 cells.
(A) Representative Western blot showing the 22 kDa band derived from the recombinant MT-SP1/matriptases expressed in COS-1 cells. Transfection was performed when cells cultured on 100 mm dishes reached approx. 90% confluence. Samples were separated by SDS/PAGE (12% polyacrylamide) under reducing conditions, and Western-blot analysis was performed using Tmc172. Lane 1, sample from COS-1 cells transfected with pcMT-SP1MycHis (25 μg); lane 2, sample from COS-1 cells transfected with pcMT-SP1wt (12.5 μg) and pSecTagHygro vector (12.5 μg); lane 3, sample from COS-1 cells transfected with pcMT-SP1wt (12.5 μg) and pSecHAI-1 (12.5 μg); lane 4, sample from COS-1 cells transfected with pSecHAI-1 (12.5 μg) and pcDNA3.1(+) vector (12.5 μg); lane 5, sample from COS-1 cells transfected with pcDNA3.1(+) vector (25 μg). (B) Representative Western blot showing the cell-surface localization of the NTF. Transfection was performed when cells cultured on 100 mm dishes reached approx. 90% confluence. Samples were separated by SDS/PAGE (16% polyacrylamide) under reducing conditions, and Western blots were probed with Tmc172. Lanes 1 and 2, the eluate and flow-through fractions respectively from streptavidin–agarose-precipitated proteins of COS-1 cells transfected with pcMT-SP1wt (12.5 μg)+pSecTagHygro vector (12.5 μg); lanes 3 and 4, eluate and flow-through fractions respectively from streptavidin–agarose-precipitated proteins of COS-1 cells transfected with pcMT-SP1wt (12.5 μg)+pSecHAI-1 (12.5 μg). Note that the biotinylated NTF (indicated by an arrow) migrated more slowly than the NTF in the flow-through fraction (indicated by an arrowhead) because of the addition of the sulpho-NHS-SS-biotin spacer.
The 22 kDa band was also detected when the MT-SP1MycHis purified from the Triton X-100 extracts of the crude membrane fraction with Ni2+-charged resin was probed with this antibody (results not shown). In contrast, purification of the 90 kDa protein from the conditioned medium with Ni2+-charged resin did not produce this signal (results not shown). This indicates that the NTF was non-covalently associated with the CTF, even after the cleavage between Gly149 and Ser150, and that the NTF can act as anchor for the CTF before the extracellular release of the CTF. A cellsurface-biotinylation technique localized the NTF to the surfaces of COS-1 cells expressing MT-SP1wt and MT-SP1wt with sHAI-1 (Figure 5B). A signal (22 kDa) was also detected in each flow-through fraction (Figure 5B), indicating that considerable amounts of the NTF exist in intracellular compartments. The N-terminal sequence of the 22 kDa fragment could not be determined.
Detection and analysis of the NTF of MT-SP1/matriptase in the jejunal enterocytes of normal adult rats
Western-blot analysis of the crude membrane samples (Triton X-100 extracts) prepared from rat jejunal mucosae and probed with Tmc172 revealed a 22 kDa band (Figure 6A, lane 1). The molecular mass was similar to that of MT-SP1wt produced in COS-1 cells (lane 2), but there was no signal when Tmc172 was preadsorbed with the antigenic peptide (lane 3).
Digestion with glycopeptidase F decreased the apparent mass to 18 kDa (Figure 6B), which is similar to the calculated mass of the NTF (16.8 kDa). Digestion with endo H, neuraminidase or O-glycosidase did not affect its mass (Figure 6B). Similar results were obtained when the crude membranes from COS-1 cells transfected with pcMT-SP1wt were used, except that endo H decreased the mass to 18 kDa in some preparations of the recombinant protein (results not shown).
Localization of MT-SP1/matriptase (NTF) in jejunal enterocytes of normal adult rats
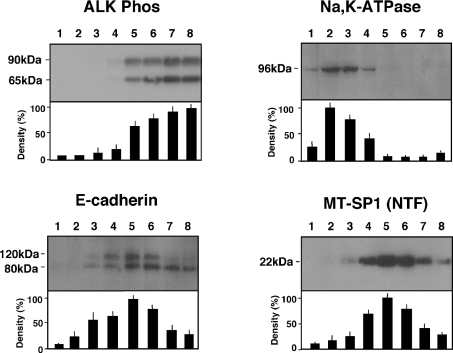
We examined the subcellular distribution of MT-SP1/matriptase (NTF) using a conventional biochemical approach. The crude membrane fraction of the rat jejunum was separated into eight fractions by ultracentrifugation in the presence of Percoll. The fractionation profile of the NTF was compared with those of other proteins expressed in enterocytes. Representative Western blots and the signal densities quantified by densitometry in each fraction are shown in Figure 7. Alkaline phosphatase, a marker for the apical membrane, was observed at 65 and 90 kDa [24], and these bands were most intense in the highest density fraction of the Percoll gradient. A monoclonal antibody directed against the α1 subunit of Na,K-ATPase, a marker of the basolateral membrane, detected an immunoreactive α1 protein band (∼96 kDa) [25]. The signal intensity was strongest in a lower-density fraction (fraction 2). E-cadherin, an adhesion molecule present in a specific region of the lateral membranes (the adherens junction), was visualized as 80 and 120 kDa bands [25]. The 80 and 120 kDa bands as well as the 22 kDa band representing the NTF of MT-SP1/matriptase were most enriched in an intermediate-density fraction (fraction 5). Linear correlation analysis revealed that the fractionation profile of the NTF was correlated with neither that of alkaline phosphatase (P=0.4771, r=0.291) nor that of Na,K-ATPase (P=0.5548, r=0.248) but was correlated significantly with that of E-cadherin (P=0.0005, r=0.9426). Neither the 90/110 kDa nor the 28 kDa band recognized by Spr992 was detected in all fractions (results not shown).
Figure 7. Percoll density gradient and Western-blot analysis of crude membranes extracted from the jejunum of normal adult rats.
Percoll density gradients were fractionated (eight fractions; fraction 8 is the bottom of the gradient and fraction 1 is the top of the gradient), the fractions were separated by SDS/PAGE [16% polyacrylamide for the NTF of MT-SP1/matriptase (MT-SP1NTF) and 12% polyacrylamide for alkaline phosphatase (ALK Phos), Na,K-ATPase and E-cadherin] and the Western blots were probed with Tmc172, anti-alkaline phosphatase antibody, anti-Na,K-ATPase antibody and anti-E-cadherin antibody respectively. The most intense signal in a fraction was given the value of 100%. The results are the means±S.D. for six different preparations.
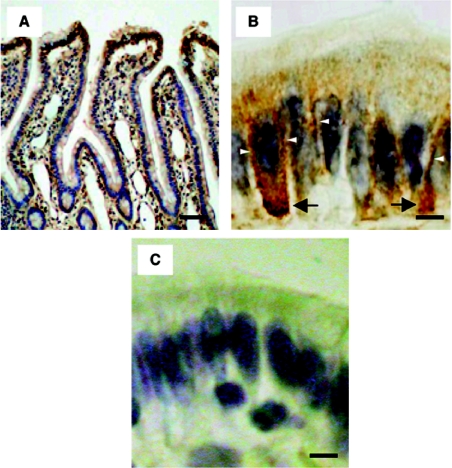
The subcellular distribution of MT-SP1/matriptase in the rat jejunum was further analysed by immunostaining with Tmc172. Positive staining occurred in the upper epithelial cells (Figure 8A); these sites are consistent with those of mRNA expression [5]. Weak-to-moderate immunoreactivity was demonstrated on the lateral membrane, but no apparent immunoreactivity was detected on the apical membrane (Figure 8B). Immunoreactivity was also visible in the cytosol, close to the basal membrane (Figures 8A and 8B), this being consistent with the existence of NTF in the intracellular compartments of the transfected COS-1 cells (Figure 5B). Staining was significantly decreased when Tmc172 was preadsorbed with the immunogenic peptide (Figure 8C). When the tissue was treated with Spr992, no apparent immunoreactivity was observed (results not shown), confirming that CTF is released from enterocytes.
Figure 8. Immunostaining of rat jejunum with Tmc172.
(A) Staining of the rat jejunum along the crypt-villus axis. (B) Staining on the villus tip. Staining on the lateral membranes and in the cytosol close to the basal membrane is indicated by open arrowheads and arrows respectively. (C) Staining on the villus tip when Tmc172 was preadsorbed with the antigenic peptide at a concentration of 1 mM. Counterstaining was with haematoxylin. Scale bars: 200 μm for (A) and 10 μm for (B, C).
DISCUSSION
The physiological roles of MT-SP1/matriptase in simple columnar epithelial cells of adult animals have been unclear. To assess these roles, we examined the subcellular distribution of this enzyme at the site of expression, such as in the enterocytes of normal adult rats. For this purpose, characterization of the post-translational processing of MT-SP1/matriptase was a prerequisite. Post-translational cleavage and the extracellular release of MT-SP1/matriptase were demonstrated by Cho et al. [26] using COS-7 cells transfected with a cDNA of epithin, a mouse orthologue of this enzyme. Cleavage between Gly149 and Ser150 was clearly demonstrated in this study by N-terminal sequencing of the CTF. Although a portion of the single-chain CTF (90 kDa) was released into the medium, other portions remained in the cells, anchored by the NTF. However, on activation of the protein facilitated by its co-expression with HAI-1 [22], the release of the single-chain CTF increased, and the activated form (the two-chain form of the CTF) was no longer associated with the cells (Figure 2). It has been shown that the tissue- and cell-specific expression of HAI-1 is consistent with the tissue- and cell-specific expression of MT-SP1/matriptase, as far as has been examined [13,27]. This strongly suggests that major portions of the single-chain CTF or the activated two-chain form are extracellularly released in vivo. This release may account for the difficulty in detecting CTF signal in the crude membranes of enterocytes. Therefore the use of antibodies to recognize the region corresponding to the CTF is probably not suitable for the determination of subcellular localization.
Probing Western blots of crude membranes from rat jejunal enterocytes with the antibody Tmc172 revealed detectable levels of the NTF. Fractionation of the crude membranes by ultracentrifugation with Percoll, followed by probing of Western blots with Tmc172, showed that the fractionation profile of the NTF correlated with that of E-cadherin. E-cadherin is stably tethered to components of the cytoskeleton through associated molecules such as catenin [28], thereby showing a distribution profile different from that of Na,K-ATPase after the isolation of intestinal epithelial cells [25]. However, it is unlikely that the NTF is stabilized by specific interactions with such molecules since the NTF (Figure 6A), but not E-cadherin, is extractable with Triton X-100. Hung et al. [29] recently reported that MT-SP1/matriptase was localized coincident with markers of adherens junctions at cell–cell contacts in cultured mammary epithelial cells. However, it is unlikely to be incorporated into the tightly bound adhesion plaques [29]. Therefore it is suggested that the NTF is localized in the perijunctional areas of polarized epithelial cells, including enterocytes. Immunostaining of the rat jejunum with Tmc172 also revealed the presence of the NTF on the lateral membranes. These results showing the presence of NTF on the lateral membranes strongly suggest the occurrence of MT-SP1/matriptase (CTF) on the basolateral side of the enterocytes, since the NTF can anchor the CTF. The basolateral occurrence is also supported by the fact that HAI-1 is exclusively localized to the lateral membranes of all tissues examined [27].
The presence of a soluble form of MT-SP1/matriptase (CTF) in human breast milk [8] suggests an apical sorting of the enzyme in mammary epithelial cells. In the present study, the NTF in the cytosol (intracellular compartments) was demonstrated in enterocytes (Figures 8A and 8B) as well as in COS-1 cells expressing MT-SP1wt (Figure 5B). One possible explanation for the signal is that the NTF is endocytosed after its dissociation from the CTF at the cell surface. Also, we cannot exclude the possibility that some newly synthesized NTFs are retained in intracellular compartments where the NTF releases the CTF. The assumption that the dissociated CTF within intracellular compartments of mammary epithelial cells is secreted in a non-vectorial fashion could account for the presence of this enzyme in milk [8]. Although we cannot rule out the possibility that enterocytes also secrete the CTF apically, the absence of the NTF in the apical membrane (Figure 8B) strongly suggests that the CTF is not present on the apical surface of the cells. The staining of apical (brush border) membranes of the rat duodenum with an antiserum raised against the protease domain of MT-SP1/matriptase is probably non-specific [17]. In fact, the signals along the villus-crypt axis are inconsistent with the expression of MT-SP1/matriptase mRNA [5]. Many of the membrane enzymes expressed in enterocytes, such as enteropeptidase, a type II transmembrane serine protease, are O-glycosylated extensively in their extracellular serine/threonine-rich domains, and this domain is involved in direct apical sorting [30]. No such domains exist in MT-SP1/matriptase. Moreover, this enzyme does not appear to be O-glycosylated as far as we examined it (Figures 4 and 6B). This also supports the proposition that MT-SP1/matriptase is an enzyme distinct from those that are sorted to the apical surface.
Most basolaterally sorted proteins on polarized epithelial cells contain sorting signals, such as the di-leucine motif, the NPXY motif or the PXXΦ motif (where X is any amino acid and Φ is an amino acid with a bulky hydrophobic group), in their cytosolic regions [15,31]. However, no typical basolateral sorting signals occur in the cytosolic region of MT-SP1/matriptase (Figure 3B). The N-terminal sequence of the unprocessed recombinant MT-SP1/matriptase (Figure 3A, lane 1) or the NTF could not be determined, indicating some modifications of the N-terminus. The predicted N-terminal amino acid sequence of rat MT-SP1/matriptase (M1GNNRGRK—) (Figure 3B) is highly homologous with that of c-Src and related proteins in which the second glycine residue is modified by myristoylation [32]. The myristoylated N-terminal region is required for the sorting of c-Src to its site of function (the cytosolic part of the adherens junction), where E-cadherin is also concentrated [33]. If the second glycine residue of MT-SP1/matriptase is myristoylated, it is possible that the N-terminal region is involved in sorting to such a site. Further studies are required to identify the specific site of and signal for the sorting of MT-SP1/matriptase.
In the present study, we have provided evidence that MT-SP1/matriptase occurs on the basolateral side of normal simple columnar epithelial cells. This localization allows the enzyme to interact with the putative substrates that are involved in cell–cell and/or cell–substratum adhesions. Activation cleavage of pro-HGF by MT-SP1/matriptase on the lateral membranes may facilitate the binding of the growth factor to its receptor, c-Met [34], which activates the intracellular signalling pathways required for the disassembly of epithelial cells [11]. Furthermore, the release of MT-SP1/matriptase allows the enzyme to come in contact with other candidate substrates such as extracellular matrix proteins and pro-urokinase-type plasminogen activator, all of which are critical for cell–substratum adhesion [12]. These processes are essential for epithelial homoeostasis, including proliferation, migration, survival, differentiation and death. Therefore MT-SP1/matriptase may be the key upstream regulator of the turnover of epithelial cells.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, the Uehara Memorial Foundation, the Asahi Glass Foundation and Japan Foundation for Applied Enzymology.
References
- 1.Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 2.Madara J. L., Trier J. S. The functional morphology of the mucosa of the small intestine. In: Johnson L. R., editor. Physiology of the Gastrointestinal Tract. 3rd edn. New York, NY: Raven Press; 1994. pp. 1577–1622. [Google Scholar]
- 3.Pritchard M. D., Watson A. J. M. Apoptosis and gastrointestinal pharmacology. Pharmacol. Ther. 1996;72:149–169. doi: 10.1016/s0163-7258(96)00102-7. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi T., Shuman M. A., Craik C. S. Reverse biochemistry: use of macromolecular protease inhibitors to dissect complex biological processes and identify a membrane-type serine protease in epithelial cancer and normal tissue. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11054–11061. doi: 10.1073/pnas.96.20.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Satomi S., Yamasaki Y., Tsuzuki S., Hitomi Y., Iwanaga T., Fushiki T. A role for membrane-type serine protease (MT-SP1) in intestinal epithelial turnover. Biochem. Biophys. Res. Commun. 2001;287:995–1002. doi: 10.1006/bbrc.2001.5686. [DOI] [PubMed] [Google Scholar]
- 6.Hooper J. D., Clements J. A., Quigley J. P., Antalis T. M. Type II transmembrane serine proteases. J. Biol. Chem. 2001;276:857–860. doi: 10.1074/jbc.R000020200. [DOI] [PubMed] [Google Scholar]
- 7.Shi Y. E., Torri J., Yiehk L., Wellstein A., Lippman M. F., Dickson R. B. Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res. 1993;53:1409–1415. [PubMed] [Google Scholar]
- 8.Lin C. Y., Anders J., Johnson M., Sang Q. A., Dickson R. B. Molecular cloning of cDNA for matriptase, a matrix degrading serine protease with trypsin-like activity. J. Biol. Chem. 1999;274:18231–18236. doi: 10.1074/jbc.274.26.18231. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.-L., Dickson R. B., Lin C.-L. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J. Biol. Chem. 2000;275:36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi T., Harris J. L., Huang W., Yan K. W., Coughlin S. R., Craik C. S. Cellular localization of membrane-type serine protease 1 and identification of protease-activated receptor-2 and single-chain urokinase-type plasminogen activator as substrates. J. Biol. Chem. 2000;275:26333–26342. doi: 10.1074/jbc.M002941200. [DOI] [PubMed] [Google Scholar]
- 11.Nusrat A., Parkos C. A., Bacarra A. E., Godowski P. J., Delp-Archer C., Rosen E. M., Madara J. L. Hepatocyte growth factor/scatter factor effects on epithelia. Regulation of intercellular junctions in transformed and nontransformed cell lines, basolateral polarization of c-met receptor in transformed and natural intestinal epithelia, and induction of rapid wound repair in a transformed model epithelium. J. Clin. Invest. 1994;93:2056–2065. doi: 10.1172/JCI117200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson P. R., Birchall I., Rosella O., Albert V., Finch C. F., Barkla D. H., Young G. P. Urokinase and the intestinal mucosa: evidence for a role in epithelial cell turnover. Gut. 1998;43:656–663. doi: 10.1136/gut.43.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberst M. D., Singh B., Ozdemirli M., Dickson R. B., Johnson M. D., Lin C. Y. Characterization of matriptase expression in normal human tissues. J. Histochem. Cytochem. 2003;51:1017–1025. doi: 10.1177/002215540305100805. [DOI] [PubMed] [Google Scholar]
- 14.List K., Haudenschild C. C., Szabo R., Chen W., Wahl S. M., Swaim W., Engelholm L. H., Behrendt N., Bugge T. H. Matriptase/MT-SP1 is required for postnatal survival, epidermal barrier function, hair follicle development, and thymic homeostasis. Oncogene. 2002;21:3765–3779. doi: 10.1038/sj.onc.1205502. [DOI] [PubMed] [Google Scholar]
- 15.Nelson J. W., Yeaman C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 2001;11:483–486. doi: 10.1016/s0962-8924(01)02145-6. [DOI] [PubMed] [Google Scholar]
- 16.Rosmann S., Hahn D., Lottaz D., Kruse M. N., Stocker W., Sterchi E. E. Activation of human meprin-alpha in a cell culture model of colorectal cancer is triggered by the plasminogen-activating system. J. Biol. Chem. 2002;277:40650–40658. doi: 10.1074/jbc.M206203200. [DOI] [PubMed] [Google Scholar]
- 17.Kishi K., Yamazaki K., Yasuda I., Yahagi N., Ichinose M., Tsuchiya Y., Athauda S. B., Inoue H., Takahashi K. Characterization of a membrane-bound arginine-specific serine protease from rat intestinal mucosa. J. Biochem. (Tokyo) 2001;130:422–430. doi: 10.1093/oxfordjournals.jbchem.a003002. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–690. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Tsuzuki S., Kokado Y., Satomi S., Yamasaki Y., Hirayasu H., Iwanaga T., Fushiki T. Purification and identification of a binding protein for pancreatic secretory trypsin inhibitor: a novel role of the inhibitor as an anti-granzyme A. Biochem. J. 2003;372:227–233. doi: 10.1042/BJ20021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakemore S. J., Aledo J. C., James J., Campbell F. C., Lucocq J. M., Hundal H. S. The GLUT5 hexose transporter is also localized to the basolateral membrane of the human jejunum. Biochem. J. 1995;309:7–12. doi: 10.1042/bj3090007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milovic V., Stein J., Ruppert S., Zeuzem S., Caspary W. F. Preparation of basolateral membrane vesicles from rat enterocytes: influence of different gradient media. Physiol. Res. 1994;43:75–81. [PubMed] [Google Scholar]
- 22.Oberst M. D., Williams C. A., Dickson R. B., Johnson M. D., Lin C. Y. The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J. Biol. Chem. 2003;278:26773–26779. doi: 10.1074/jbc.M304282200. [DOI] [PubMed] [Google Scholar]
- 23.Khatri I. A., Wang R., Forstner J. F. SEA (sea-urchin sperm protein, enterokinase and agrin)-module cleavage, association of fragments and membrane targeting of rat intestinal mucin Muc3. Biochem. J. 2003;372:263–270. doi: 10.1042/BJ20021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh K.-Y., Yeh M., Holt P. R., Alpers D. H. Development and hormonal modulation of postnatal expression of intestinal alkaline phosphatase mRNA species and their encoded isoenzymes. Biochem. J. 1994;301:893–899. doi: 10.1042/bj3010893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amerongen H. M., Mack J. A., Wilson J. M., Neutra M. R. Membrane domains of intestinal epithelial cells: distribution of Na+,K+-ATPase and the membrane skeleton in adult rat intestine during fetal development and after epithelial isolation. J. Cell Biol. 1989;109:2129–2138. doi: 10.1083/jcb.109.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho E.-G., Kim M. G., Kim C., Kim S.-R., Seong I. S., Chung C., Schwartz R. H., Park D. N-terminal processing is essential for release of epithin, a mouse type II membrane serine protease. J. Biol. Chem. 2001;276:44581–44589. doi: 10.1074/jbc.M107059200. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka H., Suganuma T., Shimomura T., Itoh H., Kitamura N., Nabeshima K., Koone M. Distribution of hepatocyte growth factor activator inhibitor type-1 (HAI-1) in human tissues: cellular surface localization of HAI-1 in simple columnar epithelium and its modulated expression in injured and regenerative tissues. J. Histochem. Cytochem. 1999;47:673–682. doi: 10.1177/002215549904700509. [DOI] [PubMed] [Google Scholar]
- 28.Woodfield R. J., Hodgkin M. N., Akhtar N., Morse M. A., Fuller K. J., Saqib K., Thompson N. T., Wakelam M. J. The p85 subunit of phosphoinositide 3-kinase is associated with beta-catenin in the cadherin-based adhesion complex. Biochem. J. 2001;360:335–344. doi: 10.1042/0264-6021:3600335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung R. J., Hsu I. W., Dreiling J. L., Lee M. J., Williams C. A., Oberst M. D., Dickson R. B., Lin C. Y. Assembly of adherens junctions is required for sphingosine 1-phosphate-induced matriptase accumulation and activation at mammary epithelial cell-cell contacts. Am. J. Physiol. Cell Physiol. 2004;286:C1159–C1169. doi: 10.1152/ajpcell.00400.2003. [DOI] [PubMed] [Google Scholar]
- 30.Zheng X., Sadler J. E. Mucin-like domain of enteropeptidase directs apical targeting in Madin-Darby canine kidney cells. J. Biol. Chem. 2002;277:6858–6863. doi: 10.1074/jbc.m109857200. [DOI] [PubMed] [Google Scholar]
- 31.Reaves B. J., Roquemore E. P., Luzio J. P., Banting G. TGN38 cycles via the basolateral membrane of polarized Caco-2 cells. Mol. Membr. Biol. 1998;15:133–139. doi: 10.3109/09687689809074524. [DOI] [PubMed] [Google Scholar]
- 32.Resh M. D. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 33.Maroun C. R., Naujokas M. A., Park M. Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program. Mol. Biol. Cell. 2003;14:1691–1708. doi: 10.1091/mbc.E02-06-0352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crepaldi T., Pollack A. L., Prat M., Zborek A., Mostov K., Comoglio P. M. Targeting of the SF/HGF receptor to the basolateral domain of polarized epithelial cells. J. Cell Biol. 1994;125:313–320. doi: 10.1083/jcb.125.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]