Abstract
The effects of antimicrobial peptides on artificial membranes have been well-documented; however, reports on the ultrastructural effects on the membranes of micro-organisms are relatively scarce. We compared the effects of histatin 5 and LL-37, two antimicrobial peptides present in human saliva, on the functional and morphological properties of the Candida albicans cell membrane. Fluorescence microscopy and immunogold transmission electron microscopy revealed that LL-37 remained associated with the cell wall and cell membrane, whereas histatin 5 transmigrated over the membrane and accumulated intracellularly. Freeze-fracture electron microscopy revealed that LL-37 severely affected the membrane morphology, resulting in the disintegration of the membrane bilayer into discrete vesicles, and an instantaneous efflux of small molecules such as ATP as well as larger molecules such as proteins with molecular masses up to 40 kDa. The effects of histatin 5 on the membrane morphology were less pronounced, but still resulted in the efflux of nucleotides. As the morphological defects induced by histatin 5 are much smaller than those induced by LL-37, but the efflux of nucleotides is similar at comparable candidacidal concentrations, we suggest that the loss of nucleotides plays an important role in the killing process.
Keywords: antimicrobial peptide, Candida albicans, electron microscopy, freeze fracture, histatin 5, LL-37
Abbreviations: CFU, colony forming units; CZE, capillary zone electrophoresis; Fmoc, fluoren-9-ylmethoxycarbonyl; IMP, intramembranous particle; PI, propidium iodide; PPB, potassium phosphate buffer
INTRODUCTION
In the human body, antimicrobial peptides, such as defensins, histatins and cathelicidins, form the first line of defence to protect tissues against micro-organisms, including bacteria, fungi and enveloped viruses. Most antimicrobial peptides have <50 residues, are polycationic and have the capacity to form amphipathic structures in which clusters of hydrophobic and hydrophilic amino acids are spatially separated. These properties promote their binding and insertion into the negatively charged phospholipid bilayers of bacteria and fungi, thereby impairing the vital membrane functions. Although the exact mechanism by which they kill bacteria is not clearly understood, it has been shown that peptide–lipid interactions leading to membrane permeation play an essential role in their activity [1]. A number of models for membrane permeation by amphipathic α-helical peptides have been described, e.g. transmembrane pore formation through a ‘barrel-stave’ mechanism and membrane disruption through a ‘carpet-like’ mechanism [2,3]. Evidence in favour of these models has been found in liposome studies, but these models may not properly reflect the complex processes involved in the killing of micro-organisms.
Numerous studies have demonstrated transmigration of antimicrobial peptides over the cytoplasmic membrane of their target cells [4–7], whereas (semi-)permanent association of antimicrobial peptides with the cytoplasmic membranes proves much harder to demonstrate than what would be expected from the killing kinetics. Discrepancies observed between the peptide-susceptibilities of artificial and biological membrane systems are further indications that, in many cases, killing of micro-organisms by antimicrobial peptides involves more than simple membrane destabilization by peptide–membrane association, e.g. histatin 5, an antimicrobial peptide from human saliva [8–10] induces release of essential compounds from its target cell, but hardly permeabilizes liposomes. For other amphipathic peptides, e.g. the human cathelicidin LL-37, in liposome models evidence in support of the carpet model [11,12] has been obtained, but studies addressing this issue in biological systems are lacking.
The aim of the present study was to examine the membrane effects of two antimicrobial peptides, histatin 5 and LL-37, using the oral yeast Candida albicans as a model organism. Histatin 5 is a weakly amphipathic peptide, secreted by the human salivary glands, that exhibits candidacidal and bactericidal activity. Its working mechanism is still a matter of debate: although it has little, if any, lytic effect in a liposome model system [8,10], in C. albicans it impairs the integrity of the cytoplasmic membrane both structurally and functionally [6,9]. Recently, Ssa1/2p heat-shock proteins on C. albicans were suggested as receptors to establish the primary interaction between C. albicans and histatin 5 [13]. How this interaction leads to subsequent membrane leakage is still unclear. As histatin 5 targets mitochondria, an involvement of respiration has been supposed, and indeed a number of studies show that an active metabolism is necessary for killing of C. albicans [7,14,15]. The other peptide used in the present study, LL-37, belongs to the cathelicidin peptide family. The cathelicidins are a group of proteins that are characterized by a highly conserved cathelin pro-sequence linked to a variable antimicrobial domain. The only human member of this family, hCAP-18, serves as a precursor for LL-37 and, to a minor extent, ALL-38 [16–18].
Besides, in neutrophils, LL-37 is expressed in numerous epithelial tissues, including skin, salivary glands and lung tissue [17,19,20]. LL-37, which has a strong tendency to adopt an amphipathic α-helical structure, is highly membrane-active and exhibits killing activity against a broad spectrum of bacteria and yeasts [21–24].
In the present study it was found that transmigration of histatin 5 over the cytoplasmic membrane of C. albicans is accompanied by efflux of vital cellular compounds, such as ATP and NAD. LL-37 was not internalized but is associated with the cell wall and the cell membrane. Freeze-fracture electron microscopy revealed that LL-37 more severely affected the structure of the cell membrane compared with histatin 5. LL-37 caused efflux of nucleotides, but also of larger molecules, including proteins with molecular masses of up to 40 kDa.
EXPERIMENTAL
Peptide synthesis, purification and labelling
LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES), histatin 5 (DSHAKRHHGYKRKFHEKHHSHRGY) and LL-37 and histatin 5 labelled with FITC were synthesized by Fmoc (fluoren-9-ylmethoxycarbonyl) chemistry with a MilliGen 9050 peptide synthesizer (MilliGen/Biosearch, Bedford, MA, U.S.A.) as described previously [10]. For the FITC labelling, peptides were extended with the linker Fmoc-L-γ-aminobutyric acid and, after detachment of the Fmoc-group, labelled overnight at room temperature (20 °C) with 30-fold excess FITC in DIPEA (di-isopropylethylamine)/DMF (dimethylformamide) before removal of the side-chain protecting groups and simultaneous detachment from the resin-support. Peptides were purified by preparative reversed-phase-HPLC. The purity of the peptides was at least 95% and the authenticity of the peptides was confirmed by ion- trap MS with an LCQ Deca XP (Thermo Finnigan, San Jose, CA, U.S.A.). The candidacidal activities of the FITC-labelled peptides were equal to those of the corresponding unlabelled peptides.
C. albicans culture
C. albicans (A.T.C.C. 10231) was cultured aerobically at 30 °C on Sabouraud dextrose agar plates. For the experiments, C. albicans was cultured overnight in Sabouraud dextrose broth (Difco, Becton Dickinson Microbiology Systems, Sparks, MD, U.S.A.) and subcultured for 2 h to yield a mid-logarithmic growth culture. Cells were washed twice in 1 mM PPB (potassium phosphate buffer; pH 7.0) and resuspended to the desired cell density.
Viability assay
The killing activities of histatin 5 and LL-37 to C. albicans were determined in a viability assay as described previously [25]. Briefly, C. albicans suspensions of 2×106 cells/ml were incubated with 2-fold serially diluted peptides, in the presence of 1.5 μM PI (propidium iodide; Molecular Probes, Eugene, OR, U.S.A.). During a 1 h incubation period, PI fluorescence was measured every 5 min at the excitation wavelength λexc 544 nm and emission wavelength λem 620 nm in a microplate fluorescence reader (Fluostar Galaxy, BMG Labtechnologies, Offenburg, Germany). After the incubation, 50 μl from selected wells was diluted 200× in PBS. The number of surviving cells was determined by plating 25 μl of this suspension on Sabouraud dextrose agar and counting the CFU (colony forming units) after 48 h of incubation at 30 °C.
Fluorescence microscopy
Fluorescence microscopy was used to study the localization of peptides in C. albicans and performed essentially as described previously [14]. C. albicans suspensions of 4×106 cells/ml in PPB were incubated with 2 or 20 μM FITC-labelled peptides for 10–60 min, and cells were examined by fluorescence microscopy (Leica DMRA, Heidelberg, Germany) and photographed with a Leica DMDL digital camera. Cell-wall labelling was performed by staining with 2 μM Calcofluor White M2R (Molecular Probes) for 30 min.
Immunoelectron microscopy
C. albicans cells were resuspended in PPB at 4×106 cells/ml and incubated with 20 μM FITC-labelled histatin 5 or 2 μM FITC-labelled LL-37 for 30 min. Cells were pelleted and fixed in 2% (w/v) paraformaldehyde and 0.5% glutaraldehyde in 0.1 mM PPB. After fixing, cells were incubated with 1% sodium metaperiodate for 15 min, and with 1% ammonium chloride for 30 min and washed with 0.1 mM PPB in between, collected in 5% (w/v) gelatin and stored in 2% paraformaldehyde in 0.1 M PPB. Cryosections were prepared and incubated with ultrasmall gold-labelled mouse anti-FITC antibodies (Aurion, Wageningen, The Netherlands). The signal was enhanced with R-gent SE-EM (Aurion) and sections were viewed in a Philips EM420 transmission electron microscope (Philips, Eindhoven, The Netherlands) equipped with an SIS MegaviewII camera (SIS, Münster, Germany).
Freeze-fracture electron microscopy
C. albicans cells were resuspended in PPB at 4×106 cells/ml. After incubation with 20 μM histatin 5, or 2 or 20 μM LL-37 for 30 min, cells were collected by centrifugation and resuspended in 0.1 M sodium phosphate buffer (pH 7.4) containing 2% paraformaldehyde and 0.5% glutaraldehyde. Cells were cryoprotected with 2.3 M sucrose in 0.1 M sodium phosphate buffer and frozen in liquid ethane at 90 K. Freeze fracturing was performed in a BAF300 (BAL-TEC, Balzers, Liechtenstein) at a vacuum of <10−5 Pa and at a temperature of 150 K. Cells were replicated with 2 nm platinum at 45° and 20 nm carbon was deposited at 90° to strengthen the replica [26]. Samples were cleansed with commercial household bleach, rinsed with distilled water and mounted on bare 300 mesh copper grids. The replicas were studied in a Philips EM-420 transmission electron microscope operated at 100 kV and equipped with an SIS MegaviewII camera.
Determination of nucleotides in C. albicans supernatants
A C. albicans suspension of 3×107 cells/ml was incubated with histatin 5 or LL-37 at 30 °C. At several time points 150 μl aliquots were taken, and centrifuged at 10000 g for 5 min. The supernatants were analysed for the presence of nucleotides (AMP, ADP, ATP, NAD and NADH) essentially as described previously [27]. In short, samples were mixed 9:1 with borate buffer (30 mM disodium tetraborate deca-hydrate, pH 9, supplemented with 1 mM EDTA and 10 mM NaCl) and analysed by CZE (capillary zone electrophoresis) with a BioFocus 2000 Capillary electrophoresis system (Bio-Rad Laboratories, Hercules, CA, U.S.A.) equipped with an uncoated fused-silica capillary of internal diameter 50 μm and a length of 50 cm. As an internal control, 20 μM 8-bromoguanosine was included. A standard test mix was prepared containing 10 μM AMP, ADP, ATP, NAD and NADH. Samples were loaded by pressure injection and separation was performed at 20 kV (cathode at the detector side) and 25 °C using the borate buffer as the electrolyte. CZE was monitored continuously at 260 nm. Peaks were quantified against the nucleotide standards using BioFocus Integrator software.
SDS/PAGE of peptide treated C. albicans supernatants
C. albicans suspensions of 3×107 cells/ml in 7 ml ammonium bicarbonate (1 mM, pH 7) were incubated with 10, 25 and 100 μM histatin 5 or LL-37 for 5 min and 60 min. Cells were centrifuged and supernatants were freeze-dried and dissolved in 400 μl water. Furthermore, samples of 75 pmol LL-37 and histatin 5 were included. Samples were analysed by SDS/PAGE. The samples were mixed with 8× concentrated sample buffer (50 mM Tris, pH 6.8, containing 2% (w/v) SDS and 10 mM dithiothreitol) and heated at 100 °C for 5 min. SDS/PAGE was performed on 16% (v/v) Tricine gels (Novex, San Diego, CA, U.S.A.) and gels were stained with Coomassie Brilliant Blue (from Phastgel® Blue R tablet, Amersham Biosciences, Uppsala, Sweden).
RESULTS
Candidacidal activities of histatin 5 and LL-37
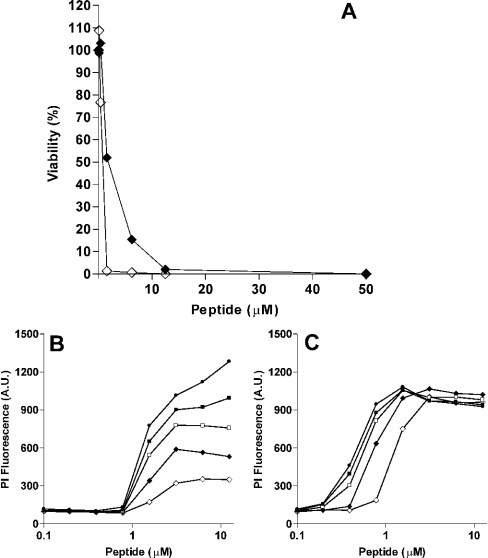
To determine the candidacidal activities of histatin 5 and LL-37, C. albicans suspensions were incubated with serial dilutions of histatin 5 or LL-37. After 1 h incubation, cells were plated on agar, and after 48 h, colonies were counted. Killing activity was expressed in terms of LC50, the concentration causing 50% reduction of viable cells. To investigate the kinetics of the killing, enhancement of PI fluorescence was measured for 60 min. The LC50 values for histatin 5 and LL-37 were 1.6 and 0.8 μM respectively (Figure 1A). After histatin 5 incubation, PI fluorescence increased over a period of time and the maximal fluorescence signal was reached after 1 h (Figure 1B), whereas LL-37 caused an immediate increase in PI fluorescence (within 5 min, Figure 1B).
Figure 1. Candidacidal activity of histatin 5 and LL-37.
(A) C. albicans cells (2×106 cells/ml PPB) were incubated for 1 h with 0–50 μM histatin 5 (◆) and LL-37 (◇). The CFU were determined as described in the Experimental section. Results are presented as percentage of the CFU relative to the control (untreated cells). (B, C) C. albicans, in the presence of 6 μM PI were incubated with histatin 5 (B) or LL-37 (C). After the addition of peptides, the PI fluorescence was monitored for 60 min (◇: 0 min, ◆: 5 min, □: 15 min, ■: 30 min, ●: 60 min incubation time). Each data point is the mean of a duplicate and curves are representative of three independent experiments.
Localization of histatin 5 and LL-37 in C. albicans
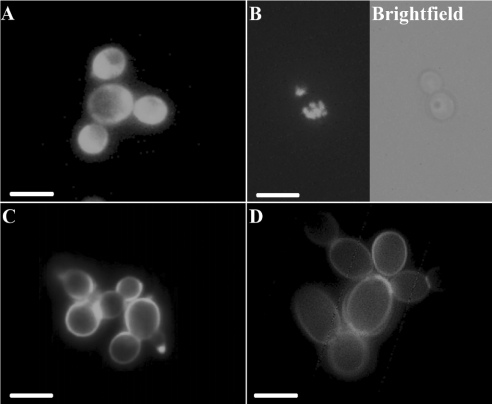
Cellular localization of histatin 5 and LL-37 in C. albicans was investigated using fluorescence microscopy. After incubation with 2 or 20 μM FITC-labelled histatin 5, in some cells uniformly dispersed staining patterns were observed in the region corresponding to the cytoplasmic space (Figure 2A), but the majority of the cells exhibited an intracellular granular fluorescence staining pattern (Figure 2B). An FITC-labelled control peptide was not internalized under these conditions (results not shown). In contrast with that of histatin 5, localization of LL-37 was confined mainly to the cell perimeter, suggesting an association with the cell wall and/or cell membrane (Figure 2C). This pattern was very similar to that of cells treated with Calcofluor White M2R, which is a specific cell-wall marker (Figure 2D). Sporadically, cells internally labelled with LL-37 were also observed. At high concentrations of LL-37 (20 μM), or after prolonged incubation times (up to 1 h), the fluorescence pattern did not change, i.e. the fluorescence remained associated with the cell perimeter.
Figure 2. Fluorescence microscopy of C. albicans treated with FITC-labelled histatin 5 and LL-37.
C. albicans cells (4×106 cells/ml PPB) were incubated with 20 μM FITC-labelled histatin 5 (A, B) or (C) LL-37 for 30 min and examined using fluorescence microscopy. (A) Diffuse cytoplasmic staining pattern as observed after incubation of C. albicans with histatin 5. (B) Granular staining pattern as seen after incubation of C. albicans with histatin 5. The right panel shows a bright field image of the same cells shown in the fluorescence micrograph. (C) Cells incubated with FITC-labelled LL-37. The perimeter of the cells was stained. (D) Cells labelled with the cell-wall specific probe Calcofluor White. Data are representative of three independent experiments. Scale bars, 5 μm.
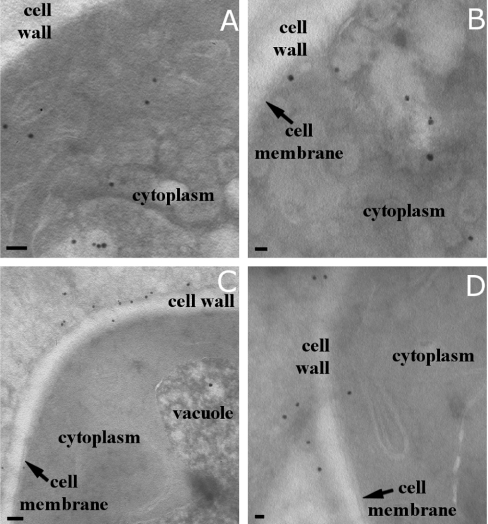
To study the localization of the peptides in more detail, immunoelectron microscopy was performed. FITC-labelled peptides were incubated with C. albicans and after fixation and cryosectioning, sections were incubated with gold-labelled anti-FITC antibodies. Gold particles were amplified with silver to increase their size to visualize better their localization. With 20 μM histatin 5, predominantly intracellular structures were labelled, but a few particles were also detected at the cell wall (Figures 3A and 3B). In cells treated with 2 μM LL-37, labelling was predominantly seen at the cell wall and cell membrane (Figures 3C and 3D).
Figure 3. Cellular localization of histatin 5 and LL-37 in C. albicans as seen with immunogold labelling.
C. albicans cells (4×106 cells/ml PPB) were incubated with 20 μM histatin 5 or 2 μM LL-37 for 30 min. Cells were fixed, sectioned and gold-labelled. Typical examples of gold labelling in histatin 5- and LL-37-treated cells are given. (A, B) In histatin 5-treated C. albicans, gold labelling is mainly found within the cells, localized both in the cytoplasm and associated with internal organelles. (C, D) In LL-37-treated cells, most gold labelling is found in association with the cell wall and cell membrane. Scale bars, 100 nm.
Effects of histatin 5 and LL-37 on the membrane morphology
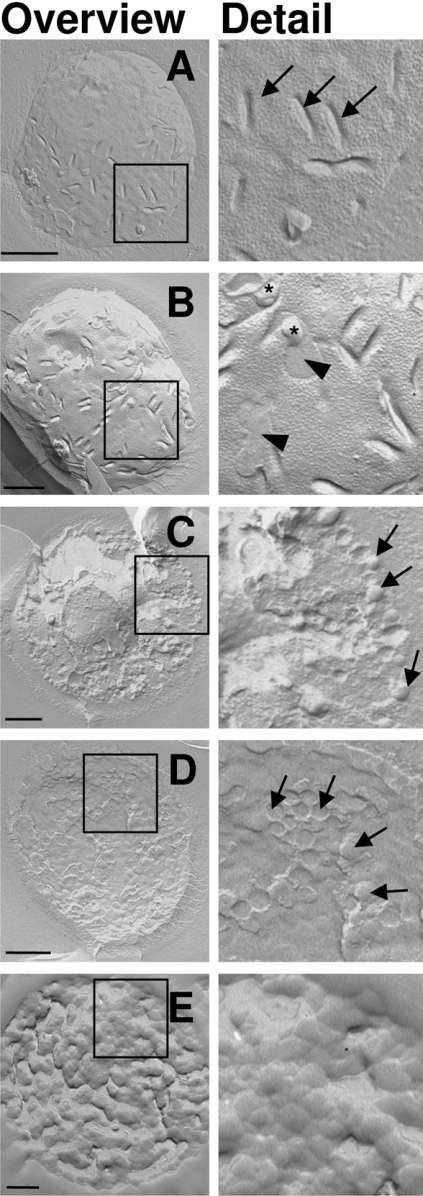
Effects of histatin 5 and LL-37 on the morphology of C. albicans membranes were studied using freeze-fracture electron microscopy. With this technique, cell fracturing occurs along the plane of least resistance, which normally is the hydrophobic interface between the inner and outer leaflet of membranes. Micrographs show either the inner leaflet (protoplasmic face) or the outer leaflet (external face) of the fractured membranes. When fractured at 150 K, approx. 95% of the untreated yeast cells were fractured along the plasma membrane, showing a uniform distribution of IMPs (intramembranous particles, Figure 4A), which are transmembrane proteins. Furthermore, trough-shaped invaginations, which are typical for yeast plasma membranes, were visible [28,29]. After treatment with 20 μM histatin 5 (Figure 4B), irregularities and blebs were visible in the cell membranes. This was accompanied by a change in membrane distribution of IMPs: whereas IMPs were evenly distributed over the membranes of the untreated control cells, after treatment with histatin 5, IMP-dense and IMP-free areas could be distinguished.
Figure 4. Ultrastructural effects of histatin 5 and LL-37 on C. albicans cell membranes.
C. albicans cells (4×106 cells/ml PPB) were incubated with 20 μM histatin 5, 2 μM LL-37 or 20 μM LL-37 for 30 min. Cells were fixed and freeze fractured. Replicas were prepared and viewed using transmission electron microscopy. (A) Control cells, showing trough-shaped invaginations (arrows) and a uniform distribution of IMPs. (B) Cells incubated with 20 μM histatin 5, in which IMP-dense and IMP-free (arrowheads) areas can be distinguished. In addition, blebs are present on the membrane (asterisks). (C, D) Among the cells incubated with 2 μM LL-37, 30% are fractured through the cytoplasm (C), whereas 60% are fractured over the membrane (D). The membrane area is filled with distinct circular membrane structures (arrows), with diameters of approx. 100 nm. Few cells show a normal membrane (5%, results not shown) and in another 5%, no recognizable membrane structures are seen (results not shown). (E) C. albicans incubated with 20 μM LL-37: all cells are fractured randomly through the cytoplasm and no structures can be distinguished, suggesting a complete disruption of the cell membrane. Data are representative of two independent experiments. Scale bars, 500 nm.
At 2 μM LL-37, which causes between 50 and 100% killing, a more drastic effect on the morphology of the cytoplasmic membranes was seen after treatment with 20 μM histatin 5 (Figures 4C and 4D). After treatment with histatin 5, virtually all cells were fractured along the cytoplasmic membrane, whereas with LL-37 only approx. 60% of the cells were fractured along the cytoplasmic membrane. In these cells, the trough-shaped invaginations and IMPs were no longer visible. Instead, the protoplasmic and the external face contained aggregates of globular structures, suggesting that at these sites the lipid bilayer had been disrupted and subsequently resealed into smaller vesicles of approx. 100 nm (Figure 4D). Approximately 30% of the cells were fractured through the cytoplasm and along the intracellular membranes, indicating a weakening or absence of the hydrophobic interface of their cytoplasmic membranes (Figure 4C). Treatment of C. albicans with 20 μM LL-37 caused a complete disappearance of the integrity of cellular membranes, as no recognizable ultrastructure could be distinguished (Figure 4E).
Effects of histatin 5 and LL-37 on cell-membrane permeability of C. albicans
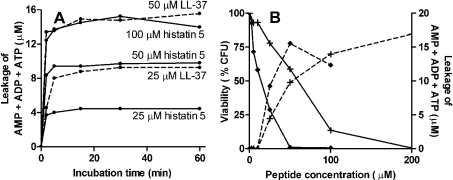
To study the extent to which the morphological changes to the cell membrane were accompanied by alterations to the membrane permeability, we analysed leakage of nucleotides, as ATP and NAD, from peptide-treated C. albicans. Suspensions of 3×107 C. albicans cells/ml were incubated with histatin 5 or LL-37, 2-fold serially diluted in phosphate buffer. At different points in time, aliquots were taken and they were analysed by CZE for the presence of nucleotides. After 60 min, additional aliquots were taken, in which the number of surviving cells was determined by plating the suspension.
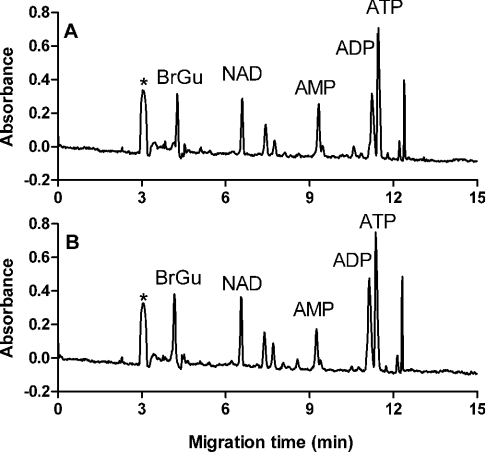
Figure 5 shows the electropherograms of supernatants of C. albicans treated for 1 h with 100 μM histatin 5 (Figure 5A) or LL-37 (Figure 5B). For peak identification, the supernatants were spiked with standard nucleotides and the corresponding peaks were identified by co-migration. Furthermore, the absorbance spectrum of sample and standard components was recorded by repeating the separation at wavelengths between 200 and 280 nm. On basis of the identical electrophoretic and spectral behaviour, several peaks in the electropherograms were identified, namely NAD, AMP, ADP and ATP. NADH was not detected in any supernatant. Several other components of unknown identity, but having an absorbance at 260 nm, were also released from the cells. Incubation with concentrations up to 10 μM histatin 5 or LL-37 caused no leakage of nucleotides (Figure 6). On treatment with histatin 5 and LL-37 concentrations at or above 25 μM, nucleotides were released directly (within 2 min, Figure 6A). The profile of compounds that were released did not change on prolonged incubations. The release profiles (i.e. the relative amount of each nucleotide species) of histatin 5-treated cells were comparable with those of LL-37-treated cells. In supernatants of untreated C. albicans, no nucleotides were detected. Concentrations of 200 μM histatin 5 and 100 μM LL-37 caused equal nucleotide leakage at 2-fold lower concentrations, but nucleotide content decreased somewhat after 1 h and 30 min respectively, probably due to a gradual degradation (hence the lower nucleotide leakage at 100 μM LL-37 compared with 50 μM LL-37 in Figure 6B).
Figure 5. CZE profiles of supernatants of peptide-treated C. albicans.
C. albicans (3×107 cells/ml PPB) incubated for 1 h with 100 μM (A) histatin 5 or (B) LL-37, were centrifuged, and supernatants were analysed by CZE. (A) CZE profile of supernatants of histatin 5-treated C. albicans. (B) CZE profile of supernatants of LL-37-treated C. albicans. Peaks corresponding to NAD, AMP, ADP and ATP are indicated. BrGu, internal standard of 8-bromoguanosine; *, buffer peak.
Figure 6. Nucleotide leakage from C. albicans after incubation with histatin 5 or LL-37.
C. albicans (3×107 cells/ml PPB) incubated with histatin 5 or LL-37 were centrifuged, and concentrations of nucleotides in the supernatants were measured by CZE. (A) Time-dependent leakage of AMP, ADP and ATP from C. albicans after incubation with histatin 5 (——) or LL-37 (- - -). (B) Peptide-induced nucleotide leakage (- - - -) of AMP, ADP and ATP from C. albicans incubated for 60 min with histatin 5 (+) or LL-37 (◆) is shown in parallel with the viability of C. albicans (——) at the peptide concentrations used. Curves are representative of three independent experiments.
After 1 h incubation, viability of the cells was determined (Figure 6B). LC50 values were 60 μM for histatin 5 and 14 μM for LL-37. These values are higher than those shown in Figure 1, as the cell density in this experiment was 15-fold higher, and consequently, at equal peptide concentrations, the cell/peptide ratio is higher.
SDS/PAGE analysis of C. albicans cell supernatants
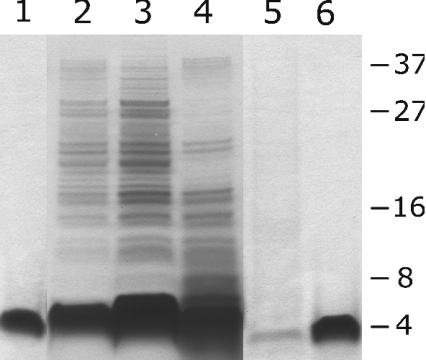
From the experiments shown in Figure 6, it is clear that both histatin 5 and LL-37 caused leakage from the cell of relatively small molecules. In view of the massive membrane disruption caused by LL-37 (Figure 4), we examined to what extent peptide treatment caused leakage of macromolecular compounds. Therefore suspensions of C. albicans (3×107 cells/ml) were incubated with 10, 25 or 100 μM histatin 5 or LL-37 for 5 and 60 min, and the supernatant was analysed by SDS/PAGE. The protein profiles of samples incubated for 60 min were essentially equal to those seen after 5 min incubation, therefore only protein profiles of samples after 60 min incubation are shown (Figure 7). In the SDS/PAGE profiles of supernatants of LL-37-treated cells, series of bands in the low-molecular-mass range were seen with apparent molecular masses up to approx. 40 kDa (Figure 7, lanes 2– 4). Except for differences in intensities, no other differences in protein bands were seen at 10, 25 and 100 μM LL-37. Supernatants of histatin 5-treated cells contained hardly any protein (Figure 7, lane 5). The profiles of both peptide-treated C. albicans samples have a band corresponding to the mass of the respective peptides (Figure 7, lane 1 and 6). The untreated supernatant contained no detectable protein (results not shown).
Figure 7. SDS/PAGE analysis of supernatants of peptide-treated C. albicans.
C. albicans (3×107 cells/ml ammonium bicarbonate buffer) cells were incubated with 10, 25 or 100 μM histatin 5 or LL-37. Cells were centrifuged after 1 h incubation and supernatants were analysed by SDS/PAGE. Lane 1, 75 pmol LL-37; lane 2, supernatant of C. albicans treated with 10 μM LL-37; lane 3, supernatant of C. albicans treated with 25 μM LL-37; lane 4, supernatant of C. albicans treated with 100 μM LL-37; lane 5, supernatant of C. albicans treated with 100 μM histatin 5; lane 6, 75 pmol histatin 5. Data are representative of three separate experiments.
DISCUSSION
The present study concentrates on the membrane-disrupting effects of the antimicrobial peptides LL-37 and histatin 5 in a biological membrane system. Histatin 5 and LL-37 kill C. albicans in the same concentration range, but target to different cell components. With fluorescence microscopy and immunoelectron microscopy, we demonstrated that LL-37 localized at the cell wall and cell membrane, whereas histatin 5 was internalized in C. albicans. We demonstrated that both peptides affected the functional properties of the cytoplasmic membrane of C. albicans, leading to instantaneous release of vital components, and subsequent cell death.
The most striking finding was that large membrane areas were fragmented into a series of adjacent vesicle-like structures with a diameter of approx. 100 nm, with exposed inner and outer leaflets. The defects seemed to have a discrete size, since only proteins with a molecular mass of <40 kDa leaked out of the cells. However, this does not support the classic pore-forming models, in which much smaller defects are predicted [30,31]. Although the defects seem to be of discrete size, results correspond more with detergent-like properties.
In a previous study, we have demonstrated that treatment with LL-37 resulted in complete permeabilization of liposomes, highlighted by the leakage of entrapped macromolecules as large as 60 kDa (trypsin) [10]. Detergent-like properties were also found by Oren et al. [11], and similar membrane disruption was found by Henzler Wildman et al. [12]. The finding that there is no leakage of proteins larger than 40 kDa remains unresolved, but the permeability of the cell wall may play a role in this.
LL-37 accumulated in large amounts in the cell wall (Figures 2 and 3). Binding to cell-wall components has been demonstrated for a number of other antimicrobial peptides [13,32–37], and a number of cell-wall components have been proposed as binding sites, such as chitin [32–35], mannan [32], β-glucan [32,35], cell wall protein 2 [36,37], and Ssa1/2p heat-shock proteins [13]. Nisin and MB-21 have increased fungicidal activity to strains or mutants lacking cell wall protein 2 [36,37]. Li et al. [13] have shown that Ssa1/2p present in the cell wall of C. albicans binds to histatin 5, and proposed that this binding is a crucial step in the killing process. In the present study, histatin 5 was only found inside the cells, indicating that the binding of histatin 5 to its putative cell-wall receptor is a transient event, followed by rapid internalization. The functional significance of the binding of LL-37 to the cell wall is not clear. It can be envisaged that accumulation of LL-37 in the cell wall results in a high local concentration in the vicinity of its cellular target, thus stimulating killing. Conversely, accumulation in the cell wall may represent irreversible entrapment of LL-37, obstructing a free passage to the membrane.
A number of studies have shown a role for LL-37 in inflammatory processes, apart from direct killing of micro-organisms [38,39]. As inflammation of host tissues can be influenced by ATP, as a ligand of purinergic receptors [40,41], the activities of LL-37 may also influence inflammatory processes in an indirect way, by means of the ATP released from C. albicans.
Acknowledgments
We thank K. Nazmi (Department of Oral Biochemistry, ACTA) for peptide synthesis, M. Valentijn-Benz (Department of Oral Biochemistry, ACTA) for technical assistance with nucleotide measurements and B. Bruyneel (Department of Molecular Cell Biology, VUmc, Amsterdam, The Netherlands). This work was financially supported by The Netherlands Institute for Dental Sciences (IOT).
References
- 1.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 2.Van't Hof W., Veerman E. C. I., Helmerhorst E. J., Nieuw Amerongen A. V. Antimicrobial peptides: properties and applicability. Biol. Chem. 2001;382:597–619. doi: 10.1515/BC.2001.072. [DOI] [PubMed] [Google Scholar]
- 3.Boman H. G. Antibacterial peptides: basic facts and emerging concepts. J. Intern. Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 4.Haukland H. H., Ulvatne H., Sandvik K., Vorland L. H. The antimicrobial peptides lactoferricin B and magainin 2 cross over the bacterial cytoplasmic membrane and reside in the cytoplasm. FEBS Lett. 2001;508:389–393. doi: 10.1016/s0014-5793(01)03100-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee D. G., Kim H. K., Kim S. A., Park Y., Park S. C., Jang S. H., Hahm K. S. Fungicidal effect of indolicidin and its interaction with phospholipid membranes. Biochem. Biophys. Res. Commun. 2003;305:305–310. doi: 10.1016/s0006-291x(03)00755-1. [DOI] [PubMed] [Google Scholar]
- 6.Ruissen A. L. A., Groenink J., Van't Hof W., Walgreen-Weterings E., van Marle J., van Veen H. A., Voorhout W. F., Veerman E. C. I., Nieuw Amerongen A. V. Histatin 5 and derivatives. Their localization and effects on the ultra-structural level. Peptides. 2002;23:1391–1399. doi: 10.1016/s0196-9781(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 7.Helmerhorst E. J., Breeuwer P., Van't Hof W., Walgreen-Weterings E., Oomen L. C., Veerman E. C. I., Nieuw Amerongen A. V., Abee T. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J. Biol. Chem. 1999;274:7286–7291. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 8.Edgerton M., Koshlukova S. E., Lo T. E., Chrzan B. G., Straubinger R. M., Raj P. A. Candidacidal activity of salivary histatins. Identification of a histatin 5-binding protein on Candida albicans. J. Biol. Chem. 1998;273:20438–20447. doi: 10.1074/jbc.273.32.20438. [DOI] [PubMed] [Google Scholar]
- 9.Koshlukova S. E., Lloyd T. L., Araujo M. W., Edgerton M. Salivary histatin 5 induces non-lytic release of ATP from Candida albicans leading to cell death. J. Biol. Chem. 1999;274:18872–18879. doi: 10.1074/jbc.274.27.18872. [DOI] [PubMed] [Google Scholar]
- 10.Den Hertog A. L., Wong Fong Sang H. W., Kraayenhof R., Bolscher J. G. M., Van't Hof W., Veerman E. C. I., Nieuw Amerongen A. V. Interactions of histatin 5 and histatin 5-derived peptides with liposome membranes: surface effects, translocation and permeabilization. Biochem. J. 2004;379:665–672. doi: 10.1042/BJ20031785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oren Z., Lerman J. C., Gudmundsson G. H., Agerberth B., Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem. J. 1999;341:501–513. [PMC free article] [PubMed] [Google Scholar]
- 12.Henzler Wildman K. A., Lee D. K., Ramamoorthy A. Mechanism of lipid bilayer disruption by the human antimicrobial peptide, LL-37. Biochemistry. 2003;42:6545–6558. doi: 10.1021/bi0273563. [DOI] [PubMed] [Google Scholar]
- 13.Li X. S., Reddy M. S., Baev D., Edgerton M. Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J. Biol. Chem. 2003;278:28553–28561. doi: 10.1074/jbc.M300680200. [DOI] [PubMed] [Google Scholar]
- 14.Ruissen A. L. A., Groenink J., Helmerhorst E. J., Walgreen-Weterings E., Van't Hof W., Veerman E. C. I., Nieuw Amerongen A. V. Effects of histatin 5 and derived peptides on Candida albicans. Biochem. J. 2001;356:361–368. doi: 10.1042/0264-6021:3560361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gyurko C., Lendenmann U., Helmerhorst E. J., Troxler R. F., Oppenheim F. G. Killing of Candida albicans by histatin 5: cellular uptake and energy requirement. Antonie Van Leeuwenhoek. 2001;79:297–309. doi: 10.1023/a:1012070600340. [DOI] [PubMed] [Google Scholar]
- 16.Bals R., Wilson J. M. Cathelicidins – a family of multifunctional antimicrobial peptides. Cell. Mol. Life Sci. 2003;60:711–720. doi: 10.1007/s00018-003-2186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehrer R. I., Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen O. E., Gram L., Johnsen A. H., Andersson E., Bangsboll S., Tjabringa G. S., Hiemstra P. S., Malm J., Egesten A., Borregaard N. Processing of seminal plasma hCAP-18 to ALL-38 by gastricsin: a novel mechanism of generating antimicrobial peptides in vagina. J. Biol. Chem. 2003;278:28540–28546. doi: 10.1074/jbc.M301608200. [DOI] [PubMed] [Google Scholar]
- 19.Gudmundsson G. H., Agerberth B., Odeberg J., Bergman T., Olsson B., Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur. J. Biochem. 1996;238:325–332. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 20.Murakami M., Ohtake T., Dorschner R. A., Gallo R. L. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 2002;81:845–850. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 21.Johansson J., Gudmundsson G. H., Rottenberg M. E., Berndt K. D., Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J. Biol. Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 22.Bals R., Wang X., Zasloff M., Wilson J. M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. U.S.A. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner J., Cho Y., Dinh N. N., Waring A. J., Lehrer R. I. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka D., Miyasaki K. T., Lehrer R. I. Sensitivity of Actinobacillus actinomycetemcomitans and Capnocytophaga spp. to the bactericidal action of LL-37: a cathelicidin found in human leukocytes and epithelium. Oral Microbiol. Immunol. 2000;15:226–231. doi: 10.1034/j.1399-302x.2000.150403.x. [DOI] [PubMed] [Google Scholar]
- 25.Van der Kraan M. I. A., Groenink J., Nazmi K., Veerman E. C. I., Bolscher J. G. M., Nieuw Amerongen A. V. Lactoferrampin: a novel antimicrobial peptide in the N1-domain of bovine lactoferrin. Peptides. 2004;25:177–183. doi: 10.1016/j.peptides.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Robards A. W., Sleytr U. B. Low temperature methods in biological electron microscopy. In: Glauert A. M., editor. Practical Methods in Electron Microscopy. Amsterdam: Elsevier; 1985. pp. 309–440. [Google Scholar]
- 27.Friess U., Lehmann R., Weigert C., Haering H. U., Voelter W., Schleicher E. Single-run determination of adenylate nucleotides, and of cellular energy status, by a simple and improved capillary electrophoretic method. Chromatographia. 2002;56:375–378. [Google Scholar]
- 28.Miragall F., Rico H., Sentandreu R. Changes in the plasma membrane of regenerating protoplasts of Candida albicans as revealed by freeze-fracture electron microscopy. J. Gen. Microbiol. 1986;132:2845–2853. doi: 10.1099/00221287-132-10-2845. [DOI] [PubMed] [Google Scholar]
- 29.Barug D., de Groot K. Effect of the imidazole derivative lombazole on the ultrastructure of Staphylococcus epidermidis and Candida albicans. Antimicrob. Agents Chemother. 1985;28:643–647. doi: 10.1128/aac.28.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuzaki K. Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta. 1999;1462:1–10. doi: 10.1016/s0005-2736(99)00197-2. [DOI] [PubMed] [Google Scholar]
- 31.He K., Ludtke S. J., Worcester D. L., Huang H. W. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys. J. 1996;70:2659–2666. doi: 10.1016/S0006-3495(96)79835-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Lucca A. J., Bland J. M., Vigo C. B., Jacks T. J., Peter J., Walsh T. J. D-cecropin B: proteolytic resistance, lethality for pathogenic fungi and binding properties. Med. Mycol. 2000;38:301–308. doi: 10.1080/mmy.38.4.301.308. [DOI] [PubMed] [Google Scholar]
- 33.Osaki T., Omotezako M., Nagayama R., Hirata M., Iwanaga S., Kasahara J., Hattori J., Ito I., Sugiyama H., Kawabata S. Horseshoe crab hemocyte-derived antimicrobial polypeptides, tachystatins, with sequence similarity to spider neurotoxins. J. Biol. Chem. 1999;274:26172–26178. doi: 10.1074/jbc.274.37.26172. [DOI] [PubMed] [Google Scholar]
- 34.Tomie T., Ishibashi J., Furukawa S., Kobayashi S., Sawahata R., Asaoka A., Tagawa M., Yamakawa M. Scarabaecin, a novel cysteine-containing antifungal peptide from the rhinoceros beetle, Oryctes rhinoceros. Biochem. Biophys. Res. Commun. 2003;307:261–266. doi: 10.1016/s0006-291x(03)01162-8. [DOI] [PubMed] [Google Scholar]
- 35.Oita S., Ohnishi-Kameyama M., Nagata T. Binding of barley and wheat alpha-thionins to polysaccharides. Biosci. Biotechnol. Biochem. 2000;64:958–964. doi: 10.1271/bbb.64.958. [DOI] [PubMed] [Google Scholar]
- 36.Dielbandhoesing S. K., Zhang H., Caro L. H., van der Vaart J. M., Klis F. M., Verrips C. T., Brul S. Specific cell wall proteins confer resistance to nisin upon yeast cells. Appl. Environ. Microbiol. 1998;64:4047–4052. doi: 10.1128/aem.64.10.4047-4052.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bom I. J., Klis F. M., de Nobel H., Brul S. A new strategy for inhibition of the spoilage yeasts Saccharomyces cerevisiae and Zygosaccharomyces bailii based on combination of a membrane-active peptide with an oligosaccharide that leads to an impaired glycosylphosphatidylinositol (GPI)-dependent yeast wall protein layer. FEMS Yeast Res. 2001;1:187–194. doi: 10.1111/j.1567-1364.2001.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 38.Tjabringa G. S., Aarbiou J., Ninaber D. K., Drijfhout J. W., Sorensen O. E., Borregaard N., Rabe K. F., Hiemstra P. S. The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J. Immunol. 2003;171:6690–6696. doi: 10.4049/jimmunol.171.12.6690. [DOI] [PubMed] [Google Scholar]
- 39.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75:39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 40.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol. Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 41.North R. A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]