Abstract
BACKGROUND:
Alcohol use disorders (AUD) are a major socioeconomic burden on society and current pharmacotherapeutic treatment options are inadequate. Aberrant alcohol use and seeking alters frontostriatal function.
METHODS:
We performed genome-wide RNA sequencing, and subsequent qPCR and receptor binding validation in the caudate-putamen of human AUD samples to identify potential therapeutic targets. We next back-translated our top candidate targets into a rodent model of long-term alcohol consumption to assess concordance of molecular adaptations in the rat striatum. Finally, we adopted rat behavioural models of alcohol intake and seeking to validate a potential therapeutic target.
RESULTS:
We found G protein-coupled receptors (GPCRs) were the top canonical pathway differentially regulated in individuals with AUD. The M4 muscarinic acetylcholine receptor (mAChR) was down regulated at the gene and protein level in the putamen, but not caudate, of AUD samples. We found concordant downregulation of the M4 mAChR, specifically on dopamine D1 receptor-expressing medium spiny neurons in the rat dorsolateral striatum. Systemic administration of the selective M4 mAChR positive allosteric modulator, VU0467154, reduced home cage and operant alcohol self-administration, motivation to obtain alcohol and cue-induced reinstatement of alcohol seeking in rats. Local microinjections of VU0467154 in the rat dorsolateral striatum reduced alcohol self-administration and cue-induced reinstatement of alcohol seeking.
CONCLUSIONS:
Collectively, these results identify the M4 mAChR as a potential therapeutic target for the treatment of AUD and D1 receptor positive medium spiny neurons in the dorsolateral striatum as a key site mediating the actions of M4 mAChR in relation to alcohol consumption and seeking.
Introduction:
Despite the socioeconomic burden of alcohol use disorders (AUD), therapeutic treatment options are limited(1). Basic neuroscience has identified targets that contribute to alcohol reward, craving and relapse(2-4); however, only an ~8% of drugs that start clinical trial will continue to market(5). Addictions emerge from aberrant learning and motivation towards drugs of abuse, in part via plasticity in the striatum and frontal cortices(6-8). The transition to aberrant seeking is hypothesized to involve a shift in recruitment of ventral striatum (accumbens) in light or social drinkers to dorsal striatum (caudate putamen) in heavy drinkers(9). Within the dorsal striatum, the putamen (dorsolateral striatum, DLS) executes behavioural control after long-term alcohol consumption in rodents(10) and humans(11). Here the cholinergic system regulates motivation for reward(12) and striatal dopamine release(13-16).
Acetylcholine (ACh) has long been implicated in reward and higher order cognitive processes(17), however, focus has historically surrounded nicotinic acetylcholine receptors (nAChRs). Recent development of novel selective drugs to target muscarinic acetylcholine receptors (mAChR) have highlighted their therapeutic potential(18-21). The M4 muscarinic acetylcholine receptor (mAChR) is a Gi/o coupled G protein-coupled receptor(22, 23), densely expressed in the striatum, on cholinergic interneurons, glutamatergic terminals and medium spiny GABAergic DRD1-expressing projection neurons(24-26). M4 mAChR KO mice show increased cocaine and alcohol self-administration(27, 28), M4 selective allosteric modulation reduces cocaine seeking(21) and genetic polymorphisms within the CHRM4 gene are linked to cocaine and heroin addiction(29).
While previous genome wide profiling has been performed on the prefrontal cortex(30), amygdala(31) and hippocampus(32, 33) of individuals with AUD, alterations in the caudate-putamen remain unknown. Therefore, we performed genome-wide RNA sequencing, RT-qPCR and receptor binding in the caudate-putamen of human AUD individuals to identify potential therapeutic targets. We then probed candidate genes in corresponding rat striatal regions (DMS and DLS) after long-term alcohol consumption and found concordance in the downregulation of the M4 mAChR. Our data suggest AUD leads to M4 mAChR downregulation within the DLS, which may facilitate the maintenance of alcohol consumption in humans and rodents after long-term alcohol consumption. We hypothesised that restoring cholinergic balance within the striatum through enhancement of M4 mAChR signalling would reduce alcohol consumption and seeking following abstinence. Together our data suggest the M4 mAChR is a potential therapeutic target for the treatment of AUD and dopamine D1 receptor positive medium spiny neurons (MSNs) in the DLS are a key site where M4 receptors are dysregulated.
Materials and Methods
Human subjects and sample analysis
Human brain samples were obtained from the New South Wales Tissue Resource Centre at the University of Sydney, Sydney. All procedures were conducted in accordance with the Australian National Health and Medical Research Council’s National Statement on Ethical Conduct in Human Research (2007), the Victorian Human Tissue Act (1982), the National Code of Ethical Autopsy Practice (2002) and the Victorian Government policies and practices in relation to post-mortem tissue. Ethics approval: NSW Health Sydney Local Health District reference number X15-0199 (prev X11-0107) & HREC/11/RPAH/147; Monash University Human Research Ethics Committee CF15/2976-2015001223. Caudate and putamen tissue samples from individuals of European descent were matched as closely as possible according to age, sex and cause of death (n=10/group). To be included as part of the study subjects had to meet the following criteria: age between 18 - 64 years, no head injury at time of death, no recent cerebral stroke, no history of other psychiatric or neurological disorders, no hepatic encephalopathy and post-mortem interval within 72 hours. The AUD cohort, subjects had to satisfy a DSM-IV diagnosis of alcohol use disorder. All subjects were “never in my lifetime” smokers (see Supplementary Figure S1 for subject demographics).
A full description of all materials and procedures used can be found in Supplementary information. RNA libraries were prepared (SureSelect Strand-Specific RNA Library Prep for Illumina Multiplexes Sequencing, Agilent Technologies, CA, USA) and sequenced using the HiSeq 2500 system rapid run mode (Illumina, CA, USA). See Supplementary Tables 4 and 5 for subject metadata, library preparation and sequencing run batching information. Quantitative Real-Time PCR (qRT-PCR) and [3H]-N-methylscopolamine (NMS) binding was used to validate targets using samples from the same AUD and healthy individuals (Supplementary Table 6 for primer sequences).
Animal subjects and analysis
All rodent subjects were adult male inbred Indiana preferring (iP) rats. All animal studies were undertaken in accordance with the Prevention Cruelty to Animals Act (2004) and carried out within the guidelines of the National Health and Medical Research Council (NHMRC) Code of Practice for the Care and Use of Animals for Experimental Purposes in Australia (2013) and approved by the Florey Animal Ethics Committee.
For RT-qPCR and RNAscope analysis rats (N=40) were divided into three groups; alcohol-naïve, alcohol-experienced and alcohol-abstinence. Rats underwent intermittent home-cage two-bottle choice for 57 sessions. Alcohol-experienced rats were sacrificed 24 h after the last ethanol access, while alcohol-abstinence rats were killed after 14 days abstinence. Alcohol-naïve groups were maintained on water. RT-qPCR was conducted in the DMS, DLS, nucleus accumbens core (AcbC) and shell (AcbSh) (n=8/group). See Supplementary Table 6 for primer sequences. The RNAscope Multiplex Fluorescent Reagent Kit (Advanced Cell Diagnostics, CA, USA) was used to detect Chrm4, Chat and Drd1 mRNA in the dorsal striatum (n=5-6/group)(34, 35). Chrm4 puncta were quantified (~Bregma 1.80 to 2.00)(36) using image J 3D object counter.
For systemic operant studies male iP rats were trained to self-administer ethanol (10% (v/v)) or sucrose (0.25–2.5% w/v) under FR3 operant conditions 5 days/week for >60 sessions(19). To test the involvement of the M4 mAChR, vehicle (10% Tween80 in water; 10 mL/kg, p.o.) or the selective M4 PAM VU0467154 was administered orally as a microsuspension in vehicle (10 or 30 mg/kg; 10 mL/kg, p.o.) 2 or 2.5 h prior to testing respectively(37). Separate cohorts of rats were tested for operant self-administration of ethanol (10 mg/kg, n=9; 30 mg/kg, n=7) or sucrose (30 mg/kg, n=8) (38, 39), progressive ratio (PR3-4, 30 mg/kg, n=10)(40, 41), or extinction followed by cue-induced reinstatement to alcohol seeking (30 mg/kg, n=8)(19). Rats were tested in a randomised counterbalanced manner after baseline responding was re-established (5 days). To assess the role of M4 mAChRs in voluntary alcohol consumption rats (n=19) were allowed to self-administer alcohol in a continuous access two-bottle choice paradigm for 11 weeks. Alcohol (10% v/v) and water consumption were measured 2, 4, 12 and 24 h from the start of the dark cycle after vehicle or VU0467154 (30mg/kg p.o.) . Two separate cohorts were examined on the rotarod after receiving vehicle or VU0467154 (30 or 100 mg/kg, p.o.) 2.5 or 3 hours prior to testing respectively (see Supplementary Information).
For intra-cranial infusions, vehicle (60% DMSO in saline, 500nL/hemisphere) or VU0467154 (600 pmol or 3 μmol/hemisphere, 500nL/hemisphere) was infused directly into the DLS via implanted cannulae directly prior to behavioural testing(19). Separate cohorts of rats then underwent self-administration (600 pmol, n=7; 3 μmol, n=14) or cue-induced reinstatement of alcohol seeking (3 μmol, n=7) as described above. Site validations were conducted by an experimenter blinded to treatment group and outcomes (Supplementary information).
Results
GPCRs are dysregulated in caudate putamen of individuals with AUD
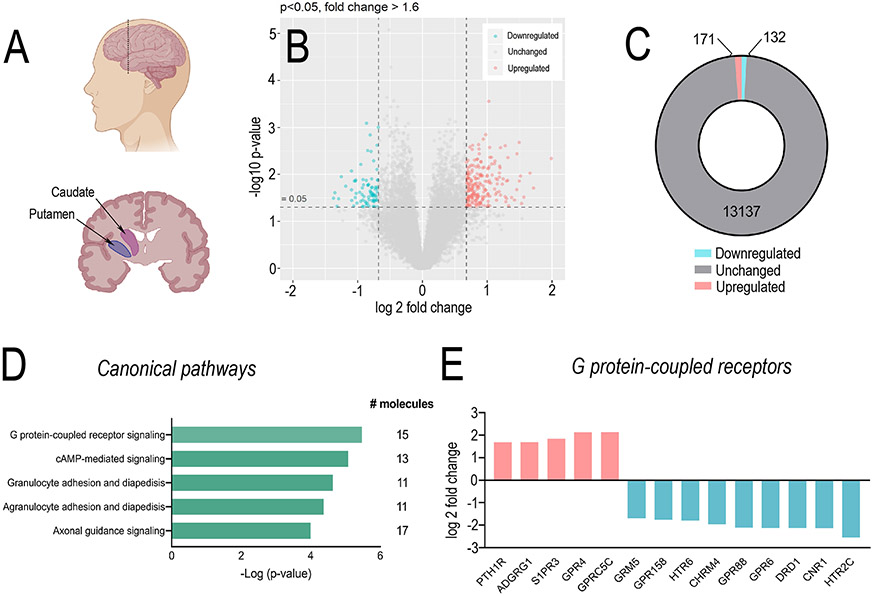
The development of AUD is hypothesized to involve recruitment of caudate putamen(9). Understanding changes induced following long-term alcohol consumption may identify targets to treat AUD. We first conducted genome-wide RNA sequencing on age and gender matched non-smoking individuals with AUD (n=10) and controls (n=10). Unpaired t-test of patient demographics revealed individuals with AUD had significantly increased alcohol intake per day, standard drinks consumed per week and number of years drinking compared to controls. A significant difference was also observed in post-mortem interval. No difference in age, BMI, ratio of brain volume/weight, brain pH level or RNA quality were observed between AUD and controls (Supplementary Figure 1).
Using the cut off (p < 0.05 and FC > 1.6) we observed 303 differentially expressed genes (DEGs) within the caudate-putamen, of which 171 genes were upregulated and 132 genes were downregulated in AUD compared to controls (Figure 1C & Supplementary Tables 1 and 2). Ingenuity pathway analysis (IPA) demonstrated G protein-coupled receptors (GPCRs) as the top differentially regulated canonical pathway in AUD (overlap 5% 15/272; p = 3.36×10−6, Figure 1D & E, Supplementary Table 3), including CHRM4 (M4 mAChR; p = 1.8×10−3, log 2 fold change (FC) = −1.96), CNR1 (cannabinoid receptor 1; p = 4.8×10−4, FC = −2.13), DRD1 (dopamine D1 receptor; p = 8.0×10−4, FC = −2.13) and GPR88 (G protein-coupled receptor 88; p = 5.7×10−3, FC = −2.10).
Figure 1. G protein-coupled receptor expression is dysregulated in individuals with AUD.
(A) Sagittal and coronal schematics of brain indicating where caudate and putamen were dissected for RNA sequencing. (B) Volcano plot and (C) summary of differentially expressed genes (DEGs) in the caudate and putamen of individuals with alcohol use disorders (AUD) compared to healthy controls showed 171 upregulated (pink) and 132 significantly downregulated (blue) genes. (D) Ingenuity Pathway Analysis of the top 300 differentially expressed genes identified the top 5 most significant canonical pathways, of which GPCRs were number 1 [overlap 5% 15/272; p = 3.36 × 10−6]. (E) Summary of differential expression of G protein-coupled receptors in individuals with AUD compared to healthy control. PTH1R (p = 3.3 × 10−4, FC = 1.69), ADGRG1 (p = 4.6 × 10−5, FC = = 1.70), S1PR3, (p = 2.2 × 10−3, FC = 1.85), GPR4 (p = 7.8 × 10−5, FC = 2.13), GPRC5C (p = 1.4 × 10−5, FC = 2.14), GRM5 (p = 1.3 × 10−2, FC = 1.69), GPR158 (p = 1.4 × 10−3, FC = 1.76), HTR6 (p = 9.3 × 10−3, FC = 1.79), CHRM4 (p = 1.8 × 10−3, FC = 1.96), GPR88 (p = 5.7 × 10−3, FC = −2.10), GPR6 (p = 1.2 × 10−2, FC = −2.13), DRD1 (p = 8.0 × 10−4, FC = −2.13), CNR1 (p = 4.8 × 10−4, FC = −2.13) and HTR2C (p = 6.0 × 10−4, FC = −2.54). n=10/treatment per region. Abbreviations; FC, fold change; ADGRG1, adhesion G-protein coupled receptor G1; GPR, G protein-coupled receptor; GRM5, glutamate metabotropic receptor 5; S1PR3, sphingosine 1 phosphate receptor 3; PTH1R, parathyroid hormone 1 receptor; HTR, 5-hydoxytryptamine receptor; CHRM4, M4 muscarinic acetylcholine receptor; DRD1, dopamine D1 receptor.
CHRM4 is dysregulated in the putamen of individuals with AUD
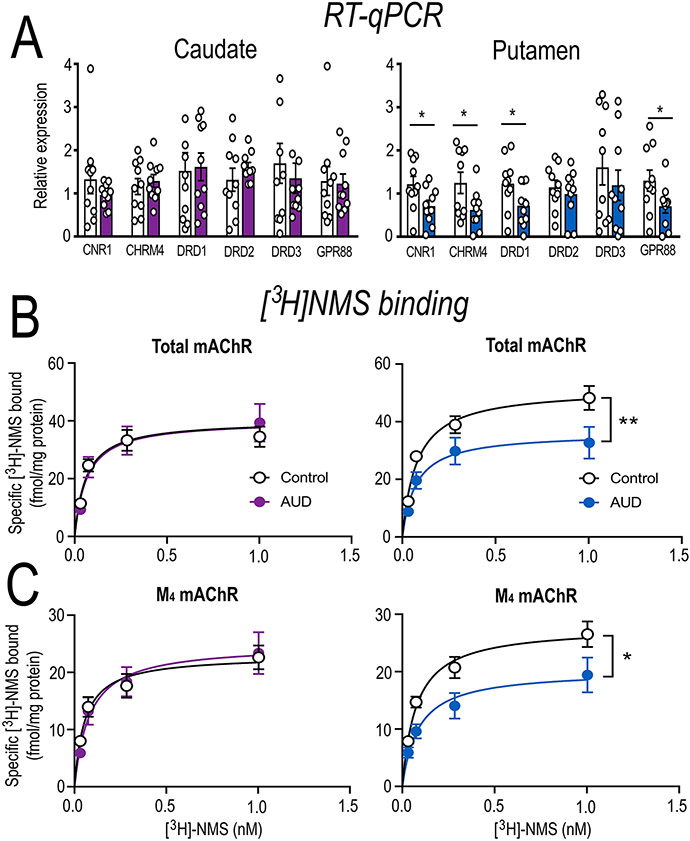
Within the caudate-putamen, long-term alcohol consumption is hypothesised to result in a shift from dorsomedial (caudate) to dorsolateral (putamen) engagement(10). Therefore, to determine whether GPCR dysregulation is localised to a specific subdivision we conducted RT-qPCR in the caudate vs. putamen from the same individuals with AUD and controls (n=10/group/region). In line with RNA sequencing, CNR1 (t = 2.25, df = 18, p = 0.037), CHRM4 (t = 2.21, df = 18, p = 0.040), DRD1 (t = 2.12, df = 18, p = 0.048) and GPR88 (t = 2.46, df = 18, p = 0.0246) were downregulated, while DRD2 (t = 0.650, df = 18, p = 0.52) and DRD3 (t = 0.772, df = 18, p = 0.45) mRNA expression were not altered. These differences were restricted to the putamen (Figure 2A), suggesting GPCR dysregulation is subregion specific in AUD. We focused on the M4 mAChR as it indirectly modulates dopamine release(13) and has been implicated in addictive behaviours(21, 28, 42). Additionally, there has been recent progress in the development of mAChR subtype selective allosteric modulators(37, 43). Therefore, we examined mAChR protein binding in the caudate vs. putamen of post-mortem tissue from the same individuals with AUD and controls using [3H]-NMS (n=10/group/region). AUD samples had decreased mAChR density in the putamen (Bmax= 51.5 fmol/mg protein control vs. 36.1 fmol/mg protein in AUD; F(1,76) = 8.153, p = 0.006, Figure 2B), but not caudate (Bmax= 37.9 fmol/mg protein control vs. 42.6 fmol/mg protein AUD; F(1,76) = 0.776, p = 0.381, Figure 2B). As M1 and M4 receptor subtypes are the most abundant in the dorsal striatum(44) we conducted assays in the presence of either the selective M1 mAChR toxin (MT-7) or the selective M4 mAChR toxin (MT-3). Individuals with AUD had decreased M4 mAChRs within the putamen (Bmax= 27.8 fmol/mg protein control vs. 20.2 fmol/mg protein AUD; F(1,76) = 5.46, p = 0.022, Figure 2C), but not caudate (Bmax= 23.0 fmol/mg protein control vs. 24.8 fmol/mg protein AUD; F(1,76) = 0.315, p = 0.576, Figure 2C). No significant differences were observed for the M1 receptor in the caudate (Bmax= 28.7 fmol/mg protein control vs. 27.6 fmol/mg protein AUD; F(1,76) = 0.033, p = 0.856), or putamen (Bmax= 35.5 fmol/mg protein control vs. 27.7 fmol/mg protein AUD; F(1,76) = 2.06, p = 0.155 (data not shown). Together these data suggest that M4 mRNA and protein is specifically downregulated within the putamen of individuals with AUD.
Figure 2. Molecular characterisation of the caudate vs putamen of individuals with AUD.
(A) RT qPCR validation was conducted independently in the caudate and putamen of healthy controls and individuals with AUD to determine region-specific dysregulation. In line with RNA sequencing data CNR1 (t = 2.25, df = 18, p = 0.037), CHRM4 (t = 2.21, df = 18, p = 0.040), DRD1 (t = 2.12, df = 18, p = 0.048) and GPR88 (t = 2.46, df = 18, p = 0.0246) were downregulated, while DRD2 (t = 0.650, df = 18, p = 0.52) and DRD3 (t = 0.772, df = 18, p = 0.45) mRNA expression were not altered in individuals with AUD. Further, this downregulation was specific to the putamen of individuals with AUD. To further explore the downregulation of corresponding M4 mAChR receptor expression, we conducted [3H]NMS saturation binding assays to determine the (B) total mAChR and (C) M4 mAChR specific binding in the caudate and putamen of healthy controls and individuals with AUD. Total mAChR binding was decreased in the putamen (F(1,76) = 8.153, p = 0.006), but not caudate (F(1,76) = 0.776, p = 0.381). In line with CHRM4 mRNA expression M4 mAChR binding was specifically decreased in the putamen (F(1,76) = 5.46, p = 0.022), but not caudate (F(1,76) = 0.315, p = 0.576) of individuals with AUD. Data expressed as mean ± SEM; n=10/group, *p < 0.05, **p < 0.01 (F-test); n.s. = not significant.
Long-term alcohol consumption/abstinence leads to dysregulation of GPCRs in the rodent striatum
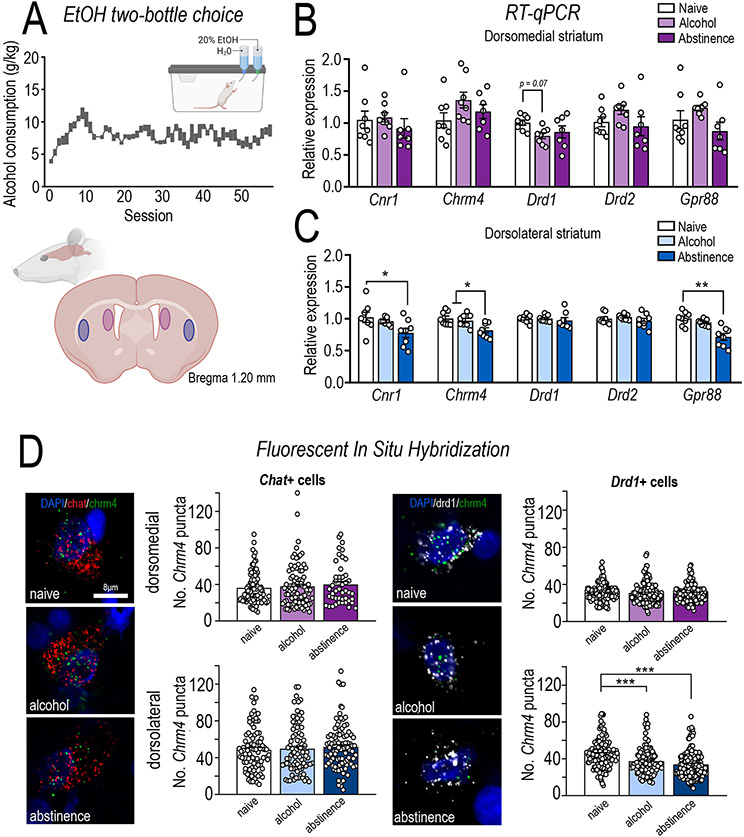
The translation between rodent and human models is a major hurdle in the development of CNS drugs(1, 2). Therefore, to further examine M4 mAChR dysregulation we reverse-translated our findings into a rodent model of long-term voluntary alcohol consumption/abstinence and assessed mRNA expression in rat DMS (caudate) and DLS (putamen) (n=8/group/region). Rats consumed an average of 7.5 ± 0.5 g/kg/session with a 71 ± 2.2 % preference for alcohol over water (Figure 3A). RT-qPCR revealed long-term alcohol consumption/abstinence lead to a reduction in Chrm4 mRNA in the DLS (F(2,21)= 6.955, p = 0.0048; Bonferroni post-hoc, naïve vs. abstinence, p = 0.0064; alcohol vs. abstinence, p = 0.0279, Figure 3C), but not DMS (Figure 3B), AcbC or AcbSh (Supplementary Figure S2). Cnr1 (F(2,21)= 3.936, p = 0.0353; naïve vs. abstinence group, p = 0.0398) and Gpr88 (F(2,21)= 15.33, p < 0.0001; naïve vs. abstinence group, p < 0.0001; alcohol vs. abstinence, p = 0.0014) were downregulated in rodent DLS in accordance with the human data (Figure 3C).
Figure 3. Molecular characterisation of the rodent dorsomedial and dorsolateral striatum following long-term alcohol consumption.
Alcohol preferring iP rats underwent long-term intermittent alcohol consumption in a two-bottle choice where they consumed (A) high levels of 20% alcohol (g/kg) across >57 sessions. Rats were randomly divided into two groups, one of which were culled 24 h after their final session (alcohol, n=8) and another which were culled 14 days after their last session (abstinence, n=8). A third group of age matched alcohol naïve rats were also included (n=8). The dorsomedial (DMS; caudate equivalent) and dorsolateral (DLS; putamen equivalent) striatum were assessed for molecular adaptations following long-term alcohol consumption and abstinence. RT-qPCR analysis was conducted in the (B) DMS and (C) DLS. In line with human post-mortem tissue from individuals with AUD, long-term alcohol consumption/abstinence in rodents decreased Chrm4 mRNA (ANOVA, F(2,21) = 6.955, p = 0.0048; Bonferroni post-hoc, naïve vs abstinence, p = 0.0064; alcohol vs. abstinence, p = 0.0279), Cnr1 (ANOVA, F(2,21) = 3.936, p = 0.0353; Bonferroni post-hoc, naïve vs. abstinence group, p = 0.0398) and Gpr88 (ANOVA, F(2,21) = 15.33, p < 0.0001; Bonferroni post-hoc, naïve vs. abstinence group, p < 0.0001; alcohol vs. abstinence, p = 0.0014) specifically in the DLS, without altering DMS mRNA expression. A trend towards DRD1 downregulated in the DMS was also observed (F (2,20) = 3.04, p = 0.0704; Bonferroni post hoc naïve vs. alcohol p = 0.0780). (D) M4 mAChRs are expressed on both direct pathway MSNs and cholinergic interneurons, therefore, to determine if Chrm4 mRNA is downregulated on direct MSNs (Drd1+) or cholinergic interneurons (Chat+) fluorescent in situ hybridization of Chrm4 expression was assessed. In line with RT-qPCR analysis, Chrm4 expression was specifically downregulated in the dorsolateral striatum, restricted to Drd1+ neurons (treatment x region interaction, F(2,841) = 13.96, p < 0.0001; Bonferroni post hoc analysis, DLS naïve vs. alcohol, p = 0.0001; naïve vs. abstinence, p = 0.0001; alcohol vs. abstinence, p = 0.0591). A ~16% reduction on Chrm4 expression was observed on Drd1+ neurons in the DLS following long-term alcohol consumption, while a ~24% reduction was observed after abstinence. No differences were observed in Chrm4 expression on Drd1+ cells in the DMS or Chat+ cells in either the DMS or DLS. Data expressed as mean ± SEM; n=70-90 Chat+ and 140-150 Drd1+ cell from 5-6 rats/treatment, *p < 0.05, **p < 0.01, ***p < 0.001.
Chrm4 mRNA is specifically downregulated on Drd1+ cells in the DLS following long-term alcohol/abstinence
In the DLS, M4 mAChRs are expressed postsynaptically on D1 MSNs and cholinergic (ChAT+) interneurons. To determine the impact of long-term alcohol consumption on Chrm4 expression on specific populations in the dorsal striatum we quantified Chrm4 expression on Chat and Drd1 expressing cells in the DMS and DLS using RNAscope (n=5-6 group/region)(34). Rats consumed an average of 6.2 ± 0.5 g/kg/session with a 61 ± 4.8 % preference for alcohol over water. Chrm4 expression on Chat+ interneurons differed between the DMS and DLS (main effect of region, F(1,480)= 30.95, p < 0.0001), but no differences were observed between naïve, alcohol or abstinence treatment groups (F(2,480)= 0.5364, p < 0.5850). In contrast, a significant reduction in Chrm4 expression on Drd1+ cells was observed only in the DLS (main effect of region, F(1,841)= 83.54, p < 0.0001; treatment, F(2, 841)= 18.27, p < 0.0001; and interaction, F(2,841)= 13.96, p < 0.0001; DLS naïve vs. alcohol, p = 0.0001; naïve vs. abstinence, p = 0.0001; alcohol vs. abstinence, p = 0.0591; Figure 3D). Chrm4 expression was reduced by ~16% on Drd1+ neurons in the DLS following long-term alcohol consumption, and ~24% following abstinence after long-term alcohol. In line with our observations in individuals with AUD, these data suggest long-term alcohol consumption/abstinence leads to a downregulation of M4 mRNA expression in the rat DLS, restricted to D1 expressing dMSNs, with no difference observed in CINs.
Administration of a selective M4 mAChR positive allosteric modulator decreases alcohol self-administration and cue-induced reinstatement of alcohol seeking in rats
Recent development of subtype selective allosteric modulators have enabled specific examination of mAChRs(37). We used the selective M4 PAM, VU0467154, to understand the role of M4 mAChRs in home-cage two-bottle choice and operant alcohol consumption and seeking behaviours. For home cage consumption, VU0467154 was administered 2.5 hours prior to dark phase onset and alcohol/water consumption measured 2, 4, 12 and 24 hours subsequently. Systemic administration of VU0467154 (30 mg/kg, p.o.) significantly reduced alcohol consumption during the dark phase [main effect of time, F(2,34) = 72.04, p < 0.0001; time x treatment interaction, F(2,34) = 4.18, p = 0.024; but effect of treatment, F(1,17) = 1.83 p = 0.195]. Bonferroni post-hoc analysis revealed a significant difference at 12 (p = 0.0137), but not 2 (p = 0.999) or 4 h (p = 0.999) into the dark phase (Supplementary Figure S3D). No difference was observed in alcohol consumption in the light phase (12-24 h) [t = 0.696, df = 17, p = 0.496], or water consumption/alcohol preference within 24 h (Supplementary Figure S3E & F).
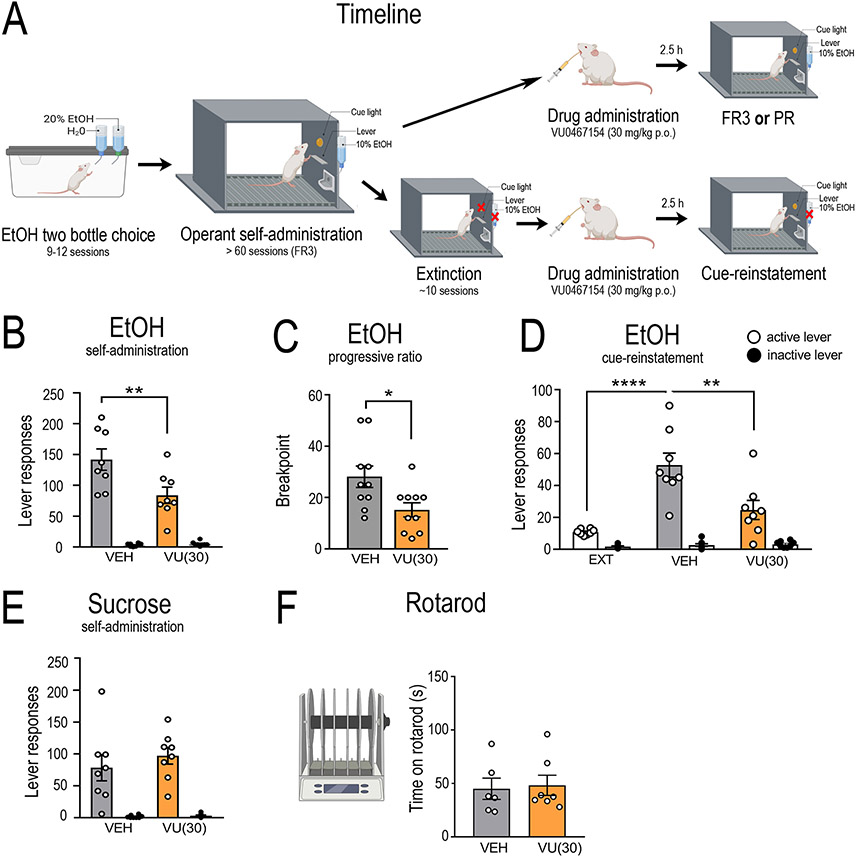
During operant self-administration sessions, rats pressed an average of 115 ± 3 (0.66 g/kg/20 min session) on the active lever and 3.5 ± 0.4 times on the inactive lever (Supplementary Figure S4). VU0467154 (10 mg/kg, p.o) failed to reduce operant self-administration (RM two-way ANOVA; main effect of lever, F(1,7)= 18.88, p < 0.0032; a trend toward main effect of treatment, F(1,7)= 4.79, p = 0.065; and interaction, F(1,7)= 0.793, p = 0.079, n=9, data not shown). An increased dose of VU0467154 (30 mg/kg, p.o.) decreased operant responding for ethanol (main effect of lever, F(1,7)= 62.74, p < 0.0001; treatment, F(1,7)= 12.16, p = 0.010; and interaction, F(1,7)= 17.09, p = 0.004; Bonferroni post hoc analysis, vehicle vs. VU0467154, p = 0.0015; n=7, Figure 4B), breakpoint for ethanol on a progressive ratio (t = 2.47, df = 9, p = 0.035; n=10, Figure 4C) and cue-induced reinstatement of alcohol seeking (main effect of lever (F(1,6)= 16.76, p < 0.0009), treatment (F(2,12)= 36.53, p = 0.0003) and interaction (F(2.12)= 13.91, p = 0.0007; extinction vs. vehicle, p = 0.0001, vehicle vs VU0467154, p = 0.0013; n=8, Figure 4D). VU0467154 did not alter latency to first lever response (Supplementary Figure S4), had no effect on sucrose self-administration (main effect of lever, F(1,14)= 147.6, p < 0.0001; no main effect of treatment, F(1,14)= 0.418, p = 0.529; or interaction F(1,14)= 0.295, p = 0.596; n=8, Figure 4E) and did not cause motor impairments at 30 mg/kg (students t-test, t = 0.234, df = 11, p = 0.819, n=6-7/group, Figure 4F). A higher dose of VU0467154 (100 mg/kg, p.o) decreased the time rats spent on the rotarod (t = 2.57, df = 16, p = 0.020, n=9/group, Supplementary Figure S5), therefore we did not test this dose further. Our operant data suggest the M4 PAM, VU0467154 has the greatest effect in decreasing the incentive properties of alcohol consumption and seeking following long-term alcohol consumption. These results also suggest this does not generalize across all appetitive substances, nor does it alter normal fluid consumption. Finally, these results suggest a therapeutic window for the M4 PAM to selectively modulate alcohol intake/seeking.
Figure 4. Systemic pharmacological manipulation of the M4 muscarinic acetylcholine receptor in alcohol consumption and seeking.
To functionally test the role of M4 mAChRs in alcohol consumption and seeking we systemically administered a selective M4 positive allosteric modulator, VU0467154, as outlined in (A). Systemic administration of VU0467154 (30 mg/kg, p.o) significantly reduced (B) active lever responding (n=8) in FR3 self-administration (treatment x lever interaction, F(1,7) = 17.09, p = 0.004; Bonferroni post hoc analysis, vehicle vs. VU0467154, p = 0.0015), (C) Breakpoint (n=10) in the progressive ratio paradigm (t = 2.47, df = 9, p = 0.035), (D) active lever responding (n=8) in cue-induced reinstatement of alcohol seeking (treatment x lever interaction, F(2.12) = 13.91, p = 0.0007; Bonferroni post hoc, extinction vs. vehicle, p = 0.0001, vehicle vs VU0467154, p = 0.0013). No effect was observed on natural reward consumption during (E) active lever responding (n=8) in sucrose self-administration (F(1,14) = 0.418, p = 0.529), or (F) ataxia (n=13) determined via performance on the rotarod (t = 0.234, df = 11, p = 0.819). Data expressed as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Dorsolateral striatum M4 mAChRs regulate alcohol consumption and cue-induced reinstatement of alcohol seeking
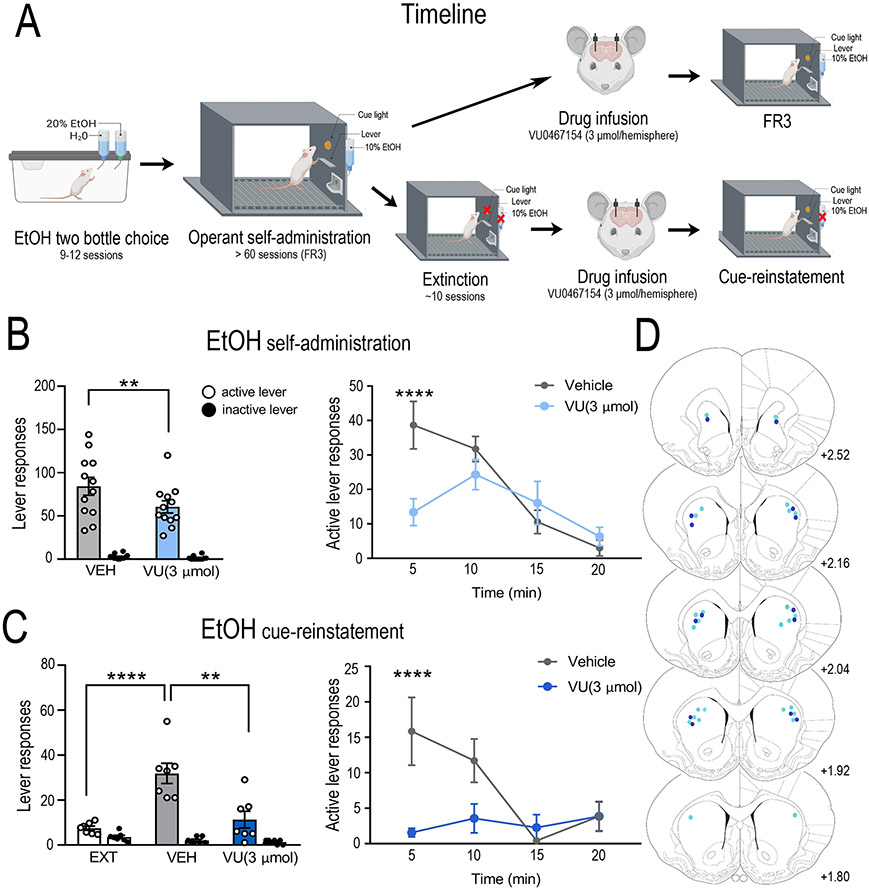
Our molecular data suggest the DLS is the main locus of M4 mAChR dysregulation. Therefore, to determine if DLS M4 mAChR’s are involved in mediating alcohol self-administration and cue-induced reinstatement of alcohol seeking we directly administered VU0467154 into the DLS. During operant self-administration sessions, rats pressed an average of 85 ± 4 (0.51 ± 0.03 g/kg/20 min session) on the active lever and 2 ± 0.2 times on the inactive lever (Supplementary Figure S5). Intra-DLS microinjections of VU0467154 (600 pmol/hemisphere) had no effect on alcohol self-administration (main effect of lever, F(1,7)= 65.93, p < 0.00001; but no effect of treatment, F(1,7)= 0.017, p = 0.897; or interaction F(1,7)= 0.011, p = 0.918, n=8, data not shown). An increased dose of VU0467154 (3 μmol/hemisphere) decreased alcohol self-administration (main effect of lever, F(1,11)= 91.45, p < 0.00001; treatment, F(1,11)= 6.69, p = 0.0253; interaction F(1,11)= 5.67, p = 0.0364; Bonferroni post hoc, active lever vehicle vs VU0467154, p = 0.0086; n=14, Figure 5B). Peak responding was significantly reduced (main effect of time, F(3,33)= 8.74, p = 0.0002; treatment F(1,11)= 6.138, p = 0.0307; and interaction F(3,33)= 8.52, p = 0.0002. vehicle vs VU0467154 at 5 min, p <0.0001, n=7, Figure 5B) without decreasing latency to first active lever press (main effect of lever, F(1,11)= 64.96, p < 0.0001; effect of treatment F(1,11)= 5.459, p = 0.039; but no interaction F(1,11)= 2.189, p = 0.1671).
Figure 5. Dorsolateral striatum pharmacological manipulation of the M4 muscarinic acetylcholine receptor in alcohol consumption and seeking.
To determine the role of dorsolateral (DLS) M4 mAChRs in alcohol consumption and seeking we microinjected the selective M4 mAChR PAM, VU0467154, into the DLS prior to self-administration (n=12) and cue-induced reinstatement (n=7) as outlined in (A). Intra-DLS VU0467154 (3 μmol/hemisphere) reduced (B) active lever responding in alcohol self-administration (treatment x lever interaction F(1,11) = 5.67, p = 0.0364; Bonferroni post hoc, active lever vehicle vs VU0467154, p = 0.0086). This reduction occurred during peak responding (treatment x time interaction F(3,33) = 8.52, p = 0.0002. Bonferroni post hoc analysis, vehicle vs VU0467154 at 5 min, p <0.0001). (C) Intra-DLS VU0467154 (3 μmol/hemisphere) also reduced active lever responding during cue-induced reinstatement of alcohol seeking (treatment x lever, F(2,12) = 11.22, p = 0.0018; Bonferroni post hoc, extinction vs. vehicle, p = 0.0004, vehicle vs. VU0467154, p = 0.0016). Decreased lever pressing was significantly reduced during peak responding (treatment x time interaction F(3,18) = 3.729, p = 0.0303. Bonferroni post hoc analysis, treatment vs. vehicle at 5 min, p = 0.0067). (D) Shows the injection sites for VU0467154 in self-administration (light blue) and cue-induced reinstatement (dark blue)(36). Data are expressed as mean ± SEM, **p < 0.01, ****p < 0.0001.
Intra-DLS VU0467154 also decreased cue-induced reinstatement (main effect of lever, F(1,6)= 122.7, p < 0.00001; treatment, F(2,12)= 10.34, p = 0.0025; interaction F(2,12)= 11.22, p = 0.0018; extinction vs. vehicle, p = 0.0004, vehicle vs VU0467154, p = 0.0016; Figure 5C). Peak responding was decreased (main effect of time, F(3,18) = 3.94, p = 0.0253; treatment F(1,6)= 7.139, p = 0.0369; and interaction F(3,18)= 3.729, p = 0.0303, treatment vs. vehicle at 5 min, p = 0.0067, Figure 5C) without decreasing latency to first active lever press (main effect of lever, F(1,6)= 32.97, p = 0.0012; no effect of treatment F(1,6)= 0.00007, p = 0.9932; or interaction F(1,6)= 3.632, p = 0.1053; Supplementary Figure S6).
Discussion
Acamprosate was the last FDA approved medication to treat AUD over 15 years ago. Consequently, there is great need for novel AUD treatments that target core pathophysiology induced by long-term alcohol consumption. Here we employed a cross-species back-translational approach to establish that a history of AUD leads to GPCR dysregulation in the putamen of humans and corresponding DLS of rats. Altered expression of GPCRs implicated preclinically and/or clinically in AUD included metabotropic glutamate 5 receptor (GRM5)(45, 46), cannabinoid 1 receptor (CNR1)(47-49), D1 dopamine receptor (DRD1)(50, 51), GPR88(52, 53) and M4 mAChR (CHRM4)(28).
The M4 mAChR is expressed within mesocorticolimbic circuitry(54, 55). Dopaminergic and cholinergic systems dynamically interact to gate and potentiate sensory inputs to the striatum(56). Endogenous cholinergic activity controls extracellular dopamine levels through a complex convergence of neurotransmitter/neuromodulator systems. Tonically active cholinergic interneurons modulate dopamine output through activation of both mAChR and nAChRs on dopamine terminals(14, 57, 58) and through the corelease of glutamate(59, 60).
Further, within the striatum, M4 mAChRs are necessary for muscarinic regulation of dopamine release(14), and the M4 PAM, VU0467154 modulates DA release indirectly through M4 receptors on D1 expressing dMSNs(16). Based on this, we pursued M4 mAChRs to further understand their role in AUD. We validated and expanded our RNA sequencing findings to demonstrate CHRM4 mRNA and M4 mAChR binding were specifically downregulated in the putamen of individuals with AUD. The putamen/DLS is a key structure executing behavioural control after long-term alcohol consumption in both rodents(10) and humans(11). Chronic exposure to ethanol alters the DLS network, strengthening its control over behaviour(61, 62). In humans, cue-induced drug craving is associated with activation of, and dopamine release in, the DLS(9, 63, 64). Further, DLS dopamine regulates alcohol seeking and an inability to disengage the DLS is associated with vulnerability to develop aberrant alcohol seeking behaviour(65). Our data in combination with our previous study(19) where microinjections of the M5 NAM, ML375, or α4β2 nAChR partial agonist, varenicline, decreased alcohol self-administration within the DLS, but not DMS, suggest this dysregulation predominates in the DLS.
Humans with AUD show persistent aberrant motivation towards alcohol consumption(66-68). The cholinergic system within the dorsal striatum has been linked to motivation for reward(12) and modulating activity of the central dopaminergic system(13). To probe the role of the M4 mAChR in alcohol consumption and seeking, we back-translated our findings to a rodent model of long-term voluntary alcohol use/abstinence. M4 mAChR downregulation was recapitulated in the corresponding DLS. Within the striatum, M4 mAChR are expressed on cholinergic interneurons and direct pathway MSNs (dMSNs). Cholinergic M4 mAChRs act via negative feedback to regulate cholinergic neuron output, while M4 mAChRs expressed post-synaptically on D1 dMSNs modulate their activity(13) as well as indirectly modulating presynaptic dopamine release(16). M4 mAChRs on these populations exert opposing functions, with deletion from cholinergic interneurons altering drug associated learning tasks, while deletion from D1-positive neurons enhanced drug seeking behaviour (42). We observed specific downregulation of Chrm4 expression on D1 dMSNs in the DLS following long-term alcohol use that persisted during abstinence. Repeated cycles of excessive alcohol intake and withdrawal potentiate glutamatergic signalling onto D1 dMSNs, which in turn drives greater alcohol seeking(69). Therefore, indirectly reducing signalling of D1 dMSNs via M4 mAChR activation may provide a nuanced mechanism to re-tune striatal function in relation to aberrant alcohol seeking.
Recent development of subtype selective allosteric modulators has allowed detailed examination of specific behavioural roles of discrete mAChRs in affective disorders (19, 43). A selective M4 PAM, VU0467154, possesses both cognitive enhancing and antipsychotic-like effects, without tolerance upon repeated dosing in rodents(37, 43). VU0467154 acts via a D1 dMSN and CB2 receptor-dependent mechanism to reduce dopamine signalling and mediate antipsychotic-like effects(16). VU0467154 administered either systemically or directly within the DLS reduced alcohol consumption, motivation for alcohol and cue-induced alcohol seeking (relapse). VU0467154 caused no alterations in water or natural reward consumption, motor control or latency to respond. Interestingly, the effect size of intra-DLS administration of VU0467154 to reduce cue-induced reinstatement of alcohol seeking was greater than that on self-administration of alcohol. This suggests a more robust role for striatal M4 mAChRs in regulating relapse behaviour, consistent with the pronounced downregulation of Chrm4 observed following abstinence after long-term alcohol consumption.
Together our data show that long-term alcohol consumption/AUD downregulates CHRM4 expression in both human and rodent, specifically on D1 MSNs in the DLS. Enhancement of M4 mAChR signalling reduces alcohol consumption and seeking, mediated at least in part through the DLS. Overall, these findings provide a strong rationale to further explore M4 mAChR PAMs as candidates for novel AUD medications. Moreover, allosteric modulation of M4 mAChRs may provide a strategy that restores cholinergic function in AUD and helps promote abstinence. VU0467154 is not a desirable candidate for clinical development with limited potency at the human M4 mAChR (70). Attempts to develop M4 PAMs with desirable efficacy and potency at the human M4 receptor are ongoing(71, 72). A number of pharmaceutical companies have active M4 PAM/agonist programs in relation to psychosis, Karuna therapeutics (KarXT), Sosei Heptares (HTL-0016878), Pfizer (Undisclosed PAM; PF-06852231) and Lundbeck/VCNDD (New generation VU PAMs). Together this opens up a genuine avenue for the treatment of AUDs by repurposing a class of molecules for which there are agents in clinical development.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “tdis paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Chemical Compound or Drug | VU0467154 (5-amino-3,4-dimethyl-N-(4-((trifluoromethyl)sulfonyl)benzyl)thieno[2,3-c]- pyridazine-6-carboxamide) | Provided by Craig Lindsley (Vanderbilt University) | N/A | |
| Commercial Assay Or Kit | RNeasy Plus Mini Kit | Qiagen | Cat No. 74136 | |
| SureSelect Strand - Specific RNA Library Preparation kit for Illumina | Aligent | Cat No. G9691A | ||
| SuperScriptII qRT-PCR kit | Invitrogen | Cat No. 10928042 | ||
| SYBR Powerup PCR kit | Qiagen | Cat No. 4368702 | ||
| RNAscope Multiplex Fluorescent Reagent Kit | Advanced Cell Diagnostics | Cat No. 320850 | ||
| Deposited Data; Public Database | Basic Local Alignment Search Tool | https://blast.ncbi.nlm.nih.gov/Blast.cgi | N/A | |
| Organism/Strain | Male inbred Indiana preferring (iP) rats | Parental stock from the late Professor T.K. Li (while at Indiana University, USA). | N/A | |
| Sequence-Based Reagent | Primers for RT-qPCR, see Table S6 | This paper | N/A | |
| Sequence-Based Reagent | drd1 RNAscope probe | Advanced Cell Diagnostics | Cat No. 317031 | |
| Sequence-Based Reagent | chat RNAscope probe | Advanced Cell Diagnostics | Cat No. 430111-C3 | |
| Sequence-Based Reagent | chrm4 RNAscope probe | Advanced Cell Diagnostics | Cat No. 456671-C2 | |
| Software; Algorithm | Subjunc aligner | - http://subread.sourceforge.net/ | version 1.4.4 - http://subread.sourceforge.net/ | |
| Software; Algorithm | EdgeR | https://www.bioconductor.org/packages/release/bioc/html/edge.html | version edgeR_3.4.2 in R studio, R version 3.0.2 - | |
| Software; Algorithm | BioMart | https://bioconductor.org/packages/release/bioc/html/biomaRt.html | version 3.10 | |
| Software; Algorithm | Partek Genomics suite | https://www.partek.com/partek-genomics-suite/ | version 6.16.0419 | |
| Software; Algorithm | qbase+ | https://www.qbaseplus.com/ | version 3.0 | |
| Software; Algorithm | Primer3 | http://bioinfo.ut.ee/primer3-0.4.0/ | version 0.4.0 | |
| Software; Algorithm | GraphPad Prism | https://www.graphpad.com/ | version 7 & 8 | |
| Software; Algorithm | Image J | NIH | version 2.0.0 | |
| Software; Algorithm | MEDPC-IV | Med Associates | version 4 |
Acknowledgements
Human tissues were received from the New South Wales Tissue Resource Centre at the University of Sydney which is supported by the University of Sydney. Research reported in this publication was supported by the National Institute of Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Number R28AA012725. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. This research was also supported by an NHMRC Project Grant to AJL & CJL (1120576) of which AJL is a Principal Research Fellow (1116930). We acknowledge the Victorian State Government Operational Infrastructure Program. JS was supported by the Thai Royal Golden Jubilee PhD Program (PHD/0253/2552) and Mahidol University. We thank Dr. Erin Campbell (Florey Institute of Neuroscience and Mental Health) for technical assistance.
Abbreviation list:
- AcbC
nucleus accumbens core
- AcbSh
nucleus accumbens shell
- ACh
acetylcholine
- AUD
alcohol use disorder
- BMI
body mass index
- CHAT
Choline Acetyl Transferase
- CHRM4
M4 muscarinic acetylcholine receptor mRNA
- CNR1
cannabinoid receptor 1 mRNA
- DEG
differentially regulated genes
- DLS
dorsolateral striatum
- DMS
dorsomedial striatum
- dMSN
direct pathway medium spiny neurons
- DMSO
dimethyl sulfoxide
- DRD1
dopamine D1 receptor mRNA
- DRD2
dopamine D2 receptor mRNA
- DRD3
dopamine D3 receptor mRNA
- EIF2A
Eukaryotic Translation Initiation Factor 2A
- FISH
fluorescent in situ hybridisation
- FR3
fixed ratio 3
- GPCR
G-protein coupled receptor
- GPR88
G-protein coupled receptor 88 mRNA
- GPR4
G-protein coupled receptor 4 mRNA
- GPR6
G-protein coupled receptor 6 mRNA
- HMBS
hydroxymethylbilane synthase
- HSP90
heatshock protein 90
- IPA
Ingenuity pathway analysis
- iP rat
Indiana preferring rat
- qRT-PCR
quantitative Real Time PCR
- mAChR
muscarinic acetylcholine receptor
- MT-3
muscarinic toxin – 3
- MT-7
muscarinic toxin – 7
- nAChR
nicotinic acetylcholine receptor
- NMS
N-methylscopolamine
- PAM
positive allosteric modulator
- PMI
post-mortem interval
- PPIA
Peptidylprolyl Isomerase A
- RIN
RNA integrity number
- RM
repeated measures
- TBP
TATA binding box protein
Footnotes
Conflict of interest statement
CWL is an inventor on patents that protect different classes of muscarinic receptor allosteric modulators, and in particular M4 PAMs. No other authors have competing financial or non-financial interests.
Data Availability
Materials, data sets and protocols are available upon reasonable request to the corresponding authors.
Bibliography
- 1.Litten RZ, Ryan ML, Falk DE, Reilly M, Fertig JB, Koob GF (2015): Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clin Exp Res. 39:579–584. [DOI] [PubMed] [Google Scholar]
- 2.Litten RZ, Falk DE, Ryan ML, Fertig JB (2016): Discovery, development, and adoption of medications to treat alcohol use disorder: goals for the phases of medications development. Alcohol Clin Exp Res. 40:1368–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahao KP, Salinas AG, Lovinger DM (2017): Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron. 96:1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LC, Lawrence AJ (2018): Investigational drug therapies in phase I and phase II clinical trials for alcohol use disorders. Exp Opin Invest Drug. 27:677–690. [DOI] [PubMed] [Google Scholar]
- 5.Arrowsmith J, Miller P (2013): Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov. 10:328–329. [DOI] [PubMed] [Google Scholar]
- 6.Everitt BJ, Robbins TW (2005): Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 8:1481. [DOI] [PubMed] [Google Scholar]
- 7.Kalivas P, Volkow N, Seamans J (2005): Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 45:647–650. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF, Volkow ND (2010): Neurocircuitry of addiction. Neuropsychopharmacology. 35:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, et al. (2010): Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 105:1741–1749. [DOI] [PubMed] [Google Scholar]
- 10.Corbit LH, Nie H, Janak PH (2012): Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol Psych. 72:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grodin EN, Sussman L, Sundby K, Brennan GM, Diazgranados N, Heilig M, et al. (2018): Neural correlates of compulsive alcohol seeking in heavy drinkers. Biol Psych Cogn Neurosci. 3:1022–1031. [DOI] [PubMed] [Google Scholar]
- 12.Pratt WE, Kelley AE (2004): Nucleus accumbens acetylcholine regulates appetitive learning and motivation for food via activation of muscarinic receptors. Behavi Neurosci. 118:730. [DOI] [PubMed] [Google Scholar]
- 13.Jeon J, Dencker D, Wörtwein G, Woldbye DP, Cui Y, Davis AA, et al. (2010): A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 30:2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, et al. (2010): Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 30:3398–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulzer D, Cragg SJ, Rice ME (2016): Striatal dopamine neurotransmission: regulation of release and uptake. Basal Ganglia. 6:123–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, et al. (2016): Antipsychotic-like effects of M4 positive allosteric modulators are mediated by CB2 receptor-dependent inhibition of dopamine release. Neuron. 91:1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cragg SJ, Exley R, Clements MA (2005): Striatal acetylcholine control of reward-related dopamine signalling. The Basal Ganglia VIII: Springer, pp 99–108. [Google Scholar]
- 18.Bender AM, Garrison AT, Lindsley CW (2018): The Muscarinic Acetylcholine Receptor M5: Therapeutic Implications and Allosteric Modulation. ACS Chem Neurosci. 10:1025–1034. [DOI] [PubMed] [Google Scholar]
- 19.Berizzi AE, Perry CJ, Shackleford DM, Lindsley CW, Jones CK, Chen NA, et al. (2018): Muscarinic M 5 receptors modulate ethanol seeking in rats. Neuropsychopharmacology. 43:1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maksymetz J, Joffe ME, Moran SP, Stansley BJ, Li B, Temple K, et al. (2019): M1 Muscarinic Receptors Modulate Fear-Related Inputs to the Prefrontal Cortex: Implications for Novel Treatments of Posttraumatic Stress Disorder. Biol Psych. 85:989–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoll K, Hart R, Lindsley CW, Thomsen M (2018): Effects of muscarinic M 1 and M 4 acetylcholine receptor stimulation on extinction and reinstatement of cocaine seeking in male mice, independent of extinction learning. Psychopharmacology. 235:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langmead CJ, Watson J, Reavill C (2008): Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Therap. 117:232–243. [DOI] [PubMed] [Google Scholar]
- 23.Matsui M, Yamada S, Oki T, Manabe T, Taketo MM, Ehlert FJ (2004): Functional analysis of muscarinic acetylcholine receptors using knockout mice. Life Sci. 75:2971–2981. [DOI] [PubMed] [Google Scholar]
- 24.Bernard V, Levey AI, Bloch B (1999): Regulation of the subcellular distribution of m4 muscarinic acetylcholine receptors in striatal neurons in vivo by the cholinergic environment: evidence for regulation of cell surface receptors by endogenous and exogenous stimulation. J Neurosci. 19:10237–10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ince E, Ciliax BJ, Levey AI (1997): Differential expression of D1 and D2 dopamine and m4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 27:357–366. [DOI] [PubMed] [Google Scholar]
- 26.Weiner DM, Levey AI, Brann MR (1990): Expression of muscarinic acetylcholine and dopamine receptor mRNAs in rat basal ganglia. Proc Nat Acad Sci. 87:7050–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DP, et al. (2011): Increased cocaine self-administration in M 4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology. 216:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de la Cour C, Sørensen G, Wortwein G, Weikop P, Dencker D, Fink-Jensen A, et al. (2015): Enhanced self-administration of alcohol in muscarinic acetylcholine M4 receptor knockout mice. Euro J Pharm. 746:1–5. [DOI] [PubMed] [Google Scholar]
- 29.Levran O, Randesi M, Peles E, Correa da Rosa J, Ott J, Rotrosen J, et al. (2016): African-specific variability in the acetylcholine muscarinic receptor M4: association with cocaine and heroin addiction. Pharmacogenomics. 17:995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD (2015): Transcriptome organization for chronic alcohol abuse in human brain. Mol Psych. 20:1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD (2012): Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 32:1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farris SP, Harris RA, Ponomarev I (2015): Epigenetic modulation of brain gene networks for cocaine and alcohol abuse. Front Neurosci. 9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Z, Yuan Q, Mash DC, Goldman D (2011): Substance-specific and shared transcription and epigenetic changes in the human hippocampus chronically exposed to cocaine and alcohol. Proc Nat Acad Sci. 108:6626–6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang F, Flanagan J, Su N, Wang L-C, Bui S, Nielson A, et al. (2012): RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn. 14:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ch'ng SS, Fu J, Brown RM, Smith CM, Hossain MA, McDougall SJ, et al. (2019): Characterization of the relaxin family peptide receptor 3 system in the mouse bed nucleus of the stria terminalis. J Comp Neurol. 527:2615–2633. [DOI] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C (2006): The rat brain in stereotaxic coordinates: hard cover edition. Elsevier. [DOI] [PubMed] [Google Scholar]
- 37.Bubser M, Bridges TM, Dencker D, Gould RW, Grannan M, Noetzel MJ, et al. (2014): Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents. ACS Chem Neurosci. 5:920–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker LC, Kastman HE, Krstew EV, Gundlach AL, Lawrence AJ (2017): Central amygdala relaxin-3/relaxin family peptide receptor 3 signalling modulates alcohol seeking in rats. Br J Pharmacol. 174:3359–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker LC, Kastman HE, Lawrence AJ (2019): Pattern of neural activation following yohimbine-induced reinstatement of alcohol seeking in rats. Euro J Neurosci. [DOI] [PubMed] [Google Scholar]
- 40.Farid WO, Lawrence AJ, Krstew EV, Tait RJ, Hulse GK, Dunlop SA (2012): Maternally administered sustained-release naltrexone in rats affects offspring neurochemistry and behaviour in adulthood. PLoS One. 7:e52812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell EJ, Flanagan JP, Walker LC, Hill MK, Marchant NJ, Lawrence AJ (2019): Anterior Insular Cortex is Critical for the Propensity to Relapse Following Punishment-Imposed Abstinence of Alcohol Seeking. J Neurosci. 39:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klawonn AM, Wilhelms DB, Lindström SH, Singh AK, Jaarola M, Wess J, et al. (2018): Muscarinic M4 Receptors on Cholinergic and Dopamine D1 Receptor-Expressing Neurons Have Opposing Functionality for Positive Reinforcement and Influence Impulsivity. Front Mol Neurosci. 11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould RW, Grannan MD, Gunter BW, Ball J, Bubser M, Bridges TM, et al. (2018): Cognitive enhancement and antipsychotic-like activity following repeated dosing with the selective M4 PAM VU0467154. Neuropharmacology. 128:492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapman KL, Vaswani D, Hendry N, Langmead CJ, Kew JN, Watson JM (2011): The muscarinic M4 receptor is the functionally predominant subtype in rat and mouse striatum as demonstrated using [35S] GTPγS binding. Euro J Pharmacol. 652:1–6. [DOI] [PubMed] [Google Scholar]
- 45.Leurquin-Sterk G, Crunelle C, Ceccarini J, de Laat B, Peuskens H, Bormans G, et al. (2016): Alcohol addiction is associated with decreased limbic mGluR5 availability: a 18F-FPEB PET study in human. J Nucl. 57:15–15. [Google Scholar]
- 46.Ceccarini J, Leurquin-Sterk G, Crunelle C, De Laat B, Bormans G, Peuskens H, et al. (2017): Recovery of decreased metabotropic glutamate receptor 5 availability in abstinent alcohol-dependent subjects. J Nucl. 58:14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, et al. (2002): Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 65:221–224. [DOI] [PubMed] [Google Scholar]
- 48.Hirvonen J, Zanotti-Fregonara P, Umhau JC, George DT, Rallis-Frutos D, Lyoo CH, et al. (2013): Reduced cannabinoid CB 1 receptor binding in alcohol dependence measured with positron emission tomography. Mol Psych. 18:916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C (2003): Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 84:698–704. [DOI] [PubMed] [Google Scholar]
- 50.Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, et al. (2016): Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Nat Acad Sci. 113:3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anokhin P, Razumkina E, Shamakina IY (2019): A Comparison of mRNA Expression of Dopamine Receptors, Tyrosine Hydroxylase, and Dopamine Transporter in the Mesolimbic System of Rats with Different Levels of Alcohol Consumption. Neurochem J. 13:137–144. [Google Scholar]
- 52.Hamida SB, Mendonça-Netto S, Arefin TM, Nasseef MT, Boulos L-J, McNicholas M, et al. (2018): Increased alcohol seeking in mice lacking Gpr88 involves dysfunctional mesocorticolimbic networks. Biol Psych. 84:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jin C, Decker AM, Makhijani VH, Besheer J, Darcq E, Kieffer BL, et al. (2018): Discovery of a potent, selective, and brain-penetrant small molecule that activates the orphan receptor GPR88 and reduces alcohol intake. J Med Chem. 61:6748–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hersch SM, Gutekunst C-A, Rees H, Heilman CJ, Levey AI (1994): Distribution of m1-m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 14:3351–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levey A, Kitt C, Simonds W, Price D, Brann M (1991): Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 11:3218–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabresi P, Picconi B, Tozzi A, Di Filippo M (2007): Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 30:211–219. [DOI] [PubMed] [Google Scholar]
- 57.Exley R, Cragg S (2008): Presynaptic nicotinic receptors: a dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 153:S283–S297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ (2012): Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 75:58–64. [DOI] [PubMed] [Google Scholar]
- 59.Rice ME, Cragg SJ (2004): Nicotine amplifies reward-related dopamine signals in striatum. Nat Neuro. 7:583. [DOI] [PubMed] [Google Scholar]
- 60.Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, et al. (2011): Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PloS one. 6:e19155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DePoy L, Daut R, Wright T, Camp M, Crowley N, Noronha B, et al. (2015): Chronic alcohol alters rewarded behaviors and striatal plasticity. Addict Biol. 20:345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Renteria R, Baltz ET, Gremel CM (2018): Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Comms. 9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garavan H, Morgan R, Mactutus C, Levitsky D, Booze R, Strupp B (2000): Prenatal cocaine exposure impairs selective attention: evidence from serial reversal and extradimensional shift tasks. Behav Neurosci. 114:725. [PubMed] [Google Scholar]
- 64.Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, et al. (2006): Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 26:6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giuliano C, Belin D, Everitt BJ (2019): Compulsive alcohol seeking results from a failure to disengage dorsolateral striatal control over behavior. J Neurosci. 39:1744–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. (2009): Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 44:372–381. [DOI] [PubMed] [Google Scholar]
- 67.Glass JM, Buu A, Adams KM, Nigg JT, Puttler LI, Jester JM, et al. (2009): Effects of alcoholism severity and smoking on executive neurocognitive function. Addiction. 104:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilcox CE, Dekonenko CJ, Mayer AR, Bogenschutz MP, Turner JA (2014): Cognitive control in alcohol use disorder: deficits and clinical relevance. Rev Neuroscience. 25:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng Y, Huang CC, Ma T, Wei X, Wang X, Lu J, et al. (2017): Distinct synaptic strengthening of the striatal direct and indirect pathways drives alcohol consumption. Biol Psych. 81:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wood MR, Noetzel MJ, Poslusney MS, Melancon BJ, Tarr JC, Lamsal A, et al. (2017): Challenges in the development of an M4 PAM in vivo tool compound: The discovery of VU0467154 and unexpected DMPK profiles of close analogs. Bioorg Med Chem Lett. 27:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Engers DW, Melancon BJ, Gregro AR, Bertron JL, Bollinger SR, Felts AS, et al. (2019): VU6005806/AZN-00016130, an advanced M4 positive allosteric modulator (PAM) profiled as a potential preclinical development candidate. Bioorg Med Chem Lett. 29:1714–1718. [DOI] [PubMed] [Google Scholar]
- 72.Temple KJ, Engers JL, Long MF, Gregro AR, Watson KJ, Chang S, et al. (2019): Discovery of a novel 3, 4-dimethylcinnoline carboxamide M4 positive allosteric modulator (PAM) chemotype via scaffold hopping. Bioorg Med Chem Lett. 29:126678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Materials, data sets and protocols are available upon reasonable request to the corresponding authors.