Abstract
Indoleamine-2, 3-dioxygenase (IDO1) and Tryptophan-2, 3-dioxygense (TDO) are heme-containing dioxygenases that catalyze the conversion of tryptophan to N-formyl-kynurenine and thus enable generation of L-kynurenine and related metabolites that govern the immune response and broadly impact human biology. Given that TDO and IDO1 activities are directly proportional to their heme contents, it is important to understand their heme delivery and insertion processes. Early studies established that TDO and IDO1 heme levels are sub-saturating in vivo and subject to change but did not identify the cellular mechanisms that provide their heme or enable dynamic changes in their heme contents. We investigated the potential involvement of GAPDH and chaperone Hsp90, based on our previous studies linking these proteins to intracellular heme allocation. We studied heme delivery and insertion into IDO1 and TDO expressed in both normal and heme-deficient HEK293T cells and into IDO1 naturally expressed in HeLa cells in response to IFN-γ, and also investigated the interactions of TDO and IDO1 with GAPDH and Hsp90 in cells and among their purified forms. We found that GAPDH delivered both mitochondrially-generated and exogenous heme to apo-IDO1 and apo-TDO in cells, potentially through a direct interaction with either enzyme. In contrast, we found Hsp90 interacted with apo-IDO1 but not with apo-TDO, and was only needed to drive heme insertion into apo-IDO1. By uncovering the cellular processes that allocate heme to IDO1 and TDO, our study provides new insight on how their activities and L-kynurenine production may be controlled in health and disease.
Keywords: IDO1, TDO, Heme protein, chaperone, immunosuppression, cancer, iron, Hsp90, GAPDH
1. Introduction
Indoleamine-2, 3-dioxygenase (EC 1.13.11.52, IDO1) and Tryptophan-2, 3-dioxygense (EC 1.13.11.11, TDO) are heme proteins that catalyze the conversion of tryptophan (Trp) to N-formyl-kynurenine [1, 2]. Their activities depend on the content of their ferrous heme, which binds dioxygen and enables its insertion into the indole ring of Trp [3, 4]. TDO is selective for L-Trp while IDO1 can accept a broader range of indole-containing substrates [5, 6]. Although IDO1 has a monomeric structure and TDO is tetrameric [7], their crystal structures indicate there are high similarities in their heme-binding environments and substrate-binding sites [8], consistent with their having similar catalytic mechanisms.
TDO expression and activity levels are highest in the liver and are upregulated by glucocorticoids and L-Trp [9, 10]. In comparison, IDO1 is constitutively expressed in some tissues such as the lung and its expression can be broadly induced by stimuli associated with immunity and inflammation including interferon-γ (IFNγ), lipopolysaccharide (LPS), and tumor necrosis factor (TNF) [11–13]. Increased TDO or IDO1 activities cause Trp depletion and increased production of metabolites of the Kynurenine (Kyn) pathway (KP), which are bioactive [14–17]. Increased IDO1 activity is thought to help downregulate several inflammatory diseases [18,19], but on the other hand, it is also associated with neurologic disorders [20,21], and an increase in IDO1 or TDO activity in cancer cells or in host dendritic cells is associated with suppression of effector T-cell responses toward tumors [22,23]. Because of this, pharmaceutical research aimed at controlling IDO1 and TDO activities has gained pace over the years.
Because IDO1 and TDO absolutely depend on their bound heme for catalysis it is fundamentally important to learn what cellular processes provide their heme and thus control their heme levels and activities. Remarkably, rodent liver TDO normally exists in a partly heme-deficient state in healthy animals [24–26], and its heme saturation level can be increased by giving the animals Trp [27, 28], boosting their heme biosynthesis [25, 26], or by injecting immune-stimulants [29, 30]. IDO1 is also predominantly expressed in its heme-free form in cells [31, 32], and like TDO can change its heme level in response to various immunologic or related stimuli [33, 34]. Although these studies established that heme levels in TDO and IDO1 are variable and subject to change, they did not identify what cellular mechanisms provide their heme or affect the dynamic changes in their heme content.
To address this gap, we turned to our recent studies on heme protein maturation that showed GAPDH allocates heme to at least two heme proteins [35,36] and that heme insertion into several heme proteins requires the cell chaperone Hsp90 [37–40]. Accordingly, we investigated the potential for GAPDH and Hsp90 involvement by utilizing IDO1-FLAG and TDO-FLAG constructs expressed in both normal and heme-deficient HEK293T cells, and studied IDO1 after naturally inducing its expression in HeLa cells with IFN-γ. We also investigated the interactions of TDO and IDO1 with GAPDH and Hsp90 in cells and among their purified forms. Our findings suggest a mechanism whereby GAPDH delivers mitochondrially-generated or exogenous heme to apo-IDO1 and to apo-TDO in cells through an interaction with these enzymes, whereas Hsp90 only interacts with apo-IDO1 to drive its heme insertion and does not interact with apo-TDO or enable its heme insertion. By identifying the cellular processes that provide and insert heme into TDO and IDO1, our study helps to explain their physiological behaviors and uncovers the mechanism whereby cellular heme allocation can control their activities in health or disease.
2. Materials and Methods
2.1. Induction of HeLa cells with IFN-γ:
HeLa cells (ATCC # CCL-2) were cultured in tissue culture treated plates containing DMEM (high glucose with L-Glutamine and Na-pyruvate) medium containing 10% FBS (normal or heme depleted) until 70% confluent. Human IFN-γ (PeproTech # 300–02) was used at a concentration of 50 ng/ml for 16 hrs to induce the expression of IDO1 in these cells. The expression of IDO1 post hIFN-γ treatment in HeLa cells was checked by western blot using anti-IDO antibody (Cell Signaling # 86630S).
2.2. 14C labeled heme production in cells and measuring radiolabelled heme counts:
GlyA-CHO cells were a gift from Dr. P. J. Stover, Cornell University, and were confirmed in-house to be glycine-auxotrophic for growth. The protocol followed for administering 14C-d-ALA to cells and IP pull-downs has been described in [42].
2.3. Plasmids, transfection and expression of human proteins in mammalian cells:
Human IDO1-FLAG (Sino Biologicals # HG11650-CF), TDO-FLAG (Sino Biologicals # HG13215-CF), HA-GAPDH-WT and HA-GAPDH-H53A were transfected in HEK293T cells (ATCC # CRL-11268) using Lipofectamine2000 (Invitrogen # 11668019). Briefly, cells were grown in DMEM medium containing either normal serum or heme depleted serum. Succinyl acetone (SA; Sigma # D-1415) which inhibits endogenous heme biosynthesis, was used at 400 μM to treat cells for 72 h in DMEM containing heme depleted serum. Cells were seeded into appropriate plates and next day were transfected with desired plasmids. This involved transfecting cells with the IDO1-FLAG or TDO-FLAG expression plasmids alone or in combination with the HA-GAPDH-WT or H53A expression plasmids. The protein expression was allowed to continue for 36 h in the presence or absence of SA, after which cycloheximide (Chx, Sigma # C7698) was used to treat cells at 5 μg/ml for 12 h to inhibit further protein synthesis. Cells were then utilized for experiments.
2.4. Transfection of siRNA and gene silencing:
Cell GAPDH protein expression was reduced using siRNA against human GAPDH mRNA. Commercially available siRNA against human GAPDH (# D-001830-01-05) and scrambled siRNA (# D-001810-10-05) were purchased from Dharmacon and used at a final concentration of 50 nM in cultures of mammalian cells of low passage number along with Lipofectamine2000. The siRNA treated cells were cultured for 48 h before they received transfections with protein expression plasmids as described above.
2.5. IDO1 and TDO activity assay:
The enzyme activity of IDO1 and TDO was measured using a colorimetric assay. The cells after treatment with Chx were treated with IDO1/TDO substrate L-Trp at 2 mM in phenol red free DMEM medium containing appropriate type of serum (normal or heme depleted) for 5 h to allow substrate utilization and L-Kynurenine product formation. The medium was collected and de-proteinized by adding an equal volume of 3% trichloroacetic acid (TCA) and incubated at 50 °C for 30 mins. The tubes were centrifuged at 9,000 g for 10 min at room temperature to precipitate proteins from the medium. Equal volumes of deproteinized sample were mixed with a 20 mg/ml solution of p-dimethyl-amino-benzaldehyde (Ehrlich’s reagent; Sigma # 109762) in glacial acetic acid at room temperature to allow the formation of a yellow-colored product. The end point absorbance of this product was measured at 492 nm (Molecular Devices) to determine the concentration of L-Kyn in the medium. A standard curve was obtained using commercial L-Kyn (Sigma # K8625) dissolved in 0.5 N hydrochloric acid in various concentrations using the exact same method.
2.6. Immuno-precipitation (IP) and western blot:
Cells were lysed using 50 mM Tris-HCl pH 7.4 buffer with 1% Triton X-100, 5 mM Na-molybdate and EDTA-free protease inhibitor cocktail (Roche). Protein concentration was measured using the Bradford method (Bio-Rad # 500–0006). IP pull-downs were performed using 1 mg of whole cell extracts with anti-FLAG antibody (Sigma # F1804) and anti-IDO antibody (Cell Signaling # 86630S). Protein G agarose beads (Millipore # 16–201) were used to pull down the antibody-protein complex. The beads were washed well with lysis buffer, proteins were boiled in Laemmli buffer, resolved onto 10% SDS-PAGE and transferred to PVDF membrane (Bio-Rad # 1620177) and probed for proteins of interest. Western blot was performed with anti-FLAG (Sigma # F1804; dilution 1:1000), anti-IDO (Cell Signaling # 86630S; dilution 1:1000), anti-Hsp90β (Enzo Life Sciences # ADI-SPA-843-D; dilution 1:1000), anti-HA (Sigma # H9658; dilution 1:2500), anti-GAPDH (Santa Cruz Biotechnology # sc-32233; dilution 1:2500) and anti-α-Tubulin (Santa Cruz Biotechnology # sc-5286; dilution 1:2500). The proteins were detected using chemiluminescence using HRP conjugated secondary antibodies of either anti-mouse (Bio-Rad # 170–6516, dilution 1:10,000) or anti-rabbit (GE Healthcare # NA9340, dilution 1:10,000) origin and ECL substrate (Thermo Scientific # 32106). The images were acquired using a chemidoc system from Bio-Rad.
2.7. Protein purification:
His6-tagged human heat shock protein 90 beta (Hsp90β) [44] and GST-tagged human GAPDH [35] were expressed in E. coli BL21 (DE3) and purified using previously reported methods respectively. Human IDO1 was cloned in pHis-parallel1 vector and purified with minor modifications for His tagged proteins as reported previously [74]. GST-tagged human TDO was purified as similar to the protocol for human GST-GAPDH [35]. During the purification of both His6-IDO1 and GST-TDO, heme was not added to the bacterial growth medium or during protein induction with IPTG. The heme bound in purified IDO1 and TDO was found to be around ten percent per mole of the total purified proteins, as measured by a heme chromogen assay [75]. No effort was made to remove this residual heme.
2.8. Fluorescence polarization measurements:
Alexafluor 647 (AF) labeling of Cys groups in purified apo- or heme-reconstituted IDO1 and TDO proteins was performed at neutral pH using methods described previously [44]. Residual fluorescence polarization measurements were performed at room temperature using methods described previously with modifications [44]. Briefly, AF-IDO1 (apo- and heme reconstituted) and AF-TDO (apo- and heme reconstituted) were diluted in 40 mM EPPS/150 mM NaCl/10% glycerol, pH 7.6 buffer to a finial concentration of 1 μM in a fluorescent 96 well micro-plate and different amount of binding partners were added into the wells. The fluorescence polarization of each well was monitored automatically on a Flexstation 3 multimode microplate reader (Molecular Devices) using excitation at 650 nm and emission at 665 nm. All titration experiments were repeated independently three times.
2.9. Statistical analyses:
All experiments were repeated at least three independent times. The results are represented as mean ± standard deviation. The statistical test used to measure significance (p-values) was one-way ANOVA in the software Graph Pad Prism (v7).
3. Results
3.1. Cellular IDO1 and TDO activities reflect their heme content
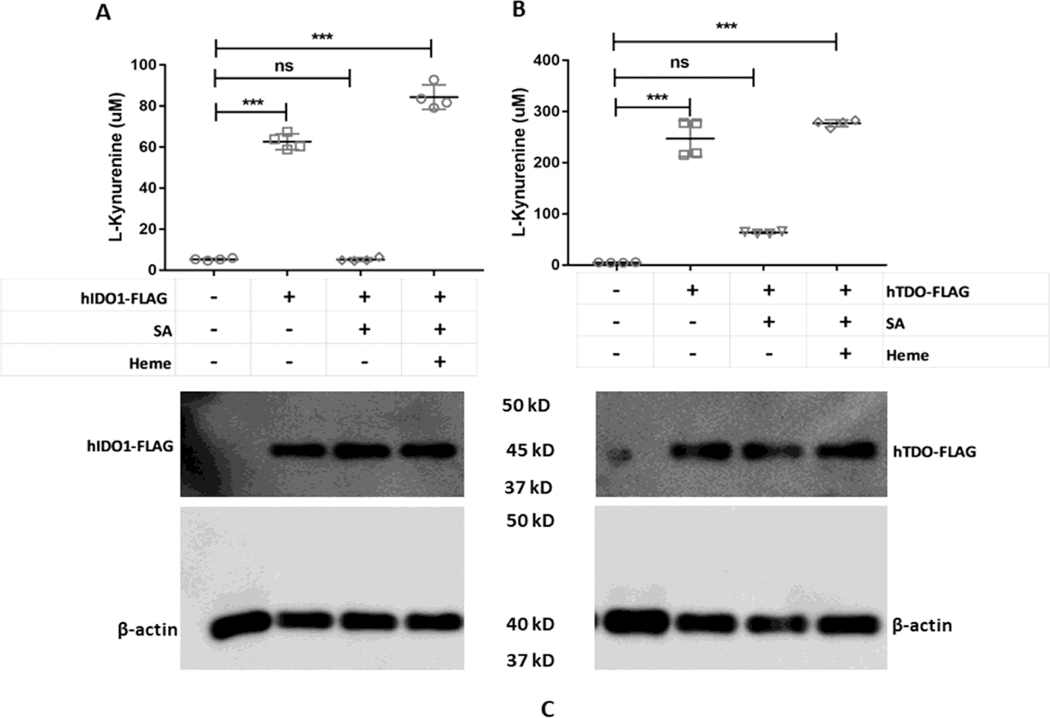
We transfected HEK293T cells, which do not naturally express detectable levels of IDO1 or TDO, to transiently express FLAG-tagged constructs of human IDO1 or TDO. Cells without FLAG-IDO1/TDO were transfected with FLAG-empty vector. As shown in Fig. 1 A and B, both enzymes were active when they were expressed in cells cultured in normal media, as judged by buildup of product Kyn over a 5 hour assay period. However, expressing IDO1 or TDO in cells that had been made heme-deficient by pre-culture with the heme biosynthesis inhibitor succinyl acetone (SA) resulted in a significantly reduced Kyn production in both cases. Adding exogenous heme to the transfected heme deficient cells restored their Kyn production. Cell expression levels of IDO1 or TDO were similar across the different experimental conditions (Fig. 1C). Heme was also added to IDO1/TDO un-transfected (empty vector transfected) and transfected cells as controls and activities of both enzymes were measured. Empty vector transfected cells had no activity of either enzyme in presence of added heme (data not shown). IDO1 transfected cells in presence of added heme had activity of 91.3±1.2 μM; n=4 (data not shown). TDO transfected cells in presence of added heme had activity of 300.61±0.9 μM; n=4 (data not shown).
Figure 1. Effects of cell heme depletion and added exogenous heme on IDO1 and TDO catalytic activities.
HEK293T cells were cultured in normal media or with SA to inhibit their heme biosynthesis and cause heme depletion prior to transfection with FLAG-tagged IDO1 or TDO expression plasmids. In some cases, cells were supplemented during the activity assays with 10 μM heme in presence of SA. (A) IDO1 activity as determined by measuring the concentration of L-Kyn in the medium. (B) TDO activity assay under similar conditions. (C) Protein expressions of IDO1 and TDO under various conditions. Activity values are the mean ± S.D. (error bars) of four measurements and are representative of four experiments each. Significance designation*** p < 0.001, one way ANOVA.
We also observed comparable results for IDO1 when its expression was induced naturally in HeLa cells by IFN-γ treatment (Fig. S1) [76]. HeLa cells without induction with IFN-γ do not express IDO1 [76]. Together, the results confirm that stable heme-deficient (apo) forms of IDO1 and TDO can build up in cells and can incorporate added heme to become active, and that the catalytic activities of both IDO1 and TDO depend on their heme content.
3.2. Catalytic activities of IDO1 and TDO are GAPDH-dependent
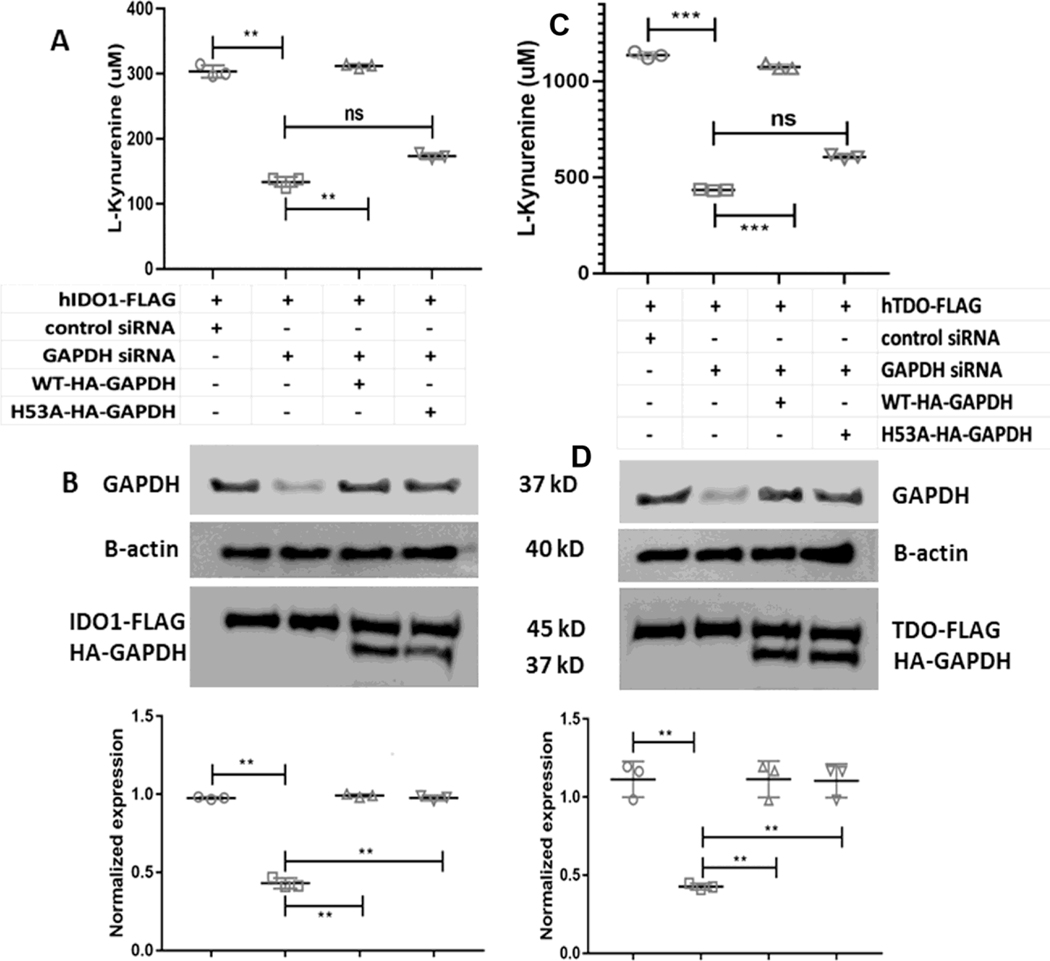
We have previously reported the heme binding properties of GAPDH [41] and the important role of His53 in this process [42]. Because heme allocation to two other heme proteins, inducible NO synthase [35,42] and soluble guanylyl cyclase (sGC) [36], depends on the expression level and heme binding ability of GAPDH, we examined if GAPDH is also involved in supporting the heme-dependent catalytic activities of IDO1 and TDO by employing our previously established siRNA knockdown and rescue strategies [36,42]. Here, we first performed targeted siRNA knockdown of GAPDH in HEK293T cells, and then transiently transfected these cells to express FLAG-tagged versions of IDO1 or TDO either alone or in combination with siRNA-resistant forms of HA-GAPDH WT or the HA-GAPDH-H53A variant which has defective heme binding, but otherwise had normal glycolytic activity [42]. The cell IDO1 and TDO activities were then determined by adding L-Trp and measuring L-Kyn production by the cultures over a subsequent 5 h period. The siRNA treatment lowered the cell GAPDH expression level by approximately 60% relative to the scrambled siRNA control (Fig. 2B & D), and this effect correlated with a decrease in the cell IDO1 and TDO activities by approximately 60% (Fig. 2A & C). Transfecting the knocked-down cells to express HA-GAPDH along with TDO or IDO1 recovered the total GAPDH protein expression level (native plus HA-tagged) to a normal level and also recovered the IDO1 or TDO enzyme activities (Fig. 2B & D). In contrast, transfecting the knocked-down cells to co-express the HA-GAPDH H53A variant [42] recovered the cell total GAPDH protein expression level (native plus HA-tagged) to a normal value but did not recover the IDO1 or TDO catalytic activities (Fig. 2A & C). Together, the results indicate that the catalytic activities of IDO1 and TDO depend on the cell GAPDH expression level and on the specific heme-binding ability of GAPDH, indicating that their activities are both GAPDH-dependent.
Figure 2. GAPDH dependence of heme delivery to apo-IDO1 and apo-TDO.
HEK293T cells were transfected to express FLAG-tagged IDO1 or TDO and were given control or GAPDH-targeted siRNA for 2 days prior to performing IDO1 and TDO activity assays. In some cases, the siRNA GAPDH-treated cells were also transfected to express siRNA-resistant constructs of HA-tagged WT GAPDH or H53A GAPDH. Top to bottom, (A) IDO1 activities under the four indicated experimental conditions; (B) representative Western blot indicating protein expressions under the four experimental conditions; quantification of GAPDH protein expression levels under the four conditions. (C) Top to bottom, TDO activities under the four indicated conditions; (D) representative Western blot indicating protein expressions under four experimental conditions; quantification of GAPDH protein expression levels under the four conditions. Activity values are the mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation *** p < 0.001, ** p < 0.01, one way ANOVA. Protein expression values are mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation ** p < 0.01, one way ANOVA.
3.3. Heme allocation to IDO1 and TDO is GAPDH-dependent
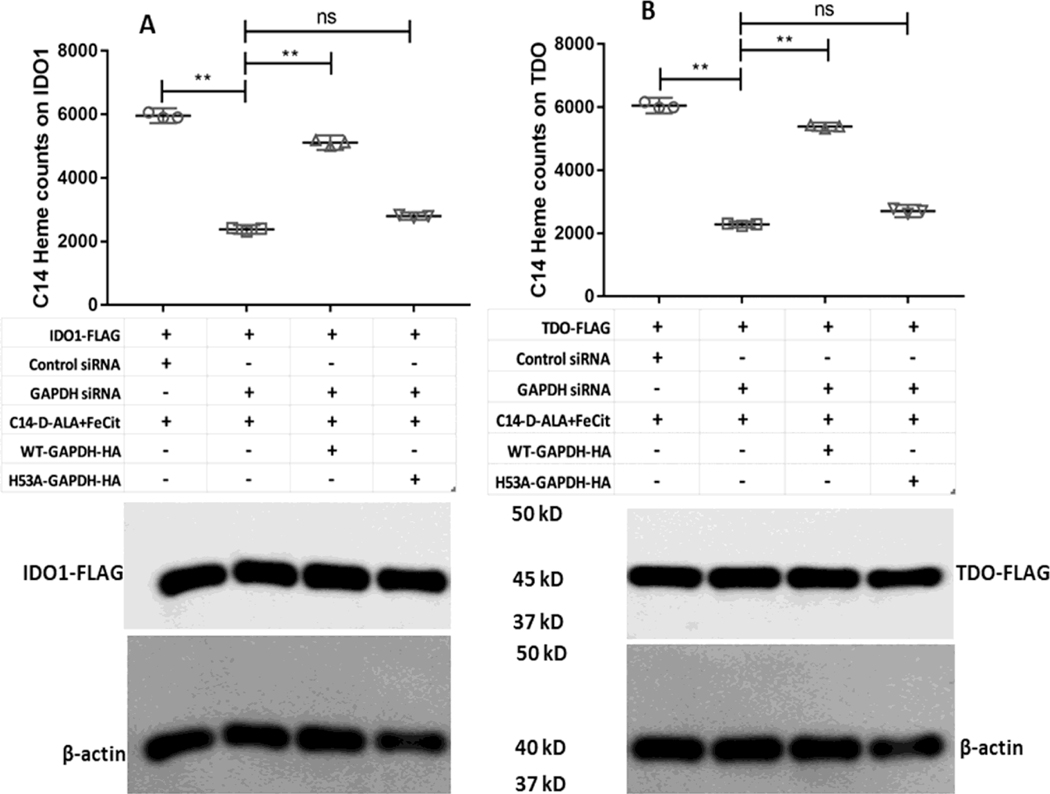
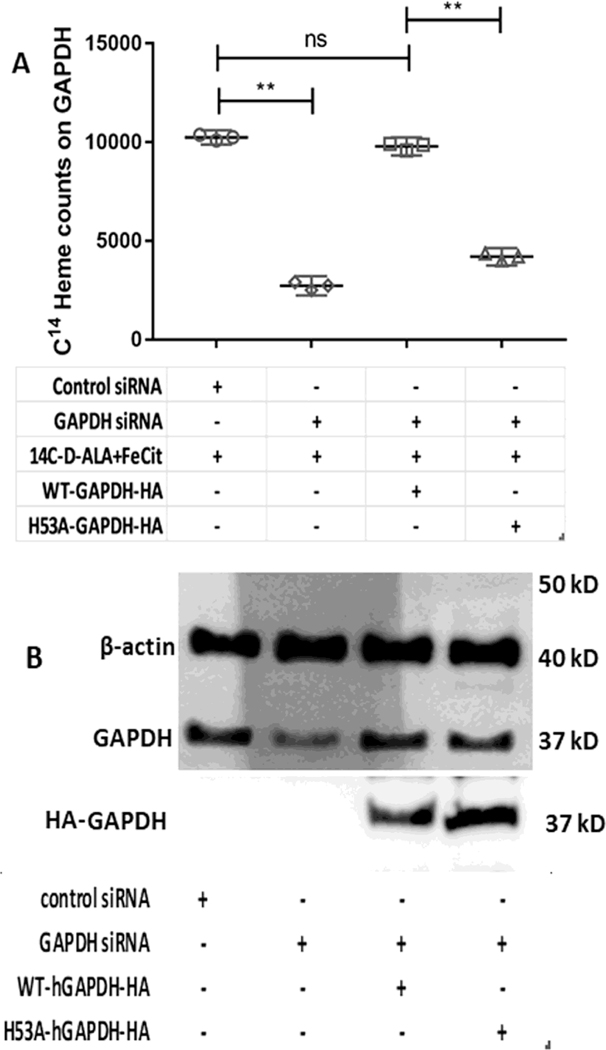
To investigate if the GAPDH-dependent IDO1 and TDO activities are related to GAPDH heme allocation, we repeated the experiments as described above but added the heme precursors 14C-d-ALA and ferric citrate to the cell cultures for two hours prior to performing the 5 h activity measurement and a subsequent cell lysis. This allowed cell mitochondria to generate 14C-heme [42], which then allowed us to measure its incorporation into the FLAG-IDO1, FLAG-TDO, HA-GAPDH WT, or HA-GAPDH H53A proteins by performing antibody pulldowns on beads. Figure 3A and B shows the 14C counts from the FLAG pulldowns. The GAPDH knockdown greatly diminished 14C-heme acquisition by FLAG-TDO or FLAG-IDO1 relative to that observed in cells that received control siRNA. Expressing HA-GAPDH WT in the knocked-down cells rescued the 14C-heme acquisition by both FLAG-IDO1 and FLAG-TDO, whereas expressing the HA-GAPDH H53A variant did not (Fig. 3A and B). These differences in 14C-heme acquisition correlated with differences in the TDO and IDO1 catalytic activities determined from L-Kyn production in the same cell cultures (Fig. S2A and B) and they also correlated with the different abilities of the two HA-GAPDH proteins to bind and accumulate the mitochondrially-generated 14C-heme in the cells (Fig. 4A). Western analyses indicated that the expression levels of FLAG-IDO1 and FLAG-TDO were similar across the various conditions (Fig. 3A and B) and also confirmed that the siRNA treatment diminished native GAPDH protein expression and show that expression of the HA-tagged GAPDH proteins in the knockdown cells restored the total GAPDH expression level (native plus HA-tagged) to a near normal value (Fig. 4B). Together, these results reveal that the basis for IDO1 and TDO activities being GAPDH-dependent reflects the ability of GAPDH to bind and allocate mitochondrial heme to apo-IDO1 and apo-TDO in the cells.
Figure 3. GAPDH dependence of endogenous 14C-labeled heme allocation to apo-IDO1 and apo-TDO.
SA-treated GlyA− CHO cells were transfected with siRNA against GAPDH and were also transfected with plasmids to express HA-WT-GAPDH, HA-H53A-GAPDH, FLAG-IDO1 and FLAG-TDO in the different samples as required. 14C-d-ALA and Fe-citrate were added to the cells after removal of SA and heme synthesis was allowed for 5 h. 14C labeled heme counts were measured on FLAG-tagged IDO1 or TDO following antibody pulldowns. (A) Counts on FLAG-IDO1 under the different indicated conditions. (B) Counts on FLAG-TDO under the different indicated conditions. The lower panels show Western blots indicating the expression levels of IDO1 and TDO in the various samples. 14C-heme count values are the mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation ** p < 0.01, one way ANOVA. Western blots are a representative image of three experiments.
Figure 4. siRNA knockdown of native GAPDH and 14C-heme binding by HA-tagged GAPDH constructs expressed in the cells.
SA-treated GlyA− CHO cells were transfected with siRNA against GAPDH and were also transfected with plasmids to express HA-WT-GAPDH, HA-H53A-GAPDH, FLAG-IDO1 and FLAG-TDO in the different samples as required. 14C-d-ALA and Fe-citrate were added to the cells after removal of SA and heme synthesis was allowed for 5 h. (A) 14C-labeled heme counts were measured on the indicated HA-GAPDH proteins following antibody pulldowns. (B) Representative Western blots indicating cell expression levels of the indicated proteins. Values in A are the mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation ** p < 0.01, one way ANOVA. Western blots are the representative image of three experiments.
3.4. Heme rescue of cell IDO1 and TDO activities show different Hsp90 requirements
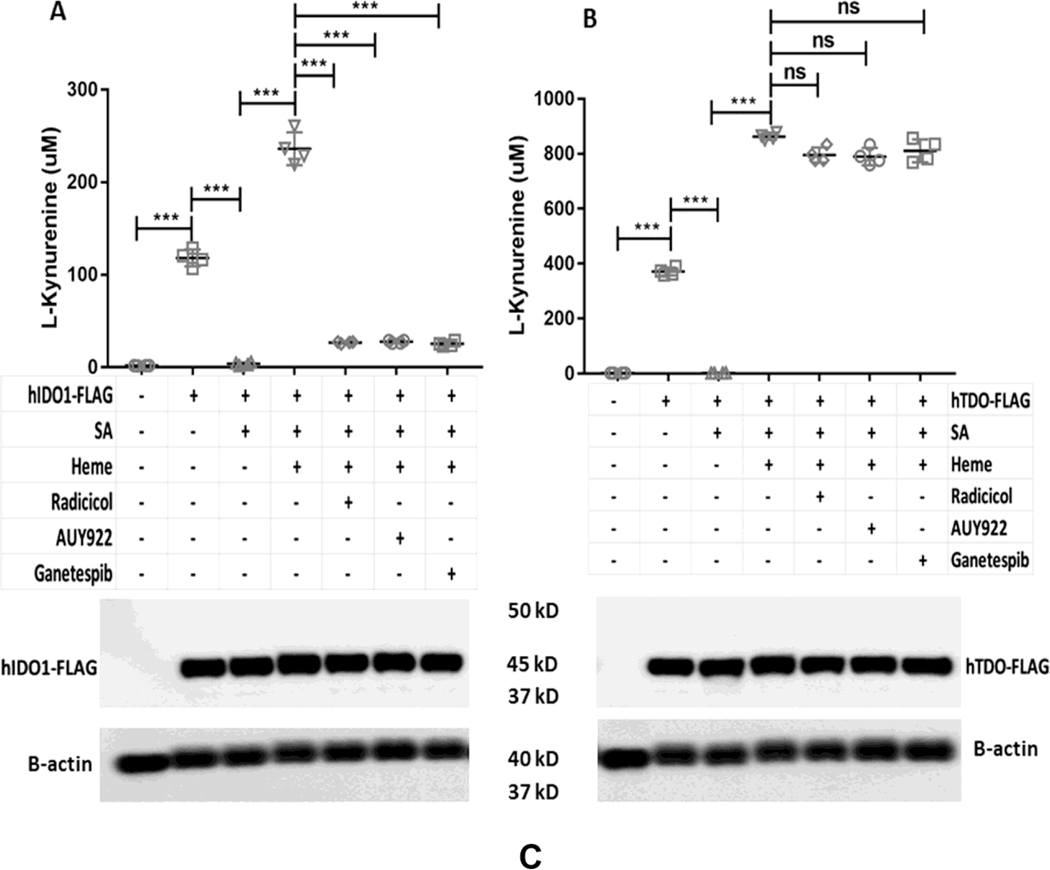
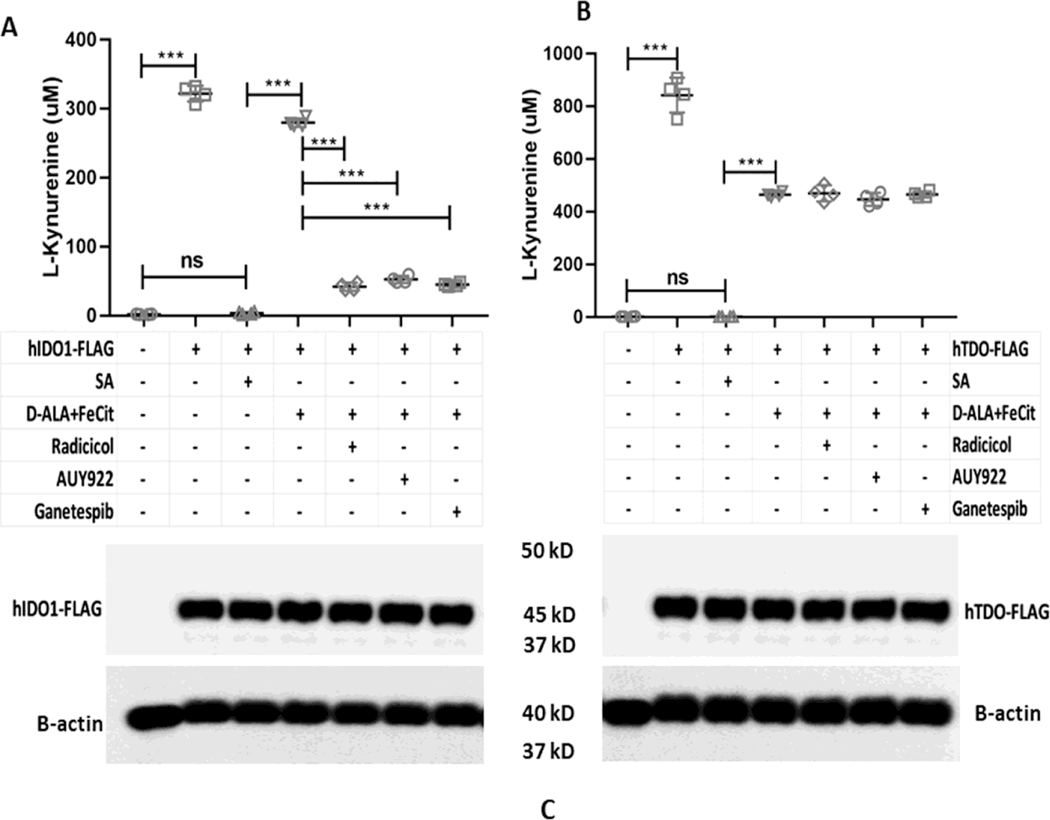
The cell chaperone Hsp90 is required for heme insertion into several different heme proteins [43]. To test if Hsp90 plays a role in IDO1 and TDO heme insertions, we examined how three different Hsp90 inhibitors (Radicicol, AUY922, and Ganetespib) would impact the ability of added hemin to rescue the catalytic activities apo-IDO1 or apo-TDO that were expressed in heme-deficient HEK293T cells. Following apo-IDO1 or apo-TDO expression, the cells received hemin plus either vehicle or Radicicol (10 μM), AUY922 (5 μM), or Ganetespib (500 nM) and were cultured in the continued presence of SA for a further 5 hrs. Fig. 5A shows that added hemin could rescue apo-IDO1 activity as judged by its boosting L-Kyn production during the 5 h period, but the rescue was prevented when each of the three Hsp90 inhibitors were present. The Hsp90 inhibitors also blocked hemin rescue of apo-IDO1 activity in a similar fashion when its expression was induced in heme-deficient HeLa cells by IFN-γ treatment (Fig. S3). In marked contrast, the three Hsp90 inhibitors did not prevent the added hemin from rescuing the activity of apo-TDO expressed in the heme-deficient HEK293T cells (Fig 5B). The cell expression levels of IDO1 or of TDO were similar across all culture conditions (Fig. 5C). The protein expressions of GAPDH and Hsp90 were similar across all samples (data not shown). Together, the results show that cell Hsp90 activity was needed for the added hemin to rescue the apo-IDO1 activity but was not needed for hemin to rescue the apo-TDO activity. We next tested how the Hsp90 inhibitors would impact the rescue of apo-IDO1 and apo-TDO activities by cell-generated heme. For this experiment, apo-IDO1 or apo-TDO were expressed in the heme-deficient HEK293T cells as before, and then their heme biosynthesis was stimulated as we have done previously [36] by adding the mitochondrial heme precursors d-ALA (1 mM) and ferric citrate (100 μM) to the cultures for 2 h in the absence of SA, followed by a further 5 h cell culture period to measure L-Kyn production. Fig. 6A shows that IDO1 activity increased in response to our triggering the endogenous cell heme biosynthesis, as expected. This increase in activity was blocked by each of the three Hsp90 inhibitors. In contrast, Fig. 6B shows that none of the Hsp90 inhibitors blocked the increase in TDO activity that occurred following the stimulation of mitochondrial heme production. The expression levels of IDO1 or of TDO were similar across all experimental conditions (Fig. 6C). The protein expressions of GAPDH and Hsp90 were similar across all samples (data not shown). Thus, cell Hsp90 activity was required for mitochondrially-generated heme to rescue the apo-IDO1 activity but not to rescue the apo-TDO activity.
Figure 5. Effect of Hsp90 inhibitors on the reconstitution of cell IDO1 and TDO activities by added exogenous heme.
HEK293T cells were cultured in normal media or with SA to inhibit their heme biosynthesis and cause heme depletion prior to transfection with FLAG-tagged IDO1 or TDO expression plasmids. In some cases, cells were supplemented with 10 μM heme in presence of SA during the assays with or without each indicated Hsp90 inhibitor. (A) IDO1 activity was determined by measuring the concentration of L-Kyn in the medium. (B) TDO activity was determined under similar conditions. (C) Protein expressions of IDO1 and TDO under various conditions. Activity values are the mean ± S.D. (error bars) of four measurements and are representative of four experiments each. Significance designation *** p < 0.001, one way ANOVA.
Figure 6. Effect of Hsp90 inhibitors on the reconstitution of cell IDO1 and TDO activities by stimulation of mitochondrial heme biosynthesis.
HEK293T cells were cultured in normal media or with SA to inhibit their heme biosynthesis and cause heme depletion prior to transfection with FLAG-tagged IDO1 or TDO expression plasmids. Media was then exchanged to remove SA prior to activity measures, in which cells were given the heme biosynthetic precursors D-ALA + Fe-citrate along with vehicle or each indicated Hsp90 inhibitor. (A) Reconstitution of IDO1 activity upon generation of endogenous heme in absence or presence of Hsp90 inhibitors. Expression of IDO1-FLAG under different experimental conditions. (B) Reconstitution of TDO activity upon generation of endogenous heme in the absence or presence of Hsp90 inhibitors. Expression of TDO-FLAG under different experimental conditions. Activity values are the mean ± S.D. (error bars) of four measurements and are representative of four experiments each. Significance designation *** p < 0.001, one way ANOVA.
3.5. Heme insertion into apo-IDO1 and apo-TDO displays different Hsp90 requirements
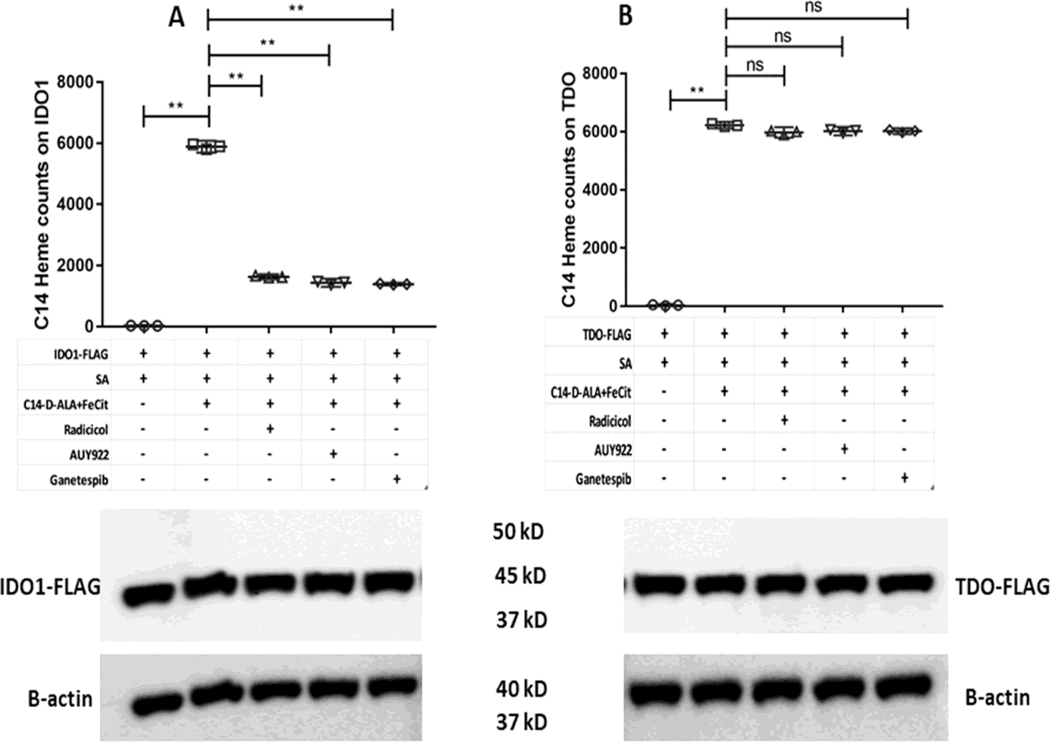
To directly investigate if the Hsp90 inhibitors impact heme incorporation into FLAG-apo-IDO1 and FLAG-apo-TDO, we repeated the experiments as described above but substituted 14C-d-ALA for its unlabeled form so that the cells could generate 14C-heme, and we then measured its incorporation into the FLAG-tagged proteins by antibody pulldowns. Fig. 7A and B shows that the cell-generated 14C-heme became incorporated into both FLAG-apo-IDO1 and FLAG-apo-TDO. The Hsp90 inhibitors blocked the 14C-heme incorporation into FLAG-apo-IDO1 (Fig. 7A) but did not block its incorporation into FLAG-apo-TDO (Fig. 7B). The L-Kyn production levels that we measured from the same cell cultures correlated with the different levels of 14C-heme incorporation (Fig. S4A and B). These results show that the Hsp90 inhibitors prevent heme rescue of IDO1 activity by blocking an Hsp90-dependent heme insertion into apo-IDO1.
Figure 7. Effect of Hsp90 inhibitors on the reconstitution of cell IDO1 and TDO heme content by mitochondrially-generated 14C-labeled heme.
SA treated GlyA− CHO cells were transfected with plasmids to express FLAG-IDO1 and FLAG-TDO as indicated. SA was removed and 14C-d-ALA and Fe-citrate were added to the cells along with the indicated Hsp90 inhibitors, and heme synthesis was allowed for 5 h. 14C-labeled heme counts on FLAG-IDO1 and FLAG-TDO were measured following their antibody pulldown from cell supernatants. (A) FLAG-IDO1 14C heme counts. (B) FLAG-TDO 14C heme counts. Lower panels show representative Western blots indicating the expression levels of IDO1 and TDO in the various samples. Values of 14C heme counts are the mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation ** p < 0.01, one way ANOVA. Western images are representative of three experiments.
3.6. Intracellular associations of IDO1 and TDO with Hsp90 or GAPDH
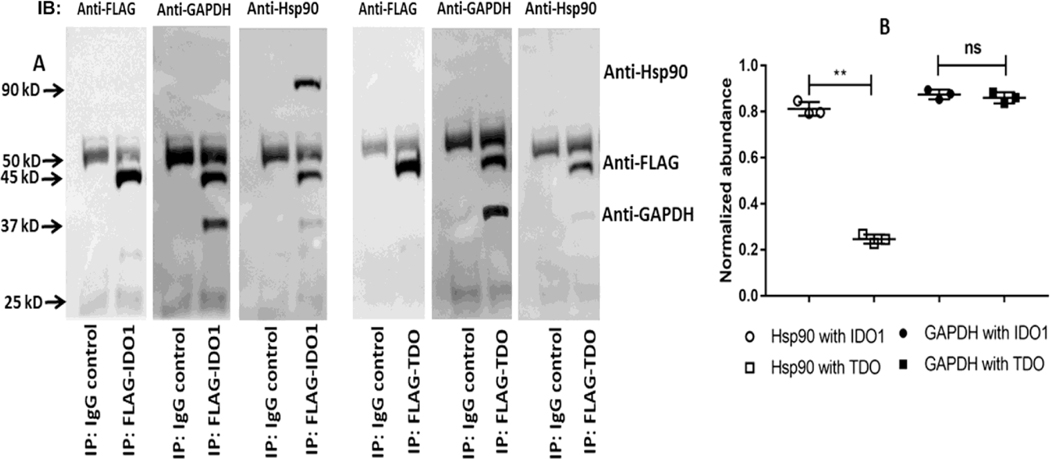
We next investigated if the FLAG-tagged IDO1 or TDO proteins associate with Hsp90 or with GAPDH in cells. Fig. 8A shows that upon pulldown with an anti-FLAG antibody, IDO1 was found associated with both Hsp90 and with GAPDH, whereas TDO was found associated only with GAPDH and not with Hsp90. The normalized abundance of GAPDH and Hsp90 associated with IDO1 or TDO is shown in Fig. 8B. This indicates that IDO1 and TDO associated with GAPDH or Hsp90 in cells in accord with their respective requirements for heme delivery and insertion.
Figure 8. Intracellular association of IDO1 or TDO with GAPDH or Hsp90.
FLAG-tagged constructs of IDO1 and TDO were expressed in HEK293T cells, and the cell supernatants were subject to pulldown using an anti-FLAG antibody. (A) Representative Western blots indicating the relative levels of Hsp90 or GAPDH associated with FLAG-IDO1 and FLAG-TDO in the pulldowns. (B) Quantification of the GAPDH and Hsp90 association levels normalized to the levels of FLAG-tagged IDO1 and TDO present in the pulldowns. Densitometry values are the mean ± S.D. (error bars) of three measurements and are representative of three experiments each. Significance designation ** p < 0.01, ns = not significant, one way ANOVA.
3.7. Binding interactions of the purified apo- or heme-reconstituted IDO1 and TDO with GAPDH or Hsp90
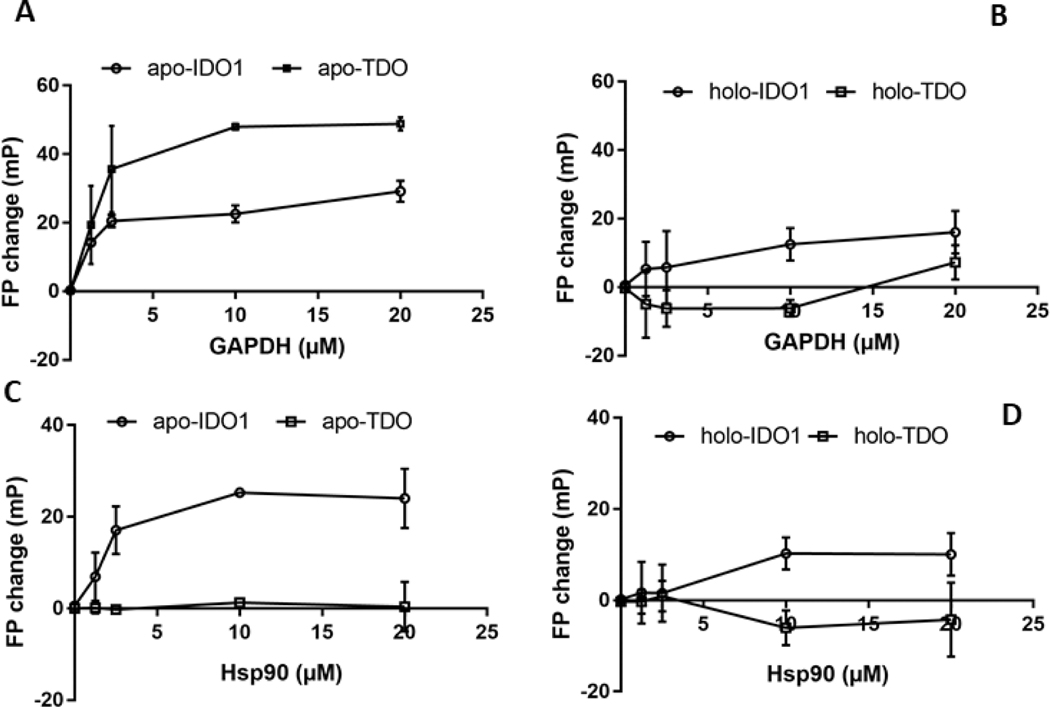
To investigate possible mechanisms of action we studied interactions between the purified proteins using an established fluorescence polarization approach [44]. Purified recombinant apo-IDO1 and apo-TDO proteins or their heme-reconstituted forms were labeled with Alexafluor 647 (spectra shown in Fig. S5), and then were titrated with increasing concentrations of purified GAPDH or Hsp90. We observed concentration-dependent increases in the level of residual fluorescence polarization when apo-IDO1 or apo-TDO was titrated with GAPDH (Fig. 9A), indicating that they both can directly bind GAPDH, with estimated Kd values of 1.5 ± 1.3 and 1.2 ± 0.4 μM, respectively. On the other hand, the heme-reconstituted forms of IDO1 and TDO showed much diminished binding affinity toward GAPDH by the same method (Fig. 9B). The fluorescence polarization data also showed that Hsp90 bound to apo-IDO1 with an estimated Kd of 2.5 ± 1.8 μM but did not bind with apo-TDO (Fig. 9C), and that the heme-reconstituted IDO1 displayed weaker affinity toward Hsp90 with estimated Kd of 9.1 ± 3.2 (Fig. 9D). Thus, apo-IDO1 and apo-TDO engaged in direct binding interactions with GAPDH or Hsp90 according to their respective requirements for these proteins in their heme delivery and insertion processes, and the heme-replete forms of both IDO1 and TDO showed diminished binding affinities relative to their apo forms.
Figure 9. Interactions of purified apo-IDO1 and TDO versus holo-IDO1 and TDO with GAPDH or Hsp90.
Fixed concentrations of Alexafluor 647-labeled IDO1 (1 μM) and TDO (1 μM tetramer) proteins were titrated with increasing amounts of GAPDH or Hsp90 and their interactions were followed as a gain in residual polarized fluorescence (FP). (A and B) The interactions of Alexafluor 647-labeled apo-IDO1 and apo-TDO or their heme replete (holo) forms with GAPDH. (C and D) The interactions of Alexafluor 647-labeled apo-IDO1 or apo-TDO or their holo forms with Hsp90. The values shown are the mean ± S.D., n=3.
4. Discussion
IDO1 and TDO generate metabolites of Trp which help to govern the immune response and impact other aspects of human biology including blood pressure regulation, emotional state, and cancer progression [20–22, 45–47]. Because the heme levels in TDO and IDO1 determine their activities, understanding their heme delivery and insertion processes is fundamentally important and may suggest new ways to manipulate their biological activities for therapeutic benefit.
We found that delivery of mitochondrially-generated or exogenous heme to human apo-IDO1 and apo-TDO in mammalian cells relies on the expression level and the specific heme-binding ability of GAPDH. Both IDO1 and TDO interacted with GAPDH in cells or when tested in purified form, and a GAPDH-heme complex transferred its heme to both apo-IDO1 and apo-TDO. Together, this indicates that the intracellular heme deliveries to IDO1 and TDO are GAPDH-dependent and may involve a direct interaction with GAPDH. In comparison, IDO1 and TDO differed regarding an Hsp90 requirement for heme insertion, with only IDO1 depending on and interacting with the Hsp90 chaperone in cells or when in purified forms. Thus, apo-IDO1 joins a growing list of heme proteins that have Hsp90-dependent heme insertions, which includes the inducible and neuronal NO synthases [37,48], sGC [49], and the globins Hb-β, Hb-γ, and Mb [39,40,43]. Remarkably, among all the heme proteins tested to date, TDO is the first to display an Hsp90-independent heme insertion. This may indicate that its heme insertion occurs independent of chaperones, or instead may involve a different chaperone than Hsp90. Thus, further work with TDO stands to broaden our understanding of the heme insertion processes that are utilized within mammalian cells.
It is important to mention that the evidence to date indicates GAPDH heme delivery is independent of its role in glycolysis and cell energy production. We previously reported that heme binding to GAPDH does not impact its dehydrogenase activity [41] and that siRNA knockdown of GAPDH expression as done here does not significantly alter cell ATP levels [42]. Moreover, the heme binding-defective H53A GAPDH variant possesses normal glycolytic dehydrogenase activity [42]. Thus, our finding that this GAPDH variant was unable to rescue heme deliveries to apo-IDO1 and apo-TDO when it was expressed in the GAPDH knockdown cells, despite it restoring the total GAPDH expression level in the cells to normal, reveals that GAPDH heme binding is the key determinant for their cellular heme deliveries independent of GAPDH glycolytic activity. The intracellular heme delivery and insertion processes of TDO and IDO1 are particularly relevant for their biology, because both enzymes exist naturally and even predominantly in their heme-deficient forms in cells and tissues and both can dynamically change their heme levels in response to diverse environmental signals. For example, investigations from over 50 years ago showed that rat liver TDO was approximately only 25 to 50% heme saturated in healthy animals [24–26], and that their liver TDO heme levels increased to 80–100% saturation within 2 h after giving the animals Trp [27,28]. Injecting the animals with either hemin or with the natural heme precursor d-ALA also increased their liver TDO heme saturation level to 90–100% within a few hours [25, 26]. These early studies were perhaps the first to demonstrate that heme proteins can naturally exist in a predominantly heme-deficient state in healthy tissue. This in turn helped birth the concept that a dynamic pool of heme is available in cells for insertion into existing subpopulations of apo-heme proteins. Other early studies found that immune stimulation could significantly impact the TDO heme level. Injecting rats or mice intraperitoneally with bacterial lipopolysaccharide caused their liver TDO to double its heme saturation level within 2 h, followed by a gradual decrease in its heme saturation back to the baseline level or below over the next 6 to 12 h period [29, 30]. This was the first indication that perturbing an animal’s physiology [in this case by immune stimulation] could cause a dynamic and transient change in the heme content of their liver TDO. More recently, IDO1 was also found to predominantly exist in its apo-form in cells and to be capable of incorporating added exogenous heme, which resulted in an increase in IDO1 activity [32]. In cultured human ovarian cancer cells, 85% of the total IDO1 was estimated to be in heme-free form and the investigators showed that this high percentage of apo-IDO1 influenced the efficacy of pharmacologic IDO1 inhibitors developed to control cancer growth [31]. Others showed that immune stimulation or exposure to an NO donor caused cell IDO1 activity or its heme level to initially increase in one study [33], and in all cases to ultimately decrease to below control levels [33,50,51]. IDO1 activity was also found to decrease in cells when activity levels of heme oxygenase 1 or NO synthase were increased [33, 34, 51]. Overall, these studies established that IDO1 also exists predominantly in heme-free form and its heme level is subject to dynamic physiologic changes. Thus, based on our current findings that heme deliveries to TDO and IDO1, and heme insertion into IDO1, depend on GAPDH and Hsp90, respectively, we propose that GAPDH and Hsp90 function in this manner is likely to determine physiologic IDO1 and TDO heme levels and activities and to orchestrate the changes in their heme contents that occur in response to the various environmental signals. This implies that a cell’s GAPDH- and Hsp90-dependent heme delivery and insertion processes are highly relevant to IDO1 and TDO functions in biology. These concepts are summarized in the model shown in Fig. 10.
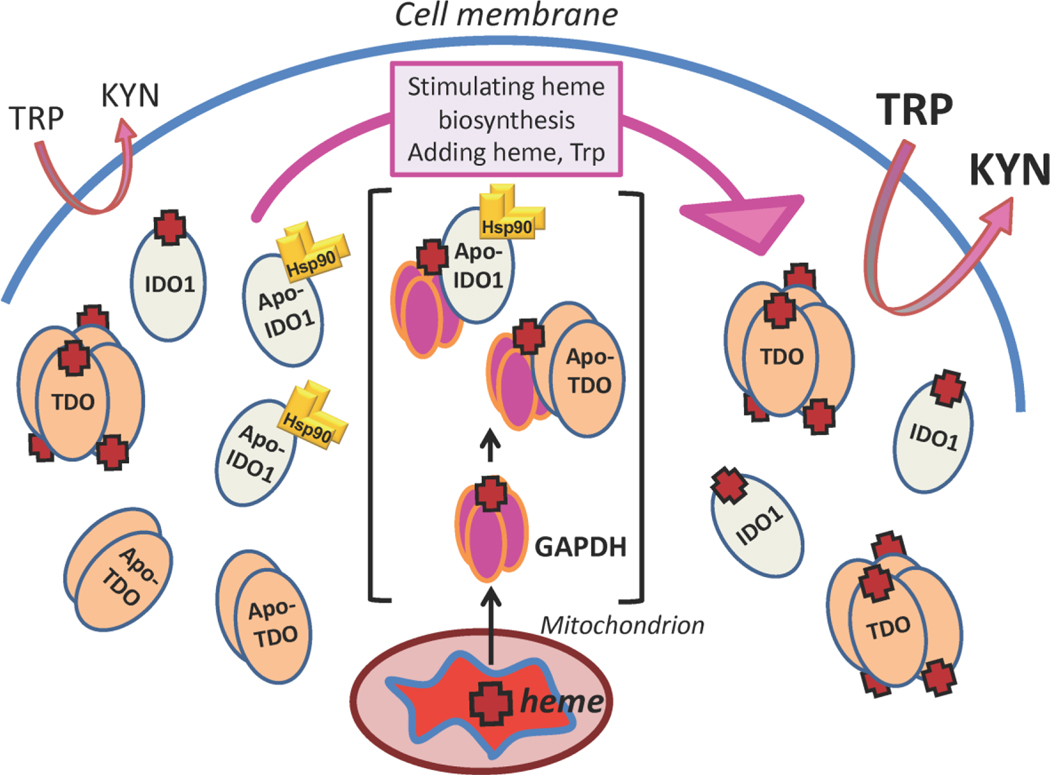
Figure 10. GAPDH and Hsp90 regulation of the heme contents and activities of IDO1 and TDO in mammalian cells.
Left side, in healthy cells significant levels of apo-TDO or apo-IDO1 exist alongside their heme-replete forms, and the apo-IDO1 is in complex with chaperone Hsp90 (gold homodimer). In this circumstance, the apo-TDO and apo-IDO1 is catalytically inactive and so the cell can convert only a relatively low amount of Trp to Kynurenine (Kyn). Center and brackets, stimulating cell heme biosynthesis, or providing the cell with exogenous heme or Trp, increases the heme contents of IDO1 and TDO through a GAPDH-dependent heme delivery to apo-IDO or apo-TDO and subsequent heme insertion that is driven either by Hsp90 (IDO1) or is independent of Hsp90 (TDO). Right side, the increased heme contents of IDO1 or TDO increase their total activities in the cell and thus allow greater conversion of Trp into Kyn.
It is interesting to consider how similarities and differences in the protein structures of IDO1 and TDO may relate to our current findings. Mature IDO1 is monomeric while mature TDO functions as a tetramer. IDO1 consists of a heme-binding domain and a smaller N-terminal domain whereas the TDO subunit has a less divided structure [8]. IDO1 utilizes its smaller N-terminal domain to help cover its heme and substrate binding region, while TDO uses for the same purpose an N-terminal region provided by an adjacent subunit in the tetramer [8]. Despite the two enzymes sharing a low sequence identity, their heme binding domains have very similar secondary and tertiary structure: They share the same fold with 10 helices and their heme binding sites superimpose along with the associated structural elements [8, 52]. Given the structural similarity between their heme binding domains, it is perhaps not surprising that IDO1 and TDO both display a common GAPDH requirement for heme delivery, but it is puzzling why they do not share a common Hsp90 requirement for heme insertion. Regarding mechanism, we found that apo-IDO1 and apo-TDO both associate with GAPDH in cells and also directly bind with GAPDH in their purified forms at low micromolar concentrations. This behavior suggests that the heme deliveries to both dioxygenases may involve their direct protein interaction with GAPDH for the heme transfer reaction, and this possibility can now be further investigated. Regarding Hsp90, the current evidence suggests that heme proteins displaying Hsp90-dependent heme insertions must bind in their apo form directly with Hsp90. This appears to stabilize their empty heme binding domain in a more exposed or less structured state to aid in the heme insertion [53, 54]. The independence of apo-TDO toward Hsp90 implies that its heme domain may already be sufficiently exposed for heme insertion, or alternatively that it is held exposed through an interaction with an unknown alternative chaperone protein. Oddly, a crystal structure for human apo-TDO showed that it has little structural deviation from the structure of heme-containing human TDO [8, 52]. Although this implies that the bound heme may have little influence on TDO structure, it is also possible that apo-TDO exists in an alternative structural form in mammalian cells. In fact, the recombinant human apo-TDO protein that was used in the aforementioned crystallographic study was shown to exist as a dimer in solution, and not as a tetramer [52]. IDO1 and TDO also contain several distinct insertion or deletion segments within their respective polypeptides [8]. In particular, there exists a significant sequence heterogeneity in the loop structure connecting their J-K α-helices, which are positioned near the proximal heme binding sites in both enzymes and provide conformational plasticity to their heme domains [55]. Whether these or other structural differences bestow IDO1 and TDO with their different Hsp90 binding affinities and requirements for heme insertion can now be further explored.
An important concept arising from our work is that anything that impacts a cell’s GAPDH and Hsp90 heme delivery and insertion functions is likely to influence the biological activities of IDO1 & TDO. For example, immune stimulation or inflammation often elevate NO biosynthesis, and NO is known to ultimately inhibit IDO1 and TDO activities [32, 33, 56]. Given that NO can inhibit GAPDH-dependent heme delivery by causing an S-nitrosation of a Cys in GAPDH [35], we speculate that elevated NO produced in inflammatory settings, possibly along with reactive oxygen species, may suppress IDO1 and TDO activities by their modifying or oxidizing GAPDH and inhibiting its heme delivery function. In turn, this may influence biological outcomes that depend on the level of IDO1 activity [50], which include the suppression of T-cell proliferation through IDO1 Trp depletion and the generation of T regulatory cells due to Kyn production by IDO1 activating the Aryl hydrocarbon receptor (AhR). It may also influence cancer progression, where increased IDO1 or TDO activities are generally associated with poorer outcomes. For example, higher IDO1 activity in plasmacytoid dendritic cells is linked to suppression of host immune surveillance [57] and to increased human tumor growth [58]. Likewise, elevated TDO activity in gliomas is linked to activation of the AhR and reduced antitumor immune responses [59]. Some human tumors show elevated TDO expression and activity, and in such cases inhibiting TDO activity restored the rejection of the TDO-expressing human tumors in mice [60]. In cancer progression, a diminished GAPDH heme delivery would be expected to suppress IDO1 and TDO activities and lead to improved outcomes. On the other hand, diminishing GAPDH heme delivery to apo-IDO1 may also lead to immune hyper-responsiveness and exacerbate allergic reactions, whose links to reduced IDO1 activity are well-established [61,62].
Similar considerations hold for inhibiting Hsp90, which is itself a target in anti-cancer pharmaceutical development and clinical trials [63, 64]. Our findings indicate that Hsp90 inhibition would suppress IDO1 activity in tissue and tumors by blocking heme insertion into apo-IDO1. Given that IDO1 is also a target for anti-cancer drug development [65], one could consider a strategy that targets both Hsp90 and IDO1 to fight cancers whose proliferation depend on increased IDO1 activity. On the other hand, our work predicts that any tumor TDO activity would be unaffected by pharmacologic Hsp90 inhibition, and thus its inhibition must rely on development of GAPDH-based or TDO-directed inhibitors [60, 66]. Dual inhibitors of IDO1 and TDO are also being developed to avoid the compensation afforded by either enzyme [65, 67, 68]. Many of these inhibitors work by binding within the IDO1 and TDO heme binding sites, and the design of heme-displacing inhibitors is currently a major focus in drug development [69–71]. Whether their efficacies could be improved by blocking upstream GAPDH and Hsp90-mediated heme allocation to apo-IDO1 or apo-TDO is a possibility that can now be considered. However, because GAPDH and Hsp90 are so broadly important to cell physiology, generally inhibiting their heme delivery and insertion processes is unlikely to be therapeutically useful. Instead, gathering new information on how one might specifically prevent GAPDH or Hsp90 interactions with these dioxygenases to impair their heme transfer and insertion processes could be a more promising approach. Our current study provides a foundation to explore these aims.
5. Conclusions and Perspectives
Identifying roles for GAPDH and Hsp90 in heme delivery and insertion into human TDO and IDO1 sheds light on their maturation processes in mammals and may help resolve how they can undergo dynamic changes in their heme contents in response to biological stimuli. GAPDH and Hsp90 participation also suggests new points to therapeutically control IDO1 and TDO in inflammation or cancer. Several new questions follow from our study: How do GAPDH and Hsp90 interact with apo-TDO and apo-IDO1 at the molecular level to perform their heme delivery and insertion functions? Are their actions modified by immune stimulation and the associated production of NO or reactive oxygen species? In a broader context, are TDO and IDO1 unusual in their existing primarily as heme-free forms in cells and tissues, or do other heme proteins significantly reside in their apo forms to undergo dynamic changes in their heme levels? This behavior is already demonstrated by at least two other heme proteins, soluble guanylate cyclase (sGC) and NADPH oxidase 5 (NOX5), whose apo forms often predominate under biological circumstances and whose heme levels can change in response to environmental factors [49,53,72,73]. Heme acquisition by apo-sGC is also GAPDH- and Hsp90-dependent [36, 38]. Whether this portends a broader existence of apo-heme proteins whose heme contents are under dynamic regulation by GAPDH, Hsp90, and environmental signals is an important fundamental question for biology and is worthy of further investigation.
Supplementary Material
Highlights.
Tryptophan metabolism by TDO and IDO1 is biomedically important and their catalytic activities are directly proportional to their heme contents.
TDO and IDO1 naturally exist with partial heme levels that can change dynamically in response to inflammation.
Cell heme allocation to TDO and IDO1 depends on the expression level and heme binding ability of GAPDH, and for IDO1 also requires actions of chaperone hsp90.
Identifying what proteins are involved in TDO and IDO1 heme acquisition provides a new way to potentially regulate their activities.
Acknowledgements
The authors would like to thank all the members of the Stuehr laboratory for their helpful discussions of the study and manuscript. This work was supported by NIH grants P01 HL081064, P01 HL103453, R01 GM130624 to Dennis J. Stuehr.
Abbreviations:
- AF
Alexa Fluor
- AhR
Aryl hydrocarbon Receptor
- ANOVA
ANalysis Of Variance
- ATP
Adenosine Tri Phosphate
- CHO
Chinese Hamster Ovary
- Chx
Cycloheximide
- d-ALA
delta-Amino Levulinic Acid
- DMEM
Dulbecco’s Modified Eagle Medium
- ECL
Enhanced chemiluminescence
- FBS
Fetal Bovine Serum
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- GST
Glutathione S-Transferase
- HA
Hemagglutinin
- Hb
Hemoglobin
- HEK293T
Human Embryonic Kidney 293 T-antigen
- HeLa
Henrietta Lacks
- HRP
Horse Radish Peroxidase
- Hsp90
Heat shock protein 90
- IB
Immuno-blot
- IDO
Indoleamine-2,3-dioxygenase
- IFN-gamma
Interferon-gamma
- IP
Immuno-precipitation
- Kyn
Kynurenine
- LPS
Lipopolysaccharide
- Mb
Myoglobin
- mRNA
messenger Ribo Nucleic Acid
- NADPH
Reduced nicotinamide adenine dinucleotide phosphate
- NO
Nitric Oxide
- PVDF
Polyvinylidene fluoride
- SA
Succinyl Acetone
- SDS-PAGE
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- sGC
soluble Guanylate Cyclase
- siRNA
small interfering Ribo Nucleic Acid
- TDO
Tryptophan 2,3-dioxygenase
- Trp
Tryptophan
- TNF
tumor necrosis factor
- WT
Wild Type
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest regarding the contents of the article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
All data are contained within this manuscript or are available from the authors.
References:
- [1].Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ, Tryptophancatabolizing enzymes - party of three, Front Immunol 5 (2014) 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raven EL, A short history of heme dioxygenases: rise, fall and rise again, J Biol Inorg Chem 22(2–3) (2017) 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Capece L, Lewis-Ballester A, Yeh SR, Estrin DA, Marti MA, Complete reaction mechanism of indoleamine 2,3-dioxygenase as revealed by QM/MM simulations, J Phys Chem B 116(4) (2012) 1401–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nienhaus K, Nienhaus GU, Different Mechanisms of Catalytic Complex Formation in Two L-Tryptophan Processing Dioxygenases, Front Mol Biosci 4 (2017) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yamamoto S, Hayaishi O, Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes, J Biol Chem 242(22) (1967) 5260–6. [PubMed] [Google Scholar]
- [6].Shimizu T, Nomiyama S, Hirata F, Hayaishi O, Indoleamine 2,3-dioxygenase. Purification and some properties, J Biol Chem 253(13) (1978) 4700–6. [PubMed] [Google Scholar]
- [7].Forouhar F, Anderson JL, Mowat CG, Vorobiev SM, Hussain A, Abashidze M, Bruckmann C, Thackray SJ, Seetharaman J, Tucker T, Xiao R, Ma LC, Zhao L, Acton TB, Montelione GT, Chapman SK, Tong L, Molecular insights into substrate recognition and catalysis by tryptophan 2,3-dioxygenase, Proc Natl Acad Sci U S A 104(2) (2007) 473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Y, Kang SA, Mukherjee T, Bale S, Crane BR, Begley TP, Ealick SE, Crystal structure and mechanism of tryptophan 2,3-dioxygenase, a heme enzyme involved in tryptophan catabolism and in quinolinate biosynthesis, Biochemistry 46(1) (2007) 145–55. [DOI] [PubMed] [Google Scholar]
- [9].Knox WE, Two mechanisms which increase in vivo the liver tryptophan peroxidase activity: specific enzyme adaptation and stimulation of the pituitary adrenal system, Br J Exp Pathol 32(5) (1951) 462–9. [PMC free article] [PubMed] [Google Scholar]
- [10].Knox WE, Auerbach VH, The hormonal control of tryptophan peroxidase in the rat, J Biol Chem 214(1) (1955) 307–13. [PubMed] [Google Scholar]
- [11].Yoshida R, Hayaishi O, Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide, Proc Natl Acad Sci U S A 75(8) (1978) 3998–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pfefferkorn ER, Guyre PM, Inhibition of growth of Toxoplasma gondii in cultured fibroblasts by human recombinant gamma interferon, Infect Immun 44(2) (1984) 211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Wachter H, Neopterin formation and tryptophan degradation by a human myelomonocytic cell line (THP-1) upon cytokine treatment, Cancer Res 50(10) (1990) 2863–7. [PubMed] [Google Scholar]
- [14].Booth ES, Basran J, Lee M, Handa S, Raven EL, Substrate Oxidation by Indoleamine 2,3-Dioxygenase: EVIDENCE FOR A COMMON REACTION MECHANISM, J Biol Chem 290(52) (2015) 30924–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Geng J, Liu A, Heme-dependent dioxygenases in tryptophan oxidation, Arch Biochem Biophys 544 (2014) 18–26. [DOI] [PubMed] [Google Scholar]
- [16].Leklem JE, Quantitative aspects of tryptophan metabolism in humans and other species: a review, Am J Clin Nutr 24(6) (1971) 659–72. [DOI] [PubMed] [Google Scholar]
- [17].Marszalek-Grabska M, Walczak K, Gawel K, Wicha-Komsta K, Wnorowska S, Wnorowski A, Turski WA, Kynurenine emerges from the shadows - Current knowledge on its fate and function, Pharmacol Ther (2021) 107845. [DOI] [PubMed] [Google Scholar]
- [18].Prendergast GC, Chang MY, Mandik-Nayak L, Metz R, Muller AJ, Indoleamine 2,3-dioxygenase as a modifier of pathogenic inflammation in cancer and other inflammation-associated diseases, Curr Med Chem 18(15) (2011) 2257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fiore A, Murray PJ, Tryptophan and indole metabolism in immune regulation, Curr Opin Immunol 70 (2021) 7–14. [DOI] [PubMed] [Google Scholar]
- [20].Mazarei G, Leavitt BR, Indoleamine 2,3 Dioxygenase as a Potential Therapeutic Target in Huntington’s Disease, J Huntingtons Dis 4(2) (2015) 109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, Rusanescu G, Yang L, Tian Y, Mao J, Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression, J Clin Invest 122(8) (2012) 2940–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hornyak L, Dobos N, Koncz G, Karanyi Z, Pall D, Szabo Z, Halmos G, Szekvolgyi L, The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy, Front Immunol 9 (2018) 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu XH, Zhai XY, Role of tryptophan metabolism in cancers and therapeutic implications, Biochimie 182 (2021) 131–139. [DOI] [PubMed] [Google Scholar]
- [24].Feigelson P, Greengard O, A microsomal iron-porphyrin activator of rat liver tryptophan pyrrolase, J Biol Chem 236 (1961) 153–7. [PubMed] [Google Scholar]
- [25].Druyan R, Kelly A, The effect of exogenous -aminolaevulinate on rat liver haem and cytochromes, Biochem J 129(5) (1972) 1095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Welch AN, Badawy AA, Tryptophan pyrrolase in haem regulation. Experiments with administered haematin and the relationship between the haem saturation of tryptophan pyrrolase and the activity of 5-aminolaevulinate synthase in rat liver, Biochem J 192(2) (1980) 403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Greengard O, Feigelson P, Relationships of the Apo-Enzyme and Coenzyme of Tryptophan Pyrrolase in Developing and Regenerating Rat Liver, Ann N Y Acad Sci 111 (1963) 227–32. [DOI] [PubMed] [Google Scholar]
- [28].Greengard O, Feigelson P, The activation and induction of rat liver tryptophan pyrrolase in vivo by its substrate, J Biol Chem 236 (1961) 158–61. [PubMed] [Google Scholar]
- [29].Berry LJ, Smythe DS, Effects of Bacterial Endotoxins on Metabolism. Vi. The Role of Tryptophan Pyrrolase in Response of Mice to Endotoxin, J Exp Med 118 (1963) 587–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bissell DM, Hammaker LE, Effect of endotoxin on tryptophan pyrrolase and delta-aminolaevulinate synthase: evidence for an endogenous regulatory haem fraction in rat liver, Biochem J 166(2) (1977) 301–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nelp MT, Kates PA, Hunt JT, Newitt JA, Balog A, Maley D, Zhu X, Abell L, Allentoff A, Borzilleri R, Lewis HA, Lin Z, Seitz SP, Yan C, Groves JT, Immune-modulating enzyme indoleamine 2,3-dioxygenase is effectively inhibited by targeting its apo-form, Proc Natl Acad Sci U S A 115(13) (2018) 3249–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Thomas SR, Salahifar H, Mashima R, Hunt NH, Richardson DR, Stocker R, Antioxidants inhibit indoleamine 2,3-dioxygenase in IFN-gamma-activated human macrophages: posttranslational regulation by pyrrolidine dithiocarbamate, J Immunol 166(10) (2001) 6332–40. [DOI] [PubMed] [Google Scholar]
- [33].Lopez AS, Alegre E, Diaz A, Mugueta C, Gonzalez A, Bimodal effect of nitric oxide in the enzymatic activity of indoleamine 2,3-dioxygenase in human monocytic cells, Immunol Lett 106(2) (2006) 163–71. [DOI] [PubMed] [Google Scholar]
- [34].Hill M, Pereira V, Chauveau C, Zagani R, Remy S, Tesson L, Mazal D, Ubillos L, Brion R, Asghar K, Mashreghi MF, Kotsch K, Moffett J, Doebis C, Seifert M, Boczkowski J, Osinaga E, Anegon I, Heme oxygenase-1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3-dioxygenase, FASEB J 19(14) (2005) 1957–68. [DOI] [PubMed] [Google Scholar]
- [35].Chakravarti R, Aulak KS, Fox PL, Stuehr DJ, GAPDH regulates cellular heme insertion into inducible nitric oxide synthase, Proc Natl Acad Sci U S A 107(42) (2010) 18004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Dai Y, Sweeny EA, Schlanger S, Ghosh A, Stuehr DJ, GAPDH delivers heme to soluble guanylyl cyclase, J Biol Chem 295(24) (2020) 8145–8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ghosh A, Chawla-Sarkar M, Stuehr DJ, Hsp90 interacts with inducible NO synthase client protein in its heme-free state and then drives heme insertion by an ATP-dependent process, FASEB J 25(6) (2011) 2049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ghosh A, Stuehr DJ, Soluble guanylyl cyclase requires heat shock protein 90 for heme insertion during maturation of the NO-active enzyme, Proc Natl Acad Sci U S A 109(32) (2012) 12998–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ghosh A, Garee G, Sweeny EA, Nakamura Y, Stuehr DJ, Hsp90 chaperones hemoglobin maturation in erythroid and nonerythroid cells, Proc Natl Acad Sci U S A 115(6) (2018) E1117–E1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ghosh A, Dai Y, Biswas P, Stuehr DJ, Myoglobin maturation is driven by the hsp90 chaperone machinery and by soluble guanylyl cyclase, FASEB J 33(9) (2019) 9885–9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hannibal L, Collins D, Brassard J, Chakravarti R, Vempati R, Dorlet P, Santolini J, Dawson JH, Stuehr DJ, Heme binding properties of glyceraldehyde-3-phosphate dehydrogenase, Biochemistry 51(43) (2012) 8514–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sweeny EA, Singh AB, Chakravarti R, Martinez-Guzman O, Saini A, Haque MM, Garee G, Dans PD, Hannibal L, Reddi AR, Stuehr DJ, Glyceraldehyde-3-phosphate dehydrogenase is a chaperone that allocates labile heme in cells, J Biol Chem 293(37) (2018) 14557–14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ghosh A, Stuehr D, Hsp90 and Its Role in Heme-Maturation of Client Proteins: Implications for Human Diseases, 2019. [Google Scholar]
- [44].Sarkar A, Dai Y, Haque MM, Seeger F, Ghosh A, Garcin ED, Montfort WR, Hazen SL, Misra S, Stuehr DJ, Heat Shock Protein 90 Associates with the Per-Arnt-Sim Domain of Heme-free Soluble Guanylate Cyclase: IMplications for Enzyme Maturation, J Biol Chem 290(35) (2015) 21615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF Jr., Hunt NH, Stocker R, Kynurenine is an endothelium-derived relaxing factor produced during inflammation, Nat Med 16(3) (2010) 279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nagy BM, Nagaraj C, Meinitzer A, Sharma N, Papp R, Foris V, Ghanim B, Kwapiszewska G, Kovacs G, Klepetko W, Pieber TR, Mangge H, Olschewski H, Olschewski A, Importance of kynurenine in pulmonary hypertension, Am J Physiol Lung Cell Mol Physiol 313(5) (2017) L741–L751. [DOI] [PubMed] [Google Scholar]
- [47].Muneer A, Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations, Clin Psychopharmacol Neurosci 18(4) (2020) 507–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Billecke SS, Draganov DI, Morishima Y, Murphy PJ, Dunbar AY, Pratt WB, Osawa Y, The role of hsp90 in heme-dependent activation of apo-neuronal nitric-oxide synthase, J Biol Chem 279(29) (2004) 30252–8. [DOI] [PubMed] [Google Scholar]
- [49].Ghosh A, Stasch JP, Papapetropoulos A, Stuehr DJ, Nitric oxide and heat shock protein 90 activate soluble guanylate cyclase by driving rapid change in its subunit interactions and heme content, J Biol Chem 289(22) (2014) 15259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].McGaha TL, Huang L, Lemos H, Metz R, Mautino M, Prendergast GC, Mellor AL, Amino acid catabolism: a pivotal regulator of innate and adaptive immunity, Immunol Rev 249(1) (2012) 135–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Kohler C, Denis-Donini S, Cesura AM, Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells, J Immunol 159(1) (1997) 419–26. [PubMed] [Google Scholar]
- [52].Meng B, Wu D, Gu J, Ouyang S, Ding W, Liu ZJ, Structural and functional analyses of human tryptophan 2,3-dioxygenase, Proteins 82(11) (2014) 3210–6. [DOI] [PubMed] [Google Scholar]
- [53].Stuehr DJ, Misra S, Dai Y, Ghosh A, Maturation, Inactivation, and Recovery Mechanisms of Soluble Guanylyl Cyclase, J Biol Chem (2021) 100336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Peng HM, Morishima Y, Pratt WB, Osawa Y, Modulation of heme/substrate binding cleft of neuronal nitric-oxide synthase (nNOS) regulates binding of Hsp90 and Hsp70 proteins and nNOS ubiquitination, J Biol Chem 287(2) (2012) 1556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Pham KN, Lewis-Ballester A, Yeh SR, Conformational Plasticity in Human Heme-Based Dioxygenases, J Am Chem Soc 143(4) (2021) 1836–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Thomas SR, Mohr D, Stocker R, Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes, J Biol Chem 269(20) (1994) 14457–64. [PubMed] [Google Scholar]
- [57].Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL, Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes, J Clin Invest 114(2) (2004) 280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ, Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase, Nat Med 9(10) (2003) 1269–74. [DOI] [PubMed] [Google Scholar]
- [59].Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M, An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor, Nature 478(7368) (2011) 197–203. [DOI] [PubMed] [Google Scholar]
- [60].Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frederick R, De Plaen E, Uyttenhove C, Wouters J, Masereel B, Van den Eynde BJ, Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase, Proc Natl Acad Sci U S A 109(7) (2012) 2497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gostner JM, Becker K, Kofler H, Strasser B, Fuchs D, Tryptophan Metabolism in Allergic Disorders, Int Arch Allergy Immunol 169(4) (2016) 203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Hu Y, Chen Z, Jin L, Wang M, Liao W, Decreased expression of indolamine 2,3-dioxygenase in childhood allergic asthma and its inverse correlation with fractional concentration of exhaled nitric oxide, Ann Allergy Asthma Immunol 119(5) (2017) 429–434. [DOI] [PubMed] [Google Scholar]
- [63].Sidera K, Patsavoudi E, HSP90 inhibitors: current development and potential in cancer therapy, Recent Pat Anticancer Drug Discov 9(1) (2014) 1–20. [PubMed] [Google Scholar]
- [64].Porter JR, Fritz CC, Depew KM, Discovery and development of Hsp90 inhibitors: a promising pathway for cancer therapy, Curr Opin Chem Biol 14(3) (2010) 412–20. [DOI] [PubMed] [Google Scholar]
- [65].Labadie BW, Bao R, Luke JJ, Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis, Clin Cancer Res 25(5) (2019) 1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].D’Amato NC, Rogers TJ, Gordon MA, Greene LI, Cochrane DR, Spoelstra NS, Nemkov TG, D’Alessandro A, Hansen KC, Richer JK, A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer, Cancer Res 75(21) (2015) 4651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Ye Z, Yue L, Shi J, Shao M, Wu T, Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications, J Cancer 10(12) (2019) 2771–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gyulveszi G, Fischer C, Mirolo M, Stern M, Green L, Ceppi M, Wang H, Bürgi B, Staempfli A, Muster W, van Waterschoot R, Gloge A, Sade H, Klaman I, Hoelzlvimmer G, Surya A, Banerjee M, Shrivastava R, Middya S, Yadav D, Basu S, Acuna G, Abstract LB-085: RG70099: A novel, highly potent dual IDO1/TDO inhibitor to reverse metabolic suppression of immune cells in the tumor micro-environment, Cancer Research 76(14 Supplement) (2016) LB-085–LB-085. [Google Scholar]
- [69].White C, McGowan MA, Zhou H, Sciammetta N, Fradera X, Lim J, Joshi EM, Andrews C, Nickbarg EB, Cowley P, Trewick S, Augustin M, von Koenig K, Lesburg CA, Otte K, Knemeyer I, Woo H, Yu W, Cheng M, Spacciapoli P, Geda P, Song X, Smotrov N, Curran P, Heo MR, Abeywickrema P, Miller JR, Bennett DJ, Han Y, Strategic Incorporation of Polarity in Heme-Displacing Inhibitors of Indoleamine-2,3-dioxygenase-1 (IDO1), ACS Med Chem Lett 11(4) (2020) 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sun L, Advances in the discovery and development of selective heme-displacing IDO1 inhibitors, Expert Opin Drug Discov 15(10) (2020) 1223–1232. [DOI] [PubMed] [Google Scholar]
- [71].Pan S, Zhou Y, Wang Q, Wang Y, Tian C, Wang T, Huang L, Nan J, Li L, Yang S, Discovery and structure-activity relationship studies of 1-aryl-1H-naphtho[2,3-d][1,2,3]triazole-4,9-dione derivatives as potent dual inhibitors of indoleamine 2,3-dioxygenase 1 (IDO1) and trytophan 2,3-dioxygenase (TDO), Eur J Med Chem 207 (2020) 112703. [DOI] [PubMed] [Google Scholar]
- [72].Ghosh A, Koziol-White CJ, Jester WF Jr., Erzurum SC, Asosingh K, Panettieri RA Jr., Stuehr DJ, An inherent dysfunction in soluble guanylyl cyclase is present in the airway of severe asthmatics and is associated with aberrant redox enzyme expression and compromised NO-cGMP signaling, Redox Biol 39 (2021) 101832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sweeny EA, Schlanger S, Stuehr DJ, Dynamic regulation of NADPH oxidase 5 by intracellular heme levels and cellular chaperones, Redox Biol 36 (2020) 101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dai Y, Schlanger S, Haque MM, Misra S, Stuehr DJ, Heat shock protein 90 regulates soluble guanylyl cyclase maturation by a dual mechanism, J Biol Chem 294(35) (2019) 12880–12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Stuehr DJ, Ikeda-Saito M, Spectral characterization of brain and macrophage nitric oxide synthases. Cytochrome P-450-like heme proteins that contain a flavin semiquinone radical, J Biol Chem 267(29) (1992) 20547–50. [PubMed] [Google Scholar]
- [76].Sherchand S, Ibana JA, Quayle AJ, Aiyar A, Cell Intrinsic Factors Modulate the Effects of IFNgamma on the Development of Chlamydia trachomatis, Journal of bacteriology & parasitology 7(4) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within this manuscript or are available from the authors.