Introduction:
Telehealth leverages technology for the remote delivery of health care, and its use has rapidly increased since the COVID-19 pandemic.1 Simultaneously, clinical investigation into the ability of telehealth, and specifically telemonitoring, to improve the care of persons with chronic liver disease has also increased. Telemonitoring, which is the use of technology to remotely monitor patients, has had a burgeoning impact on the entire clinical spectrum of liver disease and transplantation. Recent examples include a smartphone application that reduced readmissions for hepatic encephalopathy, a Bluetooth weight scale that was informative in the management of ascites, and a wearable step counter that identified patients at risk for hospitalization.2–4 Additionally, a home remote patient monitoring program was shown to improve post-transplant care.5
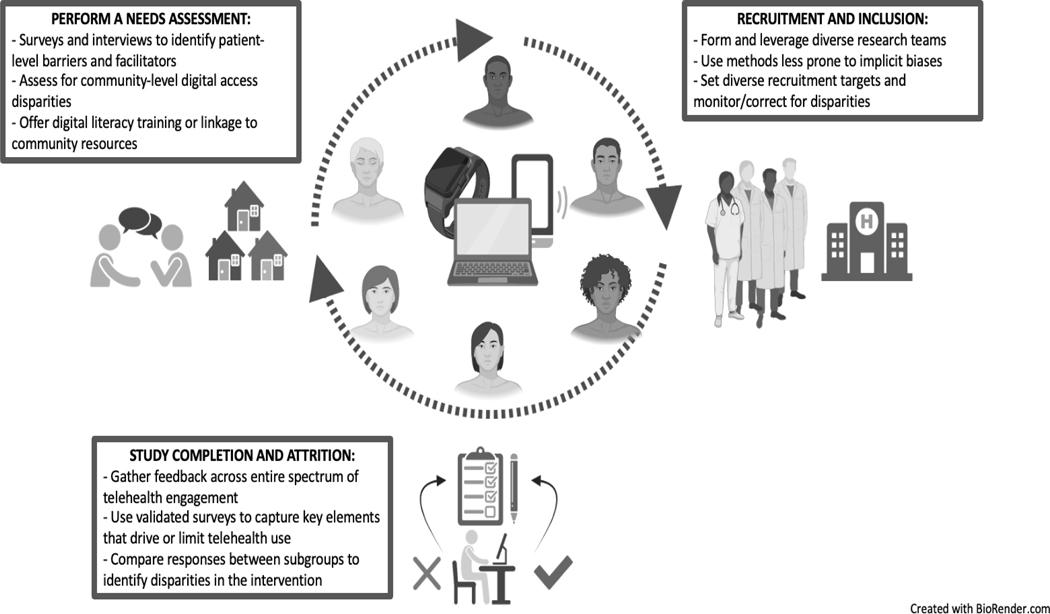
Clinical research continues to highlight opportunities for the expansion of telehealth interventions to augment and advance care. However, already there are signs of disparities within telehealth research. For example, both liver and non-liver-related telehealth studies have frequently included predominantly White (> 70%) and college-educated cohorts, required English proficiency for inclusion, and reported household incomes significantly above the national average.4–8 Additionally, non-reporting of key demographic measures is common as seen in one systematic review where cohort race, ethnicity, and language were not reported in 22%, 44%, and 50% of included studies, respectively.7 While much of these disparities are a product of larger disparities related to digital access, they need not be woven into research proposals and protocols within liver-related telehealth (telehepatology); with consequences that will have a lasting effect on clinical care. Herein, we propose a research agenda for liver-related telehealth studies to promote equity. While our focus is on research involving telemonitoring interventions, the principles apply broadly to all aspects of telehealth research. We outline research challenges and offer solutions that directly impact how we plan, recruit, conduct, and sustain telehealth interventions. The intention is to highlight opportunities in telehepatology research that will promote a realization of the promise of telehealth to deliver equitable care (Figure 1).
Figure 1:
Promoting Diversity and Inclusion in Telehepatology Research
Opportunities for Improving Telehealth Equity:
Identifying Digital Needs:
Telehealth interventions evaluated in clinical research must reflect the diverse needs of our patients. First, the means to engage in digital health are not uniform across diverse populations, therefore it is necessary to understand the degree of digital access of the local community prior to the creation, introduction, and study of telehealth interventions. Although rates of smartphone ownership among persons with liver disease are frequently 90% or higher,1,3,6 these rates are an incomplete representation of digital access. Such estimates frequently derive not from unselected cohorts of clinic patients, but are biased by inclusion criteria requiring access to participate in a technology-related study. Second, digital health tools are rendered useless without adequate proficiency to use such tools. Similar to health literacy, digital health literacy determines how patients receive, interact with, understand, and use health information in a digital format.9 Low digital literacy often preclude participation in a telehealth intervention and it can nearly double the time-burden needed to interact with the technology compared to those with higher levels of digital literacy.10 Additionally, discomfort with technology diminishes interest in, and use of, telehealth interventions.6 These factors may explain why the use of a hepatic encephalopathy-related smartphone application was low despite high initial interest.6 When digital health interventions are used that do not match the digital literacy of the target population, large groups are inevitably excluded from study.
Conceptually, a telehealth needs assessment seeks to gather information from end-users regarding barriers and facilitators to uptake and use.11 Needs assessments must be broad and multi-level in order to identify and address equity concerns in telehealth innovations. Patient surveys and semi-structured interviews are important resources to gain insights into user-centered telehealth needs and perceived barriers, such as technology comfort and self-efficacy.11 Despite high interest in telehealth tools for liver-related disease management, engagement is limited when technology comfort and confidence is low.6 Incorporating validated digital health literacy assessments provides an opportunity to recognize the digital health preparedness of potential users6,8. More simplistically, even an assessment of the technologies used in daily living provides a quick and informative surrogate of technological proficiency.12 Identification of digital literacy deficits then allows for interventions focused on digital literacy training and the provision of troubleshooting resources. Indeed, digital health training and support is an effective means for increasing the perceived competence of digital health users.13 One method of providing digital training and support is through the creation of “digital navigators” whose role on the study teams is to facilitate technology uptake and use.14 For example, confidence in the ability to participate in video-based communication reaches 78.7% in persons with cirrhosis if they know training will be available.8 Additionally, high participation rates were achieved in a post-liver transplant telemonitoring program through the use of both in-hospital and at-home digital navigation resources.5 Centers must also assess existing local community digital needs and disparities. If a significant technology disparity is found, two actions are necessary. The first is to reconsider, at that time, study protocols that rely heavily on that mode of digital access in favor of a tool that reflects the capabilities of the patient population. The second is to engage in advocacy to address digital disparities, such as ensuring equitable broadband access and affordability of digital devices.15.
Recruitment and Inclusion Deficits:
The entry point for clinical research is an invitation and subsequent acceptance to participate. Multiple reasons exist for why certain populations may have concerns regarding participation in clinical research studies; reasons extending from historical research malfeasance, communication barriers, and cultural norms and beliefs.16. As clinical research expands into patient-generated data in the virtual space, digital barriers and biases further widen disparities in research study participation. Patient portals are a cautionary case study whereby existing disparities diminish the ability to recruit diverse populations for clinical investigation. Patients who are Black, have Medicaid, have limited English proficiency, or have a high school education or lower are significantly less likely to have enrollment in the patient portal offered to them.17,18 Invitation bias frequently stems from the inaccurate assumptions that those who are from vulnerable groups have less interest in or ability to participate in telehealth.19 Early disparities in patient portal enrollment may partly explain subsequent disparities found in research examining the downstream use of another digital health tool in liver disease, telehealth visits. In observational studies where patient portal use was a prerequisite to telehealth visit engagement in hepatology clinics, telehealth visit use was more frequent in White patients and comparatively lower in patients who are Black, have Medicaid insurance, and are older.1,20 Low digital health recruitment diversity based on age, sex, race/ethnicity, and language, limit the generalizability of study findings and can propagate downstream research disparities in the role of telehealth across the spectrum of chronic liver disease.
Reshaping research recruitment strategies towards equity, requires an equity-centered approach to eliminate biases and address patient-related concerns surrounding participation. Implicit biases should be removed as barriers to the engagement in telehealth interventions. For example, switching to universal opt-out, rather than an opt-in, patient portal enrollment is one method to reduce disparities in portal enrollment and engagement; therefore, allowing for the study of the impact of portal use in diverse populations.18 Disparities are further reduced when these tools provide non-English options to serve those with limited English proficiency.18 Additionally, health policies that have expanded the use of telehealth visits must be maintained; policies that have allowed for the recruitment of persons who were previously unable to participate.15 Next, the composition of the research team is an important determinant of patient willingness to participate when recruitment is in person. Forming research teams that are reflective of, or culturally congruent, to patient populations promotes recruitment of diverse study groups.16 A diverse research team can also provide community-specific insights that promote recruitment (i.e., an understanding of community digital health resources, identifying high-yield community settings for recruitment, and partnerships with community organizations). Additionally, telehealth research is highly amenable to decentralized clinical trials that can be leveraged towards patient recruitment. These study designs remove typical recruitment barriers of transportation and missed work that disproportionally impact underserved patient populations.21 Lastly, telehealth protocols should carefully outline recruitment targets that reflect the racial/ethnic, age distribution, and socioeconomic makeup of the local community.22 Purposive, rather than convenience, sampling establishes the goal of recruitment equity early. As recruitment ensues, active surveillance of emerging recruitment disparities can drive protocol adjustments.22 Submitted work should comment on how closely the demographics of the included study population compares to the overall center demographics to increase accountability.
Study Completion and Attrition:
The rates and reasons for study attrition are as important research outcomes as the results of those fully engaging in a telehealth intervention. Attrition in non-telehealth research is influenced by income, employment, and race/ethnicity23 but there is a need to understand additional factors leading to attrition in digital health interventions. Significant proportions of those enrolled in telemonitoring interventions drop-out or minimally engage in the intervention. In a recent prospective study evaluating the relationship between steps captured by a personal activity tracker and risk of hospitalization in chronic liver disease, the device was not worn by patients (activity tracker-only group) on 46% of study inclusion days.4. When patients participating in a remote patient monitoring study for ascites management were offered the option of extending their participation past 30 days, 40% declined despite 94% of surveyed participants noting high feasibility.3 Reconciling high rates of attrition with overall positive post-study satisfaction surveys is challenging. Surveys may not adequately capture the characteristics of the telemonitoring intervention that drive or deteriorate long-term engagement.11 Additionally, it is those with high digital literacy who were satisfied enough to complete the intervention whose opinions are collected; a scenario which introduces bias and masks drivers of digital health disparities. Similarly, those who decide to discontinue participation may not have the opportunity to provide their opinions, thereby having their experiences excluded from collection as well.
High attrition despite positive patient-reported satisfaction implies bias (selection and measurement) in the feedback received, but also that the experiences using a telehealth intervention only partly explain the extent of engagement.24 Reducing selection bias in patient satisfaction surveys necessitates gathering the experiences of those across the entire spectrum of study participation, including non-users. In this manner, secondary analyses can be conducted to assess for potentially modifiable variables accounting for differential usage and engagement between groups. If vulnerable groups derive a comparatively lower benefit from a telehealth intervention, this should prompt changes that create a more equitable digital tool. Focus groups and validated digital literacy assessments8 can also be leveraged to identify factors that promoted or discouraged engagement, such as the degree of concordance between perceived digital literacy and the actual technological demands of the tool. Given the critical role of caregivers in cirrhosis care, they must be involved in these conversations as their perceptions of and experience with digital health interventions modify patient uptake and use of telemonitoring interventions.6 Telehealth satisfaction is also a broad and heterogeneous concept with numerous instruments. Use of validated instruments that capture key metrics of usability, acceptance, and overall satisfaction25 and how they relate to telehealth engagement is needed to advance telehepatology research.
Conclusion:
The promise of telehealth to provide equitable care remains achievable, however this requires an intentional approach to the design and conduct of telehealth research (Figure 1). First, the barriers patients encounter to engage in telehealth-related research are not uniform and can only be adequately understood and overcome through the careful performance of a needs assessment. Second, each step of the recruitment process - study team composition, inclusion criteria selection, and recruitment targets - requires an intentional strategy to promote the creation of diverse study cohorts. Lastly, the spectrum of engagement in a telehealth intervention is wide therefore surveying and obtaining feedback from all users and non-users is important to monitor for disparities that may be inherent to the telehealth intervention. Together, these approaches move the field towards equitable participation in telehealth research and advances care for all.
Funding:
Jeremy Louissaint is a recipient of the American Association for the Study of Liver Diseases Advanced Hepatology Award 2021. Julius Wilder has received research funding from Gilead, Janssen, and received the Clinical, Translational, and Outcomes Research Award from the American Association for the Study of Liver Diseases (AASLD). Elliot Tapper receives funding from the National Institutes of Health through NIDDK (1K23DK117055). Jorge A. Rodriguez receives funding from the National Institute of Minority Health and Health Disparities (1K23MD016439).
Conflicts of interest:
Julius Wilder has served as a consultant for Gilead, Mallinckrodt, Janssen, and Allergan. Elliot Tapper has served as a consultant to Norvartis, Axcella, and Allergan, has served on advisory boards for Mallinckrodt, Bausch Health, Kaleido, Novo Nordisk, and has received unrestricted research grants from Gilead and Valeant. Elizabeth Verna has received grant support from Salix Pharmaceuticals.
Footnotes
Disclosure:
Louissaint is the guarantor of this article
Roles
a. Concept: Louissaint, Wilder, Verna
d. Writing: Louissaint
e. Revision: Wilder, Tapper, Rodriguez, Verna
References:
- 1.Louissaint J, Gibbs JT, Lok AS, et al. Strategies to Improve Video Visit Use in Persons With Liver Disease. Gastroenterology 2021;161:1080–1084.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganapathy D, Acharya C, Lachar J, et al. The patient buddy app can potentially prevent hepatic encephalopathy-related readmissions. Liver Int 2017;37:1843–1851. [DOI] [PubMed] [Google Scholar]
- 3.Bloom P, Wang T, Marx M, et al. A Smartphone App to Manage Cirrhotic Ascites Among Outpatients: Feasibility Study. JMIR Med Inform 2020;8:e17770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin F-P, Bloomer PM, Grubbs RK, et al. Low Daily Step Count Is Associated With a High Risk of Hospital Admission and Death in Community-Dwelling Patients With Cirrhosis. Clin Gastroenterol Hepatol March 2022:S1542–3565(22)00287–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee TC, Kaiser TE, Alloway R, et al. Telemedicine Based Remote Home Monitoring After Liver Transplantation: Results of a Randomized Prospective Trial. Ann Surg 2019;270:564–572. [DOI] [PubMed] [Google Scholar]
- 6.Louissaint J, Lok AS, Fortune BE, et al. Acceptance and use of a smartphone application in cirrhosis. Liver Int 2020;40:1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman LV, Masterson Creber RM, Benda NC, et al. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Inform Assoc 2019;26:855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ismond KP, Eslamparast T, Farhat K, et al. Assessing Patient Proficiency with Internet-Connected Technology and Their Preferences for E-Health in Cirrhosis. J Med Syst 2021;45:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn P, Hazzard E. Technology approaches to digital health literacy. Int J Cardiol 2019;293:294–296. [DOI] [PubMed] [Google Scholar]
- 10.Sisodia RC, Rodriguez JA, Sequist TD. Digital disparities: lessons learned from a patient reported outcomes program during the COVID-19 pandemic. J Am Med Inform Assoc 2021;28:2265–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouquet SD, Miranda AT. Asking the Right Questions-Human Factors Considerations for Telemedicine Design. Curr Allergy Asthma Rep 2020;20:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boot WR, Charness N, Czaja SJ, et al. Computer proficiency questionnaire: assessing low and high computer proficient seniors. Gerontologist 2015;55:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley CE, Genovese N, Owsiany MT, et al. Rapid Integration of Home Telehealth Visits Amidst COVID-19: What Do Older Adults Need to Succeed? J Am Geriatr Soc 2020;68:2431–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wisniewski H, Gorrindo T, Rauseo-Ricupero N, et al. The Role of Digital Navigators in Promoting Clinical Care and Technology Integration into Practice. Digit Biomark 2020;4:119–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez JA, Shachar C, Bates DW. Digital Inclusion as Health Care - Supporting Health Care Equity with Digital-Infrastructure Initiatives. N Engl J Med 2022;386:1101–1103. [DOI] [PubMed] [Google Scholar]
- 16.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health 2014;104:e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anthony DL, Campos-Castillo C, Lim PS. Who Isn’t Using Patient Portals And Why? Evidence And Implications From A National Sample Of US Adults. Health Aff (Millwood) 2018;37:1948–1954. [DOI] [PubMed] [Google Scholar]
- 18.Ancker JS, Nosal S, Hauser D, et al. Access policy and the digital divide in patient access to medical records. Health Policy and Technology 2017;6:3–11. [Google Scholar]
- 19.Miller DP, Latulipe C, Melius KA, et al. Primary Care Providers’ Views of Patient Portals: Interview Study of Perceived Benefits and Consequences. J Med Internet Res 2016;18:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wegermann K, Wilder JM, Parish A, et al. Racial and Socioeconomic Disparities in Utilization of Telehealth in Patients with Liver Disease During COVID-19. Dig Dis Sci 2022;67:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Norman GA. Decentralized Clinical Trials: The Future of Medical Product Development?∗. JACC Basic Transl Sci 2021;6:384–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiyachati KH, Beidas RS, Lane-Fall MB, et al. Weaving Equity into the Fabric of Medical Research. J Gen Intern Med March 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henderson KH, Helmkamp LJ, Steiner JF, et al. Relationship Between Social Vulnerability Indicators and Trial Participant Attrition: Findings From the HYVALUE Trial. Circ Cardiovasc Qual Outcomes April 2022:101161CIRCOUTCOMES120007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boissy A. Patient Engagement versus Patient Experience. NEJM Catalyst May 2017. [Google Scholar]
- 25.Hajesmaeel-Gohari S, Khordastan F, Fatehi F, et al. The most used questionnaires for evaluating satisfaction, usability, acceptance, and quality outcomes of mobile health. BMC Med Inform Decis Mak 2022;22:22. [DOI] [PMC free article] [PubMed] [Google Scholar]