Abstract
Cytotoxic T-lymphocyte (CTL) responses have been implicated as playing an important role in control of human immunodeficiency virus (HIV) infection. However, it is technically difficult to demonstrate CTL responses consistently in nonhuman primate and human subjects using traditional cytotoxicity assay methods. In this study, we systematically evaluated culture conditions that may affect the proliferation and expansion of CTL effector cells and presented a sensitive method for detection of cytotoxicity responses with bulk CTL cultures. We confirmed the sensitivity and specificity of this method by demonstration of vigorous CTL responses in a simian-HIV (SHIV)-infected rhesus macaque. The expansion of epitope-specific CTL effector cells was also measured quantitatively by CTL epitope-major histocompatibility complex tetramer complex staining. In addition, two new T-cell determinants in the SIV gag region are identified. Last, we showed the utility of this method for studying CTL responses in chimpanzee and human subjects.
Cytotoxic T lymphocytes (CTL) are CD8+ αβ T lymphocytes, and their T-cell receptors (TCR) recognize small peptides of 8 to 12 amino acids presented by major histocompatibility complex (MHC) class I molecules on the cell surface (5, 13). These peptides are derived from intracellular antigens via the endogenous antigen processing and presentation pathway (15, 33). The TCR recognition of the peptide-MHC class I molecule complexes on the cell surface will trigger the cytolytic activity of CTL, resulting in the death of cells presenting the peptide-MHC class I complexes (20). Partly because of this cytotoxic function, CTL responses have been implicated as playing an important role in control of viral infection (19, 26, 41).
The importance of CTL responses in control of human immunodeficiency virus (HIV) infection has been indicated in many studies. First, appearance of vigorous CTL responses in HIV-1 or simian immunodeficiency virus (SIV)-infected subjects was found to be temporally associated with control of primary viral infection (6, 22, 23). Second, studies showed that vigorous CTL responses in HIV-infected individuals can exert strong selective pressure on the virus in the hosts to evolve escape mutants (7, 29). Third, strong T-cell immunity has been associated with effective control of viremia and prolonged prevention of disease progression in HIV-infected patients (16, 17, 31, 34). Fourth, the frequency of CTL precursors (CTLp), determined by limiting dilution assay and by CTL epitope-specific tetramer staining of T cells, has been shown to be inversely correlated with virus load in SIV-infected rhesus macaques and HIV-infected human subjects, respectively (12, 32). Last, in an SIV-infected rhesus macaque model, it has been shown in two independent studies that rhesus monkeys failed to control viral infection when their CD8+ T-cell population was depleted by administration of anti-CD8 monoclonal antibodies prior to acute infection or during chronic infection (37, 17a).
It is difficult to study CTL responses using the cytotoxicity assay in nonhuman primate and human subjects because of the polymorphic MHC background in the primate and human populations. Autologous B-lymphoid cell lines (BLCL) have to be established for each subject to serve as target cells in order to match the MHC alleles in a cytotoxicity assay. Additionally, the undefined MHC alleles in each subject make the identification and characterization of CTL epitopes in any antigen of interest a technical challenge. To circumvent these problems, recombinant vaccinia viruses are frequently used to deliver antigens (4). This type of approach entails using inactivated autologous B cells infected with vaccinia virus to provide antigen-specific restimulation for activation and expansion of memory T cells in culture and using vaccinia virus-infected autologous B cells as targets in a cytotoxicity assay (21, 28, 40). The assay is generally less sensitive and at times inconsistent because of vaccinia virus-specific background, and often only borderline responses can be demonstrated. Although many novel methods have been developed to study CD8+ T-cell responses, including gamma interferon-based ELISPOT assay and the epitope peptide-MHC class I complex tetramer staining assay (2, 24), the bulk culture cytotoxicity assay is still regarded as an important method for assessing T cells' cytotoxic function. ELISPOT and cytotoxicity assays are complementary to each other, as they are designed to evaluate different functional aspects of CD8+ T-cell response. The objective of this study is to optimize the CTL culture and cytotoxicity assay conditions for both nonhuman primates and humans. Here we show, by using unfixed autologous peripheral blood mononuclear cells (PBMC) infected with recombinant vaccinia virus for antigen-specific restimulation, that satisfactory expansion of CTL effector cells can be achieved in cultures. In addition, by using synthetic peptide pools to sensitize B lymphoid cells as targets, we can obtain consistent results with remarkable sensitivity in a cytotoxicity assay. A series of cytotoxicity experiments were conducted to further confirm the sensitivity and specificity of this configuration.
MATERIALS AND METHODS
Reagents, synthetic peptides, and recombinant vaccinia viruses.
The cell culture reagents were purchased from Gibco-BRL Life Technologies (Grand Island, N.Y.). RPMI 1640 medium, supplemented with 2 mM l-glutamine, 5 × 10−5 M β-mercaptoethanol, 5 mM HEPES, plus 25 μg of pyruvic acid, 100 U of penicillin, and 100 μg of streptomycin per ml, was designated R-10 when supplemented with 10% fetal bovine serum (FBS) and R-20 when supplemented with 20% FBS. Recombinant human interleukin-2 (IL-2) was purchased from the Cellular Products Company (Buffalo, N.Y.), and recombinant human IL-7 was from R&D Systems (Minneapolis, Minn.).
The synthetic peptides were custom ordered from the following commercial vendors: Multiple Peptide System (San Diego, Calif.), Massachusetts General Hospital Core Facility (Charlestown, Mass.), and Genesys Biotechnology Company (Houston, Tex.). All peptides were solubilized in straight dimethyl sulfoxide (DMSO) at 20 mg/ml (or 10 mg/ml if insoluble at 20 mg/ml) and stored in small aliquots at −70°C. The peptide pools were composed of an equal volume of each peptide, and the final concentration of each peptide in the pool depends on the number of peptides included in the pool. The typical concentration is in the range of 0.4 to 0.8 mg/ml. The peptides for making pools are 20-mers overlapping by 10 amino acids to cover part or all of the open reading frame of the antigen.
The generation and characterization of recombinant vaccinia viruses have been described elsewhere (11). Vac-SC was used as a wild-type control vaccinia virus, expressing only β-galactosidase under the control of the p11 promoter.
Nonhuman primate and human subjects.
Monkey MmL-3 was infected with 400 50% tissue culture infective doses (TCID50) of a nonpathogenic SIV-HIV (SHIV) composed of SIVmac239 backbone expressing the HIV-1HXBc2 rev, tat, vpu, and env genes as described earlier (27). Monkey Mm94-98, a Mamu-A*01-positive rhesus monkey infected with SIV, has also been described earlier (23). Chimpanzee 289 was infected with HIV-15016 in 1994, and chimp 316 was infected with HIV-1DH12 in 1994 and with HIV-1IIIB in 1997 (36). Both chimps are housed at the Southwest Foundation for Biological Research, San Antonio, Tex. PBMC samples from HIV-infected subjects were collected from volunteers attending clinics at the University of Pittsburgh, Mt. Sinai Hospital in New York, and the State University of New York at Stony Brook. Subjects 30495 and 402 were not treated with any antiviral therapy at the time the PBMC sample was collected, with their viral loads determined to be 9,050 and 1,728 copies/ml, respectively. Their CD4 counts were determined to be 920 and 1,247 cells/μl, respectively. Subject 802 was treated with highly active antiretroviral therapy, with her viral load and CD4 counts determined to be 1,040 copies/ml and 498 cells/μl, respectively.
Bulk culture of lymphocytes.
The procedures for establishing cultures for CTL with fresh or cryopreserved PBMC were adapted from a published method (9, 10). Briefly, 20% or another specified percentage of total PBMC were infected in a 0.5-ml volume with recombinant vaccinia virus at a multiplicity of infection (MOI) of 5 for 1 h at 37°C and then recombined with the remaining PBMC sample. The cells were washed once in 10 ml of R-10 medium and plated in a 12-well plate at approximately 5 × 106 to 10 × 106 cells/well in 4 ml of R-10 medium. Recombinant human IL-7 was added to the culture at 330 U/ml. Two or three days later, 1 ml of R-10 containing recombinant human IL-2 (rhIL-2, 100 U/ml) was added to each well. Twice weekly thereafter, 2 ml of culture medium was replaced with 2 ml of fresh R-10 medium with rhIL-2 (100 U/ml). The lymphocytes were cultured at 37°C in the presence of 5% CO2 for approximately 2 weeks and used in the cytotoxicity assay described below.
Cytotoxicity assay.
The effector cells harvested from bulk CTL cultures were tested against autologous BLCL expressing specific antigens, either through infection with recombinant vaccinia virus or by sensitization with peptides. To prepare vaccinia virus-infected targets, the BLCL cells (5 × 106 to 10 × 106 cells) were infected with recombinant vaccinia virus at an MOI of 10 in 5 ml of R-10 medium overnight at 37°C. The cells were washed once and labeled with 5 to 10 μl of Na51CrO4 (approximately 100 μCi; Amersham Life Sciences, Arlington Heights, Ill.) in 0.5 ml in a 15-ml conical tube for 1 to 2 h at 37°C. The target cells were then washed three times, enumerated, and resuspended at 5 × 104 cells/ml in R-10. To prepare the peptide-sensitized targets, the BLCL cells were washed once with R-10 medium, enumerated, and pulsed with the individual peptide (5 μg/ml) or peptide pool (about 4 to 8 μg/ml for each individual peptide) in a 0.5-ml volume. A mock target was prepared by pulsing cells with peptide-free DMSO diluent to match the DMSO concentration in the peptide-pulsed targets. From 5 to 10 μl of Na51CrO4 was added to the tubes at the same time, and the cells were incubated for 1 to 2 h 37°C. The cells were then washed three times and resuspended at 5 × 104 cells/ml in R-10 medium to be used as target cells. The cultured lymphocytes were plated with target cells at designated effector-to-target cell (E:T) ratios in triplicate in 96-well plates and incubated at 37°C for 4 h in the presence of 5% CO2. A sample of 30 μl of supernatant from each well of cell mixture was harvested onto a well of a Lumaplate-96 (Packard Instruments, Meriden, Conn.), and the plate was allowed to air dry overnight. The amount of 51Cr in the well was determined through beta-particle emission, using a plate counter from Packard Instruments. The percent specific lysis was calculated using the formula % specific lysis = (E − S)/(M − S), where E is the average counts per minute (cpm) released from target cells in the presence of effector cells, S is the spontaneous cpm released in the presence of medium only, and M is the maximum cpm released in the presence of 2% Triton X-100.
Limiting-dilution culture conditions and analyses.
The limiting-dilution analysis (LDA) protocol was modified from a previously published method (21, 22). Briefly, 10% or another specified percentage of the PBMC sample was infected with recombinant vaccinia virus expressing SIV Gag (Vac-SIVgag) at an MOI of 5 in 0.5 ml in a 15-ml conical tube for 1 h and then mixed with the remaining PBMC sample. The cells were washed once with R-10 medium and plated in 24 replicates in U-bottomed 96-well plates in serial titrations (e.g., 16,000, 12,000, 6,000, 3,000, 1,500, 750, and 500 cells/well) in a total of 100 μl of R-10 medium. Control wells containing no PBMC but only 100 μl of R-10 medium were included as a negative control. Gamma-irradiated (4,000 rads) feeder cells (50,000 cells/well of human PBMC, unless otherwise specified) were added in a volume of 100 μl to each well. Two or three days later, 50 μl of R-10 medium containing rhIL-2 at 100 U/ml was added to each well. Twice weekly thereafter, 100 μl of culture medium from each well was removed and replaced with 100 μl of fresh R-10 medium with rhIL-2 (100 U/ml). The cultures were incubated at 37°C in the presence of 5% CO2 for approximately 2 weeks. Each microculture in the 96-well plates was then split into three sets of 96-well plates with 50 μl per well for each set, and R-10 medium was used to bring the volume to 100 μl for each well. Each set was used for a cytotoxicity assay against one type of target cells (mock-, Vac-SC-, and Vac-SIVgag-infected autologous B lymphoid cells). A well was considered positive (presumably containing at least one CTLp) if the cpm value is greater than the mean of the medium control well values plus 3 standard deviations. The CTLp frequency was determined by the maximum-likelihood method (8), based on the cumulative negative hits at each cell titration, and calculated by using a spreadsheet generously provided by S. A. Kalams (Boston, Mass.).
Tetramer staining.
The procedure for tetramer staining of fresh whole blood was adopted from a published procedure (23). Briefly, phycoerythrin (PE)-conjugated tetramer Mamu-A*01/p11C complex in conjunction with fluorescent-conjugated surface marker monoclonal antibodies were used to stain 100 μl of fresh whole blood sample or about 5 × 105 cells from bulk CTL cultures. The monoclonal antibodies used in this study were anti-CD8α-PerCP (Becton Dickinson, San Jose, Calif.) and anti-rhesus monkey CD3-APC (clone FN18). The red blood cells were lysed using the kit from Becton Dickinson, and the cells were washed once with cold phosphate-buffered saline (PBS) and fixed with 1% formaldehyde in PBS. The cells were stored at 4°C for 1 day before flow cytometric analysis on a FACSCalibur (Becton Dickinson). PE-conjugated tetramer Mamu-A*01/p11C complex and anti-rhesus monkey CD3-APC were generously provided by N. Letvin (Boston, Mass.).
RESULTS
It was reported that unfixed autologous PBMC infected with recombinant vaccinia virus could be used as stimulators for human CTL cultures (9, 10, 14). To reproduce this procedure and address the concern about cytopathic effects of vaccinia virus, we first evaluated viability of unfixed PBMC infected with vaccinia virus in culture. PBMC samples from healthy human donors were infected with recombinant vaccinia virus at MOI of 0, 0.01, 0.03, 0.1, 0.3, 1, 3, and 10 for 1 h and then incubated in complete R-10 medium complemented with rhIL-2 for 1 week. The viability of the cultured lymphocytes was monitored on days 1, 3, 5, and 7. After 1 week, the supernatants and the cell lysates from each culture were assayed for vaccinia virus infection. The viability results showed that all cultures had a similar number of viable cells during the 1-week culture, regardless of the amount of vaccinia virus used for the initial infection. The results from the plaque assay confirmed that all cultures except the one at an MOI of 0 had active viral replication, with at least a 10,000- to 100,000-fold increase in virus titers. Although we could not identify the particular cell type in PBMC that was susceptible to vaccinia virus infection, the experiment did address the concern about the pathogenicity of the viral infection on PBMC in short-term cultures. In conclusion, these results confirmed the findings by other laboratories (9, 10, 14) and indicated that PBMC can survive infection with vaccinia virus in short-term cell culture.
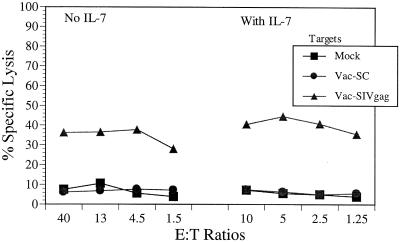
We used this procedure to detect the CTL responses of an SHIV-infected rhesus macaque. Monkey MmL-3 was infected with 400 TCID50 of a nonpathogenic SHIV composed of SIVmac239 backbone expressing the HIV-1HXBc2 rev, tat, vpu, and env genes (27). The CTL responses against SIV gag and HIV-1 env antigens have been demonstrated in this monkey with its CTL cultures stimulated with fixed autologous B lymphoid cells infected with relevant recombinant vaccinia viruses (39, 40). In the experiment shown in Fig. 1, one-tenth of the PBMC sample used for bulk culture was infected with recombinant vaccinia virus expressing SIV Gag and then recombined with the remaining PBMC in culture for 2 weeks. No procedures were used to inactivate virus. A similar culture supplemented with IL-7 was also established in parallel to evaluate the effect of IL-7 on CTL bulk cultures. The cultures were tested for cytotoxicity against autologous B lymphoid cells infected with Vac-SC (vaccinia virus control) or Vac-SIVgag or mock infected. The results of cytotoxicity assays showed that cultured CTL effectors from both sets were cytolytic against Vac-SIVgag-infected target cells, with 30 to 40% specific lysis over mock- and Vac-SC-infected target cells at all E:T ratios tested (Fig. 1). Although there seemed to be no direct benefit of IL-7 on cytolytic function of the lymphocyte effector cells, we did observe that almost twice the number of viable lymphocytes could be recovered from cultures with IL-7 compared to the ones without IL-7 (data not shown).
FIG. 1.
CTL responses demonstrated in rhesus macaque MmL-3 using unfixed autologous PBMC infected with vaccinia virus for restimulation. Ten percent of the PBMC sample from MmL-3 were infected with Vac-SIVgag and cultured in vitro with the remaining PBMC for 2 weeks with or without IL-7. The effector cells were tested in a cytotoxicity assay against autologous BLCL infected overnight with Vac-SC (vaccinia virus control) or Vac-SIVgag or mock infected.
To further optimize the conditions for bulk CTL cultures, we performed a series of LDA for CTLp frequency assessment to quantitatively define the effect of each parameter on the cytolytic function of the lymphocyte culture. The LDA cultures were established with MmL-3 PBMC, with 10% (or otherwise specified) autologous PBMC infected with Vac-SIVgag as stimulators at seven serial dilutions, and cultured under the conditions indicated in Table 1. The micro-cultures were split and tested against mock, Vac-SC, or Vac-SIVgag-infected target cells in cytotoxicity assay. The result of CTLp frequencies with 95% confidence intervals from these experiments are shown in Table 1.
TABLE 1.
CTLp frequency of MmL-3 under different culture conditions
| Culture conditions | No. of CTLp/106 PBMC (95% confidence limits)
|
||
|---|---|---|---|
| Mock | Vac-SC | Vac-SIVgag | |
| Different stimulators added | |||
| 10% autologous PBMC (unfixed) | 1 (3–16) | 9 (5–18) | 211 (163–273) |
| 10% BLCL/psoralen/UV fixed | 3 (1–9) | 5 (2–12) | 29 (19–44) |
| 10% BLCL/paraformaldehyde fixed | 4 (1–11) | 3 (1–9) | 75 (56–101) |
| Time of LDA culture | |||
| 9 days | 1 (0–8) | 4 (1–11) | 11 (6–21) |
| 14 days | 17 (10–29) | 2 (0–8) | 187 (144–241) |
| 16 days | 6 (2–14) | 1 (0–8) | 357 (271–470) |
| 21 days | 13 (7–24) | 10 (5–20) | 195 (151–253) |
| IL-2 addeda | |||
| 1× (normal regimen) | 13 (7–23) | 5 (2–12) | 116 (88–151) |
| 0.5× | 6 (3–14) | 7 (3–15) | 97 (74–128) |
| 2× | 129 (99–168) | 111 (85–145) | 433 (327–574) |
| 4× | 201 (155–260) | 165 (128–214) | 334 (255–439) |
| Irradiated feeder cells added | |||
| Human PBMC | 1 (0–8) | 14 (4–17) | 139 (76–132) |
| Rhesus PBMC | 7 (3–16) | 16 (10–27) | 61 (45–84) |
| Mouse splenocytes | 5 (2–12) | 58 (42–79) | 18 (11–29) |
| None | 2 (0–8) | 1 (0–8) | 147 (114–191) |
| IL-7 added to LDA cultureb | |||
| No | 15 (9–26) | 9 (5–19) | 157 (121–260) |
| Yes | 9 (5–19) | 5 (2–12) | 187 (145–242) |
| % of autologous PBMC infectedc | |||
| 10% | 7 (3–15) | 8 (4–16) | 197 (153–253) |
| 20% | 13 (9–24) | 7 (3–15) | 228 (177–293) |
| 100% | 22 (14–35) | 12 (7–22) | 92 (70–121) |
IL-2 feeding schedule and normal concentration are detailed in Materials and Methods.
IL-7 was added on day 0 at 330 U/ml.
The percentages indicate the portions of PBMC in the cultures that were infected with Vac-SIVgag for restimulation.
First, we compared the different restimulation methods for expansion of cytotoxic effector cells in culture. Two widely adopted antigen-specific methods use vaccinia virus-infected B lymphoid cells as stimulators, and both require fixation of stimulator cells to inactivate vaccinia virus (18, 40). To compare these two methods to the use of vaccinia virus-infected autologous PBMC (unfixed) as stimulator cells, we mixed the MmL-3 PBMC with the fixed B lymphoid stimulator cells at a 10:1 ratio and established the LDA cultures accordingly. The results showed that using autologous PBMC as stimulator cells is a better choice for activating and expanding precursor cytotoxic effector cells in culture than the other two methods. Second, we measured the kinetics of development of cytotoxic effector cells in culture. The CTLp frequency against Vac-SIVgag-infected target was almost undetectable after 9 days in culture and readily detected in the cultures after 14 days of incubation. Although CTLp can still be seen after 3 weeks in culture, we confirmed that the optimal incubation time for detecting CTL effector function is about 2 weeks, as generally used by others (21, 22). Third, we evaluated the effect of the concentration of rhIL-2 on expansion of precursor CTL in culture. The data showed that higher rhIL-2 concentrations resulted in nonspecific high background killing in the cytotoxicity assay without increasing overall sensitivity. Fourth, we replaced the human PBMC feeder cells with either allogeneic rhesus PBMC or mouse splenocytes as feeder cells in LDA cultures and showed that human PBMC are a better choice for feeder cells than rhesus PBMC or mouse splenocytes. Also we observed that MmL-3 CTLp proliferated and expanded without any feeder cells in cultures, as the equivalent CTLp frequency was detected against Vac-SIVgag-infected target cells. This observation suggests that irradiated allogeneic feeder cells are not critical for expansion of CTL effector cells in cultures if appropriate cell density is provided. Fifth, we showed in LDA culture that addition of IL-7 did not affect the cytotoxic function of the effectors, as equivalent CTLp frequencies were detected in both sets. Last, we showed that 10, 20, or even 100% autologous PBMC can be infected with Vac-SIVgag as the stimulators to provide antigen in LDA cultures. Although this does not appear to result in cytopathic effect by vaccinia virus infection on lymphocyte cultures, the benefit is not obvious by increasing the percentage of cells infected with vaccinia virus in culture. In conclusion, we determined the conditions for our bulk CTL cultures by using 20% autologous PBMC infected with vaccinia virus without fixing for antigen-specific stimulation, including IL-7 in the cultures, and culturing lymphocytes for about 2 weeks before testing in the cytotoxicity assay. Although these conditions were initially determined by the series of experiments with PBMC from one monkey (Table 1), they have been confirmed in several human LDA assays (data not shown).
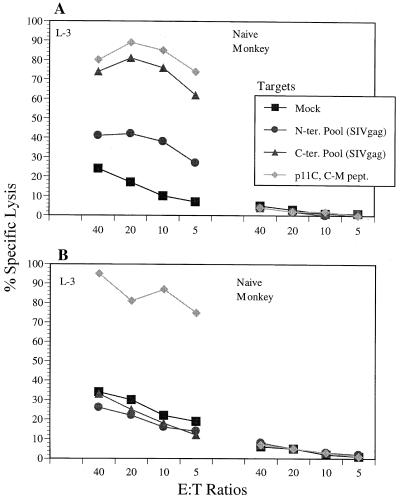
It has been shown previously that monkey MmL-3 expresses the Mamu-A*01 allele and exhibited a CTL response directed against a dominant CTL determinant, p11C, located at residues 181 to 189 of SIV Gag (1, 30). Also, we noticed that MmL-3 bulk CTL cultures, although very efficient in lysing Vac-SIVgag-infected autologous B lymphoid cells at lower E:T ratios, could lyse no more than 50% of target cells at the higher E:T ratios. We hypothesized that this “ceiling” effect of cytolysis was due to either incomplete infection of target cells by vaccinia virus or inefficient sensitization of target cells through processing and presentation of antigen expressed through the vaccinia virus vector. To improve the efficiency of target sensitization, we prepared two synthetic peptide pools covering the whole open reading frame of SIV gag. Each pool contains 25 20-mer synthetic peptides, overlapping by 10 amino acids. The N-terminal pool covers the region from residues 1 to 280 without the two peptides containing p11C sequence (residues 181 to 189), and the C-terminal pool covers residues 271 to 520. We tested autologous B lymphoid cells pulsed with both peptide pools or p11C peptide as targets in the cytotoxicity assay. As shown in Fig. 2A, MmL-3 lymphocyte effectors stimulated with vaccinia virus efficiently recognized target cells pulsed with the C-terminal peptide pool as well cells sensitized with the p11C peptide, with specific lysis of over 60% even at an E:T ratio of 5. The MmL-3 effector cells also lysed target cells pulsed with the N-terminal peptide pool, but at a much lower level (about 40% at an E:T ratio of 40). On the other hand, the lymphocyte culture from the PBMC sample of a naïve monkey did not recognize its autologous BLCL pulsed with these peptide pools or p11C peptide, demonstrating the specificity of MmL-3's responses.
FIG. 2.
Comparison of MmL-3 and a naïve monkey CTL culture restimulated with vaccinia virus or synthetic epitope peptide. (A) Twenty percent PBMC samples were infected with Vac-SIVgag and cultured with the remaining PBMC samples in culture for 2 weeks. (B) Synthetic peptide corresponding to the p11C epitope sequence was added to CTL cultures on day 0 at a concentration of 1 μg/ml, and the cultures were incubated for 2 weeks. The effector cells from both sets of cultures were harvested and tested in a cytotoxicity assay against autologous BLCL pulsed with peptide pools or peptide for 1.5 h with 51Cr label. The peptide pools contain 25 20-mer synthetic peptides overlapping by 10 amino acids, and the two peptides containing the p11C epitope sequence (residues 181 to 189) were deleted from the N-terminal peptide pool. DMSO is the solvent used for solubilizing the peptides and was used here as a mock-pulsed control.
Also in this experiment, we compared the efficiency of vaccinia virus-based restimulation versus the peptide-specific stimulation in expanding p11C peptide-specific CTLp in bulk CTL culture. The peptide p11C was added to both MmL-3 and naïve monkey lymphocyte cultures on day 0 at a concentration of 1 μg/ml. As shown in Fig. 2B, p11C peptide-based stimulation specifically expanded MmL-3 lymphocyte effector cells that recognized p11C peptide-sensitized target, with over 70% specific lysis at an E:T ratio of 5, but not the N- or C-terminal peptide pool of SIV Gag-pulsed targets. However, there is no obvious difference between percent specific lysis against p11C peptide-sensitized target cells by the vaccinia virus-stimulated culture versus p11C peptide-stimulated culture. As expected, the p11C peptide-stimulated lymphocyte culture from the naïve monkey did not recognize any autologous target cells pulsed with p11C peptide or either peptide pools.
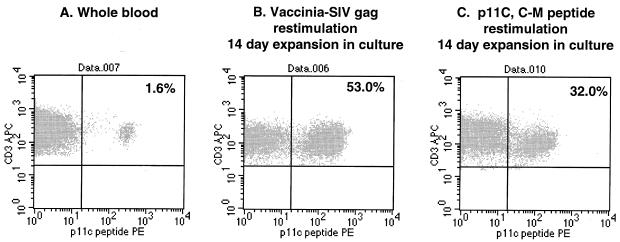
Using the Mamu-A*01/p11C tetramer complex, we examined the blood samples from MmL-3 and a naïve Mamu-A*01-positive monkey for p11C-specific T cells. As shown in Fig. 3A, the fresh staining of the whole blood sample of MmL-3 revealed a population of approximately 1.6% that was positive for tetramer staining within CD3+ and CD8+ T lymphocytes. After approximately 2 weeks in culture with stimulation with either vaccinia virus or p11C peptide, the effector cells from the cultures were estimated again using tetramer staining as described (23). The results showed that both methods achieved comparable expansion of p11C peptide-specific effector cells (53% versus 32% within the CD3+ CD8+ T-cell population). On the contrary, the naïve monkey PBMC culture, upon restimulation with Vac-SIVgag or p11C peptide, was not stained or only stained marginally positive (0 and 0.3%, respectively; results not shown).
FIG. 3.
Flow cytometric analysis of MmL-3 T cells stained with Mamu A*01/p11C tetramer complex. A fresh blood sample (A), 2-week CTL culture restimulated with Vac-SIVgag (B), and 2-week CTL culture restimulated with p11C peptide (1 μg/ml) (C) from MmL3 were stained with the tetramer complex and anti-CD3 and anti-CD8 monoclonal antibodies. The events were collected and sequentially gated for lymphocyte population (forward versus side scatter) and CD3+ CD8+ population. The percentages shown in the figure indicate the tetramer staining-positive population within the CD3+ CD8+ lymphocytes.
A similar experiment was also performed to evaluate the expansion kinetics of CTL effector cells in cultures restimulated with Vac-SIVgag versus p11C peptide. The fresh blood samples from an SIV-infected, Mamu-A*01-positive rhesus monkey, Mm94-98, showed about 1.25% of the population positive for p11C tetramer in CD3+ and CD8+ lymphocytes, whereas that from a naïve Mamu-A*01-positive monkey, 061F, had only 0.05%. Upon restimulation with either Vac-SIVgag or p11C peptide, the cultures were stained with the p11C tetramer complexes in the CD3+ CD8+ T-cell population on days 8, 10, and 14. The results shown in Table 2 clearly indicate the difference in the kinetics of the cultures restimulated with Vac-SIVgag versus p11C peptide. The vaccinia virus-restimulated culture showed a steady increase in tetramer-positive population during the 2-week culture period, reaching a peak on day 14 at 31.6%, whereas the staining of the peptide-restimulated culture showed that tetramer-positive population was 11.1% on day 8 but remained around 11 to 12% throughout the rest of period. The cultures from naïve monkey PBMC samples remained negative at all time points regardless of the restimulation method.
TABLE 2.
Kinetics of p11C tetramer-positive CTL expansion in cultures
| Monkey no. | Status | Restimulation methoda | % p11C tetramer stainingb on day of culture:
|
|||
|---|---|---|---|---|---|---|
| 0 | 8 | 10 | 14 | |||
| 94–98 | SIV+ | Vac-SIVgag | 1.25 | 9.90 | 17.73 | 31.66 |
| p11C peptide | 1.25 | 11.12 | 11.70 | 12.76 | ||
| 061F | Naïve | Vac-SIVgag | 0.05 | 0.48 | 0.24 | 0.91 |
| p11C peptide | 0.05 | 0.19 | 0.07 | 0.17 | ||
The cultures were restimulated with 20% autologous PBMC infected with Vac-SIVgag or with p11C peptide at 1 μg/ml.
The lymphocytes were gated on CD3+ and CD8+ populations.
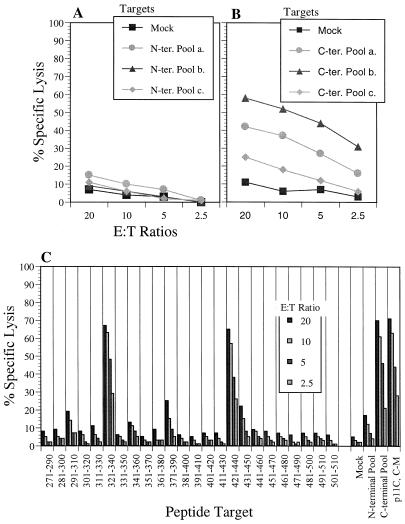
To exclude the possibility that cytotoxicity seen with peptide pool-sensitized target is due to an experimental artifact, we elected to identify the individual peptide(s) within the peptide pool that was causing the cytotoxicity. First, we delineated the CTL responses against N- and C-terminal pools with six smaller peptide pools of seven or eight peptides each (Fig. 4A, N-terminal SIV gag pools a, b, and c, and Fig. 4B, C-terminal SIV Gag pools a, b, and c). The results showed that the cytotoxic responses of MmL-3 against the N-terminal pool disappeared when the smaller pools were used to sensitize target cells (Fig. 4A), whereas the responses against the C-terminal pool persisted against the target cells sensitized with two of the three smaller pools (Fig. 4B). Further delineation with individual peptide-pulsed target cells in the cytotoxicity assay revealed two new T-cell determinants, located at residues 321 to 340 and 421 to 440 of SIV Gag, recognized by MmL-3 lymphocyte culture (Fig. 4C).
FIG. 4.
Identification of additional SIV Gag epitopes recognized by MmL-3 CTL. Cultured MmL-3 lymphocytes restimulated with Vac-SIVgag were assayed against autologous BLCL pulsed with six smaller N-terminal (A) and C-terminal peptide pools covering (B) the SIV gag open reading frame or SIV Gag individual peptides within the C-terminal pool (C) in the cytotoxicity assay. The bars in panel C in each column represent the four E:T ratios used in the experiment (20, 10, 5, and 2.5). The data shown are representative of three similar experiments. Mock, negative control (DMSO).
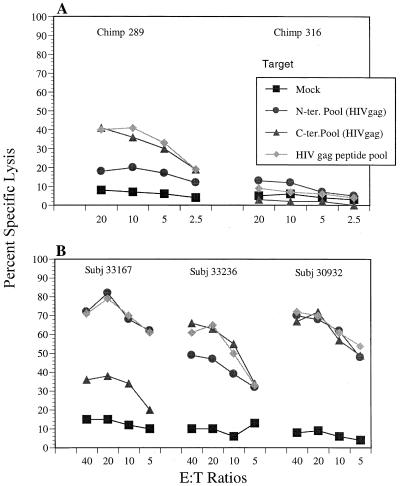
To evaluate whether these culture conditions and cytotoxicity assay procedures are suitable for studying other primate or human CTL responses, we tested the procedures with PBMC samples from chimpanzees and human subjects infected with HIV-1. Chimp 289 was infected with HIV-15016 in 1994, and chimp 316 was infected with HIV-1DH12 in 1994 and HIV-1IIIB in 1997 (36; K. Murthy, personal communication). We used two peptide pools which correspond to the N- and C-terminal halves of the HIV-1 Gag sequence, each containing 25 20-mer synthetic peptides overlapping by 10 amino acids. We also tested an HIV Gag peptide pool that was a combination of both the N- and C-terminal pools. The results in Fig. 5A showed that the CTL culture from chimp 289 recognized and lysed autologous B lymphoid cells pulsed with the HIV Gag peptide and C-terminal pools, but only marginally lysed the cells sensitized with the N-terminal peptide pool. No significant cytotoxicity can be detected with the CTL cultures of chimp 316 PBMC. As shown in Fig. 5B, the CTL cultures from three HIV-infected human subjects all recognized the peptide pool-sensitized target cells. It should be noted that percent lysis of the target cells pulsed with the HIV Gag peptide pool matches the higher percent lysis of target cells pulsed with the N- or C-terminal pools.
FIG. 5.
CTL responses demonstrated with cultured lymphocytes from HIV-infected chimpanzee (A) and human (B) subjects. CTL cultures restimulated with Vac-HIVgag were tested against autologous BLCL pulsed with N-terminal, C-terminal, or complete HIV Gag peptide pools or mock pulsed at the indicated E:T ratios in the standard cytotoxicity assay.
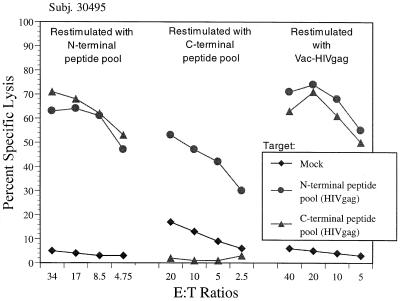
Individual synthetic peptide has been commonly used for restimulation, and the peptide pools have also been used for this purpose. We tested this possibility with the lymphocyte cultures of an HIV-infected human subject. The cultures were restimulated with the N-terminal peptide pool, C-terminal peptide pool, or Vac-HIVgag. The results in Fig. 6 showed that the culture expanded with the N-terminal peptide pool recognized both N- and C-terminal peptide pool-sensitized target cells, whereas the C-terminal peptide pool-stimulated culture only recognized the corresponding target. The Vac-HIVgag-restimulated culture recognized both pool-sensitized target cells. These data raised concerns about using a synthetic peptide pool as the antigen source for in vitro culture restimulation because of apparent loss of specificity of cytotoxic responses.
FIG. 6.
Specificity of CTL cultures restimulated with peptide pools versus vaccinia virus. Lymphocyte cultures from an HIV-infected subject were restimulated with HIV Gag N- or C-terminal peptide pools or Vac-HIVgag, as indicated, for 2 weeks and tested against autologous B cells pulsed with HIV Gag N-terminal and C-terminal peptide pools or mock pulsed (DMSO) at the indicated E:T ratios in the standard cytotoxicity assay.
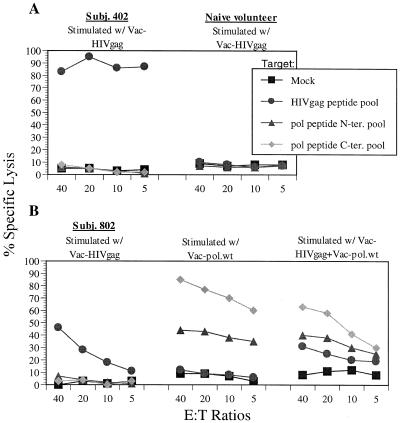
To further confirm the specificity of our bulk CTL assay configuration, we tested more human samples. First, the lymphocyte cultures from an HIV-1-infected subject and a naïve volunteer were tested against autologous BLCL pulsed with the HIV Gag peptide pool or Pol N-terminal and C-terminal peptide pools or mock pulsed (DMSO) (Fig. 7A). Since both CTL cultures were stimulated with Vac-HIVgag, cytolytic responses against target cells sensitized with the HIV Gag pool, but not the two Pol peptide pools were detected with the lymphocyte cultures of subject 402, an HIV-infected individual. There were no nonspecific responses against targets pulsed with the two Pol peptide pools, and no cytotoxicity was seen with CTL cultures from the naïve volunteer against any target cells pulsed with the HIV Gag or Pol peptide pools. Second, the PBMC from an HIV-infected individual were restimulated with Vac-HIVgag, Vac-pol, or a combination of both, and the corresponding CTL cultures were tested against autologous BLCL pulsed with HIV Gag or two Pol peptide pools (Fig. 7B). The CTL cultures expanded with Vac-HIVgag or Vac-pol demonstrated the specific cytotoxicity against target cells pulsed with the HIV Gag pool and the two HIV Pol peptide pools, respectively. The culture expanded with both vaccinia viruses lysed all three targets but not mock-pulsed target cells.
FIG. 7.
Specificity of CTL responses in HIV-infected human subjects. Lymphocyte cultures restimulated with the indicated vaccinia virus were tested against autologous BLCL pulsed with HIV Gag peptide pool or the HIV Pol N-terminal or C-terminal peptide pool or mock pulsed (DMSO) at the indicated E:T ratios in the standard cytotoxicity assay. (A) Cytotoxicity responses of lymphocyte cultures from HIV-1-infected subject 402 and a naïve volunteer; (B) cytotoxicity responses of lymphocyte cultures from HIV-1-infected subject 802.
DISCUSSION
In this study, we evaluated the parameters associated with expansion of CTL precursors in bulk cultures in vitro and presented a sensitive method for detecting cytotoxic effector functions in primate and human PBMC samples. The method combines vaccinia virus-based restimulation and peptide pool-based target sensitization, and the experiments presented in this study confirm the sensitivity and specificity of this configuration for assaying CTL responses in primate and human subjects. This method has been used for studying hundreds of out-bred nonhuman primates that have been immunized with our vaccine candidates, and we have noted remarkable consistency for detecting CTL responses in many longitudinal studies (unpublished data).
The infection of PBMC by vaccinia virus was confirmed by plaque assay using the cell lysates and the supernatants from 1-week PBMC cultures. Although the exact titers were not determined in this study, active viral replication was unquestionable, as the infectious virus titer in these cultures detected in a plaque assay far exceeded the initial infection dose (at least 1,000-fold higher than the input infection dose). It is unclear at this point why the vaccinia viruses do not lyse the lymphocytes in cultures. As the CTLp against Vac-SIVgag target was still detectable even when all MmL-3 PBMC (100% PBMC sample) were infected with Vac-SIVgag before being plated in LDA culture (Table 1), one can only assume that not all lymphocytes are susceptible to vaccinia virus infection, since it is known that vaccinia virus infection will ultimately shut down host cell functions (4). This assumption is also supported by the fact that we could not detect any antigen expression in the lymphocyte cultures after infection with recombinant vaccinia virus, by either intracellular staining or Western blot analysis (results not shown). Thus, it is unclear at this point what types of cells are infected and serve as host for viral replication in lymphocyte cultures.
There are some considerations that favor using unfixed PBMC infected with vaccinia virus for restimulation. First, there is no concern about BLCL-related high background lysis that many considered nonspecific lysis caused by expanding effector cells in culture in the presence of B lymphoid cells, thus eliminating the need for inclusion of unlabeled target cells in the CTL assay to reduce the background. Second, live and infectious vaccinia virus in the culture can provide a continuous supply of antigen to drive T effector cell proliferation and expansion (Table 2), eliminating the need for additional stimulator cells during the 2-week culture period. This is also supported by the LDA results, showing that 2 weeks in culture are optimal for detecting CTLp with vaccinia virus as the restimulation reagent (Table 1). Third, and most important, is that the unfixed vaccinia virus-infected PBMC method is equally if not more efficient for activation and expansion of p11C epitope-specific CTLs compared to stimulation with a synthetic peptide of p11C. Tetramer staining provided a unique quantitative means to track p11C-specific effector expansion in both cultures (Fig. 3 and Table 2).
The bulk culture CTL assay is used to measure the cytotoxic function of the expandable memory CD8+ T-cell population. The activation and proliferation of memory CD8+ T cells and efficient expansion of cytotoxic effector cells in culture are critical for detecting CTL response in the cytotoxicity assay. Monkey MmL-3 has been infected with a nonpathogenic SHIV for over 2 years, and its immune status is considered stable. Its CTLp frequency against autologous BLCL infected with Vac-SIVgag has been consistently detected in the range of 120 to 240 per 106 PBMC over a 1-year period in our study (Table 1) (unpublished results). Thus, MmL-3 provided an ideal sample source for evaluation of different parameters associated with expansion of CTLp in culture. The results in Table 1 presented the optimal conditions in our laboratory for expansion of CTLp cells in LDA microculture, and the conditions are generally applicable to bulk CTL cultures. It should be noted that the benefit of using IL-7 is not reflected by the results from either bulk CTL assay (Fig. 1) or LDA (Table 1). However, we favor IL-7 simply based on the observation that more viable effector cells can be recovered from the lymphocyte cultures with IL-7. Other groups have reported similar observations (K. Weinhold, personal communication).
The sensitivity of this CTL assay configuration hinges on using peptide pools to sensitize target cells. The “ceiling” effect in percent specific lysis of Vac-SIVgag-infected target cells by MmL-3 effector cells indicates the limitation of using vaccinia virus to present antigen for target cells (Fig. 1). This limitation could be explained by the fact that not all BLCL are effectively infected with vaccinia virus even at a relatively high MOI. The intracellular staining of BLCL infected with Vac-HIVgag at an MOI of 10 showed maximally about 60% cells expressing p24 antigen after overnight incubation (results not shown). This limitation is compounded by the fact that intracellular antigens have to be processed in order to be presented properly by MHC class I molecules on the cell surface, further reducing the number of functional epitope peptide-MHC class I complexes for TCR recognition (33, 42). It has been demonstrated that a full-length version of influenza virus nucleoprotein expressed through a vaccinia virus yielded only about 30 copies of an H-2Kd-restricted CTL epitope per cell, while the minigene version of this epitope expressed through a vaccinia virus produced about 55,000 copies per cell (3). This observation exemplifies one of the rate-limiting factors associated with the endogenous antigen processing and presentation (42). It is known that the density of functional peptide-MHC class I complexes on the cell surface will affect the efficiency of TCR recognition (25). Supplying synthetic peptide exogenously certainly bypassed these limitations, which are inherent in the vaccinia virus delivery system.
The peptides in the pools used in this study are 20-mers, which is by no means the optimal size for MHC class I molecule presentation. From crystallographic structure studies of MHC class I molecules (5), we know that the grooves in which peptides are bound are closed ended and are unlikely to be able to accommodate the 20-mer peptides used in this study. It is perceivable that 20-mer peptides in the pools are processed to smaller peptides in the culture medium during the 1 to 2 h of incubation. It has been demonstrated that a COOH-terminal dipeptidase in FBS can trim a longer peptide to the optimal size in the culture medium for antigen presentation (38). It is also possible that short peptide intermediates exist in the pools as incomplete products from peptide synthesis process, and these short versions are the ones presented by MHC class I molecules. Nonetheless, it has been shown in many studies that longer peptides can sensitize target cells for CTL recognition at concentrations ranging from 1 to 10 μg/ml (1), and the concentrations of the individual peptides in the pools used in this study are certainly within this range.
It is clear that the antigens expressed through vaccinia viruses are appropriately processed and presented to memory T cells in CTL cultures. This type of endogenously presented antigen is highly specific for expansion and proliferation of relevant cytotoxic effector cells in cultures. On the contrary, the cytotoxic effectors activated and expanded with peptide pool-based restimulation showed high levels of promiscuous killing against targets sensitized by peptide pools (Fig. 6). It has been reported that variant peptides of an Epstein-Barr virus CTL epitope with only one amino acid conserved for TCR contact can activate the memory T cells in culture (35). Nonetheless, the results from a series of experiments serve to address the concerns about the artificiality of this method for detection of CTL responses. First, we identified the peptide determinants responsible for the cytotoxicity seen against the C-terminal SIV Gag pool-sensitized target by MmL-3 effector cells. Two new T-cell determinants, located at residues 321 to 340 and 421 to 440 of SIV Gag, were reported in this study. Second, we showed that PBMC samples from naïve subjects would not recognize any peptide pool-sensitized targets after being restimulated in culture with vaccinia virus. Third, we demonstrated that only lymphocytes from the cultures exposed to a particular antigen expressed through vaccinia virus would recognize the cognate peptide pool-sensitized targets in the cytotoxicity assay (Fig. 7).
In summary, we present here a new configuration of restimulation and target sensitization in a cytotoxicity assay for detecting CTL responses in primate and human subjects. The method has been tested for over a year in our laboratory and used to examine the CTL responses from hundreds of primate and human subjects with remarkable consistency in results.
ACKNOWLEDGMENTS
We thank N. L. Letvin and M. Kuroda of Harvard Medical School for PE-p11C C-M tetramer complex and APC-anti-rhesus CD3 monoclonal antibody. We are also grateful for the spreadsheet for calculating CTLp frequency that S. A. Kalams of Harvard Medical School generously provided. Special thanks to C. Rinaldo of University of Pittsburgh, R. Steigbigel of SUNY at Stony Brook, and J. Jacobson of Mt. Sinai Hospital in New York for supplying HIV-infected PBMC samples.
REFERENCES
- 1.Allen T M, Sidney J, del Guercio M F, Glickman R L, Lensmeyer G L, Wiebe D A, DeMars R, Pauza C D, Johnson R P, Sette A, Watkins D I. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol. 1998;160:6062–6071. [PubMed] [Google Scholar]
- 2.Altman J D, Moss P A H, Goulder P J R, Barouch D H, McHeyzer Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. . (Erratum, 280:1821.) [PubMed] [Google Scholar]
- 3.Anton L C, Yewdell J W, Bennink J R. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 4.Bennink J R, Yewdell J W. Recombinant vaccinia viruses as vectors for studying T lymphocyte specificity and function. Curr Top Microbiol Immunol. 1990;163:153–184. doi: 10.1007/978-3-642-75605-4_6. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkman P J. MHC restriction in three dimensions: a view of T cell receptor/ligand interactions. Cell. 1997;89:167–170. doi: 10.1016/s0092-8674(00)80195-6. [DOI] [PubMed] [Google Scholar]
- 6.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Wei X, Horwitz M S, Peffer N, Meyers H, Nelson J A, Gairin J E, Hahn B H, Oldstone M B, Shaw G M. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- 8.de St. Groth S F. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari G, Berend C, Ottinger J, Dodge R, Bartlett J, Toso J, Moody D, Tartaglia J, Cox W I, Paoletti E, Weinhold K J. Replication-defective canarypox (ALVAC) vectors effectively activate anti-human immunodeficiency virus-1 cytotoxic T lymphocytes present in infected patients: implications for antigen-specific immunotherapy. Blood. 1997;90:2406–2416. [PubMed] [Google Scholar]
- 10.Ferrari G, Humphrey W, McElrath M J, Excler J L, Duliege A M, Clements M L, Corey L C, Bolognesi D P, Weinhold K J. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–1401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu T-M, Mylin L M, Schell T D, Bacik I, Russ G, Yewdell J W, Bennink J R, Tevethia S S. An endoplasmic reticulum-targeting signal sequence enhances the immunogenicity of an immunorecessive simian virus 40 large T antigen cytotoxic T-lymphocyte epitope. J Virol. 1998;72:1469–1481. doi: 10.1128/jvi.72.2.1469-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallimore A, Cranage M, Cook N, Almond N, Bootman J, Rud E, Silvera P, Dennis M, Corcoran T, Stott J, McMichael A, Gotch F. Early suppression of SIV replication by CD8+ nef-specific cytotoxic T cells in vaccinated macaques. Nat Med. 1995;1:1167–1173. doi: 10.1038/nm1195-1167. [DOI] [PubMed] [Google Scholar]
- 13.Garcia K C, Degano M, Stanfield R L, Brunmark A, Jackson M R, Peterson P A, Teyton L, Wilson I A. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 14.Gardner J, Khanna R, Sherritt M A, Suhrbier A. Use of recombinant vaccinia to restimulate antigen specific human peripheral blood cytotoxic T lymphocytes. J Virol Methods. 1997;65:105–109. doi: 10.1016/s0166-0934(96)02173-8. [DOI] [PubMed] [Google Scholar]
- 15.Germain R N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 16.Harrer T, Harrer E, Kalams S A, Barbosa P, Trocha A, Johnson R P, Elbeik T, Feinberg M B, Buchbinder S P, Walker B D. Cytotoxic T lymphocytes in asymptomatic long-term nonprogressing HIV-1 infection: breadth and specificity of the response and relation to in vivo viral quasispecies in a person with prolonged infection and low viral load. J Immunol. 1996;156:2616–2623. [PubMed] [Google Scholar]
- 17.Haynes B F, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 17a.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Zhang L, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson R P, Glickman R L, Yang J Q, Kaur A, Dion J T, Mulligan M J, Desrosiers R C. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8:472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- 20.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 21.Kalams S A, Johnson R P, Dynan M J, Hartman K E, Harrer T, Harrer E, Trocha A K, Blattner W A, Buchbinder S P, Walker B D. T cell receptor usage and fine specificity of human immunodeficiency virus 1-specific cytotoxic T lymphocyte clones: analysis of quasispecies recognition reveals a dominant response directed against a minor in vivo variant. J Exp Med. 1996;183:1669–1679. doi: 10.1084/jem.183.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda M J, Schmitz J E, Charini W A, Nickerson C E, Lifton M A, Lord C I, Forman M A, Letvin N L. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- 24.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V, McMichael A J. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanzavecchia A, Lezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- 26.Letvin N L. Progress in the development of an HIV-1 vaccine. Science. 1998;280:1875–1880. doi: 10.1126/science.280.5371.1875. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Salvato M S, Pauza C D, Li J, Sodroski J, Manson K, Wyand M, Letvin N, Jenkins S, Touzjian N, Chutkowski C, Kushner N, LeFaile M, Payne L G, Roberts B. Utility of SHIV for testing HIV-1 vaccine candidates in macaques. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12:99–106. doi: 10.1097/00042560-199606010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Lubaki M N, Egan M A, Siliciano R F, Weinhold K J, Bollinger R C. A novel method for detection and ex vivo expansion of HIV type 1-specific cytolytic T lymphocytes. AIDS Res Hum Retroviruses. 1994;10:1427–1431. doi: 10.1089/aid.1994.10.1427. [DOI] [PubMed] [Google Scholar]
- 29.McMichael A J, Phillips R E. Escape of human immunodeficiency virus from immune control. Annu Rev Immunol. 1997;15:271–296. doi: 10.1146/annurev.immunol.15.1.271. [DOI] [PubMed] [Google Scholar]
- 30.Miller M D, Yamamoto H, Hughes A L, Watkins D I, Letvin N L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J Immunol. 1991;147:320–329. [PubMed] [Google Scholar]
- 31.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 32.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 33.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 34.Pontesilli O, Klein M R, Kerkhof Garde S R, Pakker N G, de Wolf F, Schuitemaker H, Miedema F. Longitudinal analysis of human immunodeficiency virus type 1-specific cytotoxic T lymphocyte responses: a predominant gag-specific response is associated with nonprogressive infection. J Infect Dis. 1998;178:1008–1018. doi: 10.1086/515659. [DOI] [PubMed] [Google Scholar]
- 35.Reali E, Guerrini R, Marastoni M, Tomatis R, Masucci M G, Traniello S, Gavioli R. A single specific amino acid residue in peptide antigens is sufficient to activate memory CTL: potential role of cross-reactive peptides in memory T cell maintenance. J Immunol. 1999;162:106–113. [PubMed] [Google Scholar]
- 36.Robert Guroff M, Kaur H, Patterson L J, Leno M, Conley A J, McKenna P M, Markham P D, Richardson E, Aldrich K, Arora K, Murty L, Carter L, Zolla Pazner S, Sinangil F. Vaccine protection against a heterologous, non-syncytium-inducing, primary human immunodeficiency virus. J Virol. 1998;72:10275–10280. doi: 10.1128/jvi.72.12.10275-10280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 38.Sherman L A, Burke T A, Biggs J A. Extracellular processing of peptide antigens that bind class I major histocompatibility molecules. J Exp Med. 1992;175:1221–1226. doi: 10.1084/jem.175.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Voss G, Letvin N L. Definition of human immunodeficiency virus type 1 gp120 and gp41 cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I alleles in simian-human immunodeficiency virus-infected rhesus monkeys. J Virol. 1996;70:7335–7340. doi: 10.1128/jvi.70.10.7335-7340.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voss G, Li J, Manson K, Wyand M, Sodroski J, Letvin N L. Human immunodeficiency virus type 1 envelope glycoprotein-specific cytotoxic T lymphocytes in simian-human immunodeficiency virus-infected rhesus monkeys. Virology. 1995;208:770–775. doi: 10.1006/viro.1995.1209. [DOI] [PubMed] [Google Scholar]
- 41.Yang O O, Kalams S A, Rosenzweig M, Trocha A, Jones N, Koziel M, Walker B D, Johnson R P. Efficient lysis of human immunodeficiency virus type 1-infected cells by cytotoxic T lymphocytes. J Virol. 1996;70:5799–5806. doi: 10.1128/jvi.70.9.5799-5806.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yewdell J W, Bennink J R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]