ABSTRACT
Dermatomyositis (DM) is an inflammatory disease of the muscles and skin. Severe gastrointestinal (GI) involvement, characterized by GI bleeding and perforation secondary to underlying vasculopathy, is rarely seen. We describe a case of newly diagnosed DM in a 75-year-old woman who presented with a rash and muscle weakness. She then had sudden onset of hematemesis and was found to have duodenal ulcers due to leukocytoclastic vasculitis from her DM. Our aim was to highlight the need for recognition of GI involvement in adults with DM.
KEYWORDS: dermatomyositis, leukocytoclastic vasculitis, GI bleed
INTRODUCTION
Dermatomyositis (DM) is an inflammatory myopathy characterized by proximal muscle weakness and cutaneous manifestations. The annual incidence rate of DM varies between 1.0 and 15 per million.1,2 The etiology of DM is unknown, but Human leukocyte antigen genetic predisposition, drugs, and certain infections have been implicated.3 Classical cutaneous signs of DM include Gottron papules, heliotrope rash, and shawl sign.3–6 The pathophysiology of DM is predicted to be an immunological attack against vessels in the muscle secondary to complement and membrane attack complex, which infiltrate the endothelium.3 This inflammation leads to muscular hypoxic injury causing perifascicular atrophy.3,7 Diagnosis involves muscle and skin biopsies, which show perivascular and perimysial inflammatory infiltrates.3,7 Treatment is with corticosteroids and/or intravenous immunoglobulin (IVIG) in severe cases.2,8,9
Extramuscular manifestations of DM include pulmonary, cardiac, and gastrointestinal (GI) involvement, with dysphagia as the most well-recognized GI symptom. However, severe GI involvement presenting as ulcers, GI bleeding, and perforation has also been rarely reported.10 We describe a woman with DM who developed coffee-ground emesis secondary to DM-related vasculitis.
CASE REPORT
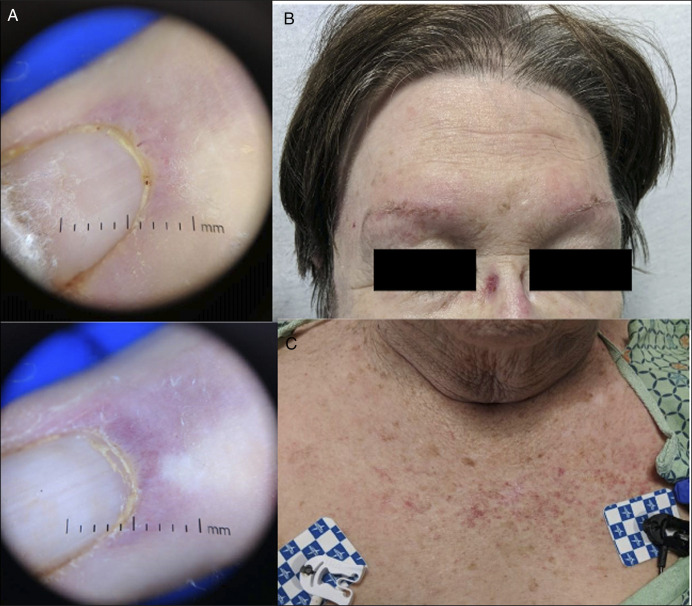
A 75-year-old woman presented after 4 months of myalgias and a nondescriptive rash of her brows, nasal bridge, and chest (Figure 1). She also described one month of progressive proximal upper and lower extremity weakness. An outpatient electromyogram revealed myopathy. A week later, she was admitted with rapid muscle weakness and jaw claudication. Laboratory results were notable for creatine kinase of 737, C-reactive protein 44, erythrocyte sedimentation rate 65, and elevated antinuclear matrix protein 2 (NXP-2) antibodies.
Figure 1.
(A) Thick, hyperkeratotic cuticles and telangiectasias of the nail. (B) Heliotrope rash. (C) Shawl sign, a patchy V-shaped rash on chest with purple and red discolorations.
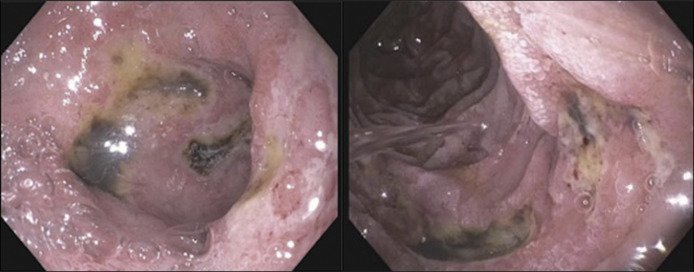
The following evening, the patient had 300 cc of coffee-ground emesis. She denied abdominal pain, melena, or hematochezia but reported taking 800–1200 mg ibuprofen daily for the past year. Computed tomography revealed thickened edematous hyperattenuating bowel wall with fat stranding in the duodenum. Esophagogastroduodenoscopy showed multiple cratered duodenal ulcers with pigmented material (Figure 2). Helicobacter pylori stool antigen and fasting gastrin were negative. We initially suspected the ulcers to be from nonsteroidal anti-inflammatory drug use. However, biopsies revealed vascular changes consistent with leukocytoclastic vasculitis (LCV) in the setting of potential DM (Figure 3). Immunostaining for cytomegalovirus and herpes simplex virus was negative. A thigh punch biopsy confirmed DM. She received 5 days of IV methylprednisolone and 5 doses of IVIG. Repeat esophagogastroduodenoscopy 2 weeks later showed ulcer resolution. Patient follows with rheumatology and has had no further GI complications.
Figure 2.
Duodenal ulcers with pigmented spots.
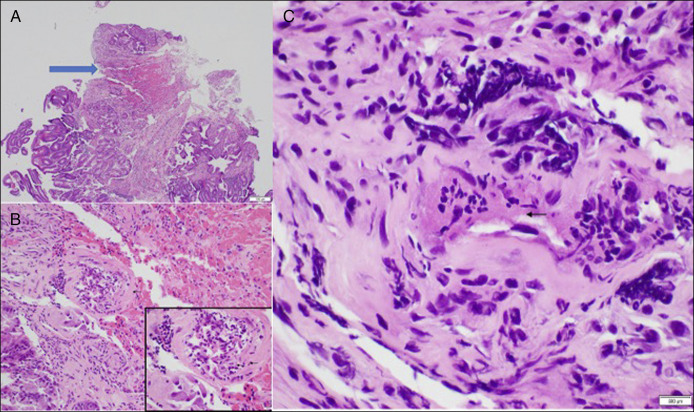
Figure 3.
Skin biopsy: (A) Low magnification (10×) of ulcer (blue arrow) and extravasated red blood cells. (B) 40× swelling of the vessel wall with transmural infiltration of neutrophils with presence of nuclear dust (60× inset). (C) Fibrinoid necrosis is present(60×) (arrow).
DISCUSSION
GI involvement in DM ranges from abdominal pain, vomiting, and diarrhea to motility issues or dysphagia.10–13 More severe GI complications include ulcers, bleeding, vasculopathy and vasculitis, and even perforation. It has been reported that juvenile DM, for unclear reasons, has a higher association with earlier and more severe GI involvement.12,14–16
Our patient had hematemesis attributable to duodenal ulcers. Furthermore, as her skin and muscle biopsies confirmed DM and our endoscopy biopsies were characteristic of LCV, we gained confidence in attributing her constellation of symptoms to systemic inflammation secondary to her DM. LCV is a small-vessel vasculitis primarily affecting the skin. The pathophysiology involves immune complex deposition in the small blood vessels, leading to inflammation and damage. Both cutaneous and GI vasculitis have been described in relation to DM. This patient's nonsteroidal anti-inflammatory drug use was a curious confounder and may have exacerbated the formation of her ulcers. To our knowledge, however, nonsteroidal anti-inflammatory drugs do not directly induce LCV. In our patient, LCV was thought to cause her ulcers, histologically due to the presence of fibrinoid necrosis, neutrophils in the vessel walls, and apoptosis and extravasation of red blood cells—all in the setting of DM with her skin biopsy also demonstrating LCV.
Only a few cases with similar presentations to our patient exist in the literature. An 18-year-old patient had melena attributed to GI vasculitis from DM and was treated with IVIG and steroids.8 A 24-year-old woman with DM was found to have perforation from gastric and small bowel ulcers. She required a total gastrectomy, and histology demonstrated vasculitis and obliterative endarteritis.13 A retrospective analysis reported 3 of 188 patients presented with severe GI manifestations of DM.12 Endoscopy revealed upper GI ulcers that were complicated by bleeding and perforation, most of which proved fatal.12
Furthermore, our patient was positive for anti-NXP2, which is a myositis-specific antibody associated with a specific clinical phenotype of DM. Notably, the relationship between severe GI involvement and NXP2 has been explored in juvenile DM and adults.17,18 In 2024 analysis, 10 of 56 adult patients with NXP2 presented with GI involvement, mostly perforation, and histology demonstrated vasculitis. They showed promising therapeutic signals with the use of vedolizumab, an anti-α4β7-integrin monoclonal antibody.17 Four patients received vedolizumab over a median of 20 weeks. These patients showed improvement in GI symptoms, reduced glucocorticoid use, and enhanced overall outcomes, with one patient avoiding surgical intervention. These data suggest that vedolizumab can be a valuable addition to the standard care regimen for patients with anti-NXP2-positive DM and severe GI involvement, particularly when traditional treatments such as steroids and IVIG are inadequate. As such, our case supports the importance of monitoring for GI symptoms in patients with NXP2 DM and highlights the potential utility of myositis-specific antibody testing as both a prognostic marker and a guide for targeted therapeutic strategies.
In summary, we present this case to emphasize that severe GI complications, especially true vasculitis, are rare but possible in adult DM. Abdominal pain or GI bleeding should raise a suspicion for the development of severe GI manifestations and prompt expedited management to prevent consequences such as perforation, particularly in anti-NXP2-positive patients. We have found benefit from the use of IVIG and steroids, though other immunomodulators such as methotrexate and azathioprine have also been found to be helpful.12
DISCLOSURES
Author contributions: Study concept and design: S. Min, DW Yen, GL Bongiovanni. Drafting of the manuscript: S. Min, DW Yen. Critical revision of the manuscript: GL Bongiovanni. DW Yen is the article guarantor.
Financial disclosure: None to report.
Previous presentation: This abstract was presented as a poster at the ACG Annual Scientific Meeting in Vancouver, BC, Canada, October 20-25, 2023.
Informed consent was obtained for this case report.
Contributor Information
Debra W. Yen, Email: yendw@ucmail.uc.edu.
Gail L. Bongiovanni, Email: bongiogl@ucmail.uc.edu.
REFERENCES
- 1.Kronzer VL, Kimbrough BA, Crowson CS, Davis JM, Holmqvist M, Ernste FC. Incidence, prevalence, and mortality of dermatomyositis: A population-based cohort study. Arthritis Care Res. 2023;75(2):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choy EHS, Isenberg DA. Treatment of dermatomyositis and polymyositis. Rheumatology. 2002;41(1):7–13. [DOI] [PubMed] [Google Scholar]
- 3.Qudsiya Z, Waseem M. Dermatomyositis. In: StatPearls. StatPearls Publishing; (http://www.ncbi.nlm.nih.gov/books/NBK558917/) (2022). Accessed March 1, 2023. [Google Scholar]
- 4.Dalakas MC. Pathogenesis and therapies of immune-mediated myopathies. Autoimmun Rev. 2012;11(3):203–6. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti SS, Bhattacharjee A, Gambhir IS. Vasculitic ulcers in a young female with dermatomyositis: An unusual case. J Case Rep. 2017;7(1):75–7. [Google Scholar]
- 6.Lowry CA, Pilkington CA. Juvenile dermatomyositis: Extramuscular manifestations and their management. Curr Opin Rheumatol. 2009;21(6):575–80. [DOI] [PubMed] [Google Scholar]
- 7.De Paepe B. Vascular changes and perifascicular muscle fiber damage in dermatomyositis: Another question of the chicken or the egg that is on our mind. Ann Transl Med. 2017;5(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marie I, Levesque H, Cailleux N, Courtois H, Guédon C. Intravenous immunoglobulin for treatment of gastro- intestinal haemorrhage in dermatomyositis. Ann Rheum Dis. 2001;60(7):723–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selva-O’Callaghan A, Martínez-Costa X, Solans-Laque R, Mauri M, Capdevila JA, Vilardell-Tarrés M. Refractory adult dermatomyositis with pneumatosis cystoides intestinalis treated with infliximab. Rheumatology (Oxford, England). 2004;43(9):1196–7. [DOI] [PubMed] [Google Scholar]
- 10.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD. Gastrointestinal manifestations in systemic autoimmune diseases. Mædica. 2011;6(1):45–51. [PMC free article] [PubMed] [Google Scholar]
- 11.Laskin BL, Choyke P, Keenan GF, Miller FW, Rider LG. Novel gastrointestinal tract manifestations in juvenile dermatomyositis. J Pediatr. 1999;135(3):371–4. [DOI] [PubMed] [Google Scholar]
- 12.Matas-Garcia A, Milisenda JC, Espinosa G, et al. Gastrointestinal involvement in dermatomyositis. Diagnostics. 2022;12(5):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eshraghi N, Farahmand M, Maerz LL, Campbell SM, Deveney CW, Sheppard BC. Adult-onset dermatomyositis with severe gastrointestinal manifestations: Case report and review of the literature. Surgery. 1998;123(3):356–8. [PubMed] [Google Scholar]
- 14.Takeda T, Fujisaku A, Jodo S, Koike T, Ishizu A. Fatal vascular occlusion in juvenile dermatomyositis. Ann Rheum Dis. 1998;57(3):172–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhaskaran H, Balan S. Unusual cause for intestinal perforation in juvenile dermatomyositis. BMJ Case Rep. 2019;12(8):e229395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mamyrova G, Kleiner DE, James-Newton L, Shaham B, Miller FW, Rider LG. Late-onset gastrointestinal pain in juvenile dermatomyositis as a manifestation of ischemic ulceration from chronic endarteropathy. Arthritis Rheum. 2007;57(5):881–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y, Gu L, Chen J, et al. Severe gastrointestinal involvements in patients with adult dermatomyositis with anti-NXP2 antibody. RMD Open. 2024;10(1):e003901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Ma X, Zhou Z, et al. Gastrointestinal perforation in anti-NXP2 antibody-associated juvenile dermatomyositis: Case reports and a review of the literature. Pediatr Rheumatol Online J. 2021;19(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]