Abstract
Newborns are very susceptible to infections because their immune systems are not fully developed and react to antigen exposure preferentially with unresponsiveness. UV-inactivated herpes simplex virus type 1 (HSV-1) represents such an antigen and does not induce an immune response in neonates. In contrast, protective T cells were primed in newborn mice by a single replicative cycle of DISC HSV-1 given once within 24 h of birth. Each of the HSV-1-primed CD4+ or CD8+ T cells induced in wild-type or interferon-deficient mice conferred resistance to naive animals exposed to a lethal virus challenge. Inactivated HSV-1, injected at variable doses up to 104 times that of DISC HSV-1, was ineffective in inducing any detectable immune responses in neonates. Thus, the capacity of HSV-1 to replicate once, but not the number of virus particles per se, was decisive in inducing protective T-cell-associated immunity in newborn mice.
Individuals exposed to antigens in utero or shortly after birth have been believed to develop antigen-specific tolerance preferentially (7, 10, 43, 60), although exceptions to this “rule” are known (41). Recent investigations have revised the idea that antigenic exposure in neonatal life is a tolerogenic rather than an immunogenic event. Ridge et al. (48) argued that the neonatal immune system, though not fully mature, might respond actively to antigens when presented in adequate amounts. The requirements appear to be appropriate antigen dosage, strong simulation of the innate immune response, and repeated immunizations. However, with the exception of live virus vaccines (50, 55) and some recent DNA vaccination studies (34), the immune responses obtained in neonates have been in general T-helper 2 (Th2)-biased types, which persisted after adult boosting (2, 4, 6, 26). Because the immune system is not fully developed in neonates, it remains difficult to induce an immune response in these individuals that is adequate to control virus infections.
Gamma interferon (IFN-γ), IFN-α, and IFN-β are believed to be required for the induction of a Th1-biased immune response associated with a powerful cytotoxic-T-lymphocyte (CTL) response and immunoglobulin isotype switch to immunoglobulin G2a (IgG2a) in mice (12, 28, 61, 63). Herpes simplex virus (HSV) is a strong inducer of the Th1-type immune responses. CTLs and CD4+ T cells capable of producing IFN-γ are required for the control of this virus in its latent stage in ganglia (29, 30, 46). Surprisingly, in mice without functional IFN receptors, HSV type 1 (HSV-1) or other alphaherpesviruses, but not inactivated virus or HSV-1 amplicon, when applied at peripheral sites, induce the production of virus-specific IgG2a, IgG2b, and IgG3 (18, 58). Thus, replication of HSV-1 appeared to trigger the induction of a Th1-mediated immune response by IFN-dependent and IFN-independent mechanisms. In neonates, the induction of a Th1 immune response is difficult to achieve because of the stringent requirements to stimulate antigen-presenting cells (in particular, dendritic cells) and the low number of cells able to produce only limited amounts of IFN-γ and interleukin-12 (IL-12) (3, 15, 48, 52). The powerful stimulation of the innate immune response by replicating HSV-1 appeared to be a promising approach to apply to neonates to stimulate a protective Th1-mediated immune response.
Infants of many species are very susceptible to infection with wild-type (wt) HSV-1 (5; for a review, see references 31 and 37). To overcome the low resistance of neonatal mice against this virus, humoral and cellular components of the innate immunity, as well as HSV-1-specific antibodies (Abs) and T-cell populations, have been transferred to these mice (25). In general, not one but several components of the innate or specific immunity are required to arm the neonatal immune system against infections with HSV-1. This may explain why maternal HSV-1-specific Abs and antiviral drugs have additive effects to protect newborns against fatal infections with this virus (23, 65). Abs may suppress the development of cellular or humoral immunity (51, 53). This modulatory effect of the maternal humoral immunity needs to be addressed using neonatal vaccination studies.
Despite the wealth of information that is available on the immune response of adults against HSV-1 (31, 57), little is known of active neonatal immunity against this virus (13, 24). We have chosen neonatal mice as an experimental model to study the immune responses to HSV-1 because of the relative immaturity of this species at birth. Mice have to mature for 1 week to reach the degree of immune maturity of humans, and thus vaccines given to mice at birth are a stringent test for the neonatal immune system (34). To conduct such experiments, we have used an HSV-1 variant able to complete a single cycle of virus replication (DISC [disabled infectious single cycle]) (35, 58). The DISC viral genome does not contain the gene encoding glycoprotein H (gH), which is essential for infection. This virus thus requires a gH-expressing cell line for multicycle growth but can complete a single cycle of infection in noncomplementing cells. This virus does not cause disease in adults.
Here we report that mice with or without functional IFN systems given a single immunization with DISC HSV-1 24 h after birth develop a Th1 immune response. Furthermore, immunized mice resisted an intraperitoneal (i.p.) injection with a lethal dose of wt HSV-1 without showing any signs of disease.
MATERIALS AND METHODS
Animals, cells, and viruses.
Female, 7- to 10-week-old 129Sv/Ev (H-2b), C57BL/6, BALB/c, and newborn mice less than 24 h old were obtained from BRL, Füllinsdorf, Switzerland. Congenic mice with gene-targeted disruptions of the IFN receptor IFN-α/β and IFN-γ (AG129) were also used (18, 61). The AG129 mice were obtained from R. M. Zinkernagel, University of Zurich. Vero cells (ATCC, Rockville, Md.), HSV-1 gH-expressing Vero cells (35), H-2b thymoma cells (EL-4), and gB-expressing fibroblast cells (MC57) (11, 62) were grown in complete Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (58). DISC HSV-1, a gH deletion mutant capable of completing a single cycle of infection, was propagated on F1 cells (35, 58). HSV-1 strain F was obtained from B. Roizman (University of Chicago) and propagated on Vero cells (14). All virus preparations were purified by ultracentrifugation on a sucrose density gradient, and the virus titer was determined as described previously (58). Inactivation of virus was done by β-propiolactone treatment or UV radiation. The presence of intact virus particles was verified by electron microscopy.
Immunization protocols.
Newborn 129Sv/Ev C57BL/6 or BALB/c mice were immunized within 24 h of birth with 107 PFU of DISC HSV-1; newborn AG129 mice were inoculated with 105 PFU in 50 μl of DMEM medium or, as a control, with 50 μl of DMEM medium alone. The immunization dose was split into two portions for simultaneous i.p. and subcutaneous injections (55). Some animals were boosted i.p. with 107 PFU of DISC HSV-1 in 100 μl of DMEM medium 3 weeks after priming and, 10 days later, all animals were analyzed for the induction of cellular and humoral immune responses or challenged with wt HSV-1 strain F. For some experiments, mothers were immunized i.p. with 107 PFU of DISC HSV-1 before gestation and boosted i.p. 10 days before delivery with the same dose of virus.
Assessment of CTLs.
Spleen cells were assayed for the presence of CTL activity as described elsewhere (58). Briefly, a single cell suspension from spleen cells was prepared from immunized or control animals with an H-2b haplotype and assayed after restimulation in vitro for 5 days using UV-irradiated HSV-1 gB-expressing MC57 (11, 58, 62). EL-4 target cells were pulsed for 1 h with 3 μg of gB-specific H-2b-restricted peptide SSIEFARL (single-letter amino acid code) per ml and analyzed by a standard 4-h 51Cr release cytotoxicity assay. Spontaneous 51Cr release was consistently <15% in a 4-h assay (58).
In some cases CTL were restimulated in vivo in 3-week-old neonatally primed mice by i.p. injection of 107 PFU of DISC HSV-1 or 2 × 108 PFU of HSV-1 strain F. CTL activity was determined by directly incubating the splenocytes isolated as described above as effector cells with the gB peptide-loaded EL-4 cells as target cells and analyzed in a 6-h 51Cr release cytotoxicity assay.
Serology.
Enzyme-linked immunosorbent assay (ELISA) was performed, and Ab titers were determined as previously described (58) using peroxidase-conjugated polyclonal anti-mouse IgG1, IgG2a, IgG2b, and IgG3 Abs or polyclonal anti-human IgG (Southern Biotechnology, Birmingham, Ala.). Sera obtained from immunized and control animals were analyzed for HSV-1 neutralization using standard methods (58).
Western immunoblot analysis.
Sera were analyzed for the presence of polyreactive Ab by Western blot analysis using radioactively labeled cell lysates subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose for immunostaining as described elsewhere (1, 58).
Cell transfer experiment.
CD4+ and CD8+ T cells were isolated from pooled lymph nodes and spleens of 4-week-old mice infected once with 107 PFU of DISC HSV-1 during the first 24 h after birth. MACS colloidal supermagnetic anti-mouse CD4 and CD8 microbeads (Miltenyi Biotec) were used for positive selection of CD4+ and CD8+ T cells according to the manufacturer's instructions. Then, 107 highly purified (>95%) CD4+ or CD8+ T cells were injected intravenously into 4-week-old syngeneic recipients, and the animals were challenged 24 h later.
Virus challenge.
At 4 weeks of age the mice were challenged i.p. with 2 × 108 PFU of HSV-1 strain F in 100 μl of DMEM medium. The doses of HSV-1 strain F given i.p. that cause lethal infections in 50% of the animals (LD50) have been determined in age-matched 129Sv/Ev or C57BL/6 mice and AG129 mice and were found to be 106 and <10 PFU, respectively. The animals were examined daily for signs of disease, and the surviving animals were counted 21 days after the challenge.
RESULTS
CTLs are primed in neonatal mice injected once with DISC HSV-1, but not with inactivated virus: role of maternal Ab and IFN in CTL priming.
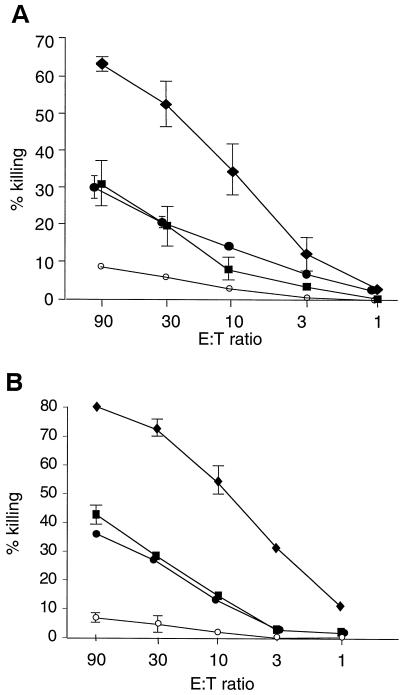
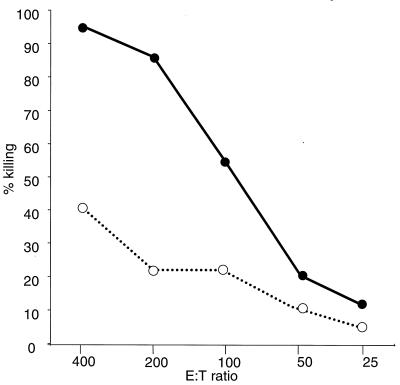
Newborn individuals are very susceptible to infection with HSV-1, but the virus can induce a powerful immune response in adults (22, 46). HSV-1 variants such as DISC HSV-1 are able to complete only one cycle of replication and are immunogenic but do not induce disease (35, 40, 58). Therefore, DISC HSV-1 appeared ideal for immunological studies in neonates. Newborn 129Sv/Ev or C57BL/6 mice were injected within 24 h of birth with 107 PFU of DISC HSV-1 or UV-inactivated virus or they were mock treated and left with their mothers for 3 weeks. During this time all animals stayed healthy and no difference in weight gain was observed between the groups. At 3 weeks of age, one group of mice was injected (i.p.) with 107 PFU of DISC HSV-1; one group was mock treated; and all mice were analyzed at 4 weeks of age. CTL activity was detected in restimulated splenocytes from mice injected with DISC HSV-1 at birth. Boosting the primed animals at 3 weeks of age enhanced the CTL activity. DISC HSV-1-vaccinated 129Sv/Ev or C57BL/6 mice had similar CTL activity. Mice injected with inactivated virus and mock-treated mice had similar CTL activities (Fig. 1A). In further experiments, newborn mice were injected with 100 times the standard dose (i.e., 109 PFU) of inactivated DISC HSV-1 and boosted with the same amount of virus at 3 weeks of age. No significant CTL activity was detected in these mice. The data indicate that it is not the number of HSV-1 particles per se but the capacity to replicate at least once that was decisive for CTL induction in neonatal mice.
FIG. 1.
(A) HSV-1–gB-specific CTL activity after restimulation in vitro. 129Sv/Ev mice (five animals per group) were injected with 107 PFU of DISC HSV-1 within 24 h of birth in the presence (●) or absence (⧫ and ■) of maternal Abs to HSV-1. One group of virus-primed mice (⧫) was boosted at 3 weeks of age with 107 PFU of DISC HSV-1. A fourth group of neonatal mice was injected with 107 PFU of UV-inactivated HSV-1 and boosted at 3 weeks of age with the same antigen load (○). At 4 weeks of age, splenocytes were isolated from all mice, restimulated in vitro, and analyzed for HSV-1-specific CTL activity in a 4-h 51Cr release assay. The results are expressed as the lysis of gB-loaded target cells at various effector/target (E:T) ratios. The lysis of peptide-loaded or non-peptide-loaded target cells by restimulated splenocytes obtained from UV-inactivated DISC HSV-1 or mock-injected mice was similar. Spontaneous 51Cr release was <20%. (B) Mice with deleted IFN receptors (AG129 mice; five animals per group) were injected with 105 PFU of DISC HSV-1 but otherwise treated as described for the 129Sv/Ev mice. CTL analysis and symbols are as in panel A.
Virus-neutralizing maternal Abs may suppress active neonatal immune responses against the same infectious agent (53, 54). To test the influence of maternal Ab for CTL priming, neonatal mice delivered by HSV-1-immune mothers were injected with 107 PFU of DISC HSV-1 within 24 h of birth. The CTL activity in splenocytes was detected 4 weeks after priming, despite an HSV-1-specific neutralization titer of 60 to 120 per ml in the serum of the mothers at the time of delivery and a titer of 30 to 60 in the 4-week-old offspring (Fig. 1A, Table 1). Therefore, neutralizing maternal Abs specific to HSV-1 antigen did not appreciably influence the priming of CTLs in neonates.
TABLE 1.
HSV-1-specific antibody titers of murine seraa
| Time postchallenge (days) | Group | No. of mice | Mean neutralization titer ± SEMb | Mean ELISA titer (IgG) ± SEMb |
|---|---|---|---|---|
| 0 | 1 | 8 | <4 | <50 |
| 2 | 8 | <4 | <50 | |
| 3 | 8 | 30 ± 15 | 1,250 ± 150 | |
| 7 | 1 | 8 | 60 ± 25 | 3,200 ± 480 |
| 2c | 1 | ND | ND | |
| 3 | 8 | 30 ± 18 | 1,600 ± 240 | |
| 21 | 1 | 8 | 200 ± 50 | 12,500 ± 2,250 |
| 2c | 1 | ND | ND | |
| 3 | 8 | 60 ± 35 | 3,350 ± 855 |
129Sv/Ev mice were vaccinated at birth with 107 PFU of DISC HSV-1 (group 1), 107 PFU of UV-inactivated virus (group 2), or 107 PFU of DISC HSV-1 in the presence of maternal Ab specific to HSV-1 (group 3). All animals were challenged at 4 weeks of age with HSV-1 (200 LD50).
Serum dilution ± the standard error of the mean. ND, not determined.
Only one animal survived in this group.
We had previously noted that HSV-1 or other alphaherpesviruses induce CTL-mediated and virus-specific IgG Abs of all isotypes in adult mice lacking IFN receptors for both IFN-α/β and IFN-γ (AG129 mice) (18), arguing for a potent IFN-independent stimulation of the Th1 immune pathway. To test whether this pathway was also operative in neonates, newborn AG129 mice were injected within 24 h of birth with DISC HSV-1 and analyzed at 4 weeks of age. A virus dose of 105 PFU was found to be optimal for these experiments. CTL activity similar to wild-type mice was detected. Boosting the primed animals with DISC HSV-1 at 3 weeks of age could enhance CTL activity. Thus, maternal Abs did not influence the induction of CTL-mediated immunity. Injection of UV-inactivated DISC HSV-1 did not induce significant CTL activity (Fig. 1B). Moreover, doses of up to 109 PFU (>104 times the standard dose used) did not induce significant CTL activity. Therefore, replication competent DISC HSV-1, but not UV-inactivated virus, enable CTL priming in newborns that have no functional receptors for IFN-α/β or IFN-γ.
DISC HSV-1-primed neonates do not induce detectable Ab to HSV-1 but do develop a Th1-associated Ab response after a booster injection.
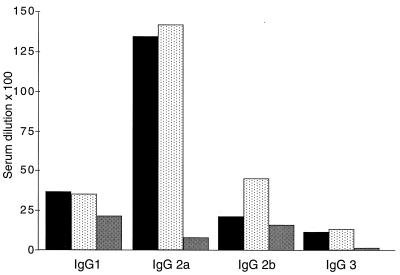
Neonates vaccinated with DISC HSV-1 induced CTL immunity efficiently. We therefore expected to find HSV-1-specific Abs 4 weeks after priming neonatal mice with DISC HSV-1. However, in more than 50 sera obtained from these mice, Ab to HSV-1 antigen was not detected by ELISA nor by a virus neutralization assay (Table 1). When mice were boosted 3 weeks after neonatal priming, the sera analyzed at 4 weeks of age contained Abs of all IgG isotypes against HSV-1 antigen, a finding typical for a Th1 immune response (Fig. 2). These data were confirmed with neonatal priming experiments using BALB/c mice (Fig. 2).
FIG. 2.
HSV-1-antigen-specific ELISA. Groups of five 129Sv/Ev (■) or BALB/c mice (░⃞) were injected at birth with 107 PFU or five AG129 (▩) mice were injected with 105 PFU of DISC HSV-1, and then the animals boosted at 3 weeks of age with the same amounts of virus used for priming. At 4 weeks of age, the sera were analyzed by ELISA (the mean titer is given as the serum dilution × 10−2).
We next analyzed whether neonatal AG129 mice were also capable of producing virus-specific Abs after vaccination with HSV-1. Groups of neonatal AG129 mice were injected with 105 PFU of DISC HSV-1. Some mice were boosted at 3 weeks of age with the same dose of virus, and the sera of all mice were analyzed at 4 weeks of age for the presence of Abs against HSV-1 antigen. Abs were only detected in sera of mice boosted at 3 weeks of age. Therefore, like the adult AG129 mice, infant mice devoid of a functional IFN system were also capable of producing Abs of IgG2a, IgG2b, and IgG3 isotypes specific to HSV-1 antigen. However, the Ab titers were lower compared to wild-type animals, and IgG1 Abs dominated the serological immune response in HSV-1-vaccinated AG129 animals. Therefore, similar to adult AG129 mice, an IFN-independent mechanism of both CTL induction (Fig. 1B) and of an immunoglobulin isotype switch to IgG2a, IgG2b, and IgG3 is operative in neonatal mice infected with DISC HSV-1 (18).
Protective activity of serum Ab from DISC HSV-1-immunized mice.
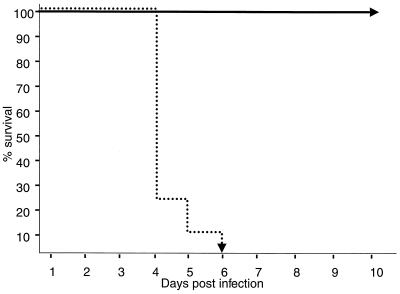
Newborn mice injected once within 24 h of birth did not produce significant Abs to HSV-1 antigen (Fig. 2). It was still possible that the detection of minute amounts of in vivo protective Ab had been missed by the in vitro assays used, as shown previously (42). To exclude this possibility, 500-μl aliquots of sera from 4-week-old animals that had been either vaccinated at birth with DISC HSV-1 or from animals vaccinated at birth and boosted at 3 weeks of age were transferred to groups of naive age-matched animals. This was followed by a challenge with HSV-1 strain F. Sera from animals that received a booster vaccination at 3 weeks of age were fully protective. Naive animals that had received sera from neonatally primed animals or sera from mock-immunized animals were not protective (Fig. 3). We conclude that a single immunization with DISC HSV-1 does not induce detectable Abs against HSV-1 antigen, as determined in vitro as well as in vivo experiments.
FIG. 3.
Serum transfer and challenge experiments. Two groups of eight 129Sv/Ev mice were injected at birth with 107 PFU of DISC HSV-1. One group was boosted at 3 weeks of age with the same amount of virus. At 4 weeks of age, all mice were bled, and the sera in each group were pooled. Pooled serum (500 μl/mouse) from singly vaccinated mice (…) or boosted mice (—) was transfused i.p. to two groups of eight naive, 4-week-old mice. One day later, the transfused animals were challenged i.p. with 2 × 108 PFU of HSV-1 strain F (200 LD50). The survival of mice after this lethal challenge is shown.
A single neonatal immunization with DISC HSV-1 protects mice from lethal HSV-1 infection.
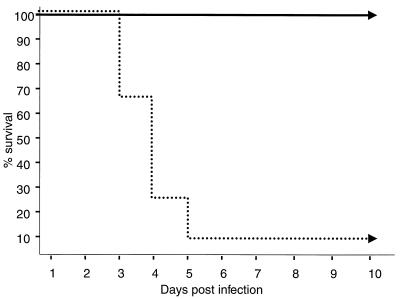
Although mice vaccinated within 24 h of birth had no detectable HSV-1 antigen-specific Ab at 4 weeks of age, primed T cells were present (Fig. 1). In the next experiments, we determined whether the HSV-1-primed T cells could protect mice against a lethal challenge with HSV-1 strain F. Mice born to HSV-1-antigen-naive mothers were primed with 107 PFU of DISC HSV-1 or appropriate controls within 24 h of birth. They were left with their mothers until 4 weeks of age. At this time point, groups of eight mice were challenged in three individual experiments with 200 LD50s of HSV-1 strain F (Fig. 4). The results show clear protection of the 4-week-old mice vaccinated once with DISC HSV-1 within 24 h of birth. None of the 24 vaccinated animals challenged in the three separate experiments showed any signs of disease, and all mice survived more than 14 days. In contrast, three groups of eight mice injected with UV-inactivated DISC HSV-1, eight mock-treated mice, or eight naive control mice died within 4 to 6 days, and only one of these twenty-four mice survived the virus challenge (Fig. 4).
FIG. 4.
Challenge experiments of DISC HSV-1-vaccinated mice. Three groups of eight 129Sv/Ev mice were injected at birth with 107 PFU of DISC HSV-1. A fourth group was injected with 107 PFU of UV-inactivated virus. Group 5 was mock injected, and group 6 was left untouched. At 4 weeks of age the animals were challenged with 2 × 108 PFU of HSV-1 strain F (200 LD50) in three separate experiments. The data from the individual challenge experiments from the three DISC HSV-1-vaccinated groups (—) and groups 4 to 6 (…) were pooled. The survival of mice after lethal challenge is shown.
Wt 129Sv/Ev mice or C57BL/6 mice vaccinated with DISC HSV-1 at birth survived a lethal challenge with HSV-1 strain F for more than 50 days. In contrast, IFN-deficient mice vaccinated at birth or as adult animals with DISC HSV-1 died between 7 and 35 days after the challenge (data not shown). Therefore, CTL and virus-specific neutralizing Abs induced by vaccination in IFN-deficient mice control acute infection with HSV-1 for a short time, but a functional IFN system is absolutely necessary for long-term survival.
To further characterize the CTL response activated in vivo by the challenge injection with HSV-1 strain F, spleen cells from neonatally vaccinated mice were analyzed 4 days after the virus challenge for their capacity to directly kill gB-peptide-loaded EL-4 target cells. Efficient killing of target cells was observed when splenocytes from DISC HSV-1-vaccinated mice, but not from mice injected neonatally with UV-inactivated virus, were used (Fig. 5). Therefore, neonatally DISC HSV-1-primed T-cells were restimulated in vivo by HSV-1 strain F.
FIG. 5.
Ex vivo HSV-1–gB-specific CTL assay. Groups of three mice were vaccinated at birth with either 107 PFU of DISC HSV-1 (—) or UV-inactivated virus (…). At 4 weeks of age, mice were challenged with 2 × 108 PFU of HSV-1 strain F (200 LD50). At 4 days after the challenge, splenocytes were isolated from all mice and analyzed directly for HSV-1-specific CTL activity in a 6-h 51Cr release assay on EL-4 target cells loaded with gB-peptide. Lysis of non-peptide-loaded target cells has been subtracted from the data shown. The values of individual mice from one of three similar experiments are shown.
Following challenge with HSV-1 strain F, the sera of the surviving animals were analyzed by HSV-1 neutralization and ELISA (Table 1). In the sera of mice that were vaccinated once at birth with DISC HSV-1, significant Abs were first detected 7 days after the virus challenge. The amounts of Ab increased, as determined 21 days after the challenge by virus neutralization and by ELISA analysis. HSV-1-specific Ab recognized a broad spectrum of different Abs, as analyzed by Western blot analysis (data not shown). The data confirm that B cells had not been primed neonatally (Fig. 2) and suggested that CD4+ or CD8+ T cells were responsible for the induction of immune protection against virus challenge.
Both CD4+ and CD8+ T cells from neonatally vaccinated mice transferred to naive recipients confer protection against a lethal virus challenge.
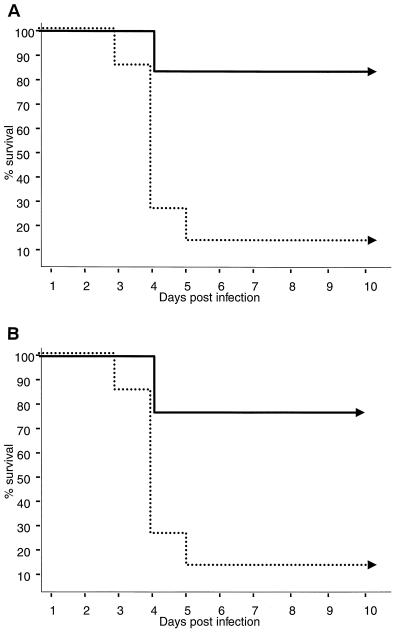
To identify the T-cell population that was responsible for the induction of immune protection, cell transfer experiments were performed, followed by virus challenge. Groups of neonatal mice were vaccinated once with DISC HSV-1, and at 4 weeks of age CD8+ and CD4+ T cells were isolated and transferred to naive age- and sex-matched recipients. Approximately 70 to 90% of the mice that had received CD4+ or CD8+ T cells from DISC HSV-1-vaccinated mice survived the virus challenge. Cells isolated from naive mice were ineffective (Fig. 6). Thus, a single vaccination within 24 h of birth induced CD8+ and CD4+ T cells able to confer protection to naive recipients.
FIG. 6.
CD4+ and CD8+ T-cell transfer and challenge experiments. Two groups of 16 mice were vaccinated at birth with either 107 PFU of DISC HSV-1 (—) or left as controls (…). CD4+ (A) or CD8+ (B) T cells from spleens and lymph nodes were purified by using magnetic beads at 4 weeks of age. Aliquots (107 cells/mouse) of the four different cell populations were injected intravenously into eight age- and sex-matched mice per group. One day after the cell transfer, mice were challenged with 2 × 108 PFU of HSV-1 strain F (200 LD50). The survival of mice after lethal challenge is shown.
DISCUSSION
A single vaccination with DISC HSV-1, within 24 h of birth, induced CD8+ or CD4+ T cells able to protect naive mice against a lethal challenge with HSV-1 strain F. Because UV-inactivated, but otherwise intact, virus particles injected at doses of 102 to 104 times that of DISC HSV-1 were ineffective, we concluded that one round of virus replication was the key element for the induction of this cellular immune response. Therefore, HSV-1 replication is linked to immunogenicity that leads to Th1-mediated immune responses by IFN-dependent and IFN-independent mechanisms (Fig. 1 and 2). These data support and extend our previous observations that in both adult and newborn mice, alphaherpesviruses can induce CTL and an IFN-independent switch to all IgG isotypes. Furthermore, herpesvirus-primed and in vitro-restimulated splenocytes produced high amounts of IFN-γ but negligible IL-4 (18).
The protective function of the transferred CD8+ T cells in vivo (Fig. 6B) might be direct cytolytic activity by these cells as analyzed by ex vivo CTL assays (Fig. 5) or indirectly by the secretion and stimulation of inflammatory cytokines (17). These may provide support for the recruitment of immune cells, notably natural killer (NK) cells, to the site of infection (20, 59). The critical role of CD8+ T cells and the cytokines released by these cells at later stages of infections with HSV-1 is well documented (29, 30, 56). Transfer of HSV-1-activated CD4+ T cells in naive animals (Fig. 6A) and induction of protection upon lethal virus challenge was shown previously for adult mice (9, 38) and for neonatal mice (25). Since CD4+ T cells have no known direct cytolytic activity against HSV-1-infected cells, the effect must be indirect. The mechanism includes increased upregulation of IFN-α/β and IFN-γ (27), recruitment and activation of macrophages and NK cells (21, 59), induction of nitric oxide and tumor necrosis factor alpha (21, 44), and the rapid help of B cells for the production of neutralizing Abs (Table 1). Clearly, antiviral CD8+ T cells and CD4+ T cells induced by neonatal vaccination are the master effector cells against lethal HSV-1 infection. However, how and when are these cells induced in neonates?
The successful induction of CTLs or CD8+ T cells in neonates has been reported after DNA vaccination (8, 33), for influenza viruses and retroviruses (41, 50), with live Sendai virus vaccines (55) but not with live attenuated measles virus (6). Our own attempts to DNA vaccinate neonates for CTL induction that is specific against HSV-1 gB with either the BAC-VAC system (58), genes encoding gB (19), or Sindbis virus-based vectors containing gB (19) failed (data not shown). After DNA vaccination, antigens can be found for weeks after their introduction, and thus the actual priming of naive T cells could happen at a later time point. However, recent data indicate that dendritic cells considered crucial for immune induction need to be directly transfected for successful priming (45). This implies that priming occurs immediately after DNA vaccination by the directly transfected dendritic cells. Because of the inadequate development of the immune system immediately after birth, DNA vaccination may not be successful in neonates. This may not apply to infections with small amounts of virus able to replicate in neonates until recognized by the more mature immune system (50). In contrast to replicating virus, live viruses able to replicate only once have a limited time span available to prime naive T and B cells in neonates. Neonatal mice vaccinated with a single dose of replication-deficient Sendai virus produced adult-like virus-specific Ab titers and CTLs (55). Although neonatal mice vaccinated once with DISC HSV-1 within the first 24 h developed protective CD8+ and CD4+ T cells, Ab against HSV-1 antigen was not detected by any of the test systems used. Importantly, mice vaccinated once with DISC HSV-1 at 10 days rather than within 24 h of birth developed HSV-1-antigen-specific Abs when analyzed 4 weeks later (unpublished observations). Therefore, in contrast to mice infected at birth with Sendai virus and able to produce antigen-specific Abs, the production of Ab against DISC HSV-1 required the presence of a more mature immune system. The reason for this is presently unclear and requires further investigation.
HSV-1 encodes more than 70 genes, classified as immediate-early (IE), early, and late genes that are transcribed in a temporal fashion (for a review, see references 31 and 47). However, the genes activated in a given cell infected by the virus and the signals transduced within the cell are poorly understood and may require microarray analysis as was successfully done for cytomegalovirus (66). Therefore, the molecular mechanism of the powerful T-cell-based immune response in neonates against DISC HSV-1 is unknown but must require virus replication for the following reasons: (i) inactivated virus particles were ineffective, as shown here (Fig. 1 and 4) and previously (40); (ii) mutant viruses that are able to replicate only part of the HSV-1 genome (IE and early genes) were able to induce T-cell-dependent immunoglobulin class switching (39); (iii) HSV-1 amplicon or HSV-1 variants that have defects in the IE and early genes that block virus replication are apathogenic and do not induce immune responses (32, 58); and (iv) only replication-competent alphaherpesviruses induce CTL and IgG class switch in an IFN-independent mechanism (18).
The data presented here have clinical and practical implications. An equally strong T-cell-based immune response could be induced by a simple vaccination in neonates in the presence or absence of maternal Abs (Fig. 1). This novel concept of preventive medicine may not only be useful in industrialized countries with advanced possibilities for HSV-1 diagnosis and drug treatment but, because of its relative low costs, the concept may also be highly beneficial for countries where diagnosis and drug treatment is a prohibitive economic factor (16, 36, 49, 64).
ACKNOWLEDGMENTS
We thank Pascale Koebel from the Basel Institute of Immunology for excellent technical assistance.
This work was supported by the Kanton of Zürich and the Swiss Federal Office for Education and Research (European Union Network no. ERBFMRXCT960053).
REFERENCES
- 1.Ackermann M, Braun D K, Pereira L, Roizman B. Characterization of herpes simplex virus 1 alpha proteins 0, 4, and 27 with monoclonal antibodies. J Virol. 1984;52:108–118. doi: 10.1128/jvi.52.1.108-118.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins B. Development of neonatal Th1/Th2 function. Int Rev Immunol. 2000;19:157–171. doi: 10.3109/08830180009088503. [DOI] [PubMed] [Google Scholar]
- 3.Adkins B. T-cell function in newborn mice and humans. Immunol Today. 1999;20:330–335. doi: 10.1016/s0167-5699(99)01473-5. [DOI] [PubMed] [Google Scholar]
- 4.Adkins B, Nassiri M. Apoptosis of murine neonatal T cells. Int Rev Immunol. 1999;18:465–484. doi: 10.3109/08830189909088494. [DOI] [PubMed] [Google Scholar]
- 5.Andervont H B. Activity of herpetic virus in mice. J Infect Dis. 1929;44:383. [Google Scholar]
- 6.Barrios C, Brawand P, Berney M, Brandt C, Lambert P H, Siegrist C A. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 7.Billingham R E, Brent L, Medawar P B. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 8.Bot A, Antohi S, Bot S, Garcia-Sastre A, Bona C. Induction of humoral and cellular immunity against influenza virus by immunization of newborn mice with a plasmid bearing a hemagglutinin gene. Int Immunol. 1997;9:1641–1650. doi: 10.1093/intimm/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 9.Brehm M A, Bonneau R H, Knipe D M, Tevethia S S. Immunization with a replication-deficient mutant of herpes simplex virus type 1 (HSV-1) induces a CD8+cytotoxic T-lymphocyte response and confers a level of protection comparable to that of wild-type HSV-1. J Virol. 1997;71:3534–3544. doi: 10.1128/jvi.71.5.3534-3544.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnet F M, Fenner F. The production of antibodies. London, England: Macmillan; 1949. The production of antibodies; pp. 102–105. [Google Scholar]
- 11.Cose S C, Kelly J M, Carbone F R. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Waal Malefyt R. The role of type I interferons in the differentiation and function of Th1 and Th2 cells. Semin Oncol. 1997;24:S9–94–S9–98. [PubMed] [Google Scholar]
- 13.Diamond C, Mohan K, Hobson A, Frenkel L, Corey L. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J. 1999;18:487–489. doi: 10.1097/00006454-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 15.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 16.Ghebrekidan H, Ruden U, Cox S, Wahren B, Grandien M. Prevalence of herpes simplex virus types 1 and 2, cytomegalovirus, and varicella-zoster virus infections in Eritrea. J Clin Virol. 1999;12:53–64. doi: 10.1016/s0928-0197(98)00064-6. [DOI] [PubMed] [Google Scholar]
- 17.Grob P, Baumann S, Ackermann M, Suter M. A system for stable indirect immobilization of multimeric recombinant proteins. Immunotechnology. 1998;4:155–163. doi: 10.1016/s1380-2933(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 18.Grob P, Schijns V E, van den Broek M F, Cox S P, Ackermann M, Suter M. Role of the individual interferon systems and specific immunity in mice in controlling systemic dissemination of attenuated pseudorabies virus infection. J Virol. 1999;73:4748–4754. doi: 10.1128/jvi.73.6.4748-4754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hariharan M J, Driver D A, Townsend K, Brumm D, Polo J M, Belli B A, Catton D J, Hsu D, Mittelstaedt D, McCormack J E, Karavodin L, Dubensky T W, Jr, Chang S M, Banks T A. DNA immunization against herpes simplex virus: enhanced efficacy using a Sindbis virus-based vector. J Virol. 1998;72:950–958. doi: 10.1128/jvi.72.2.950-958.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karupiah G, Xie Q W, Buller R M, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 21.Kodukula P, Liu T, Rooijen N V, Jager M J, Hendricks R L. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J Immunol. 1999;162:2895–2905. [PubMed] [Google Scholar]
- 22.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohl S. Additive effects of acyclovir and immune transfer in neonatal herpes simplex virus infection in mice. Infect Immun. 1983;39:480–482. doi: 10.1128/iai.39.1.480-482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohl S. The immune response of the neonate to herpes simplex virus infection. In: Rouse B T, Lopez C, editors. Immunobiology of herpes simplex virus. Boca Raton, Fla: CRC Press; 1989. pp. 121–129. [Google Scholar]
- 25.Kohl S, Thomas J W, Loo L S. Defective production of anti-herpes simplex virus antibody by neonatal mice. Reconstitution with Ia+macrophages and T helper lymphocytes from nonimmune adult syngeneic mice. J Immunol. 1986;136:3038–3044. [PubMed] [Google Scholar]
- 26.Kovarik J, Siegrist C A. Immunity in early life. Immunol Today. 1998;19:150–152. doi: 10.1016/s0167-5699(97)01230-9. [DOI] [PubMed] [Google Scholar]
- 27.Leib D A, Harrison T E, Laslo K M, Machalek M A, Moorman N J, Virgin H W. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J Exp Med. 1999;189:663–672. doi: 10.1084/jem.189.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leibson H J, Gefter M, Zlotnik A, Marrack P, Kappler J W. Role of gamma-interferon in antibody-producing responses. Nature. 1984;309:799–801. doi: 10.1038/309799a0. [DOI] [PubMed] [Google Scholar]
- 29.Liu T, Khanna K M, Chen X, Fink D J, Hendricks R L. CD8+T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu T, Tang Q, Hendricks R L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez Z, Arvin A M, Ashley R. Immunity to herpesvirus infections in humans. In: Roizman B, Whitley R J, Lopez Z, editors. The human herpesviruses. New York, N.Y: Raven; 1993. pp. 397–425. [Google Scholar]
- 32.Marshall K R, Lachmann R H, Efstathiou S, Rinaldi A, Preston C M. Long-term transgene expression in mice infected with a herpes simplex virus type 1 mutant severely impaired for immediate-early gene expression. J Virol. 2000;74:956–964. doi: 10.1128/jvi.74.2.956-964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert P H, Siegrist C A. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci USA. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez X, Li X, Kovarik J, Klein M, Lambert P H, Siegrist C A. Combining DNA and protein vaccines for early life immunization against respiratory syncytial virus in mice. Eur J Immunol. 1999;29:3390–3400. doi: 10.1002/(SICI)1521-4141(199910)29:10<3390::AID-IMMU3390>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.McLean C S, Erturk M, Jennings R, Challanain D N, Minson A C, Duncan I, Boursnell M E, Inglis S C. Protective vaccination against primary and recurrent disease caused by herpes simplex virus (HSV) type 2 using a genetically disabled HSV-1. J Infect Dis. 1994;170:1100–1109. doi: 10.1093/infdis/170.5.1100. [DOI] [PubMed] [Google Scholar]
- 36.Mostad S B, Kreiss J K, Ryncarz A J, Mandaliya K, Chohan B, Ndinya-Achola J, Bwayo J J, Corey L. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- 37.Nahmias A J, Keyserling H L, Kerrick G M. Herpes simplex. In: Remington J S, Klein J O, editors. Infection of the fetus and newborn infant. W. B. Philadelphia, Pa: Saunders; 1983. pp. 636–678. [Google Scholar]
- 38.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt-2+T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen L, Knipe D M, Finberg R W. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- 40.Nguyen L H, Knipe D M, Finberg R W. Replication-defective mutants of herpes simplex virus (HSV) induce cellular immunity and protect against lethal HSV infection. J Virol. 1992;66:7067–7072. doi: 10.1128/jvi.66.12.7067-7072.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nossal G J V. The immunological response of fetal mice to influenza virus. Aust J Exp Biol Med Sci. 1957;35:549–558. doi: 10.1038/icb.1957.57. [DOI] [PubMed] [Google Scholar]
- 42.Ochsenbein A F, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel R M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 43.Owen R D. Immunogenetic consequences of vascular ananstomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 44.Paludan S R, Ellermann-Eriksen S, Mogensen S C. NF-κB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-gamma-induced nitric oxide production in macrophages. J Gen Virol. 1998;79:2785–2793. doi: 10.1099/0022-1317-79-11-2785. [DOI] [PubMed] [Google Scholar]
- 45.Porgador A, Irvine K R, Iwasaki A, Barber B H, Restifo N P, Germain R N. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posavad C M, Koelle D M, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 47.Preston C M. Repression of viral transcription during herpes simplex virus latency. J Gen Virol. 2000;81(Pt 1):1–19. doi: 10.1099/0022-1317-81-1-1. [DOI] [PubMed] [Google Scholar]
- 48.Ridge J P, Fuchs E J, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 49.Ryncarz A J, Goddard J, Wald A, Huang M L, Roizman B, Corey L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J Clin Microbiol. 1999;37:1941–1947. doi: 10.1128/jcm.37.6.1941-1947.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarzotti M, Robbins D S, Hoffman P M. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726–1728. doi: 10.1126/science.271.5256.1726. [DOI] [PubMed] [Google Scholar]
- 51.Seiler P, Brundler M A, Zimmermann C, Weibel D, Bruns M, Hengartner H, Zinkernagel R M. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J Exp Med. 1998;187:649–654. doi: 10.1084/jem.187.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegrist C A. Vaccination in the neonatal period and early infancy. Int Rev Immunol. 2000;19:195–219. doi: 10.3109/08830180009088505. [DOI] [PubMed] [Google Scholar]
- 53.Siegrist C A, Barrios C, Martinez X, Brandt C, Berney M, Cordova M, Kovarik J, Lambert P H. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998;28:4138–4148. doi: 10.1002/(SICI)1521-4141(199812)28:12<4138::AID-IMMU4138>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 54.Siegrist C A, Lambert P H. Maternal immunity and infant responses to immunization: factors influencing infant responses. Dev Biol Stand. 1998;95:133–139. [PubMed] [Google Scholar]
- 55.Siegrist C A, Saddallah F, Tougne C, Martinez X, Kovarik J, Lambert P H. Induction of neonatal Th1 and CTL responses by live viral vaccines: a role for replication patterns within antigen presenting cells? Vaccine. 1998;16:1473–1478. doi: 10.1016/s0264-410x(98)00111-x. [DOI] [PubMed] [Google Scholar]
- 56.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steiner I. Human herpes viruses latent infection in the nervous system. Immunol Rev. 1996;152:157–173. doi: 10.1111/j.1600-065x.1996.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 58.Suter M, Lew A M, Grob P, Adema G J, Ackermann M, Shortman K, Fraefel C. BAC-VAC, a novel generation of (DNA) vaccines: a bacterial artificial chromosome (BAC) containing a replication-competent, packaging-defective virus genome induces protective immunity against herpes simplex virus 1. Proc Natl Acad Sci USA. 1999;96:12697–12702. doi: 10.1073/pnas.96.22.12697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanigawa M, Bigger J E, Kanter M Y, Atherton S S. Natural killer cells prevent direct anterior-to-posterior spread of herpes simplex virus type 1 in the eye. Investig Ophthalmol Vis Sci. 2000;41:132–137. [PubMed] [Google Scholar]
- 60.Traub E. Factors influencing the peristance of choriomeningitis virus in the blood of mice after clinical recovery. J Exp Med. 1938;68:229–250. doi: 10.1084/jem.68.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van den Broek M F, Muller U, Huang S, Zinkernagel R M, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunol Rev. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 62.Vasilakos J P, Michael J G. Herpes simplex virus class I-restricted peptide induces cytotoxic T lymphocytes in vivo independent of CD4+T cells. J Immunol. 1993;150:2346–2355. [PubMed] [Google Scholar]
- 63.Wang Z E, Reiner S L, Zheng S, Dalton D K, Locksley R M. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitley R, Arvin A, Prober C, Corey L, Burchett S, Plotkin S, Starr S, Jacobs R, Powell D, Nahmias A, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324:450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 65.Whitley R J. Neonatal herpes simplex virus infections: is there a role for immunoglobulin in disease prevention and therapy? Pediatr Infect Dis J. 1994;13:432–439. [PubMed] [Google Scholar]
- 66.Zhu H, Cong J P, Mamtora G, Gingeras T, Shenk T. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc Natl Acad Sci USA. 1998;95:14470–14475. doi: 10.1073/pnas.95.24.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]