Supplemental Digital Content is Available in the Text.
Key Words: CB1 agonist, THC, cannabis, population pharmacokinetics, Δ9-tetrahydrocannabinol
Abstract
Background:
Unusually high variability in blood Δ9-tetrahydrocannabinol (THC) concentrations have been observed in subjects inhaling similar cannabis products over similar time periods when consumption is ad libitum. This makes simple gravimetric dose estimation a poor predictor of THC exposure. Population pharmacokinetic analyses of blood THC concentration versus time data are routinely used to estimate pharmacokinetic parameters. The aim of this study was to estimate the inhaled dose of THC in occasional and daily users of high potency market cannabis.
Methods:
Blood THC concentrations were measured for 135 minutes from 29 participants who either smoked high concentration flower or inhaled concentrates ad libitum during a 15-minute session. Frequent blood samples were obtained over the following 135 minutes.
Results:
The estimated central and rapidly equilibrating volumes of distribution of a 3-compartment model were 19.9 ± 1.2 and 51.6 ± 4.7 L whereas the intercompartmental clearances were 1.65 ± 0.14 and 1.75 ± 0.10 L/min, respectively. Covariate-adjusted analysis revealed that the estimated inhaled THC dose was considerably less among occasional users compared with daily users.
Conclusions:
Three-compartment pharmacokinetics of THC did not differ among the 3 user groups, and the early phase (first 135 minutes postinception of inhalation) kinetics were similar to those previously described after smoking low potency cannabis products. Therefore, inhaled THC dose can be estimated from pharmacokinetic data and covariate-driven adjustments can be used to estimate THC doses, based on the participant cannabis usage pattern (occasional versus daily), improving the accuracy of THC exposure estimates compared with those derived from weighed THC content alone.
INTRODUCTION
The use of cannabis among adults in the United States continues to rise,1,2 with more states legalizing its use for recreational and medical purposes.3 Despite this widespread use, the pharmacokinetics (PK) of the main psychoactive compound of cannabis, Δ9-tetrahydrocannabinol (THC), remains incomplete. Owing to federal restrictions on the source of cannabis products available to research institutions, most previous clinical trials used low-concentration cannabis made available from the National Institute on Drug Abuse (NIDA) Drug Supply Program and often adhered to paced smoking regimens intended to minimize variability in participants' delivered THC dose.4 Such uniformity was necessary to apply descriptive pharmacokinetic analyses (eg, Cmax, Tmax, Area-Under-the-Curve, and terminal half-life). However, the cannabis supplied by NIDA for these studies differed considerably, in cultivar and THC content, from that being sold in the current retail and medical marketplace.1,5,6
The investigation of THC pharmacokinetics in studies using NIDA-supplied cannabis has been constrained by the Drug Enforcement Agency (DEA) requirements that the participants consume identical combusted or vaporized cannabis products over a relatively uniform time interval.4,7–9 Most cannabis consumers self-administer, ad libitum, in a naturalistic manner. Cannabis plant material and concentrates may differ not only in THC content but also in moisture content, terpene profile, legal and illicit excipients, and residues (such as solvents, pesticides, or herbicides) that may influence the perceived quality (eg, harshness or irritancy) of the inhaled smoke or vapor. The temperature delivered by different pipes, vaping devices, and smoking techniques may also affect volatilization of cannabinoids and other constituents,7 which may contribute to relegating the starting point of most pharmacokinetic analyses, that is, the drug dose administered to unknowable variability. Yet, in a recent Request for Applications aimed at defining the pharmacokinetics and pharmacodynamics (PK/PD) of cannabinoids, NIDA stipulated that “results should be reported using the Standard THC Unit (STU), defined as 5 milligrams of THC (5 mg THC)”10 without first defining the imprecision and utility of undertaking the study of THC PK/PD with an assumed dose.
Assessing systemic exposure to THC is recognized as a key component to research examining the public health implications of cannabis consumption.11 Cannabis legalization has increased the diversity of delivery methods and potency of products available which affects public health outcomes,12 yet characterizing exposure with external techniques (ie, gravimetric and frequency) is difficult.13
Because of the diversity of cannabis delivery methods, observed user variability of inhalation efficiency, and titrated effects,9,14 a more accurate methodology for estimating THC dose is needed. It has been observed that distribution pharmacokinetics for a given drug show little interindividual variability in healthy adults because these processes are determined by physiological factors, such as tissue mass and blood flow,15 and physicochemical factors such as tissue:blood partitioning and protein binding.16 These principles allow for the construction of physiologically based pharmacokinetic models17 and form the basis of target-controlled infusions of anesthetic drugs18 in which plasma drug concentrations are held stationary by continuously adjusting the drug infusion rate to account for ongoing drug distribution. Therefore, it should be feasible to combine blood/plasma THC concentration data with a pharmacokinetic analysis to estimate the inhaled dose of THC.
To better understand real-world THC pharmacokinetics, a study should ideally allow research participants to bring their preferred cannabis product to a clinical laboratory and consume it in their usual manner. This study evaluated the pharmacokinetics of THC in occasional and daily cannabis users who smoked or vaped their preferred, commercially available cannabis product according to their usual pattern of consumption. It was hypothesized that these more denser, precisely timed blood THC concentrations versus time data would demonstrate, through a population pharmacokinetic analysis, small interindividual variability of parameters characterizing drug early distribution, and that the inhaled dose could be estimated and would show larger interindividual variability.
MATERIALS AND METHODS
Participants
We recruited 30 healthy adults (aged 21–55 years). Key inclusion criteria included cannabis use on a daily or weekly basis and willingness to consume a minimum of at least 2 inhalations of cannabis flower or concentrate products (eg, by dabbing or vaporizing wax or oil) containing less than 2% CBD. Participants were recruited into 3 groups: (1) occasional use characterized by an average of at least 2 days per month and no more than 3 days per week in the 90 days before enrollment, with typical use of flower products, (2) daily use with typical use of flower products, and (3) daily use with typical use of concentrate products (eg, vape pens or “dabs”).
Participants were asked to refrain smoking or vaping inhaled cannabis products for at least 8 hours before the data collection study visit and ingesting edible cannabis products for at least 12 hours before the data collection study visit. This study was approved by the Colorado Multiple Institutional Review Board (Protocol 20-0949).
Cannabis Use Procedures
Participants were asked to supply a product purchased legally in Colorado, which require state laboratory-verified labeling of THC concentration, with total THC (THC and tetrahydrocannabinolic acid) concentrations ranging from 15% to 30% for flower products and 60%–90% for extract/concentrate products. The product package details were recorded. Participants were instructed to weigh the initial and residual cannabis containing product placed or contained within the smoking or vaping device before and after smoking or vaping using a digital scale provided by the study staff, and the weight before and after use was recorded.
During a 15-minute interval, participants in the user groups were instructed to smoke or vape ad libitum “the amount you most commonly use for the effect you most commonly desire.” Start time was recorded as the inception of the first inhalation. The number of inhalations was counted, and the end time was noted as the last inhalation, which was no more than 15 minutes after the start time. Participants informed the research team which inhalation was intended to be their final inhalation.
Blood Collection
An upper arm vein was cannulated with an 18-gauge catheter for withdrawal of blood samples. Whole blood samples of approximately 5 mL were collected into tubes containing 100 mg sodium fluoride and 20 mg potassium oxalate additive at the following time points: baseline (minutes before the study's first inhalation); approximately 1, 5, and 10 minutes after the final inhalation; and then 30, 60, 90, and 145 minutes after the inception of the first inhalation. The actual midpoint of the blood withdrawal time relative to either the first or final inhalation of cannabis was recorded for pharmacokinetic analyses.
Sample Analysis
Reagents and Supplies
All cannabinoid standards and internal standards were purchased from Cerilliant (Round Rock, TX). Captiva EMR-Lipid columns (1 mL, 40 mg) were purchased from Agilent Technologies (Santa Clara, CA). Chromatography was performed with a 3.0 × 50 mm, 2.7 μm raptor biphenyl column (Restek, Inc., Bellefonte, PA).
Calibrators, Quality Controls, and Internal Standard Preparation
Matrix-matched calibrators and controls were prepared by the addition of appropriate volumes of methanolic stock standards mixes (0.01, 0.1, or 1.0 mcg/mL, or 10 mcg/mL of THC) to 100 µL of cannabinoid free blood. These were used to produce calibrators for THC at 0.5, 1, 5, 10, 50, 100, and 500 ng/mL. Quality controls samples were prepared at 5 and 10 ng/mL for each analyte. Quality control samples were run after every 20 subject samples with an expected accuracy of ±20%.
Cannabinoids Analysis by LC-MS/MS
Blood samples and matrix-matched standards and quality controls were prepared for LC-MS/MS analysis by protein precipitation and lipid removal. 10 μL of the internal standard solution was added to 100 μL of blood sample and mixed in a microcentrifuge tube, and then 600 μL of acetonitrile/methanol (85%/15%) was added and vortexed for 30 seconds to precipitate proteins. Samples were centrifuged and supernatants transferred to Captiva EMR-Lipid columns for lipid removal. Eluents were collected into a clean glass test tube and dried under nitrogen at 45°C. Eluents were reconstituted in 75 µL of water/methanol (50%/50%) with 0.1% acetic acid and transferred to autosampler vials for LC-MS/MS analysis.
Samples were analyzed with an Agilent 1290 UHPLC coupled to an Agilent 6460 triple quadruple mass spectrometer equipped with an Agilent Jet Stream electrospray ionization source (Agilent, Santa Clara, CA). Quantitation was performed using linear regression with 7-point calibration curves from 0.5 ng/mL to 500 ng/mL. Multiple cannabinoids were quantitated; however, only the values for THC are presented herein.
Each participant's baseline blood THC concentration was subtracted from the measured THC concentrations before pharmacokinetic analysis.
Pharmacokinetic Analyses
The pharmacokinetic analysis was performed using Phoenix NLME 8.3.4 with the FOCE ELS algorithm (Certara, Princeton, NJ). A 3-compartment pharmacokinetic model was used in keeping with models for rapid intravenous infusion and inhalation of lipid soluble, central nervous system–acting drugs such as ketamine19–21 and THC9,22 and in which the elimination clearance and slow equilibrating volume of distribution were fixed to previous estimates22 because no terminal phase pharmacokinetic data were collected in this study of 2.5 hours duration. In addition, we performed a 2-compartment analysis without linking to terminal phase pharmacokinetic estimates. The inhalation dose was arbitrarily assumed, for modeling purposes, to consist of a 60 mg THC joint smoked with 25% efficiency or bioavailability (ie, 15 mg fully bioavailable by inhalation). An individual's ad libitum inhaled dose was, therefore, estimated as a multiple of this assumed dose, Finhaled. For instance, Finhaled estimates of 0.5, 1.0, and 1.5 would correspond to estimated inhaled doses of 7.5 mg, 15 mg, and 22.5 mg, respectively. Variability of Finhaled may reflect deviations from the measured weight of the cannabis product consumed, the actual inhalation efficiency, or both.
Model parameters (θj, theta, for model parameter j) were assumed to be log-normally distributed across the population with a central, typical value (θTV) allowing for assessment of between subject variability (ηj,I, eta, for individual i and model parameter j) such that:
| (1) |
The residual within-subject error was calculated using the relative error method. Models were selected based on inspection of the data fits, standard errors of the parameter estimates (SEE) a decrease in the objective function, −2·Loglikelihood (−2LL), by 6.63 for addition of a model parameter to be statistically significant (χ2, P ≤ 0.01). THC concentration data were fit to a 3-compartment model. To accomplish this and to compare the current results to previous pharmacokinetic analyses, which were performed with plasma THC concentration measurements, the THC blood:plasma concentration ratio of 0.63 was applied to the measured blood THC concentrations.23 Values below the lower limit of quantitation (ie, <0.5 ng/mL) were pharmacokinetically analyzed according to the M3 method of Beal.24
Potential model covariates were sought through a stepwise search in which potential covariates were added when the −2·Loglikelihood decrease was at the P < 0.05 significance level (χ2-test; 3.84) and removal did not result in an increase of the −2·Loglikelihood at the P < 0.01 significance level (χ2-test; 6.63). Potential covariate effects of participant demographics that were continuous variables (ie, age and body mass index) were added to the base model in the following manner:
| (2) |
where θTV is the typical value for the parameter, Vardemographic is the continuous demographic variable, θcov is the parameter which quantifies the covariate effect, and ηi is a random variable describing the variance between the individual (θi) and population mean (θTV). Categorical variables (ie, sex and category of usual use pattern, such as occasional flower, daily concentrate, and daily flower) were added in the following manner:
| (3) |
In addition, a modeled analysis was conducted, in which the pattern of use was dichotomized (daily use versus occasional use) without regard to whether flower or concentrate was consumed in the dosing protocol.
The “weighed cannabis inhalation dose” (estimated by multiplying the product's labeled THC concentration times the measured weight difference preinhalation to postinhalation) was treated as a continuous covariate:
| (4) |
RESULTS
A total of 30 participants completed this study. Twenty were daily users, among which 10 consumed inhaled cannabis concentrate products and 10 smoked cannabis flower. One participant in the occasional use group had no detectable THC in their blood at the first or subsequent blood draws, and was dropped from the analysis, resulting in 29 participants (Table 1) with highly variable cannabis usage patterns (see Table, Supplemental Digital Content, http://links.lww.com/TDM/A754).
TABLE 1.
Participant Demographic Characteristics and Cannabis Use History
| Total | Daily, Flower | Daily, Concentrate | Occasional, Flower | |
| N = 29 | n = 10 | n = 10 | n = 9 | |
| Gender | ||||
| Male | 10 (34.5%) | 5 (50%) | 3 (30%) | 2 (22.2%) |
| Female | 19 (65.5%) | 5 (50%) | 7 (70%) | 7 (77.8%) |
| Age | ||||
| Mean (SD) | 32.8 (7.6) | 34.7 (8.3) | 32.2 (6.5) | 31.4 (7.8) |
| 21–29 | 12 (41.4%) | 4 (40%) | 3 (30%) | 5 (55.6%) |
| 30–39 | 12 (40%) | 3 (30%) | 6 (60%) | 3 (33.3%) |
| 40–49 | 3 (10%) | 2 (20%) | 1 (1%) | 0 (0%) |
| 50–53 | 2 (6.7%) | 1 (10%) | 0 (0%) | 1 (11.1%) |
| Race | ||||
| White | 25 (86.2%) | 9 (90%) | 8 (80%) | 8 (88.9%) |
| Black/African American | 3 (10.3%) | 1 (10%) | 2 (20%) | 0 (0%) |
| Other/no response | 1 (3.4%) | 0 (0%) | 0 (0%) | 1 (11.1%) |
| Ethnicity | ||||
| Hispanic/Latino | 5 (17.2%) | 1 (10%) | 3 (30%) | 1 (11.1%) |
| Non-Hispanic/Latino | 22 (75.9%) | 8 (80%) | 7 (70%) | 7 (77.8%) |
| Declined to respond | 2 (6.9%) | 1 (10%) | 0 (0%) | 1 (11.1%) |
| Body mass index (BMI) | ||||
| Mean (SD) | 26.9 (6.2) | 26.6 (6.4) | 27.8 (7.5) | 26.3 (4.5) |
| Age at first use | ||||
| Mean (SD) | 15.6 (2.5) | 15.2 (2.5) | 14.4 (2.2) | 17.3 (2.1) |
| Days used (past 30 days) | ||||
| Mean (SD) | 22.8 (9.8) | 28.8 (1.8) | 29.7 (0.5) | 9.1 (3.8) |
| Times used per day | ||||
| Mean (SD) | 3.7 (2.8) | 4.6 (3.5) | 5.2 (1.4) | 1.2 (0.2) |
The average percent THC (wt/wt) of the products used in this study was 39.4%, which was 22.0% and 23.8% in the 2 groups using flower, and 72.1% in the group using concentrate products. The average number of inhalations taken during the consumption period was 9.5 over an average of 5.4 minutes Table 2 presents additional data on participants' observed cannabis consumption and perceived drug effect by group.
TABLE 2.
Descriptive Summary of Participant Cannabis Consumption
| Total | Daily, Flower | Daily, Concentrate | Occasional, Flower | |
| N = 29 Mean (SD) |
n = 10 Mean (SD) |
n = 10 Mean (SD) |
n = 9 Mean (SD) |
|
| % Total THC (w/w) | 39.8 (26.1) | 22.0 (4.9) | 72.1 (18.7) | 23.8 (4.1) |
| Weight change (mg) | 178.5 (203.6) | 288.9 (266.6) | 50.0 (34.8) | 198.8 (169.8) |
| No. of inhalations | 9.5 (6.3) | 12.1 (5.7) | 6.4 (5.1) | 10.1 (6.9) |
| Consumption time (min) | 5.4 (3.6) | 7.6 (2.5) | 4.1 (3.3) | 4.3 (3.9) |
| Preconsumption drug effect (mm)* | 1.4 (2.4) | 0.6 (1.4) | 1.5 (2.9) | 2.1 (2.7) |
| Postconsumption, drug effect (mm)* | 74.9 (18.5) | 85.0 (9.4) | 77.9 (16.5) | 60.4 (20.5) |
The drug effect questionnaire asked, “Do you feel a drug effect right now?” Participants marked on a 100-mm line visual analog scale from “not at all (0 mm)” to “extremely (100 mm).”
There were 203 blood samples collected from 29 research participants. Two occasional users had a total of 6 blood samples, in both cases at later collection points, for which the THC concentrations were below the limit of quantitation. Figure 1 shows the measured blood THC concentration versus time data for all 29 participants included in the analysis, color-coded according to their reported use patterns.
FIGURE 1.
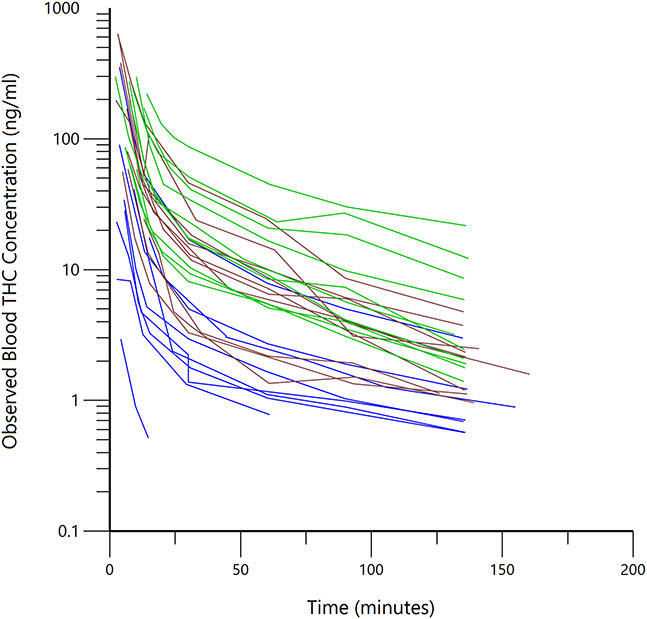
Observed blood THC concentration versus time, beginning with the first postinhalation blood sample, for all 29 subjects (each line connects the measured concentrations from one subject) after subtraction of the baseline (before inhalation) blood THC concentration. Blue lines are occasional flower users, brown lines are daily concentrate users, and green lines are daily flower users.
Figure 2 represents the final 3-compartment THC model. Table 3 includes the population pharmacokinetic parameter estimates. The value of the objective function (−2·Loglikelihood) of the 3-compartment pharmacokinetic model before inclusion of covariates was 1213.34. The 2-compartment pharmacokinetic analysis −2·Loglikelihood was 1219.1. There was a significant correlation (r2 = 0.97) of the individual Finhaled parameter estimates of the 2- versus 3-compartment analyses with a slope of 1.41, indicating a systematic 40% underestimation of the inhaled dose when the terminal phase is not accounted for by inclusion of a deep peripheral compartment. Therefore, subsequent results will refer to only the 3-comparment population pharmacokinetic analyses. No demographic covariates (ie, age, body weight, or sex), applied to any of the parameter estimates, reduced the objective function by more than 3.84 and, therefore, did not reach statistical significance in the forward segment of the stepwise covariate selection process. Inclusion of pattern of use (daily versus occasional) resulted in a −2·Loglikelihood of 1202.11, showing a significant (P < 0.001) reduction of 11.23 (see Figure 1, Supplemental Digital Content, http://links.lww.com/TDM/A755). Further delineation of usage pattern to include daily concentrate and daily flower use was significant at a lower level and did not further decrease the −2·Loglikelihood, so it was not included in the final model. Neither weighed dose nor inhalation device used showed a significant reduction in −2·Loglikelihood in the forward step. The variance (ω2) of the estimated Finhaled parameter (ie, the dose estimate) was 1.92 ± 0.022 without the usage pattern covariate and was 0.82 ± 0.23 with the covariate, indicating that approximately 50% of the interindividual variance of inhaled, bioavailable THC was explained by the usage pattern. Diagnostic plots appear as Supplemental Digital Content (see Figure 2, http://links.lww.com/TDM/A755).
FIGURE 2.
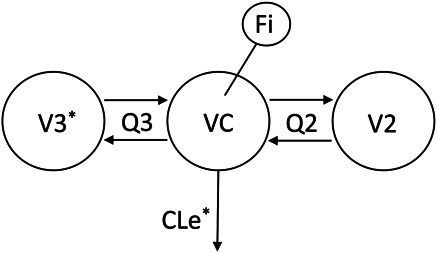
Final compartmental model used to fit plasma THC concentrations versus time. Drug is inhaled into VC of a 3-compartment THC model with rapidly and slowly equilibrating peripheral compartments V2 and V3, intercompartmental clearances Q2 and Q3, and elimination clearance CLe. Fi is the inhaled dose estimated as the fraction of a “standard” 15 mg absolute bioavailable THC dose. The *s signify model parameters which were fixed to the typical value estimates of those parameters from a previous study.22
TABLE 3.
Parameters of the Final Pharmacokinetic Model
| Typical value ± SEE | ω2 ± SEE | Shrinkage | |
| VC (L) | 17.9 ± 1.20 | 0.044 ± 0.044 | 0.29 |
| V2 (L) | 51.6 ± 4.66 | 0.16 ± 0.16 | 0.10 |
| V3* (L) | 3327 | ||
| Q2 (L/min) | 1.65 ± 0.14 | 0.13 ± 0.046 | 0.25 |
| Q3 (L/min) | 1.75 ± 0.10 | ||
| CLe* (L/min) | 0.72 | ||
| Fi | 0.12 ± 0.03 | 0.82 ± 0.02 | 0.004 |
| Covariate (daily user) | 1.79 ± 0.29 | ||
| δ | 0.15 ± 0.02 |
Parameters fixed to values from Sempio et al.22
δ, proportional (relative) intrasubject variability; ω2, intersubject variability; CLe, elimination clearance of THC; Covariatedaily user, value of the covariate for the estimated inhaled THC dose for both concentrate and flower users where the default is Covariateoccasional user = 0; Fi, fraction of nominal inhaled (bioavailable) THC ‘dose’ (15 mg); Q2 and Q3, intercompartmental clearances to the fast and slow compartments, respectively; SEE, standard error of the estimate; VC, V2, and V3, volumes of the central, fast, and slow equilibrating THC compartments, respectively.
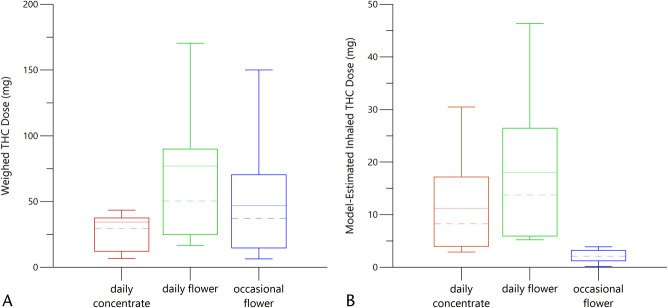
The mean weighed, used THC dose was 53.0 ± 58.5 mg, whereas the estimated inhaled amount of THC in all participants was 11.1 ± 11.1 mg. The daily users of concentrates had smaller weighed doses than the daily or occasional flower users (Fig. 3A). However, the estimated inhaled doses of THC were similar for both the daily users of concentrates and flower whereas the estimated inhaled THC doses were much smaller for the occasional users (Fig. 3B), mirroring the relationships among the observed concentration versus time profiles presented in Figure 1.
FIGURE 3.
A, Box plots of weighed THC doses with colors coded to match Figure 1. The dashed lines are the median values; the solid lines are the mean values. The ends of the “box” are the 25th and 75th percentiles. The whiskers show the lowest data value still within 1.5 IQR of the lower quartile and the highest value within 1.5 IQR of the upper quartile, whereas IQR is the interquartile range. B, Box plots of model-estimated inhaled THC doses with colors coded to match Figure 1. The dashed lines are median values; the solid lines are mean values. The ends of the “box” are the 25th and 75th percentiles. The whiskers show the lowest data value still within 1.5 IQR of the lower quartile and the highest value within 1.5 IQR of the upper quartile, whereas IQR is the interquartile range.
The weight of the consumed cannabis product multiplied by the product labeled THC concentration for a given inhalation event was a very poor predictor of the actual, inhaled THC amount or exposure, as presented in Figure 4.
FIGURE 4.
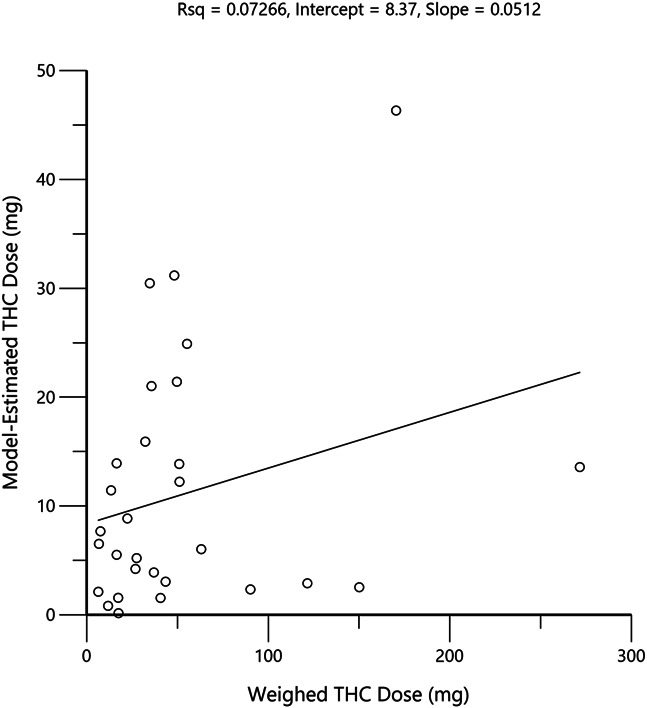
Plot of weighed THC doses and model-estimated inhaled THC doses for all subjects. Circles represent the values for each individual and the black line is the linear regression through the origin.
DISCUSSION
We described the population pharmacokinetics of THC in 29 subjects during the first 135 minutes after ad libitum inhalation of participant-selected Colorado commercial market cannabis flower and concentrates. Despite the vast differences in product composition and concentration between this study and 2 previous THC population pharmacokinetic analyses (15%–30% THC for cannabis flower and 60%–90% THC for the concentrates in this study—versus 1.75%, 3.55%, and 9% THC cannabis flower as modeled by Sempio et al,22 from the cannabis clinical trial published by Huestis et al4 as well as the study by Alvarez et al,25 the corrected blood-to-plasma and blood THC concentrations in this study were similar to those observed in the earlier studies in which the inhalation consumption of low concentration, NIDA-sourced cannabis,22 and THC and CBD powder added to tobacco25 were both rigidly controlled by prompted paced inhalation.
Because there were no blood samples obtained beyond 2.25 hours after inhalation of cannabis in this study, we fixed the values of the deep pharmacokinetic compartment (V3) and elimination clearance (Cle) according to Sempio et al. The pharmacokinetic parameters estimated from the 2 hours of plasma THC concentration versus time data correspond well with previous parameter estimates9,22 for the VC, V2, Q2, and Q3 of THC, although these previous studies included time points beyond 12 hours and this study did not include a formal washout period; instead subtracting baseline blood THC concentrations from all measurements to allow direct comparison with these previous studies9,22 and the THC plasma concentration profiles among our subjects without regard for their baseline starting point. Furthermore, using a parsimonious, reduced model (ie, 2-compartment), while fitting plasma THC concentration versus time data, resulted in a marked systematic underestimation of the inhaled dose (r2 = 0.97, slope = 1.41). This difference is in the same direction as the ratio of the sum of clearances for the respective models (1.83), suggesting a smaller dose is estimated for the 2-compartment model in which concentration versus time data is confined to the early distribution phase before full model identifiability of the ongoing rapid distribution to deep compartments and elimination clearance is apparent. Thus, it is reasonable to use the current THC population pharmacokinetic model parameters to simulate plasma and blood THC concentration profiles, and their variability in similar populations, of various market-available cannabis products for the first 2 hours after consumption when neuropsychological effects are most apparent.14,26 Further study and simulation may be required to best determine precise requirements for estimating the inhaled dose from limited concentration versus time data.
There was a significant difference in the estimated inhaled THC dose based on product labeling and precannabis to postcannabis product weight change between users of cannabis flower and concentrate, with the concentrate group using much less weighed dose. Despite this difference in dose, similar blood THC concentrations were observed for the 2 hours after their inhalation sessions in the 2 daily user groups, regardless of whether flower or concentrate was inhaled. The occasional cannabis flower users had similar weighed THC doses as the daily cannabis flower users. However, the model-estimated inhaled THC dose between the occasional users and both the 2 daily user groups was lower. This suggests that the occasional users are either much less efficient in their ability to extract the active drug from the cannabis product or may self-titrate to smaller inhaled doses and effects. Similar results between daily and occasional users were found by Alvarez et al,25 despite that study's rigidly prompted and paced smoking regimen. This difference associated with cannabis use history was verified by the population pharmacokinetic analysis because occasional versus daily cannabis use (without regard to daily use of flower or concentrate) was a statistically significant covariate for the estimates of THC inhaled dose. The parameter estimates for the covariate of usage pattern (occasional = 0, daily = 1.79 ± 0.67) translates to an approximate 5-fold difference in inhaled THC consumption on the day of the study. No other tested covariate met statistical inclusion criterion in the stepwise analysis for modeling of THC compartment volumes, clearances, and dose.
The interindividual variance terms, ω2, clearly indicate that the biggest contributor to variance among research participants is the estimated inhaled dose. This variability far exceeds any of the pharmacokinetic parameters found in this study. Nonetheless, the derived parametric indicators, along with observation of the raw data presented in Figures 1 and 4, are consistent with the notion that plasma or blood THC concentration data can reliably be used to estimate the inhaled THC dose. A previous study, using only 2 plasma THC concentration measurements after an unwitnessed cannabis use session,27 was able to estimate inhaled dose, but required many pharmacokinetic modeling assumptions. The current results with detailed pharmacokinetic data for approximately 2.25 hours, beginning approximately 60 seconds after a witnessed last inhalation, demonstrates both the feasibility and improved accuracy of using modeled pharmacokinetic data to estimate the THC dose.
The typical inhaled THC dose for daily users estimated in this study (10.78 mg) is only slightly larger than the assumed (fixed) value of 8.40 mg used by Sempio et al22 arrived at by assuming a bioavailability of 0.25 for a 33.6 mg low-concentration (3.55%) NIDA cannabis cigarette.28,29 Further studies with larger sample sizes and stratification according to dosage form may allow for covariate refinements of inhaled dose estimates according to product form (ie, flower versus concentrate) and method of inhalation (eg, joint, bowl, vape pen, and dabbing).
In this pharmacokinetic study of 29 subjects, focusing on the time of onset and dissipation of THC neuropsychological effects (ie, within 2.25 hours after inhalation), the estimates of the population pharmacokinetic model parameters most affecting the early time course of plasma and blood THC concentrations were verified and refined. The estimated interindividual variances for the central and rapidly equilibrating volumes of distribution along with the variance estimate for rapid intercompartmental clearance are similar to those reported by Sempio et al22 and Alvarez et al25 and demonstrate, in this larger sample, that the variability of early phase pharmacokinetics among research participants may be small. This observation is supported by Figure 1 in which the shapes of the individual observed blood THC concentrations are similar. Thus, it would be reasonable to apply Bayesian estimation techniques30 to sparse data (1–3 blood or plasma THC concentrations of known intervals) to both estimate the relevant pharmacokinetic parameters for the individual and use the Bayesian parameter estimates to simulate a full blood or plasma THC concentration versus time profile from the time of first inhalation to 2.25 hours postinhalation. Further studies and application of these concepts could have both clinical and forensic interests.
This population pharmacokinetic study of THC had several unique features: enrolled participants represented a wide range of cannabis usage patterns, varying from 2 times per week to 12 times per day; they used different cannabis products (flower and concentrates) from the commercial Colorado market, and the cannabis inhalation pattern on the study visit was ad libitum. This design yielded a diverse dose range which, when combined with an adaptable, but uniform and relatively dense blood sampling regimen, allowed comparison of pharmacokinetics across various patterns of usage and estimated inhaled THC dose. However, the participants in this study were mostly young adult White females (aged 21–39 years), so extrapolations to other populations will benefit from further study.
Certain study limitations were related to the regulatory environment regarding clinical research with this DEA Schedule I drug. The investigators were unable to take possession of and temporarily store a sample of the cannabis products self-supplied by the subjects for independent analysis; however, the product package label was the sole source for THC potency information. Baseline whole blood THC concentrations were subtracted from all subsequent postsmoking measurements. This introduced a small error (<5% over the 2 hours measurement interval), based on the assumption that after a minimum of 8 hours of cannabis abstinence, all subjects would be in the terminal elimination pharmacokinetic phase of THC, which is associated with a 30–100-h half-life.9,22,25,31
CONCLUSION
This population pharmacokinetic analysis of 2.25 hours of blood THC concentration versus time data following ad libitum inhalation of high potency cannabis flower and concentrate products compared closely with the findings of previous THC pharmacokinetic modeling9,22,25 and descriptive data.7,8 This study demonstrates how this population-based pharmacokinetic approach facilitates estimation of inhaled THC dose from the pattern of blood THC concentrations over time. Furthermore, the population pharmacokinetic analysis suggests that covariate-driven adjustment to weighed THC dose based on a subject's cannabis usage pattern (occasional versus daily) greatly improves the accuracy of estimating the inhaled THC dose.
Supplementary Material
Footnotes
Supported by the National Institutes of Health (R01 DA049800).
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.drug-monitoring.com).
Contributor Information
George S. Wang, Email: george.wang@childrenscolorado.org.
Greg Dooley, Email: gregory.dooley@colostate.edu.
Ashley Brooks-Russell, Email: ashley.brooks-russell@cuanschutz.edu.
Julia Wrobel, Email: julia.wrobel@emory.edu.
Sarah Limbacher, Email: sarah.limbacher@cuanschutz.edu.
Michael Kosnett, Email: michael.kosnett@cuanschutz.edu.
REFERENCES
- 1.Vergara D, Bidwell LC, Gaudino R, et al. Compromised external validity: federally produced cannabis does not reflect legal markets. Sci Rep. 2017;7:46528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Administration SAaMHS. NSDUH Detailed Tables. Available at: https://www.samhsa.gov/data/sites/default/files/reports/rpt29394/NSDUHDetailedTabs2019/NSDUHDetailedTabsTOC2019.htm#toc2019. Accessed November 12, 2023. [Google Scholar]
- 3.National Conference of State Legislatures. State Medical Cannabis Laws. Washington, DC: National Conference of State Legislatures; 2023. Available at: https://www.ncsl.org/health/state-medical-cannabis-laws. Accessed April 17, 2023. [Google Scholar]
- 4.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. [DOI] [PubMed] [Google Scholar]
- 5.Goodman S, Wadsworth E, Leos-Toro C, et al. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int J Drug Pol. 2020;76:102658. [DOI] [PubMed] [Google Scholar]
- 6.MPG Consulting. 2019 Regulated Marijuana Market Update. Denver, CO: MPG Consulting; 2020. [Google Scholar]
- 7.Spindle TR, Cone EJ, Schlienz NJ, et al. Acute pharmacokinetic profile of smoked and vaporized cannabis in human blood and oral fluid. J Anal Toxicol. 2019;43:233–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newmeyer MN, Swortwood MJ, Barnes AJ, et al. Free and glucuronide whole blood cannabinoids' pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: identification of recent cannabis intake. Clin Chem. 2016;62:1579–1592. [DOI] [PubMed] [Google Scholar]
- 9.Heuberger JA, Guan Z, Oyetayo OO, et al. Population pharmacokinetic model of THC integrates oral, intravenous, and pulmonary dosing and characterizes short- and long-term pharmacokinetics. Clin Pharmacokinet. 2015;54:209–219. [DOI] [PubMed] [Google Scholar]
- 10.Department of Health and Human Services. Pharmacokinetics (PK) and Pharmacodynamics (PD) of THC in Cannabis and Cannabis Products (R01—Clinical Trial Optional). Department of Health and Human Services; 2021. Available at: https://grants.nih.gov/grants/guide/rfa-files/RFA-DA-22-028.html. Accessed September 8, 2021. [Google Scholar]
- 11.Martin-Willett R, Helmuth T, Abraha M, et al. Validation of a multisubstance online Timeline Followback assessment. Brain Behav. 2020;10:e01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cinnamon Bidwell L, YorkWilliams SL, Mueller RL, et al. Exploring cannabis concentrates on the legal market: user profiles, product strength, and health-related outcomes. Addict Behav Rep. 2018;8:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prince MA, Conner BT, Pearson MR. Quantifying cannabis: a field study of marijuana quantity estimation. Psychol Addict Behav. 2018;32:426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bidwell LC, Ellingson J, Karoly HC, et al. Association of naturalistic administration of cannabis flower and concentrates with intoxication and impairment. JAMA Psychiatry. 2020;77:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krejcie TC, Henthorn TK, Shanks CA, et al. A recirculatory pharmacokinetic model describing the circulatory mixing, tissue distribution and elimination of antipyrine in dogs. J Pharmacol Exp Ther. 1994;269:609–616. [PubMed] [Google Scholar]
- 16.Garzone PD, Atkinson AJ, Jr. In search of physiologically based distribution volume estimates for macromolecules. Clin Pharmacol Ther. 2012;92:419–421. [DOI] [PubMed] [Google Scholar]
- 17.Rostami AA, Campbell JL, Pithawalla YB, et al. A comprehensive physiologically based pharmacokinetic (PBPK) model for nicotine in humans from using nicotine-containing products with different routes of exposure. Sci Rep. 2022;12:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struys MM, De Smet T, Glen JI, et al. The history of target-controlled infusion. Anesth Analg. 2016;122:56–69. [DOI] [PubMed] [Google Scholar]
- 19.Henthorn TK, Avram MJ, Dahan A, et al. Combined recirculatory-compartmental population pharmacokinetic modeling of arterial and venous plasma S (+) and R (–) ketamine concentrations. Anesthesiology. 2018;129:260–270. [DOI] [PubMed] [Google Scholar]
- 20.Kamp J, Olofsen E, Henthorn TK, et al. Ketamine pharmacokinetics: a systematic review of the literature, meta-analysis, and population analysis. Anesthesiology. 2020;133:1192–1213. [DOI] [PubMed] [Google Scholar]
- 21.Jonkman K, Duma A, Olofsen E, et al. Pharmacokinetics and bioavailability of inhaled esketamine in healthy volunteers. Anesthesiology. 2017;127:675–683. [DOI] [PubMed] [Google Scholar]
- 22.Sempio C, Huestis MA, Mikulich‐Gilbertson SK, et al. Population pharmacokinetic modeling of plasma Δ9‐tetrahydrocannabinol and an active and inactive metabolite following controlled smoked cannabis administration. Br J Clin Pharmacol. 2020;86:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karschner EL, Schwope DM, Schwilke EW, et al. Predictive model accuracy in estimating last Δ9-tetrahydrocannabinol (THC) intake from plasma and whole blood cannabinoid concentrations in chronic, daily cannabis smokers administered subchronic oral THC. Drug Alcohol Dependence. 2012;125:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodynamics. 2001;28:481–504. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez JC, Hartley S, Etting I, et al. Population pharmacokinetic model of blood THC and its metabolites in chronic and occasional cannabis users and relationship with on-site oral fluid testing. Br J Clin Pharmacol. 2021;87:3139–3149. [DOI] [PubMed] [Google Scholar]
- 26.Spindle TR, Cone EJ, Schlienz NJ, et al. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw Open. 2018;1:e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sempio C, Bidwell C, Hutchison K, et al. Using population pharmacokinetic modeling to estimate exposure to Δ9-tetrahydrocannabinol in an observational study of cannabis smokers in Colorado. Ther Drug Monit. 2021;43:536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. [DOI] [PubMed] [Google Scholar]
- 29.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. II. Models for the prediction of time of marijuana exposure from plasma concentrations of delta 9-tetrahydrocannabinol (THC) and 11-nor-9-carboxy-delta 9-tetrahydrocannabinol (THCCOOH). J Anal Toxicol. 1992;16:283–290. [DOI] [PubMed] [Google Scholar]
- 30.Brocks DR, Hamdy DA. Bayesian estimation of pharmacokinetic parameters: an important component to include in the teaching of clinical pharmacokinetics and therapeutic drug monitoring. Res Pharm Sci. 2020;15:503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson EVA, Agurell S, Hollister LE, et al. Prolonged apparent half-life of Δ1-tetrahydrocannabinol in plasma of chronic marijuana users. J Pharm Pharmacol. 1988;40:374–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.