Abstract
Zoopharmacognosy is the study of the self-medication behaviors of non-human animals that use plant, animal or soil items as remedies. Recent studies have shown that some of the plants employed by animals may also be used for the same therapeutic purposes in humans. The aim of this study was to determine the antioxidant and antibacterial activity of Ceiba pentandra, Myrianthus arboreus, Ficus subspecies (ssp.) and Milicia excelsa bark crude extracts (BCE), plants consumed by western lowland gorillas (Gorilla gorilla gorilla) in Moukalaba-Doudou National Park (MDNP) and used in traditional medicine, and then to characterize their phytochemical compounds. DPPH (2,2-Diphenyl-1-Picrylhydrazyl), phosphomolybdenum complex and β-carotene bleaching methods were used to assess antioxidant activity. Antimicrobial susceptibility testing was performed using the diffusion method, while minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were assessed using the microdilution method. The highest level of total phenolics was found in Myrianthus arboreus aqueous extract [385.83 ± 3.99 mg [gallic acid equivalent (GAE)/g]. Total flavonoid (134.46 ± 3.39) mg quercetin equivalent (QE)/100 g of extract] were highest in Milicia excelsa, tannin [(272.44 ± 3.39) mg tannic acid equivalent (TAE)/100 g of extract] in Myrianthus arboreus and proanthocyanidin [(404.33 ± 3.39) mg apple procyanidins equivalent (APE)/100 g of extract] in Ceiba pentandra. Ficus ssp. (IC50 1.34 ±3.36 μg/mL; AAI 18.57 ± 0.203) ethanolic BCE and Milicia excelsa (IC50 2.07 ± 3.37 μg/mL; AAI 12.03 ± 0.711) showed the strongest antioxidant activity. Myrianthus arboreus ethanolic BCE (73.25 ± 5.29) and Milicia excelsa aqueous BCE (38.67 ± 0.27) showed the strongest percentage of total antioxidant capacity (TAC). Ceiba pentandra ethanolic BCE (152.06 ± 19.11 mg AAE/g) and Ficus ssp aqueous BCE (124.33 ± 39.05 mg AAE/g) showed strongest relative antioxidant activity (RAA). The plant BCE showed antimicrobial activity against multidrug resistant (MDR) E. coli (DECs) isolates, with MICs varying from 1.56 to 50 mg/mL and inhibition diameters ranging from 7.34 ± 0.57 to 13.67 ± 0.57mm. Several families of compounds were found, including total phenolic compounds, flavonoids, tannins and proanthocyanidins were found in the plant BCEs. The plant BCEs showed antioxidant activities with free radical scavenging and antimicrobial activities against 10 MDR E. coli (DECs) isolates, and could be a promising novel source for new drug discovery.
1. Introduction
Natural medicines from plants have been used to enhance human and veterinary health since time immemorial, as revealed in ancient tales, scriptures and other historical literature [1]. This practice is experiencing a resurgence [1, 2]. The World Health Organization (WHO) estimates that approximately 80% of the world’s population uses medicinal plants (MPs) for their health and care needs [3]. Recent studies have focused on the potential to develop antioxidant and antimicrobial drugs from plants [4–6]. These antioxidants, for example, reduce the incidence of many metabolic diseases [7]. Antioxidants also reduce the incidence of chronic inflammation by reinforcing immunity [8], which would ultimately contribute to the efficacy of antimicrobial therapy. Antimicrobial agents are also used as antibiotics to control infections in the human body, but can cause many side effects, especially by increasing reactive oxygen species (ROS) [9]. ROS are very dangerous to human health and well-being and can contribute to the development of cancer [10]; further, they are aggravating factors for the emergence of various other metabolic diseases [11]. Finally, the use of these antimicrobial agents as antibiotic drugs can lead to resistance selection pressure in microorganisms such as bacteria [12].
Antimicrobial resistance is considered by the WHO to be one of the world’s three greatest threats to human health because of the extensive spread of antibiotic-resistant bacteria (ARB) and antibiotic resistance genes (ARGs) [13]. Infectious diseases caused by multidrug-resistant (MDR) bacteria affect millions of people worldwide [14]. Furthermore, many zoonoses caused by pathogenic microorganisms such as enterobacteria (e.g.; MDR Escherichia coli), have been a human public health problem for decades [15]. E. coli is a Gram-negative bacteria and gut commensal in animals, including non-human primates (NHPs) [16]. The close phylogenetic relationship between humans and other hominids, combined with a rapidly expanding human–animal interface, enables pathogen transmission across species, leading to morbidity and mortality in great ape populations throughout the world [17, 18]. Several studies have shown that wildlife [19, 20], including wild primates such as western lowland gorillas (Gorilla gorilla gorilla), could transmit this kind of pathogens to humans [21, 22], or that humans could transmit the pathogens to wildlife [23, 24]. This situation has great potential for the discovery of new antimicrobial agents [25, 26]. Many recent studies show that natural products from medicinal plants or plants consumed by animals continue to play a role in drug discovery and development [27, 28].
Zoopharmacognosy is the study of non-human animals self-medicating, using plants, animals and natural compounds, such as soil, as a preventative or direct medicinal cure to regain health in their natural habitat [2, 29]. Most great apes, including western lowland gorillas (Gorilla gorilla gorilla) have a predominantly frugivorous diet [30, 31]. However, bark is the main fallback food for gorillas [32]. These feeding practices appear to be beneficial to the well-being and health of these animals but also to those of humans [33]. The use of great ape pharmacopoeia or zoopharmacognosy is a very promising strategy for management of human diseases because of the phylogenetic proximity of humans and great apes [34]. Several studies have shown that plants from the diet of great apes, including western lowland gorilla, are also used as MPs by healers in traditional African medicine [35, 36].
In view of the physiological (pathological, infectious) state of the gorillas in the PNMD, linked to the presence of potentially pathogenic enterobacteria, multi-resistant to antimicrobials used in human therapy, including MDR enterobacteria such as MDR E. coli (DECs) obtained in a previous study, how did these animals manage to host and control these microorganisms? In this study, we hypothesized that "the immunity-enhancing consumption of certain plant items (such as bark) by gorillas could be responsible for their ability to host and control these infectious microorganisms without developing serious disease".
Gabon, with its exceptional biodiversity, constitutes a vast reservoir of unexplored potential active biomolecules [37]. This study aims to evaluate the chemical composition (secondary metabolites families), antioxidant and antimicrobial activities of four plant species consumed by gorillas living in MDNP to control microbial infections within their communities and, used as traditional MPs by healers in Gabon. Ceiba pentandra [38, 39], Myrianthus arboreus [40, 41], Ficus ssp [42] and Milicia excelsa [43, 44] are the four plants selected for this study on the basis of ethnobotanical and ethnopharmacological surveys carried out among local populations. The ethnopharmacological activities of these four plants in traditional medicine have already been reported in recent literature.
2. Material and methods
2.1. Study area and field research authorization
Sampling of the bark of four plants consumed by western lowland gorillas was carried out under field research authorization N° 003/20/DG/JBLD/N° 306, from August 1 to 11, 2022 in MDNP, during the daily monitoring of gorillas by observing plants items they consumed in their natural environment during this period, using the non-invasive method as previously described [45]. Research Institute for Tropical Ecology (IRET) provided all the necessary authorizations to carry out this study on the MDNP site. No special permits were required, due to the scientific and technical agreements established between the Centre National de Recherches Scientifiques et Techniques (CENAREST) and the Centre Interdisciplinaire de Recherches Médicales de Franceville (CIRMF).
2.2. Data and sample collection
A survey was carried out among 27 inhabitants of the village of Doussala, including a number of traditional healers and herbalists, both men and women, known to the local peoples, using a questionnaire, as previously described by Obiang et al. [46]. Fresh bark plant samples collected were identified by a team of botanists leading by Prof. Brama Ibrahim from the Department of Biology, Faculty of Sciences of the University of Science and Technology of Masuku (USTM) in Franceville (Gabon). Voucher specimens of Ceiba pentandra (BRLU/4618), Myrianthus arboreus (BRLU-LBV/8324), Ficus ssp (USTM/#443) and Milicia excelsa (USTM/739) has been deposited at the herbarium of the same institute. The choice of the bark of the selected plants was made based on local traditional medicinal use and the fact that gorillas also consumed them.
2.2.1. Therapeutic indications
Descriptive statistical methods were used to analyze the ethnopharmacological survey data and various quantitative indices, including use value (UV) and relative frequency of citation (RFC). Data were reported in proportions and percentages [46].
Use Value (UV) and Relative Frequency of Citation (RFC) were calculated according to the following formula:
2.3. Treatment of plant material
2.3.1. Extraction
From 100 g of bark powder of each selected plant, an extraction by maceration under agitation was carried out for 72 h with 2 L of each solvent (ethanol 99.8% and water). After filtration of the two (ethanolic and aqueous) maceration, using Whatman N°1 filter paper, the aqueous extract was directly lyophilized, while the ethanolic extract was concentrated and dried in an oven.
2.4. Preliminary phytochemical screening
Each plant BCE was tested for the presence of flavonoids, coumarins, tannins, total phenolics, catechin tannins, gallic tannins, cyanidins, alkaloids, oses or holosides, saponosides, sterols or triterpenoids, anthracenosides, cardiotonic heterosides and reducing sugar as described previously [47, 48].
2.5. Quantitative phytochemical analysis
2.5.1. Total phenol content
To determine the total phenol content, the Folin-Ciocalteu method was used [49]. Absorbance was measured at 735 nm. Phenolic compounds were expressed as mg gallic acid equivalents (GAE) /dry weight of extract.
2.5.2. Total flavonoid content
Aluminum trichloride method was used to determine the flavonoid content and absorbance was measured at 435 nm. Flavonoid content was expressed in quercetin equivalent (QE) [50].
2.5.3. Tannin content
Tannin content was determined using the method described previously by Sima-Obiang et al. [51]. Absorbance was measured at 525 nm and tannic acid was used as a standard. Tannin contents were expressed in mg of tannic acid equivalent (TAE)/g of dry extract.
2.5.4. Proanthocyanidin content
Proanthocyanidins were determined using the HCl-Butanol method [52]. Absorbance was read at 550 nm and apple procyanidin was applied as standard. Proanthocyanidin levels were expressed in apple procyanidins equivalent (APE)/g of dry extract.
2.6. Bioactive properties of bark crude extracts of selected species consumed by western lowland gorilla
2.6.1. Antioxidant activity of bark crude extracts of selected species consumed by western lowland gorilla
2.6.1.1. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Capacity. The method described by Scherer and Godoy [53], based on the DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical test, was used to determine the Antioxidant Activity Index (AAI). Briefly, DPPH solution was prepared by dissolving 10 mg of DPPH powder in 200 mL methanol ([DPPH] = 0.05 mg/mL). 400 μL of each of the eight BCE (at 1mg/mL concentration) were added to 1.6 mL of methanol to obtain a stock solution. Cascade dilutions to 1/2 were made from the stock solution into test tubes containing 1 mL of methanol beforehand. Then, 1 mL of DPPH was added to each of the tubes. Absorbencies were measured at 517 nm after 30 min incubation at room temperature in the dark against a blank. Ascorbic acid (vitamin C) was used as reference. The ability to scavenge DPPH radical (RSA) was calculated by the following equation:
A = Absorbance at 517 nm. The IC50 (concentration providing 50% inhibition) of BCE and standards was determinate using regression curves in the linear range of concentrations. The AAI was then calculated as follows:
2.6.1.2. Phosphomolybdenum complex method for total antioxidant capacity. Spectrophotometric evaluation of total antioxidant activity was carried out through the formation of a phosphomolybdenum complex. The assay was based on the reduction of Mo (VI) to Mo (V) and subsequent formation of a green phosphate/Mo (V) complex in acid pH [54, 55]. A total volume of 0.3 mL of each bark crude extract (at 5mg/mL concentration) dissolved in methanol was added to 3 mL of reagent solution (0.6 mol/L H2SO4, 28 mmol/L Na3PO4 and 4 mmol/L ammonium molybdate). The mixtures were incubated at 95°C for 90 min the cooled to room temperature. The absorbance was measured at 695 nm. The total antioxidant activity of each plant BCE was expressed as the number of equivalence of ascorbic acid (mg AAE/g).
2.6.1.3. β-Carotene bleaching assay. The β-carotene–linoleic acid model system is prepared by dissolving 0.5 mg β-carotene in 1 mL chloroform followed by the addition of 40 μL linoleic acid and 500 μL Tween-20. A rotary evaporator was used to completely evaporate the chloroform added into the system. To the resulting solution, 100 mL of oxygenated distilled water is added followed by vigorous shaking. 500 μL of BCE (at 1mg/mL concentration) or antioxidant solution of the reference (Ascorbic acid; 1mg/mL concentration) was added to 2.5 mL of the previous emulsion. The absorbance was measured at 490 nm before and after heat treatment with regular time intervals for 48 h. The measurement of the absorbance continued until the color of β-carotene disappears [56].
The bleaching analysis of β-carotene was calculated as follows:
2.6.2. Antimicrobial activity of bark crude extracts of selected species consumed by western lowland gorilla
2.6.2.1. Bacterial strains tested growth conditions and inoculums standardization. Ten MDR E. coli (DECs) isolates obtained in a previous study [57] were used to assess the plant studied BCE antimicrobial activity. The cultures were held at 4°C on Muller-Hinton agar (bioMérieux, France). Colonies from 24-h cultures were used to make the inoculum suspension. The colonies were vortexed for 15 s after being suspended in sterile saline (0.9% NaCl). The turbidity of a 0.5 McFarland Norm (equivalent to 1–5 108 CFU/mL) was used as density setting [58].
2.6.2.2. Antimicrobial activities. To test the antimicrobial activity of BCEs and the antimicrobial susceptibility, antibiograms were performed using the Kirby–Bauer disk diffusion method, according to the CLSI (Clinical and Laboratory Standards Institute) protocols [59]. The agar was suspended in distilled water, heated to complete dissolution autoclaved at 121°C, and poured into Petri dishes. Whatman paper discs (6 mm) were inoculated with suspensions (108 CFU/mL) and dispersed on the surface of Mueller-Hinton agar plates (bioMérieux, France) [60]. Then, the discs were impregnated with 20 μL of each bark crude plant extracts. All plates were incubated for 24 h at 37°C. The diameters of the inhibition zones were determined after incubation. Antimicrobial activity was estimated after incubation at 37°C for 24 h by measuring the zone of inhibition against the tested microorganisms. Antimicrobials tested were amoxicillin/clavulanic acid (AMC, 30 μg) and gentamycin (GEN, 10 μg). Breakpoints provided by the CLSI were used for the designation of isolates as resistant (R), intermediately susceptible (IS) or susceptible (S). For subsequent data analysis, the isolates with an I result were grouped with the isolates that gave an R result and defined as resistant. Multidrug-resistant isolates were identified based on the definition of MDR as bacteria that are resistant to three or more classes of antimicrobial agents [61, 62].
2.6.2.3. Minimum Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) assays. In microplate wells (96 wells), 10 μL of each dilution of plant bark crude extracts varying from 30 mg/mL to 0.014 mg/mL were combined with Mueller Hinton broth (bioMérieux, France) (170 μL) and bacterial inoculums (20 μL) and set to a final microbial concentration of 5 × 105 CFU/mL according to NCCLS standards methods, with some modifications [63]. The ethanol content in each well was less than 3.5% in an overall amount of 200 μL. For the negative control, the same percentage of ethanol was used. The MIC is the lowest concentration that does not emit a red color after 2 h of incubation. To assess MBC, a portion of each well where the concentrations are > or = (MIC) was sub-cultured on Muller-Hinton agar (MHA) (bioMérieux, France) and incubated for 24 h at 37°C. The MBC is described as the extract concentration at which 99.9% of the inoculated bacteria were destroyed [64].
2.7. Data analysis
All tests were performed as triplicate and the results are showed present as the mean. Excel software for Microsoft was used to analyze data. Analysis of variance (ANOVA) followed by Student’s tests were used to test for significant differences between means. Differences were considered statistically significant at p < 0.05. Adobe Illustrator software was used to plot the histograms. The Pearson correlation was determined between the antimicrobial and antioxidant activity of plant BCE and total phenolic, flavonoid, proanthocyanidin and tannin content. To visualize the correlation data, a heatmap of three colors: red (r = ─1), white (r = 0) and blue (r = -1) analysis was plotted with the packages Gplot using R 4.0.2.
2.8. Inclusivity in global research
Additional information regarding the ethical, cultural, and scientific considerations specific to inclusivity in global research is included in the S1 Checklist.
3. Results
3.1. Ethnobotanical and ethnopharmacological survey
Western lowland gorilla living in MDNP consumed 27 plants [30]. Local people use different parts of these plants (bark, root, fruit and leaves) in medicinal preparation (maceration, decoction, lotion, pomade and infusion) (Table 1). The cross-referenced results of ethnobotanical and ethnopharmacological surveys on the traditional use of plants by traditional healers in their pharmacopoeia to treat various human illnesses enabled us to select the four plants consumed by western lowland gorillas living in MDNP for this study. This information was recovered from autochthone Vungu people, living in Doussala village in MDNP.
Table 1. Plant species consumed by gorillas and used by humans to treat various diseases including diarrhea in southeast Gabon.
| Species | Family | Local name in Vungu | Part used | Preparations | Route of administration | Indication | UV | RFC | Nature |
|---|---|---|---|---|---|---|---|---|---|
| Caloncoba welwitschii | Flacourtiaceae | myanmongom | Leave, root, bark, fruit, seed | Decoction, macération, infusion | Oral administration | asthma, heavy, painful periods, harmless madness, biliary disorders | 0,19 | 0,02 | Tree |
| Cissus dinklagei | Verbenaceae | eéko / léko | Fruit, liana, leave | Raw, dried, cooked | Oral administration, ocular route | myopia, yellow fever, diarrhoea, stomach pain | 0,12 | 0,02 | Tree |
| Ceiba pentandra. | Malvaceae | Mufuma | Fruit, bark, fruit, root, leave | Decoction, macération, infusion | Oral administration, baths | colic, diarrhoea, inflammation, poisoning, fatigue, diurétique, hydropisia | 0,22 | 0,0352 | Tree |
| Cola sp. | Sterculiaceae | fudi | Seed, fruit | Mastication | Oral administration | stimulant, appetite suppressant | 0,07 | 0,0315 | Tree |
| Dichostema glaucescens | Euphorbiaceae | Dibule / Mabule | Bark | maceration, powdered | Oral administration | bad luck, purification bath, nurturing galactogen, emetic, diarrhoea | 0,15 | 0,016 | Tree |
| Diospyros mannii | Ebenaceae | Emba | Fruit, leave, seed, bark | bark shavings, | Cutaneous application, lotions | chest pain | 0,04 | 0,026 | Tree |
| Diospyros sp. | Ebenaceae | mufi nzi | Fruit, leave, seed | Decoction, macération, drink | Oral administration | albumin regulation, cysticercosis, intestinal parasites, toxoplasmosis, allergies, emphysema | 0,22 | 0,02 | Tree |
| Diospyros spp. | Ebenaceae | nemba / ilalaba | Bark, root, stem, fruit | Decoction, macération | Oral administration | antibacterial, antifungal, diarrhoea | 0,07 | 0,02 | Tree |
| Duboscia macrocarpa | Tiliaceae | Moupighi | Fruit, leave, seed, bark | Decoction, macération, infusion | Oral administration | toothache, coughing, tuberculose, vermifuge, stomach pain | 0,19 | 0,026 | Tree |
| Eriocoelum macrocarpum | Sapindaceae | Dibotsa | Bark, root | Decoction | Oral administration | female infertility, food cooking liquid | 0,08 | 0,0178 | Tree |
| Ficus ssp | Moraceae | divevenguengui | Fruit, leave, seed, bark | Decoction, macération, infusion, latex | Oral administration, cutaneous application, lotions | painful periods, diarrhoea, uterine care, local application | 0,12 | 0,0352 | Tree |
| Hexalobus crispifl orus | Annonaceae | tsago | Leave, root, bark, fruit | Decoction, macération | Oral administration | venereal diseases, paludisme, wounds, boils, fever, muscle pain, arthritis, convulsion | 0,3 | 0,0315 | Tree |
| Irvingia gabonensis | Irvingiaceae | andok / Mwiba | Fruit, leave, seed, bark | Raw, dried, decoction, eaten, fresh, macération | Oral administration, cutaneous application, lotions | deconstipant, diarrhoea or dysentery, toothache, astringent | 0,15 | 0,013 | Tree |
| Klainedoxa gabonensis | Irvingiaceae | mougoma | Fruit, leave, seed, bark | Raw, dried, decoction, eaten, fresh | Oral administration, cutaneous application, lotions | analgesic, venereal disease, diarrhoea, sterility, impotence | 0,12 | 0,026 | Tree |
| Lannea welwitschii | Anacardiaceae | gongo | Fruit, leave, seed | Decoction, macération | Oral administration | AIDS, opportunistic diseases, respiratory tract infections, toothache, hypertension | 0,19 | 0,02 | Tree |
| Meiocarpidium lepidotum | Annonaceae | depeyrie | Bark, root, fruit | Decoction, macération | Oral administration | fever, purgative, abdominal pain, worm infections in babies | 0,15 | 0,016 | Tree |
| Milicia excelsa | Moraceae | Kambal / Iroko | Leave, root, bark, fruit | Decoction, macération, infusion, latex | Oral administration, cutaneous application, lotions, ocular route | filariose, schizophrenia, diarrhoea, infertility, fortifiant | 0,19 | 0,0352 | Tree |
| Myrianthus arboreus | Moraceae | dibimbi / mububa | Fruit, leave, seed, bark | Raw, dried, decoction, macération | Oral administration | infertility, stomach ulcer, sore, gonorrhea, hypertension, cough, anemia | 0,26 | 0,0352 | Tree |
| Panda oleosa | Pandaceae | ovaga | Leave, root, bark, fruit, seed | Decoction, macération, infusion | Oral administration | burns, diarrhoea, dysmenorrhea, milk purification for nursing mothers, analgésique | 0,19 | 0,016 | Tree |
| Pycnanthus angolensis | Myristicaceae | mulomba | Fruit, leave, liana, seed, bark | Decoction, eaten, macération, drink | Oral administration, cutaneous application, lotions | stomach pain, diarrhoea, enema, madness, epilepsy, gastritis | 0,19 | 0,0315 | Tree |
| Ricinodendron heudelotii | Euphorbiaceae | ndjoé | Fruit, leave, seed, bark | Decoction, macération | Oral administration | AIDS, opportunistic diseases, respiratory tract infections, toothache, hypertension, stomach pain | 0,22 | 0,0178 | Tree |
| Sacoglottis gabonensis | Humiriaceae | Ozouga | Fruit, bark, fruit | Decoction, macération | Oral administration | AIDS, opportunistic diseases, venereal diseases | 0,12 | 0,0123 | Tree |
| Synsepalum dulcifi cum | Sapotaceae | Fruit | Raw, dried, cooked | Oral administration | suppress sensations of acidity and bitterness | 0,04 | 0,026 | Tree | |
| Staudtia gabonensis | Myristicaceae | mobé / mibé | Fruit, bark | Decoction, macération | Oral administration | haemostatic, intestinal worms, anemia, menstruation | 0,15 | 0,0178 | Tree |
| Trichilia prieureana | Meliaceae | Fruit, leave, seed, bark | Decoction, macération, infusion | Oral administration, cutaneous application, lotions | venereal diseases, fever, coughs, constipation, poisoning, ascites, aphrodisiac, lumbago, rheumatism | 0,34 | 0,013 | Tree | |
| Uapaca guineensis | Euphorbiaceae | Assam | Fruit, bark, fruit, root | Decoction, macération | Oral administration | intestinal parasitism, anti-abortive, aphrodisiac, restorative for young mothers, leprosy, gastrointestinal disorders, diarrhoea | 0,22 | 0,02 | Tree |
| Xylopia guintasii | Annonaceae | M’voma | Bark, root | Decoction, eaten, macération, drink, powdered | Oral administration, cutaneous application, lotions | broncho-pneumonic affections, febrile pains, knot-like swellings, treatment of pyorrhoea, ulcers | 0,19 | 0,013 | Tree |
UV: Use Value indicates the relative importance of uses of plant species. RFC: Relative Frequency of Citation indicates the local importance of each species.
Table 2 shows the results of ethnobotanical and ethnopharmacological surveys about traditional uses of most cited Ceiba pentandra L., Myrianthus arboreus P. Beauv., Ficus ssp. and Milicia excelsa Welw C.C. berg. bark by traditional healers in their pharmacopoeia to treat various human diseases, plants consumed by western lowland gorillas living in MDNP. These informations were recovered from autochthone people, called Vungus, living in Doussala village in MDNP and the relevant literature review.
Table 2. Phytochemical screening of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa.
| Secondary metabolites | Ceiba Pentandra | Myrianthus arboreus | Ficus ssp | Milicia excelsa | |||||
|---|---|---|---|---|---|---|---|---|---|
| AE | EE | AE | EE | AE | EE | AE | EE | ||
| Saponins | ++ | + | ++ | + | ++ | + | - | - | |
| Tannin gallic | +++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | |
| Tannin catechin | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| Total phenolics | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | |
| Total flavonoids | ++ | ++ | ++ | + | - | - | ++ | ++ | |
| Reducing sugars | +++ | ++ | +++ | ++ | + | ++ | ++ | ++ | |
| Alkaloids | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | |
| Anthracenosides | +++ | ++ | ++ | ++ | ++ | ++ | - | - | |
| Coumarins | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | |
| Sterols and Triterpenoids | + | + | + | - | + | - | + | - | |
| Oses and holosides | +++ | ++ | - | + | + | ++ | ++ | ++ | |
| Cardiac glycosides | +++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | |
| Digitoxins | +++ | ++ | +++ | + | +++ | ++ | +++ | ++ | |
| Gitoxins | - | - | - | - | ++ | ++ | ++ | ++ | |
| Gitoxins genins | - | - | +++ | ++ | |||||
| Cyanidins | +++ | ++ | +++ | ++ | - | - | +++ | ++ | |
| Flavonols | - | - | - | - | - | - | - | - | |
| Flavones | +++ | + | ++ | + | - | - | - | - | |
| Flavonones | - | - | - | - | - | - | ++ | ++ | |
+++ = very abundant; ++ = abundant; + = not abundant,— = not detected. EE: Ethanol extract; AE: Aqueous extract. Color intensity/foam observed was used to indicate the phytochemicals abundance.
3.2. Extraction yields
Extraction yields varied according to the solvents used. Water proved more efficient with higher yields than ethanol.
3.3. Preliminary phytochemical screening
Phenolic compounds, alkaloids, flavonoids, tannin gallic, anthracenosides, reducing sugar, coumarins, sterols and triterpenes, oses and holosides, cyanidines, cardiac glycosides and saponins were found in almost all plant BCEs tested (Table 4).
Table 4. Inhibition zones (mm) diameters induced by Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE against MDR E. coli (DECs).
| MDR E. coli (DECs) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Species | E32 | E40 | E41 | E8 | E4 | E7 | E37 | E50 | E49 | E10 |
| Ceiba Pentandra | ||||||||||
| AE | 8.67 ± 0.57 | 9.34 ± 0.57 | 8.34 ± 0.57 | 8.67 ± 0.57 | 9.34 ± 0.57 | 13.67 ± 0.57 | 10.34 ± 0.57 | 13.34 ± 0.57 | 8.34 ± 0.57 | 9.67 ± 0.57 |
| EE | 9 ± 0.57 | 9.67 ± 0.57 | 9.67 ± 0.57 | 11.67 ± 0.57 | 10.34 ± 0.57 | 11.67 ± 0.57 | 10.34 ± 0.57 | 9.34 ± 0.57 | 8.34 ± 0.57 | 10.34 ± 0.57 |
| Myrianthus arboreus | ||||||||||
| AE | 7.34 ± 0.57 | 8.34 ± 0.57 | ND | ND | ND | ND | ND | ND | ND | ND |
| EE | 8.67 ± 0.57 | 9 ± 0.57 | ND | ND | ND | 10.67 ± 0.57 | 10.34 ± 0.57 | 10.34 ± 0.57 | 10 ± 0.57 | ND |
| Ficus ssp | ||||||||||
| AE | ND | ND | 8.67 ± 0.57 | 8 ± 0.57 | 8 ± 0.57 | 8 ± 0.57 | 9.67 ± 0.57 | 8.34 ± 0.57 | 10.4 ± 0.57 | ND |
| EE | 9 ± 0.57 | 10.34 ± 0.57 | 10.67 ± 0.57 | 7.34 ± 0.57 | 9.67 ± 0.57 | 7.67 ± 1.54 | ND | ND | 8.34 ± 0.57 | 9 ± 0.57 |
| Milicia excelsa | ||||||||||
| AE | ND | ND | ND | ND | ND | ND | ND | ND | 9.34 ± 0.57 | 8.67 ± 1.54 |
| EE | 8.67 ± 0.57 | ND | 8.34 ± 0.57 | 8.67 ± 0.57 | 9.34 ± 0.57 | 13.67 ± 0.57 | 10.34 ± 0.57 | 13.34 ± 0.57 | 8.67 ± 0.57 | 9.67 ± 0.57 |
| Standards | ||||||||||
| AMC | 9 ± 1 | 9.34 ± 1.15 | 17.67 ± 0.57 | 8 ± 0 | 14.67 ± 0.57 | 8 ± 0 | 8 ± 0 | 9 ± 1 | 9.34 ± 1.15 | 8 ± 0 |
| GEN | 8 ± 0 | 9.67 ± 1.57 | 18 ± 2 | 8 ± 0 | 18 ± 3.60 | 10 ± 1 | 10.34 ± 0.57 | 9.34 ± 1.52 | 9 ± 1 | 8 ± 0 |
| Control | ||||||||||
| Ethanol (98%) | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Water | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
Values are expressed as means ± SD; ND: not determined, EE: Ethanol extract; AE: Aqueous extract.
3.4. Antioxidant potential of bark crude extracts of selected species consumed by western lowland gorilla
The results of antioxidant potential of the four plant bark crude extracts are shown in Figs 1 and 2.
Fig 1. Total phenolic and flavonoid content of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCEs.
(a): Total phenolic content of plant bark crude aqueous extracts. (b): Total phenolic content of plant bark crude ethanolic extracts. (c): Total flavonoid content of plant bark crude aqueous extracts. (d): Total flavonoid content of plant bark crude ethanolic extracts. a p < 0.05 compared to Ceiba pentandra BCE in the same column; b p < 0.05 compared to Myrianthus arboreus BCE in the same column; c p < 0.05 compared to Ficus ssp BCE in the same column; GAE = gallic acid equivalent; QE = quercetin equivalent; TAE = tannic acid equivalent; APE = apple procyanidins equivalent.
Fig 2. Total proanthocyanidin and tannin content of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE.
(e): Total proanthocyanidin content of plant bark crude aqueous extracts. (f): Total proanthocyanidin content of plant bark crude ethanolic extracts. (g): Total tannin content of plant bark crude aqueous extracts. (h): Total tannin content of plant bark crude ethanolic extracts. a p < 0.05 compared to Ceiba pentandra BCE in the same column; b p < 0.05 compared to Myrianthus arboreus BCE in the same column; c p < 0.05 compared to Ficus ssp BCE in the same column; GAE = gallic acid equivalent; QE = quercetin equivalent; TAE = tannic acid equivalent; APE = apple procyanidins equivalent.
3.5. Antioxidant activity of bark crude extracts of selected species consumed by western lowland gorilla
3.5.1. DPPH radical scavenging activity
The AAI of the bark crude extracts from Ceiba pentandra ranged from 9.3 ± 1.28 to 10.17 ± 3.45. For Myrianthus arboreus it ranged 11.81 ± 0.30 to 13.72 ± 6.88. From Ficus ssp it ranged 7.11 ± 1.16 to 19.45 ± 2.98, and from Milicia excelsa it ranged 7.11 ± 4.64 to 11.98 ± 3.56 (Table 3). These values are lower than the AAI of Ascorbic acid (23.16 ± 20.26).
Table 3. Antioxidant activities of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE determined by DPPH, Phosphomolybdenum complex and β-Carotene bleaching assays using L-ascorbic acid as a positive control.
| Sample | DPPH IC50 (mg/mL) | DPPH AAI | PM TAC (mg AAE/g) | BCB RAA (%) |
|---|---|---|---|---|
| Ceiba Pentandra | ||||
| AE | 2.73 ± 0.35 | 9.3 ± 1.28 | 54.73 ± 12.48 | 37.07 ± 0.44 |
| EE | 2.65 ± 0.87 | 10.17 ± 3.45 | 48.66 ± 12.96 | 73.25 ± 5.29 |
| Myrianthus arboreus | ||||
| AE | 2.12 ± 0.05a | 11.81 ± 0.30a | 152.06 ± 19.11a | 36.47 ± 0.64a |
| EE | 2.18 ± 1.12a | 13.72 ± 6.88a | 104.33 ± 63.25a | 36.35 ± 0.44a |
| Ficus ssp | ||||
| AE | 6.15 ± 0.24a,b | 7.11 ± 1.16a,b | 90.6 ± 49.77a,b | 38.67 ± 0.27a,b |
| EE | 1.31 ± 0.20a,b | 19.45 ± 2.98a,b | 92.93 ± 20.50a,b | 29.83 ± 0.20a,b |
| Milicia excelsa | ||||
| AE | 6.15 ± 6.14a,b,c | 7.11 ± 4.64a,b,c | 124.33 ± 39.05a,b,c | 34.87 ± 0.35a,b,c |
| EE | 2.25 ± 0.79a,b,c | 11.98 ± 3.56a,b,c | 104.6 ± 36.61a,b,c | 31.79 ± 0.57a,b,c |
| Ascorbic acid | 1.73 ± 1.20a,b,c,d | 23.16 ± 20.26a,b,c,d | 100.00 ± 1.78a,b,c,d |
DPPH: (2,2-Diphenyl-1-Picrylhydrazyl); IC50: the concentration of extracts reducing 50% of DPPH; AAI: Antioxidant Activity Index; PM TAC: Phosphomolybdenum Total Antioxidant Capacity; BCB RAA: β-Carotene Bleaching Relative Antioxidant Activity. Values are expressed as means ± SD. a p < 0.05 compared to aqueous and ethanolic extracts of Ceiba Pentandra. in the same column; b p < 0.05 compared to aqueous and ethanolic extracts of Myrianthus arboreus in the same column; c p < 0.05 compared Ficus ssp in the same column; d p < 0.05 compared Milicia excelsa in the same column.
3.5.2. Phosphomolybdenum complex assay
Myrianthus arboreus aqueous extract showed the highest total antioxidant activity with 152.06 ± 19.11 mg AAE/g and Ceiba pentandra aqueous extract showed the lowest value with 54.73 ± 12.48 mg AAE/g (Table 3).
3.5.3. β-Carotene bleaching assay
The β-carotene bleaching assay results (Table 3) and β-carotene bleaching kinetics (Fig 3) showed that the highest relative antioxidant activity (RAA) was shown by Ceiba pentandra ethanolic extract (RAA = 73.25 ± 5.29) and Ficus ssp aqueous extract (RAA = 38.67 ± 0.27). Ficus ssp and Myrianthus arboreus ethanolic extract showed lower activities (RAA = 31.79 ± 0.57).
Fig 3. β-carotene bleaching kinetics of (0.5 mg) at 490 nm from Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE (500 μL) and Ascorbic acid.
(a): Plant aqueous BCE. (b): Plant ethanolic BCE. Each value is the mean of three analyses.
3.6. Antimicrobial activity of bark crude extracts of selected species consumed by western lowland gorilla
The ethanolic BCE tested showed more significant activity against MDR E. coli (DECs) than the aqueous BCE. Of the four plants tested, all extracts were active against at least one MDR E. coli (DECs) isolate (Table 4 and Fig 4). Ceiba pentandra aqueous BCE showed remarkable activity against all the tested MDR E. coli (DECs) isolates with inhibition zones ranging from 8.34 ± 0.57 mm to 13.67 ± 0.57 mm (Table 4). Ceiba pentandra ethanolic BCE also showed reasonable activity against all the tested MDR E. coli (DECs) isolates with inhibition zones ranging from 8.34 ± 0.57 mm to 11.67 ± 0.57 mm. Ficus ssp aqueous and ethanolic BCE showed activity against MDR E. coli (DECs) isolates with inhibition zones ranging from 8.34 ± 0.57 mm to 10.4 ± 0.57 mm and 7.67 ± 1.54 mm to 10.67 ± 0.57 mm respectively. Myrianthus arboreus aqueous BCE showed activity only against two MDR E. coli (DECs) isolates with inhibition zones ranging from 7.34 ± 0.57 mm to 8.34 ± 0.57 mm. Myrianthus arboreus ethanolic BCE showed activity against six tested MDR E. coli (DECs) isolates with inhibition zones ranging from 8.67 ± 0.57 mm to 10.67 ± 0.57 mm. Milicia excelsa Welw C.C berg. aqueous BCE were the least active. Ethanol (98%) and water were used as control and did not have any effect on all studied MDR E. coli (DECs) isolates.
Fig 4. Diameters of the inhibition zones (mm) produced by Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE test against MDR E. coli (DECs) isolates.
MIC values were between 1.56 mg/mL and 50 mg/mL, and MBC values were between 3.12 mg/mL and >50 mg/mL (Table 5).
Table 5. Minimal inhibitory (MIC) and minimal bactericidal (MBC) concentration (mg/mL) of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCE.
| E32 | E40 | E41 | E8 | E4 | E7 | E37 | E50 | E49 | E10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| Ceiba Pentandra | ||||||||||||||||||||
| AE | 12.5 | 25 | 6.25 | 12.5 | 12.5 | 25 | 12.5 | 25 | 6.25 | 12.5 | 1.56 | 3.12 | 3.12 | 6.25 | 1.56 | 3.12 | 12.5 | 25 | 6.25 | 12.5 |
| EE | 6.25 | 12.5 | 6.25 | 12.5 | 6.25 | 12.5 | 1.56 | 3.12 | 3.12 | 6.25 | 1.56 | 3.12 | 3.12 | 6.25 | 6.25 | 12.5 | 12.5 | 25 | 3.12 | 6.25 |
| Myrianthus arboreus | ||||||||||||||||||||
| AE | 25 | 50 | 12.5 | 25 | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ |
| EE | 12.5 | 25 | 6.25 | 12.5 | ─ | ─ | ─ | ─ | ─ | ─ | 3.12 | 6.25 | 3.12 | 6.25 | 3.12 | 6.25 | 3.12 | 6.25 | ─ | ─ |
| Ficus ssp | ||||||||||||||||||||
| AE | ─ | ─ | ─ | ─ | 12.5 | 25 | 12.5 | 25 | 12.5 | 25 | 12.5 | 25 | 6.25 | 12.5 | 12.5 | 25 | 3.12 | 6.25 | ─ | ─ |
| EE | 6.25 | 12.5 | 3.12 | 6.25 | 3.12 | 6.25 | 25 | 50 | 6.25 | 12.5 | 25 | 50 | ─ | ─ | ─ | ─ | 12.5 | 25 | 6.25 | 12.5 |
| Milicia excelsa | ||||||||||||||||||||
| AE | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | 6.25 | 12.5 | 12.5 | 25 |
| EE | 12.5 | 25 | ─ | ─ | 12.5 | 25 | 12.5 | 25 | 6.25 | 12.5 | 1.56 | 3.12 | 3.12 | 6.25 | 1.56 | 3.12 | 12.5 | 25 | 6.25 | 12.5 |
| Ethanol (98%) | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ |
| Water | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ | ─ |
MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration, EE: Ethanol extract; AE: Aqueous extract; ─: Not effective.
3.7. Correlations analysis
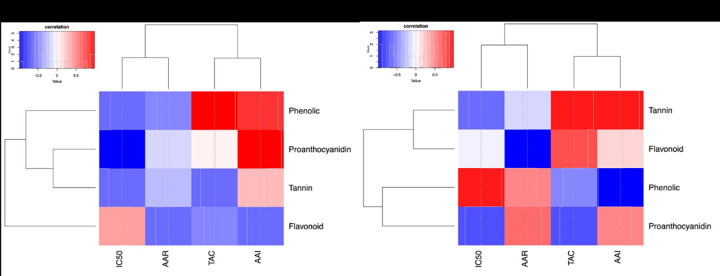
There was a positive correlation between the overall total phenolic, flavonoid, proanthocyanidin and tannin content and antimicrobial activity and a negative correlation with antioxidant activity assessed by DPPH IC50, PM TAC and BCB RAA (Figs 5 and 6).
Fig 5. Heat map illustrating the correlation between total phenolic, flavonoid, proanthocyanidin and tannin content with the assessed antimicrobial activities.
(a): Plant aqueous BCE. (b): Plant ethanolic BCE.
Fig 6. Heat map illustrating the correlation between total phenolic, flavonoid, proanthocyanidin and tannin content with the assessed antioxidant activities.
(c): Plant aqueous BCE. (d): Plant ethanolic BCE.
4. Discussion
The use of MPs for their pharmacological properties and biological activities is a practice that is increasingly reported across the world [65]. The WHO estimates that more than 25% of prescription drugs derive from MPs [66]. Research on and development of evidence-based phytomedicines is a priority for Africa, including Gabon [67]. Zoopharmacognosy is an original approach that could achieve these objectives [68]. Since the introduction of zoopharmacognosy as a scientific discipline, many drugs that are now in use have been found by studying animal self-medication behaviors [2]. Great apes use plants to heal themselves and thus control their parasitemia, viremia and bacteremia [69]. Moreover, traditional healers use plants items usually consumed by great apes for their pharmacological properties and biological activities [70]. However, these traditional uses of plants are not based on Western science and analysis [71].
The results in this study showed a negative correlation between extraction yield and solvent. The percentage yield of the different solvents obtained for the four plant bark extracts studied can be compared to those reported in the literature. For Ceiba pentandra, studies report a yield of 0.34% of the ethanolic extract [72], and 9% for the aqueous bark extract [73]. For Myrianthus arboreus, the yield of the ethanolic extract was 9.87% [74], and 0.05% for leaf aqueous extract [75]. For Milicia excelsa the yield .65% for stem bark ethanolic extract [76]. There are no reports on the yield of Ficus ssp. The differences observed between the results of this study and those in the literature could be explained by the many factors taken into account during the extraction process [77].
Preliminary phytochemical screening revealed the presence of several classes of secondary metabolites in the bark extracts of the four plants consumed by gorillas living in MDNP. These analyses suggest that the four plants bark investigated are good sources of natural products, secondary metabolites endowed pharmacological properties and biological activities. Similar results have been obtained in other studies of the phytochemical content and antimicrobial activities of C. pentandra extracts [78, 79]. For Myrianthus arboreus, studies in Cameroon [80] and Ghana [81] showed comparable results to those obtained here. One study showed only the presence of phenolic compounds and reducing sugars [82]. Another detected phenolic compounds and flavonoids [83]. Ficus spp are used as medicine to reduce the risk of cancer and heart disease [84]. Comparable results to those obtained here have been reported for Ficus species in Tunisia [85], Turkey [86] and Italy [87]. For Milicia excelsa BCE, a study in Nigeria, showed the presence of tannins, alkaloids, flavonoids and saponins in Nigeria [88]. Flavonoids and phenolic compounds were also found in Nigeria [89]. The secondary metabolites found in the BCE of the four plants studied possess pharmacological properties and are endowed with biological activities, suggesting that they can be used in traditional medicine as MP by healers and in pharmaceutical products [90, 91].
Traditional African healers make use of MPs to treat microbial diseases without western scientific basis [92, 93]. The results for total phenolic, flavonoid and tannin content obtained here in Ceiba pentandra are consistent with those reported in recent studies [38, 73]. For Myrianthus arboreus, total phenolic and flavonoid content [94], proanthocyanidins content [95] and tannins content [81] have been found as in this study. Recent work on the genus Ficus has also revealed the presence of total phenolic compounds and total flavonoids [96], and total tannins [97]. Similar results to those obtained here have been reported for Milicia excelsa extracts [98]. Therefore, the use of these four plant species in traditional medicine could be attributed to the high content of phenolic [99], flavonoids (flavones and flavonones) [100], tannins [101] and proanthocyanidins [102] compounds which are known for their antimicrobial, antioxidant, anticancer, antidiarrheal, anti-inflammatory, antimalarial, anti-cytotoxic and antispasmodic activities. The phenolic, flavonoid tannin and proanthocyanidin compounds found in this study may justify their bioactive effects for diarrheal diseases, those related to oxidative stress caused by ROS and diseases related to MDR E. coli (DECs).
Several studies have been conducted on MPs and their extracts with the aim of determining their antioxidant capacity, total phenolic content and characterizing the respective phenolic components [103]. Most mentioned plant secondary metabolites associated with antioxidant activity are alkaloids, sulfur compounds, terpene/terpenoids, essential oil, carotene/carotenoid, polyphenol/phenol, flavonoid, tannin, and coumarin [104]. However, variations exist, even among plant species belonging to the same genus. This could be attributed to differences in the extraction method during sample preparation [105], differences in harvest time [106], differences in the variety of the analyzed sample [107], as well as differences in the climatic and soil conditions and their origins [108]. Antioxidant compounds from MPs or nutritional plants display numerous beneficial effects such as a remarkable scavenger ability against various radical species [109]. As the latter explain their action through different mechanisms, usually, various tests should be explored to fully estimate the antioxidant capacity of bark crude extracts for example [110]. Extraction of plant by solvent is a commonly used method to obtain antioxidants. However, no single solvent can extract all the secondary metabolites with antioxidant activities from plant because of its variation in solubility and polarity [111, 112]. In the present study, solvents with different polarities including water and ethanol and were used as solvents to extract antioxidants from Ceiba pentandra L., Myrianthus arboreus P. Beauv., Ficus ssp and Milicia excelsa Welw C.C berg. bark. The most natural antioxidants are multifunctional. Therefore, a reliable antioxidant evaluation protocol requires different antioxidant activity assessments to take into account various mechanisms of antioxidant action [113].
In this study, DPPH assay showed that the radical scavenging activity of the BCEs is dose-dependent. All plant BCE studied here have DPPH radical scavenging activities. Ficus ssp ethanolic BCE showed a DPPH scavenging activity (IC50) (1.31 ± 0.20 mg/mL) higher than Ficus brevibracteata leaves with a IC50 value of 1.30 μg/ml (85.18 ± 0.22% of DPPH scavenging) [114], and lower than Ficus variegata stem bark ethanolic extract with 91% of DPPH scavenging [115]. Studies show a high relative scavenging activity (RSA) (about 50% of DPPH scavenging and 73.7 ± 8.4% of DPPH scavenging) [80, 116, 117] and Myrianthus arboreus aqueous extract has antioxidant activities [75]. In addition, antioxidant activities results obtained in the present study for Myrianthus arboreus are close to those observed in a previous study, which obtained a IC50 value of 13.3 μg/mL for the ethanolic extract [95]. For Ceiba pentandra ethanolic BCE, we observed similar antioxidant activities to those in a previous study, where the DPPH radical scavenging activity of ethanolic Ceiba pentandra leaves extract increased in dose dependent manner ranging from 10–50 μg/ml [118]. For Ceiba pentandra aqueous BCE, studies have obtained comparable results from aqueous extracts of stem bark [119] which induced also a concentration-dependent radical scavenging activity on DPPH, trunk [120] and leaf bark extracts [121]. Milicia excelsa showed radical scavenging activity on DPPH [122, 123]. Previous studies highlighted total antioxidant activities using phosphomolybdenum total antioxidant activity assay of Ficus nota [124], Ficus carica which possessed lower reducing ability with EC50 value of 39 μg/ml [125], Ficus sycomorus with EC50 value of 25 μg/ml [125] and Ficus Benghalensis methanolic extract with a IC50 value for phosphor-molybdenum of 31.84 ± 0.12 μg/ml [126]. A recent study showed Myrianthus arboreus aqueous root bark extract (40.3 ± 3.9 mg TE/g) and ethanolic extract (161.1 ± 11.9 mg TE/g) total antioxidant activities using phosphomolybdenum total antioxidant activity assay [95]. Sinha et al. reported Bombax ceiba (Ceiba pentandra) flowers phenolic extract total antioxidant activities using phosphomolybdenum complex assay [127]. There have been no reports on the effects of Milicia excelsa BCE using this assay. The β-carotene bleaching activities of all plant BCE depended on concentration. The antioxidant activity was observed in order of Ceiba pentandra.> Myrianthus arboreus> Ficus ssp> Milicia excelsa. There are no reports in the literature on the antioxidant activities of the BCEs of the four plants we studied using β-carotene bleaching assay. Plant antioxidants are a natural reservoir of bioactive compounds and play important roles in plant acclimation and adaptation to environmental challenges, and are also beneficial to human health [104]. Recent work on pollen extracts of three plants from the Palestinian pharmacopoeia reported antioxidant activities of flavonoids compounds such as flavones and flavonols in these extracts [48]. The antioxidant capacities of these all secondary compounds support the human body’s battle against diseases by absorbing free radicals and chelating metal ions that could catalyze the production of ROS, which facilitates lipid peroxidation [128]. Antioxidants break radical chain reactions, preventing oxidative stress-related damage [129]. In brief, antioxidants can act according to two major mechanisms, either by transfer of hydrogen atom or by electron transfer [130].
However, in this study, regarding the three methods used (DPPH radical capacity and Phosphomolybdenum complex for total antioxidant capacity and β-Carotene bleaching assay), ethanolic extracts of Ceiba pentandra L., Ficus ssp and Milicia excelsa Welw C.C berg. excepted for Myrianthus arboreus P. Beauv., presented relatively higher antioxidant activity than the aqueous ones. In a practical way, plant extracts antioxidant activity depends on several factors, for example: the concentration of the extracts, the method of evaluation, the sensitivity of the antioxidants to the temperature of the test, and the water- or fat-soluble nature of the antioxidant [131, 132].
Regarding antimicrobial activity, previously published reports report that the inhibitory activity of MPs extracts against Gram-positive and Gram-negative bacteria has been widely highlighted in the literature [133], and that MPs extracts with MIC values less than 100 mg/mL can be considered to have very good antimicrobial activity [134, 135]. In addition, the antimicrobial activity of these MPs extracts could be different toward various kinds of bacteria and different kinds of extract [136, 137]. This can be explained by the highest resistance of Gram-negative bacteria due to the complexity of their cell wall, containing a double membrane as opposed to the unique glycoprotein/teichoic acid membrane of Gram-positive bacteria [138]. In light of these indications, the antimicrobial activity of the bark crude extracts from the four plants studied and consumed by western lowland gorillas, against MDR E. coli (DECs) isolates, varied with different extraction solvents.
Results of the antimicrobial activities from the four plant BCEs investigated against MDR E coli (DECs), found in this study are comparable to those reported in the literature. Ceiba pentandra ethanolic extract showed antimicrobial activities against E. coli (an organism frequently implicated in gastroenteritis and pelvic inflammation) with a zone of inhibition value of 10.55 ± 1.45 mm and showed a MIC value of 12.5 mg/ml against E. coli [72]. A previous study also showed that Ceiba pentandra ethanolic extract was highly active against E. coli and that the antimicrobial activity of leaf extract increased as the concentration of ethanolic extract of Ceiba pentandra leaves increased [118]. Parulekar et al. [78] reported moderate antimicrobial activities from aqueous extract and strong antimicrobial activities ethanolic extract against E. coli, which increased as the concentration of the extracts increased; and Njokuocha et al. [79] also reported antimicrobial activities of Ceiba pentandra extracts. Ceiba pentandra aqueous extract has shown antimicrobial activities against Gram-negative bacteria, including E. coli [78, 79]. A recent study showed that the mean zone of inhibition of Myrianthus arboreus was zero for E. coli ATCC 25922 with aqueous and ethanolic extracts. However, for clinical MDR isolates, the Myrianthus arboreus inhibition zone diameter value was 0.4–2.2 mm for ethanolic extracts, showing low antimicrobial activities [82]. Ethanolic extracts of taxa belonging to the genus Ficus have been studied for their antimicrobial potential [139]. Biologically, Milicia excelsa Welw C.C berg. extract antimicrobial activities against enterobacteria have been demonstrated [44]. The flavonoid neocyclomorusin isolated from Milicia excelsa extract exhibited antimicrobial activity against K. pneumoniae ATCC11296 and E. cloacae BM47, with MIC values of 4 μg/mL each [43]. Padayachee et al. reported zero antimicrobial activity of Milicia excelsa extract against E. coli [140].
Many of these bioactive compounds are believed to have been used by plants and their parts, during their evolution, to protect against bacteria and are responsible for antimicrobial activity [141]. A possible mechanism for this phytochemical activity may be either through inhibiting the growth of microbes, inducing cellular membrane perturbations, interference with certain microbial metabolic processes, or modulation of signal transduction or gene expression pathways. However, these mechanisms may all occur at the same time as a result of the synergistic effect between the compounds [142]. The roles of secondary metabolites are relatively straightforward; for instance, they participate in general protective roles (antioxidant, free radical scavenging, UV light absorbing, and antiproliferative agents) and protect the plant from herbivorous animals (grazing) including different pathogenic microorganisms such as bacteria, fungi, and viruses. They also manage interplant relationships, acting as allelopathic defenders of the plant’s growing space against competitor plants [143].
Several phytochemicals have already been identified using GC-MS or HPLC-MS for Ceiba pentandra [118, 120], for Myrianthus arboreus [41, 95] and for Milicia excelsa [43]. These bioactive components are known to exhibit medicinal property [144]. In addition, in the current study, a positive correlation was observed between total phenolic, flavonoid, proanthocyanidin and tannin content with antimicrobial and antioxidant activity. The antioxidant activity of an extract or compound is often associated with their redox proprieties, which allow them to act as reducing agents [145]. Several other studies have also reported positive correlations between plant extracts secondary metabolites such as phenolic [146], flavonoid [147], proanthocyanidin [148], tannin [149] and antimicrobial activity.
Biodiversity contributes significantly towards human livelihood and development and thus plays a predominant role in the well-being of the global population [150]. Valorization of plants with medicinal value is a world challenge that meets the objectives of biodiversity conservation [104, 151]. The latter could involve the study of phytochemistry, pharmacological properties, antioxidant and antimicrobial activities of plants consumed by non-human animals, including great apes such as western lowland gorilla [2, 152]. Additionally, new drug discovery from natural sources involve a multifaceted approach combining botanical, phytochemical, biological, and molecular techniques [153, 154]. These bioprospecting practices have implications for medicine, environment, economy, public health, and culture [155]. Unfortunately, the potential benefits of plant-based medicines have led to unscientific exploitation of natural resources, a phenomenon that is being observed globally. The decline in biodiversity and loss due to species extinction is largely the result of the rise in the global population, rapid and sometimes unplanned industrialization, indiscriminate deforestation, overexploitation of natural resources, illegal trade, pollution, and finally global climate change [155, 156]. Therefore, it is of utmost importance that biodiversity is preserved, to provide future structural diversity and compounds for the sustainable development of human civilization [153, 157]. This becomes even more important for low and middle-income nations, where well-planned bioprospecting coupled with non-destructive commercialization could help in the conservation of biodiversity, ultimately benefiting humankind in the long run [153, 157].
5. Conclusion
The results of this study, which examined the antioxidant and antimicrobial activity of Ceiba pentandra, Myrianthus arboreus, Ficus ssp and Milicia excelsa BCEs, plants are consumed by western lowland gorilla living in MDNP and used in traditional medicine by Gabonese healers, revealed some important facts. Indeed, all plant BCEs studied showed antioxidant and antimicrobial activities. The asymptomatic nature of theses gorillas with regard MDR E. coli (DECs) could be explained by their consumption of the bark of the four plants tested. The scientific results obtained during pharmacological analyses could justify the use of these plants in the traditional pharmacopoeia against various human diseases. The BCEs of the four plants studied could be promising sources for new bioactive molecules discovery in the pharmaceutical, cosmetics and food industries. One of the potential challenges of this study was to address the issue of potential alternative solutions to the problem of antimicrobial resistance, using a zoopharmacognosy approach.
These results show that the BCE of these plants could be used as an effective treatment for diseases caused by free radicals and diseases caused by antimicrobial-resistant bacterial strains. Then, all this founding could comfort the self-medication hypothesis of non-human animals, including great apes. The results of our study suggest that all plant BCEs studied could potentially be candidate improved traditional medicines (ITMs) in the application of new therapeutic protocols against infectious diseases of bacterial origin.
In addition, the identification of all plant BCEs studied bioactive compounds, using HPLC-MS or a LC-MS/MS and molecular network approach, would add quality to the results obtained in our study. This approach is a valuable tool for revealing the metabolomes of plants extracts, groups secondary metabolites into molecular families based on their spectral similarities, and identify known compounds in order to focus on unknown compounds that may potentially be of biological interest.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors would like to acknowledge Doctoral School of Tropical Infectious Diseases of Franceville (EDR), Interdisciplinary Center for Medical Research of Franceville (CIRMF) and University of Sciences and Technology of Masuku (USTM) of Franceville. We also acknowledge Jean Bernard LEKANA-DOUKI and Jacques LEBIBI, Managing, Christophe NGOKOMAKA, mission vehicle driver and Peter MOMBO, Doussala station trackers team leader and all his collaborators.
Data Availability
All relevant data can be found in the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Giannenas I., et al., The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives, in Feed additives. 2020, Elsevier. p. 1–18. [Google Scholar]
- 2.Domínguez-Martín E.M., et al., Zoopharmacology: a way to discover new cancer treatments. Biomolecules, 2020. 10(6): p. 817. doi: 10.3390/biom10060817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahoua A.R.C., et al., Antimicrobial potential of 27 plants consumed by chimpanzees (Pan troglodytes verus Blumenbach) in Ivory Coast. BMC Complementary and Alternative Medicine, 2015. 15(1): p. 1–12. doi: 10.1186/s12906-015-0918-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sivakanthan S., et al., Antioxidant and antimicrobial applications of biopolymers: A review. Food Research International, 2020. 136: p. 109327. doi: 10.1016/j.foodres.2020.109327 [DOI] [PubMed] [Google Scholar]
- 5.Bordean M.-E., et al., Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants, 2023. 12(3): p. 596. doi: 10.3390/antiox12030596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kandyliari A., et al., Development of Dairy Products Fortified with Plant Extracts: Antioxidant and Phenolic Content Characterization. Antioxidants, 2023. 12(2): p. 500. doi: 10.3390/antiox12020500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y.-J., et al., Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules, 2015. 20(12): p. 21138–21156. doi: 10.3390/molecules201219753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mrityunjaya M., et al., Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Frontiers in immunology, 2020. 11: p. 2337. doi: 10.3389/fimmu.2020.570122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh S., et al., Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implications for multidrug resistance. International journal of molecular sciences, 2019. 20(10): p. 2468. doi: 10.3390/ijms20102468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raut P.K., et al., Growth of breast cancer cells by leptin is mediated via activation of the inflammasome: Critical roles of estrogen receptor signaling and reactive oxygen species production. Biochemical pharmacology, 2019. 161: p. 73–88. doi: 10.1016/j.bcp.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 11.Panieri E. and Santoro M., ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell death & disease, 2016. 7(6): p. e2253–e2253. doi: 10.1038/cddis.2016.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramalingam A.J., History of antibiotics and evolution of resistance. Research J. Pharm. and Tech, 2015. 8(12): p. 1719–1724. [Google Scholar]
- 13.Talebi Bezmin Abadi A., et al., World Health Organization report: current crisis of antibiotic resistance. BioNanoScience, 2019. 9: p. 778–788. [Google Scholar]
- 14.Behzadi P., et al., The role of gram-negative bacteria in urinary tract infections: current concepts and therapeutic options. Advances in Microbiology, Infectious Diseases and Public Health, 2020: p. 35–69. [DOI] [PubMed] [Google Scholar]
- 15.Kahn R., Clouser D., and Richt J., Emerging infections: a tribute to the one medicine, one health concept. Zoonoses and Public Health, 2009. 56(6‐7): p. 407–428. doi: 10.1111/j.1863-2378.2009.01255.x [DOI] [PubMed] [Google Scholar]
- 16.Bailey C. and Mansfield K., Emerging and reemerging infectious diseases of nonhuman primates in the laboratory setting. Veterinary pathology, 2010. 47(3): p. 462–481. doi: 10.1177/0300985810363719 [DOI] [PubMed] [Google Scholar]
- 17.Estrada A., et al., Impending extinction crisis of the world’s primates: Why primates matter. Science advances, 2017. 3(1): p. e1600946. doi: 10.1126/sciadv.1600946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunay E., et al., Pathogen transmission from humans to great apes is a growing threat to primate conservation. EcoHealth, 2018. 15: p. 148–162. doi: 10.1007/s10393-017-1306-1 [DOI] [PubMed] [Google Scholar]
- 19.Rabinowitz P., Scotch M., and Conti L., Human and animal sentinels for shared health risks. Veterinaria Italiana, 2009. 45(1): p. 23. [PMC free article] [PubMed] [Google Scholar]
- 20.Quammen D., Ebola: the natural and human history of a deadly virus. 2014: WW Norton & Company. [Google Scholar]
- 21.Köndgen S., et al., Pandemic human viruses cause decline of endangered great apes. Current Biology, 2008. 18(4): p. 260–264. doi: 10.1016/j.cub.2008.01.012 [DOI] [PubMed] [Google Scholar]
- 22.Calvignac-Spencer S., et al., Wild great apes as sentinels and sources of infectious disease. Clinical Microbiology and Infection, 2012. 18(6): p. 521–527. doi: 10.1111/j.1469-0691.2012.03816.x [DOI] [PubMed] [Google Scholar]
- 23.Carne C., et al., The risk of disease to great apes: simulating disease spread in orang-utan (Pongo pygmaeus wurmbii) and chimpanzee (Pan troglodytes schweinfurthii) association networks. PloS one, 2014. 9(4): p. e95039. doi: 10.1371/journal.pone.0095039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmerman D.M., et al., Great ape health watch: Enhancing surveillance for emerging infectious diseases in great apes. American journal of primatology, 2022. 84(4–5): p. e23379. doi: 10.1002/ajp.23379 [DOI] [PubMed] [Google Scholar]
- 25.Vila J., Moreno-Morales J., and Ballesté-Delpierre C., Current landscape in the discovery of novel antibacterial agents. Clinical Microbiology and Infection, 2020. 26(5): p. 596–603. doi: 10.1016/j.cmi.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 26.Iskandar K., et al., Antibiotic Discovery and Resistance: The Chase and the Race. Antibiotics, 2022. 11(2): p. 182. doi: 10.3390/antibiotics11020182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasool A., et al., Medicinal plants: Role, distribution and future. J Pharmacogn Phytochem, 2020. 9(2): p. 2111–2114. [Google Scholar]
- 28.Christaki S., et al., Recent advances in plant essential oils and extracts: Delivery systems and potential uses as preservatives and antioxidants in cheese. Trends in Food Science & Technology, 2021. 116: p. 264–278. [Google Scholar]
- 29.Chamberlin B., What is Zoopharmacognosy. Academia Letters, 2021: p. 2. [Google Scholar]
- 30.Takenoshita Y., Ando C., and Yamagiwa J., Fruit phenology of the great ape habitat in the Moukalaba-Doudou National Park, Gabon. African Study Monographs. Supplementary Issue., 2008. 39: p. 23–39. [Google Scholar]
- 31.Nguelet F.L.M., et al., Etude de la relation entre l’abondance des grands mammifères frugivores et celle des fruits dans le Parc National de Moukalaba-Doudou, Gabon. International Journal of Biological and Chemical Sciences, 2016. 10(5): p. 1969–1982. [Google Scholar]
- 32.Yamagiwa J. and Basabose A.K., Diet and seasonal changes in sympatric gorillas and chimpanzees at Kahuzi–Biega National Park. Primates, 2006. 47(1): p. 74–90. doi: 10.1007/s10329-005-0147-7 [DOI] [PubMed] [Google Scholar]
- 33.Bye Z.L., et al., What Role Do Plant-Based Diets Play in Supporting the Optimal Health and Well-being of Canadians? A Scoping Review. Advances in Nutrition, 2021. 12(6): p. 2132–2146. doi: 10.1093/advances/nmab061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huffman M.A., Self-Medicative Behavior in the African Great Apes: An Evolutionary Perspective into the Origins of Human Traditional Medicine: In addition to giving us a deeper understanding of our closest living relatives, the study of great ape self-medication provides a window into the origins of herbal medicine use by humans and promises to provide new insights into ways of treating parasite infections and other serious diseases. BioScience, 2001. 51(8): p. 651–661. [Google Scholar]
- 35.Cousins D. and Huffman M.A., Medicinal properties in the diet of gorillas: an ethno-pharmacological evaluation. African study monographs, 2002. 23(2): p. 65–89. [Google Scholar]
- 36.Maloueki U., et al., Propriétés ethnomédicinales et pharmacologiques des plantes consommées par les bonobos (Pan paniscus) à Bolobo, République Démocratique du Congo: végétation herbacée terrestre. 2015, Ethnopharmacologia. [Google Scholar]
- 37.Obiang C.S., et al., Antimicrobial, antioxidant, anti-inflammatory and cytotoxic study of extracts of Guibourtia tessmanii (harms) J. Léonard from Gabon. Clinical Phytoscience, 2021. 7(1): p. 1–8. [Google Scholar]
- 38.Afzal S., et al., A Comparative Analysis of Antimicrobial, Antibiofilm and Antioxidant Activity of Silver Nanoparticles Synthesized from Erythrina Suberosa Roxb. and Ceiba Pentandra. Journal of Oleo Science, 2022. 71(4): p. 523–533. [DOI] [PubMed] [Google Scholar]
- 39.Silué G.N.A., et al., Anti-inflammatory and antioxidant activities assessment of an aqueous extract of Ceiba pentandra (L.) Gaertn (Malvaceae). RPS Pharmacy and Pharmacology Reports, 2023. 2(4): p. rqad037. [Google Scholar]
- 40.Kasangana P.B., et al., Root bark extracts of Myrianthus arboreus P. Beauv.(Cecropiaceae) exhibit anti-diabetic potential by modulating hepatocyte glucose homeostasis. Journal of ethnopharmacology, 2018. 211: p. 117–125. doi: 10.1016/j.jep.2017.09.017 [DOI] [PubMed] [Google Scholar]
- 41.García-Pérez M.-E., Kasangana P.-B., and Stevanovic T., Bioactive Molecules from Myrianthus arboreus, Acer rubrum, and Picea mariana Forest Resources. Molecules, 2023. 28(5): p. 2045. doi: 10.3390/molecules28052045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simsek E., Kilic D., and Caliskan O., Phenotypic variation of fig genotypes (Ficus carica L.) in the eastern Mediterranean of Turkey. Genetika, 2020. 52(3): p. 957–972. [Google Scholar]
- 43.Oke-Altuntas F., et al., Bioactivity evaluation of cudraxanthone I, neocyclomorusin and (9βh)-3β-acetoxylanosta-7, 24-diene isolated from Milicia excelsa Welw. CC Berg (Moraceae). Medicinal Chemistry Research, 2016. 25(10): p. 2250–2257. [Google Scholar]
- 44.Biwôlé J.J.E., et al., Iroko wood (Milicia excelsa CC berg), a good candidate for high-speed rotation-induced wood dowel welding: An assessment of its welding potential and the water resistance of its welded joints. International Journal of Adhesion and Adhesives, 2023. 123: p. 103360. [Google Scholar]
- 45.Mbehang Nguema P.P., et al., High level of intrinsic phenotypic antimicrobial resistance in enterobacteria from terrestrial wildlife in Gabonese national parks. PLoS One, 2021. 16(10): p. e0257994. doi: 10.1371/journal.pone.0257994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obiang C.S., et al., Ethnopharmacological Study and Antibacterial Activities of Some Plants Used in Traditional Medicine for the Treatment of Diarrheal Diseases in Gabon. Traditional and Integrative Medicine, 2022: p. 386–401. [Google Scholar]
- 47.Aiyegoro O.A. and Okoh A.I., Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC complementary and alternative medicine, 2010. 10(1): p. 1–8. doi: 10.1186/1472-6882-10-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadeq O., et al., Phytochemical screening, antioxidant and antibacterial activities of pollen extracts from micromeria fruticosa, achillea fragrantissima, and phoenix dactylifera. Plants, 2021. 10(4): p. 676. doi: 10.3390/plants10040676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hossain M.A., et al., Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pacific journal of tropical biomedicine, 2013. 3(9): p. 705–710. doi: 10.1016/S2221-1691(13)60142-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Do Q.D., et al., Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. Journal of food and drug analysis, 2014. 22(3): p. 296–302. doi: 10.1016/j.jfda.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sima-Obiang C., et al., Phytochemical screening, phenolic compounds content, antioxidant, anti-inflammatory, and antimicrobial properties of Pachylobus balsamifera Guillaum from Gabon. Phytothérapie, 2018. 16(S1): p. S65–S73. [Google Scholar]
- 52.Ngoua-Meye-Misso R.-L., et al., Phytochemical screening, antioxidant, anti-inflammatory and antiangiogenic activities of Lophira procera a. Chev.(Ochnaceae) medicinal plant from Gabon. Egyptian Journal of Basic and Applied Sciences, 2018. 5(1): p. 80–86. [Google Scholar]
- 53.Scherer R. and Godoy H.T., Antioxidant activity index (AAI) by the 2, 2-diphenyl-1-picrylhydrazyl method. Food chemistry, 2009. 112(3): p. 654–658. [Google Scholar]
- 54.Prieto P., Pineda M., and Aguilar M., Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical biochemistry, 1999. 269(2): p. 337–341. doi: 10.1006/abio.1999.4019 [DOI] [PubMed] [Google Scholar]
- 55.Kubola J. and Siriamornpun S., Phenolic contents and antioxidant activities of bitter gourd (Momordica charantia L.) leaf, stem and fruit fraction extracts in vitro. Food chemistry, 2008. 110(4): p. 881–890. doi: 10.1016/j.foodchem.2008.02.076 [DOI] [PubMed] [Google Scholar]
- 56.Prieto M., et al., β-Carotene assay revisited. Application to characterize and quantify antioxidant and prooxidant activities in a microplate. Journal of Agricultural and Food Chemistry, 2012. 60(36): p. 8983–8993. [DOI] [PubMed] [Google Scholar]
- 57.Oyaba Yinda L.E.D., et al., Phylogenetic groups, Pathotypes and antimicrobial resistance of Escherichia coli isolated from Western lowland Gorilla Faeces (Gorilla gorilla gorilla) of Moukalaba-Doudou National Park (MDNP). Pathogens, 2022. 11(10): p. 1082. doi: 10.3390/pathogens11101082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falciglia M.D., et al., In Vitro Antimicrobial Activity Evaluation of a Novel Fitostimoline® Plus Spray Formulation. International Journal of Microbiology, 2021. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CLSI., Performance Standards for Antimicrobial Susceptibility Testing. 31st ed; 2020. 2020.
- 60.Imtara H., Elamine Y., and Lyoussi B., Honey antibacterial effect boosting using Origanum vulgare L. essential oil. Evidence-Based Complementary and Alternative Medicine, 2018. 2018. doi: 10.1155/2018/7842583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bamunusinghage N.P., et al., Antimicrobial Resistance Patterns of Fecal Escherichia coli in Wildlife, Urban Wildlife, and Livestock in the Eastern Region of Sri Lanka, and Differences between Carnivores, Omnivores, and Herbivores. The Journal of Wildlife Diseases, 2022. 58(2): p. 380–383. doi: 10.7589/JWD-D-21-00048 [DOI] [PubMed] [Google Scholar]
- 62.Ibrahim M., Bilal N., and Hamid M., Increased multi-drug resistant Escherichia coli from hospitals in Khartoum state, Sudan. African health sciences, 2012. 12(3): p. 368–375. doi: 10.4314/ahs.v12i3.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peterson L.R. and Collins S.M., Bactericidal testing for infectious disease therapy: the why and how to measure if we kill the bugs. Clinical Microbiology Newsletter, 2000. 22(20): p. 153–157. [Google Scholar]
- 64.El-Ashmawy I.M., Al-Wabel N.A., and Bayad A.E., Achillea fragrantissima, rich in flavonoids and tannins, potentiates the activity of diminazine aceturate against Trypanosoma evansi in rats. Asian Pacific Journal of Tropical Medicine, 2016. 9(3): p. 228–234. doi: 10.1016/j.apjtm.2016.01.032 [DOI] [PubMed] [Google Scholar]
- 65.Szopa A., Ekiert R., and Ekiert H., Current knowledge of Schisandra chinensis (Turcz.) Baill.(Chinese magnolia vine) as a medicinal plant species: a review on the bioactive components, pharmacological properties, analytical and biotechnological studies. Phytochemistry Reviews, 2017. 16(2): p. 195–218. doi: 10.1007/s11101-016-9470-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fowler M.W., Plants, medicines and man. Journal of the Science of Food and Agriculture, 2006. 86(12): p. 1797–1804. [Google Scholar]
- 67.Ngwa W., et al., Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules, 2020. 25(11): p. 2707. doi: 10.3390/molecules25112707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Greene A.M., et al., Asian elephant self-medication as a source of ethnoveterinary knowledge among Karen mahouts in northern Thailand. Journal of ethnopharmacology, 2020. 259: p. 112823. doi: 10.1016/j.jep.2020.112823 [DOI] [PubMed] [Google Scholar]
- 69.Krief S., et al., Bioactive properties of plant species ingested by chimpanzees (Pan troglodytes schweinfurthii) in the Kibale National Park, Uganda. American Journal of Primatology: Official Journal of the American Society of Primatologists, 2006. 68(1): p. 51–71. doi: 10.1002/ajp.20206 [DOI] [PubMed] [Google Scholar]
- 70.Masi S., et al., Unusual feeding behavior in wild great apes, a window to understand origins of self-medication in humans: role of sociality and physiology on learning process. Physiology & behavior, 2012. 105(2): p. 337–349. doi: 10.1016/j.physbeh.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 71.Patwardhan B., et al., Ayurveda and traditional Chinese medicine: a comparative overview. Evidence-based complementary and alternative medicine, 2005. 2(4): p. 465–473. doi: 10.1093/ecam/neh140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anosike C., et al., Phytochemical screening and antimicrobial activity of the petroleum ether, methanol and ethanol extracts of Ceiba pentandra stem bark. Journal of Medicinal Plants Research, 2012. 6(46): p. 5743–5747. [Google Scholar]
- 73.Osuntokun O., et al., Assessment of antimicrobial and phytochemical properties of crude leaf and bark extracts of Ceiba pentandra on selected clinical isolates found in Nigerian teaching hospital. Journal of Bacteriology and Mycology Open Access, 2017. 4(1): p. 17–23. [Google Scholar]
- 74.Olonode E.T., Aderibigbe A.O., and Bakre A.G., Anti-nociceptive activity of the crude extract of Myrianthus arboreus P. Beauv (Cecropiaceae) in mice. Journal of ethnopharmacology, 2015. 171: p. 94–98. doi: 10.1016/j.jep.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 75.Mvondo M.A., et al., The leaf aqueous extract of Myrianthus arboreus P. Beauv.(Cecropiaceae) improved letrozole-induced polycystic ovarian syndrome associated conditions and infertility in female Wistar rats. BMC Complementary Medicine and Therapies, 2020. 20: p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abiola A.L., et al., Acute Toxicity and Sedative-hypnotic Effects of Ethanol Stem Bark Extract and Fractions of Milicia excelsa (Moraceae) in Mice. Adv. Pharmacol. Pharm., 2020. 8: p. 11–18. [Google Scholar]
- 77.Mohammedi H., et al., The effect of solvents and extraction procedure on the recovery of phenolic compounds and the antioxidant capacity of Algerian Bassia muricata L. extracts. Chemistry Journal of Moldova, 2019. 14(2): p. 79–89. [Google Scholar]
- 78.Parulekar G., Antibacterial and phytochemical analysis of Ceiba pentandra (L.) seed extracts. Journal of Pharmacognosy and Phytochemistry, 2017. 6(3): p. 586–589. [Google Scholar]
- 79.Njokuocha R.C. and Ewinike A., Antibacterial and phytochemical properties of crude leaf extract of Moringa oleifera Lam., Pterocarpus santalinoides L’Herit DC and Ceiba pentandra L. on some clinical bacteria isolates in Nigeria. Journal of Complementary and Alternative Medical Research, 2020. 10(4): p. 1–15. [Google Scholar]
- 80.Biapa P., et al., Phytochemical studies and antioxidant properties of four medicinal plants used in Cameroon. African Journal of Traditional, Complementary and Alternative Medicines, 2007. 4(4): p. 495–500. doi: 10.4314/ajtcam.v4i4.31243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Agyare C., et al., Wound healing and anti-infective properties of Myrianthus arboreus and Alchornea cordifolia. Med. Chem, 2014. 4(7): p. 533–539. [Google Scholar]
- 82.Owusu E., et al., Antimicrobial activity of selected medicinal plants from a sub-Saharan African country against bacterial pathogens from post-operative wound infections. Medical Sciences, 2021. 9(2): p. 23. doi: 10.3390/medsci9020023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fankam, A.G., et al., Antioxidant and antifungal activities of Myrianthus arboreus P. Beauv.(Moraceae), Allanblackia gabonensis Pellegr.(Clusiaceae) and three other Cameroonian medicinal plants.
- 84.Eissa S., et al., Molecular Genetic Studies on (Ficus Palmata & Ficus Carica Subsp Rupestris.) Under the Effect of Gamma Radiation by Tissue Culture Techniques. Al-Azhar Journal of Agricultural Research, 2021. 46(2): p. 128–138. [Google Scholar]
- 85.Lazreg-Aref H., et al., Chemical composition and antibacterial activity of a hexane extract of Tunisian caprifig latex from the unripe fruit of Ficus carica. Pharmaceutical Biology, 2012. 50(4): p. 407–412. doi: 10.3109/13880209.2011.608192 [DOI] [PubMed] [Google Scholar]
- 86.Paşayeva L., Özalp B., and Fatullayev H., Potential enzyme inhibitory properties of extracts and fractions from fruit latex of Ficus carica-based on inhibition of α-amylase and α-glucosidase. Journal of Food Measurement and Characterization, 2020. 14(5): p. 2819–2827. [Google Scholar]
- 87.Toma C.C., et al., Ficus carica SSP Dottato Buds by Intercropping Different Species: Metabolites, Antioxidant Activity and Endogenous Plant Hormones (IAA, ABA). Rev. Chim, 2017. 68: p. 1628–1631. [Google Scholar]
- 88.Udegbunam S., et al., Evaluation of herbal ointment formulation of Milicia excelsa (Welw) CC berg for wound healing. African journal of biotechnology, 2013. 12(21). [Google Scholar]
- 89.Ayepola, O.O., A.F. Samson, and O. Onile-Ere, In vitro Antioxidant and Anti-staphylococcal Activity of Bixa orellana Linn. and Milicia excelsa Welw.
- 90.Huang Y.-H., et al., The traditional uses, secondary metabolites, and pharmacology of Eleutherococcus species. Phytochemistry Reviews, 2022. 21(4): p. 1081–1184. [Google Scholar]
- 91.ElNaggar M.H., et al., The old world salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: a review. Journal of Enzyme Inhibition and Medicinal Chemistry, 2022. 37(1): p. 2036–2062. doi: 10.1080/14756366.2022.2102005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rigat M., et al., Traditional and alternative natural therapeutic products used in the treatment of respiratory tract infectious diseases in the eastern Catalan Pyrenees (Iberian Peninsula). Journal of Ethnopharmacology, 2013. 148(2): p. 411–422. doi: 10.1016/j.jep.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 93.Hajdu Z. and Hohmann J., An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. Journal of ethnopharmacology, 2012. 139(3): p. 838–857. [DOI] [PubMed] [Google Scholar]
- 94.Harley B.K., et al., Flavanols and triterpenoids from Myrianthus arboreus ameliorate hyperglycaemia in streptozotocin-induced diabetic rats possibly via glucose uptake enhancement and α-amylase inhibition. Biomedicine & Pharmacotherapy, 2020. 132: p. 110847. [DOI] [PubMed] [Google Scholar]
- 95.Kasangana P.B., Haddad P.S., and Stevanovic T., Study of polyphenol content and antioxidant capacity of Myrianthus arboreus (Cecropiaceae) root bark extracts. Antioxidants, 2015. 4(2): p. 410–426. doi: 10.3390/antiox4020410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alzahrani F.O., et al., Total phenol, flavonoid, and individual phenolic acid contents and antioxidant and cytotoxic activities of methanol extracts from Ficus cordata ssp. salicifolia. Canadian Journal of Plant Science, 2021. 101(4): p. 517–524. [Google Scholar]
- 97.Sumi S.A., et al., Investigation of the key pharmacological activities of Ficus racemosa and analysis of its major bioactive polyphenols by HPLC-DAD. Evidence-Based Complementary and Alternative Medicine, 2016. 2016. doi: 10.1155/2016/3874516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adebayo M.A., et al., Evaluation of anti-diarrheal activity of methanol root bark extract of Milicia excelsa (Welw) C. C Berg (Moraceae) in rats. Drug Research, 2019. 69(08): p. 439–444. [DOI] [PubMed] [Google Scholar]
- 99.Tungmunnithum D., et al., Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines, 2018. 5(3): p. 93. doi: 10.3390/medicines5030093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dirar A., et al., Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. South African Journal of Botany, 2019. 120: p. 261–267. [Google Scholar]
- 101.Mahomoodally M.F., Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evidence-based complementary and alternative medicine, 2013. 2013. doi: 10.1155/2013/617459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Navarro M., et al., Proanthocyanidin Characterization, Antioxidant and Cytotoxic Activities of Three Plants Commonly Used in Traditional Medicine in Costa Rica: Petiveria alliaceae L., Phyllanthus niruri L. and Senna reticulata Willd. Plants, 2017. 6(4): p. 50. doi: 10.3390/plants6040050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gonçalves S., et al., Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. European Food Research and Technology, 2019. 245: p. 753–762. [Google Scholar]
- 104.Llauradó Maury G., et al., Antioxidants in plants: A valorization potential emphasizing the need for the conservation of plant biodiversity in Cuba. Antioxidants, 2020. 9(11): p. 1048. doi: 10.3390/antiox9111048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Silva B.N., et al., Chemical profile and bioactivities of extracts from edible plants readily available in Portugal. Foods, 2021. 10(3): p. 673. doi: 10.3390/foods10030673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Farhat M.B., et al., Antioxidant potential of Salvia officinalis L. residues as affected by the harvesting time. Industrial Crops and Products, 2014. 54: p. 78–85. [Google Scholar]
- 107.Erdogan-Orhan I., et al., Sage-called plant species sold in Turkey and their antioxidant activities. Journal of the Serbian Chemical Society, 2010. 75(11): p. 1491–1501. [Google Scholar]
- 108.Yesiloglu Y., Sit L., and Kilic I., In vitro antioxidant activity and total phenolic content of various extracts of Satureja hortensis L. collected from Turkey. Asian Journal of Chemistry, 2013. 25(15): p. 8311. [Google Scholar]
- 109.Ruskovska T., Maksimova V., and Milenkovic D., Polyphenols in human nutrition: from the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability–an overview and perspective. British Journal of Nutrition, 2020. 123(3): p. 241–254. doi: 10.1017/S0007114519002733 [DOI] [PubMed] [Google Scholar]
- 110.Pellegrini N., et al., Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. The Journal of nutrition, 2003. 133(9): p. 2812–2819. doi: 10.1093/jn/133.9.2812 [DOI] [PubMed] [Google Scholar]
- 111.Sun T. and Ho C.-T., Antioxidant activities of buckwheat extracts. Food chemistry, 2005. 90(4): p. 743–749. [Google Scholar]
- 112.Iqbal S. and Bhanger M., Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chemistry, 2007. 100(1): p. 246–254. [Google Scholar]
- 113.Wong S.P., Leong L.P., and Koh J.H.W., Antioxidant activities of aqueous extracts of selected plants. Food chemistry, 2006. 99(4): p. 775–783. [Google Scholar]
- 114.Moncayo S., et al., Preliminary phytochemical screening for antioxidant activity and content of phenols and flavonoids of 18 species of plants native to western Ecuador. Trends in Phytochemical Research, 2021. 5(2): p. 93–104. [Google Scholar]
- 115.Kusuma I.W., Arung E.T., and Kim Y.-u, Antimicrobial and antioxidant properties of medicinal plants used by the Bentian tribe from Indonesia. Food Science and Human Wellness, 2014. 3(3–4): p. 191–196. [Google Scholar]
- 116.Ilecholubo A.P., Okpachi A.C., and Ocholi E.D., Phytochemical constituents and Antioxidant properties of Aqueous leaf extract of Myrianthus arboreus. Int. J. Biomed. Sci. Eng., 2017. 5(1): p. 9. [Google Scholar]
- 117.Konan Y., et al., Antioxidant activity and total phenolic content of nine plants from Côte d’Ivoire (West Africa). Journal of Applied Pharmaceutical Science, 2014. 4(8): p. 036–041. [Google Scholar]
- 118.Bhavani R., Bhuvaneswari E., and Rajeshkumar S., Antibacterial and Antioxidant activity of Ethanolic extract of Ceiba pentandra leaves and its Phytochemicals Analysis using GC-MS. Research Journal of Pharmacy and Technology, 2016. 9(11): p. 1922–1926. [Google Scholar]
- 119.Fofié C.K., et al., Hypoglycemic properties of the aqueous extract from the stem bark of Ceiba pentandra in dexamethasone-induced insulin resistant rats. Evidence-Based Complementary and Alternative Medicine, 2018. 2018. doi: 10.1155/2018/4234981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nguelefack T.B., et al., Multimodal α-glucosidase and α-amylase inhibition and antioxidant effect of the aqueous and methanol extracts from the trunk bark of Ceiba pentandra. BioMed research international, 2020. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Aji A., et al., Biosynthesis of gold nanoparticles using Kapok (Ceiba pentandra) leaf aqueous extract and investigating their antioxidant activity. Journal of Molecular Structure, 2022. 1270: p. 133906. [Google Scholar]
- 122.Ekere N., Okparanozie T., and Agbo M., Effects of solvents on the in-vitro antioxidant activity of Dennettia tripetala G. Baker and Milicia excelsa (Welw.) C. Berg root extracts. Iranian Journal of Health Sciences, 2018. [Google Scholar]
- 123.Akinpelu L.A., et al., Phytochemical estimations and antihypoxic effect of ethanol leaf extract of Milicia excelsa (Moraceae) in mice. GSC Biological and Pharmaceutical Sciences, 2020. 10(2): p. 024–029. [Google Scholar]
- 124.Latayada F.S. and Uy M.M., Screening of the antioxidant properties of the leaf extracts of Philippine medicinal plants Ficus nota (Blanco) Merr., Metroxylon sagu Rottb., Mussaenda philippica A. Rich., Inocarpus fagifer, and Cinnamomum mercadoi Vidal. Bull Environ Pharmacol Life Sci, 2016. 5(3): p. 18–24. [Google Scholar]
- 125.Abdel-Aty A.M., et al., Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatalysis and Agricultural Biotechnology, 2019. 20: p. 101199. [Google Scholar]
- 126.Yadav Y.C., et al., In-vitro antioxidant activity of methanolic extraction of Ficus Benghalensis L. latex. Pharmacologyonline, 2011. 1: p. 140–148. [Google Scholar]
- 127.Sinha S., et al., Antioxidant and choline esterase inhibitory activity of phenolic rich extracts from Bombax ceiba l. flowers. Free Radicals and Antioxidants, 2018. 8(2): p. 135–140. [Google Scholar]
- 128.Niki E., Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biology and Medicine, 2010. 49(4): p. 503–515. doi: 10.1016/j.freeradbiomed.2010.04.016 [DOI] [PubMed] [Google Scholar]
- 129.Da Pozzo E., et al., Antioxidant and antisenescence effects of bergamot juice. Oxidative medicine and cellular longevity, 2018. 2018. doi: 10.1155/2018/9395804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Santos-Sánchez N.F., et al., Antioxidant compounds and their antioxidant mechanism. Antioxidants, 2019. 10: p. 1–29. [Google Scholar]
- 131.Kartal N., et al., Investigation of the antioxidant properties of Ferula orientalis L. using a suitable extraction procedure. Food chemistry, 2007. 100(2): p. 584–589. [Google Scholar]
- 132.Pukalskas A., et al., Isolation, identification and activity of natural antioxidants from horehound (Marrubium vulgare L.) cultivated in Lithuania. Food chemistry, 2012. 130(3): p. 695–701. [Google Scholar]
- 133.Yuan G., et al., Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Scientific reports, 2021. 11(1): p. 10471. doi: 10.1038/s41598-021-90035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuete V., Potential of Cameroonian plants and derived products against microbial infections: a review. Planta medica, 2010. 76(14): p. 1479–1491. doi: 10.1055/s-0030-1250027 [DOI] [PubMed] [Google Scholar]
- 135.Kuete V., et al., Antibacterial activities of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complementary and Alternative Medicine, 2012. 12(1): p. 1–8. doi: 10.1186/1472-6882-12-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Radünz M., et al., Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food chemistry, 2019. 276: p. 180–186. doi: 10.1016/j.foodchem.2018.09.173 [DOI] [PubMed] [Google Scholar]
- 137.Bussmann R.W., et al., Minimum inhibitory concentrations of medicinal plants used in Northern Peru as antibacterial remedies. Journal of ethnopharmacology, 2010. 132(1): p. 101–108. doi: 10.1016/j.jep.2010.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ndamane Y., et al., Antibacterial effectiveness of Tetradenia riparia extract, a plant traditionally used in the Eastern Cape Province to treat diseases of the respiratory system. 2013. [Google Scholar]
- 139.Tkachenko H., et al., Preliminary in vitro screening of the antibacterial activity of leaf extracts from various Ficus species (Moraceae) against Yersinia ruckeri. Fisheries & Aquatic Life, 2019. 27(1). [Google Scholar]
- 140.Padayachee T. and Odhav B., Antimicrobial activity of plant phenols from Chlorophora excelsa and Virgilia oroboides. African Journal of Biotechnology, 2013. 12(17). [Google Scholar]
- 141.Ashmawy N.A., et al., Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microbial pathogenesis, 2018. 121: p. 331–340. doi: 10.1016/j.micpath.2018.05.032 [DOI] [PubMed] [Google Scholar]
- 142.Omojate Godstime C., et al., Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens–a review. J Pharm Chem Biol Sci, 2014. 2(2): p. 77–85. [Google Scholar]
- 143.Tahara S., A journey of twenty-five years through the ecological biochemistry of flavonoids. Bioscience, biotechnology, and biochemistry, 2007. 71(6): p. 1387–1404. doi: 10.1271/bbb.70028 [DOI] [PubMed] [Google Scholar]
- 144.Olukemi O.A., et al., The use of selected Nigerian natural products in management of environmentally induced free radical skin damage. Pakistan Journal of Biological Sciences, 2005. 8(8): p. 1074–1077. [Google Scholar]
- 145.Diouf P.N., Stevanovic T., and Cloutier A., Antioxidant properties and polyphenol contents of trembling aspen bark extracts. Wood Science and Technology, 2009. 43: p. 457–470. [Google Scholar]
- 146.Weerakkody N.S., et al., In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control, 2010. 21(10): p. 1408–1414. [Google Scholar]
- 147.Tian Y., et al., Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food research international, 2018. 106: p. 291–303. doi: 10.1016/j.foodres.2017.12.071 [DOI] [PubMed] [Google Scholar]
- 148.Navarro-Hoyos M., et al., Proanthocyanidin characterization and bioactivity of extracts from different parts of Uncaria tomentosa L.(cat’s claw). Antioxidants, 2017. 6(1): p. 12. doi: 10.3390/antiox6010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yahia Y., et al., Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PloS one, 2020. 15(5): p. e0232599. doi: 10.1371/journal.pone.0232599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sandifer P.A., Sutton-Grier A.E., and Ward B.P., Exploring connections among nature, biodiversity, ecosystem services, and human health and well-being: Opportunities to enhance health and biodiversity conservation. Ecosystem services, 2015. 12: p. 1–15. [Google Scholar]
- 151.Niesenbaum R.A., The integration of conservation, biodiversity, and sustainability. 2019, MDPI. p. 4676. [Google Scholar]
- 152.Krishnaswamy S., Bioprospecting, botany, biodiversity, and their impact on botanical drug development. Botanical Drug Products: Recent Developments and Market Trends, 2018. 39. [Google Scholar]
- 153.Sen T. and Samanta S.K., Medicinal plants, human health and biodiversity: a broad review. Biotechnological applications of biodiversity, 2014: p. 59–110. [DOI] [PubMed] [Google Scholar]
- 154.Ozturk M. and Hakeem K.R., Plant and Human Health, Volume 2: Phytochemistry and Molecular Aspects. 2019: Springer. [Google Scholar]
- 155.Alves R. and Alves H.N., The faunal drugstore: Animal-based remedies used in traditional medicines in Latin America. Journal of ethnobiology and ethnomedicine, 2011. 7(1): p. 1–43. doi: 10.1186/1746-4269-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mukherjee J., Biotechnological Applications of Biodiversity. 2015: Springer. [Google Scholar]
- 157.Shukla V. and Kumar N., Environmental Concerns and Sustainable Development. 2020: Springer. [Google Scholar]