Abstract
Background
Noncommunicable diseases (NCDs) are a global health challenge. The complex etiology of NCDs involves genetic, environmental, and lifestyle factors, including dietary habits. Chronic latent metabolic acidosis has been associated with an increased risk of NCDs. Alkalizing diets and mineral water consumption have shown promise in improving acid-base balance and potentially impacting NCDs.
Methods
In this randomized controlled intervention study, the effect of drinking 1,500–2,000 mL of mineral water daily on acid-base balance was evaluated. Ninety-four healthy participants were divided into two groups: one consumed mineral water with a high bicarbonate and sodium content (HBS, n = 49) and the other consumed mineral water with a low bicarbonate and sodium content (LBS, n = 45). Changes in venous blood gas and urinary acid-base parameters were measured over a short-term (3 days) and long-term (28 days) intervention period. Potential renal acid load (PRAL) and nutrient intake were calculated at baseline and after 28 days.
Results
HBS water consumption led to increased urinary pH (24-hour urine and spontaneous urine, both p < 0.001) and bicarbonate levels (p < 0.001), accompanied by reduced titratable acids (p < 0.001) and ammonium (p < 0.001), resulting in a lower renal net acid excretion (p < 0.001). These changes occurred in the short term and persisted until the end of the study. LBS consumption showed no significant effects on urinary pH but led to a slight decrease in bicarbonate (p < 0.001) and NH4+ (p < 0.001), resulting in a slight decrease in NAE (p=0.011). Blood gas changes were modest in both groups. Mineral water consumption in the HBS group altered dietary intake of sodium and chloride, contributing to changes in PRAL values.
Conclusion
The study demonstrates that the consumption of mineral water high in bicarbonate and sodium (1,500 mL–2,000 mL/day) can positively influence urinary acid-base parameters and reduce NAE, suggesting potential benefits in maintaining acid-base balance without adverse effects on human health. These findings highlight the importance of mineral water composition in acid-base regulation. This trial is registered with DRKS00025341.
1. Introduction
Noncommunicable diseases (NCDs) are a major global health challenge, affecting millions of people worldwide. They are among the leading causes of death and disability-adjusted life years (DALYs) in the world [1, 2]. The etiology of these diseases is complex and multifaceted, involving an interplay of genetic predisposition, environmental exposures, and lifestyle factors, particularly dietary habits. Several studies have shown a significant association between chronic latent metabolic acidosis and an increased risk of developing or progressing NCDs such as osteoporosis, type 2 diabetes, and even mental disorders or cancer [3–7]. Prolonged adherence to an acid-forming diet results in a sustained disturbance of acid-base balance, leading to chronic latent acidosis. Therefore, maintaining a balanced acid-base status through dietary and lifestyle modifications may have a positive impact on health [4, 8].
In this context, dietary patterns rich in alkalizing components have demonstrated their ability to exert a favorable influence on human acid-base balance [9–12]. Furthermore, the consumption of mineral water has emerged as a promising way to improve acid-base balance [10, 13]. The bioactive components present in mineral water, which include essential minerals such as calcium, magnesium, potassium, and sodium, are known to improve the potential renal acid load (PRAL) and counteract the acidogenic components in the diet [10, 14]. Some mineral waters also contain high levels of bicarbonate, which is also a natural component of the body's bicarbonate buffering system and plays a key role in neutralizing acids and maintaining the body's acid-base balance [15, 16]. Consistent with these findings, consumption of bicarbonate-rich mineral water and a low PRAL value have been shown to exhibit beneficial effects on markers of acid-base metabolism [10].
Nevertheless, the existing body of evidence on the effect of mineral water on urinary and blood parameters related to acid-base status remains limited. Previous studies have focused on mineral waters with different bicarbonate concentrations and PRAL values or just alkalizing water. They also mainly investigated the effects of mineral water consumption over longer time periods (2–4 weeks). The present intervention study was carried out with the aim of determining the influence of two different mineral waters on acid-base balance in healthy, omnivorous adults, with comprehensive evaluations conducted over both short-term (3 days) and long-term (4 weeks) intervention periods. These waters were characterized by either a very high bicarbonate content or a very low PRAL value (HBS) or a relatively low bicarbonate content and a moderate PRAL value (LBS).
2. Materials and Methods
2.1. Study Design
This parallel-group randomized controlled intervention study was designed to compare the influence of mineral water very high in bicarbonate and high in sodium (HBS) with mineral water low in bicarbonate and sodium (LBS) on urinary and blood parameters of the acid-base-status. The study was carried out at the Institute of Food Science and Human Nutrition at Leibniz University Hanover in Germany. It was conducted between June 2021 and May 2022.
2.2. Subjects
Ninety-four healthy subjects, ranging in age from 30 to 65 years, were recruited from the general population of Hanover and Hildesheim. Primary inclusion factors were an omnivorous diet and a BMI within the range of 20.0 to 29.9 kg/m2. Subjects with chronic conditions such as manifest cardiovascular or renal diseases, as well as those under medical treatment for hypertension, were not considered for participation.
2.3. Ethical Approval
The study was conducted in accordance with the ethical standards described in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Medical Chamber of Lower Saxony (Hanover, Germany) (BO/24/2021). After being fully informed of the purpose of the study and any potential adverse effects, all participants gave informed consent. The study was registered in the German Clinical Trial Register (DRKS00025341, https://drks.de/search/en). In adherence to established protocols for the documentation of clinical trials, the CONSORT (Consolidated Standards of Reporting Trials) checklist was implemented to ensure transparency and comprehensiveness in explaining both the methodology and results of the trial (Supplementary S1).
2.4. Procedure and Test Products
The study consisted of a screening and a 4-week intervention phase. The intervention included an initial examination at baseline (t0) and two further examinations after 3 days (t3) and after 4 weeks of mineral water consumption (t28). Short-term effects were investigated at t3, while long-term effects were determined at t28. Participants received the bottles of the test water for the entire study period on the first day of the intervention (t0), immediately after the examinations. Alternatively, the water was delivered to their homes in advance. Participants who received the water in advance were instructed not to start drinking it until the end of the examinations on the first examination day (t0).
Participants were randomly assigned by an independent researcher to one of the two intervention groups using stratified randomization according to the covariates sex and age (in descending order). Subjects assigned to the high bicarbonate and sodium group (HBS, n = 49) consumed a mineral water with a very high bicarbonate content (4,368 mg/L) and a high sodium content (1,708 mg/L). Conversely, those assigned to the low bicarbonate and low sodium group (LBS, n = 45) consumed a mineral water with a low bicarbonate (228 mg/L) and a low sodium (8.4 mg/L) content. The composition of the test products is shown in Table 1. Over the course of the 4-week intervention, participants were instructed to consume a minimum of 1,500 mL and a maximum of 2,000 mL of the provided test water daily. Additional fluencies as tap water in its pure form, tea, coffee, mixture of juice and tap water were allowed. Besides that, subjects were advised to maintain their usual diet and maintain their physical activity level. To monitor the consumption of the test water, participants were asked to complete a daily protocol documenting the amount of water consumed daily. This protocol was used to check compliance with the study protocol and to calculate the amount of daily test water consumption after the intervention period. Moreover, compliance was checked by a question in the questionnaire at the end of the study. Participants were considered compliant if they drank 1,500 to 2,000 mL of the respective mineral water on at least half of the intervention days. In addition, consumption of less than 1 liter per day on more than 2 intervention days resulted in exclusion from the analysis.
Table 1.
Mineral content and PRAL of the test products.
| Mineral | Test product | |
|---|---|---|
| HBS mineral water | LBS mineral water | |
| HCO3− (mg/L) | 4368 | 228 |
| Na+ (mg/L) | 1708 | 8.4 |
| Cl− (mg/L) | 322 | 11 |
| SO42− (mg/L) | 174 | 15 |
| K+ (mg/L) | 110 | 2.3 |
| Ca2+ (mg/L) | 90 | 67.5 |
| Mg2+ (mg/L) | 11 | 6.9 |
| P (mg/L) | 0.0∗∗ | 0.0∗∗ |
| PRAL (mEq/L) | −63.07 | −0.89 |
HBS = high bicarbonate and sodium, LBS = low bicarbonate and sodium. PRAL = potential renal acid load according to the formula proposed by Wynn et al. [14]. ∗∗Phosphate content below detection limit.
In order to minimize the impact of circadian rhythm-related fluctuations, all appointments for participants were set at approximately the same time within a consistent morning time frame, spanning from 6:00 to 10:00 a.m. Moreover, the study period began on Monday for all participants. The examination days (t0, t3, t28) were structured as follows: After completion of the current health status questionnaire, measurements of anthropometric characteristics were performed, followed by blood sampling.
2.5. Urine Sampling and Biochemical Measurements
In order to assess the effect of water consumption on urinary acid-base parameters, participants were instructed to collect 24-hour urine samples on the day before each examination day and spontaneous urine samples on the examination days (t0, t3, and t28). All participants were given detailed written instructions for collecting the 24-hour urine samples and were provided with preservative-free plastic containers (Sarstedt AG and Co. KG, Nümbrecht, Germany). Participants were instructed to begin the collection the day before their scheduled examination, beginning after voiding and excluding the first morning urine. The collected urine included the morning sample of the following day. Subjects were asked to record both the start and end times of the collection on the plastic containers. To check the completeness of the 24-hour urine collection, urinary creatinine excretion was measured. Upon arrival at the research institute, urine volume was measured and urine samples were immediately mixed. They were then divided into aliquots, and stored at −22°C until analysis. One aliquot (1 urine tube) was stored at 5°C until transferred to an external laboratory (LADR, Laboratory Network Hanover) for further analysis. After the arrival at the research institute, subjects were asked to collect another urine sample, which served as spontaneous urine.
Urine pH was measured in both spontaneous urine and 24-hour urine samples using a pH meter (Mettler-Toledo, Giessen, Germany; Sartorius, Göttingen, Germany) at the Research Institute. Titratable acids (TA), ammonium (NH4+), and bicarbonate (HCO3−) were measured in 24-hour urine specimens according to the 3-step titration method by Lüthy [17]. These urine parameters were used to calculate renal net acid excretion (NAE) according to the following equation: NAE = TA − HCO3− + NH4+.
2.6. Anthropometric Measurements
Anthropometric measurements were conducted at baseline (t0) and the end of the intervention period (t28). Height was measured with a stadiometer (Seca GmbH and Co. KG, Hamburg, Germany). Body weight was measured by the use of a digital scale (Kern and Sohn GmbH, Balingen-Frommern, Germany). BMI was calculated by dividing weight in kilograms by the square of height in meters. Waist and hip circumferences were measured at the end of a regular exhalation using a nonstretch tape.
2.7. Blood Sampling and Biochemical Measurements
To quantify biochemical indicators of acid-base status, venous blood samples were collected from each participant after a 12-hour overnight fast using EDTA, serum, and NaF citrate tubes (Sarstedt AG and Co. KG, Nümbrecht, Germany). Blood gas analysis was carried out at the research institute right after blood sampling. Blood pH, partial pressure of carbon dioxide (pCO2), base excess (BE), and bicarbonate (HCO3−) were analyzed using the epoc® Blood Analysis System (Siemens Healthcare GmbH, Erlangen, Germany). The remaining blood samples were stored at ≈5°C and transferred to an accredited and certified laboratory (LADR, Laboratory Network Hanover) for analysis.
2.8. Dietary Assessment and PRAL Calculation
To evaluate any potential dietary changes that may have occurred during the intervention, the participants' dietary intake was assessed before (t0) and at the end of the intervention period (t28) using 3-day food records. In order to directly compare nutritional intake and urinary output, participants recorded the type and amount of all foods and beverages consumed over three consecutive days (Friday, Saturday, and Sunday) directly before the first day of the examination (Monday). To take into account different mineralization of tap water, specific tap water composition of every participant was entered in the database. The composition of the participants' tap water was determined on the basis of the drinking water analysis of the water company that supplies the participants' particular area of residence. Nutrient intake was calculated from food and beverage intake (3 days). Moreover, the main fluid intake (beverages and soups) was evaluated at t28 to assess fluid balance. Nutrient and energy intake were estimated using the software PRODI 6.12 Expert® (Nutri-Science GmbH, Freiburg, Germany).
To evaluate the potential renal acid load (PRAL) of the nutrition, PRAL values of the food and beverages consumed were calculated using the formula proposed by Remer and Manz (PRAL1995) [18]. The PRAL value of the mineral water is calculated according to the formula adapted by Wynn [14].
Despite this, the following assumption was used to calculate the amount of salt ingested: NaCl = 40% Na and 60% Cl. Therefore, the calculation equation for salt was: Cl (mg) × 100/60 = NaCl (mg).
2.9. Statistical Analysis
Sample size calculation was made for the primary aim of the study (NAE) considering a statistical power of 80% and a hypothesized effect size above 1.0. An a priori sample size calculation revealed that 36 participants (18 for each water group) would be required to detect a statistical difference of p < 0.05 in a parallel group design.
Distribution of all data was assessed using Shapiro–Wilk test and Q-Q-plots. Non-normally distributed data were transformed using the natural logarithm (ln) or square root (sqrt) transformation, whichever was more appropriate. For data that exhibited non-normal distribution after transformation, nonparametric testing was used. Data are presented as mean ± standard deviation or median and interquartile range (IQR), depending on the distribution. The primary outcome measured is NAE. All other outcomes are secondary outcomes.
Differences in baseline characteristics between the two intervention groups were analyzed using the unpaired t-test or Mann–Whitney U test. Nominal variables were tested using Fischer's exact test.
In terms of nutritional data, between-group differences were analyzed using unpaired t-test or Mann–Whitney U test, while within-group differences were analyzed using paired t-test or Wilcoxon test. Moreover, a two-way repeated measures ANOVA was computed to detect intervention effects (interactions between time and group), despite the partial absence of normal distribution and the partial presence of heteroscedasticity. According to Bortz [19], variance analyses are considered robust against violation of the normal distribution assumption with a sufficiently large sample size (central limit theorem, n > 50), and inhomogeneous variances are not a problem with approximately equal group sizes. In addition, repeated-measures analyses of variance (rmANOVA) followed by Bonferroni correction for multiple testing (pairwise testing) were also performed between baseline (t0), interim (t3), and final (t28) examinations to compare inner-group differences. Two-sided p values <0.05 were considered as statistically significant. All statistical analyses were performed using SPSS Software for Windows (Version 28.0.1.0; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Study Population
The allocation of participants to the two intervention groups is shown in Figure 1. Ninety-four participants (67 women, 27 men) were included in this study, but 9 subjects were excluded from the evaluation due to dropouts and missing values (forgotten samples, illness or other personal reasons). One subject in the HBS group withdrew from the study due to gastrointestinal discomfort. No participant was excluded due to insufficient water consumption. Therefore, data from 85 participants (HBS group: n = 43, LBS group: n = 42) were analyzed. In terms of 24-hour urine data, there was one more missing data in the HBS group, resulting in 42 evaluable 24-hour urine samples.
Figure 1.
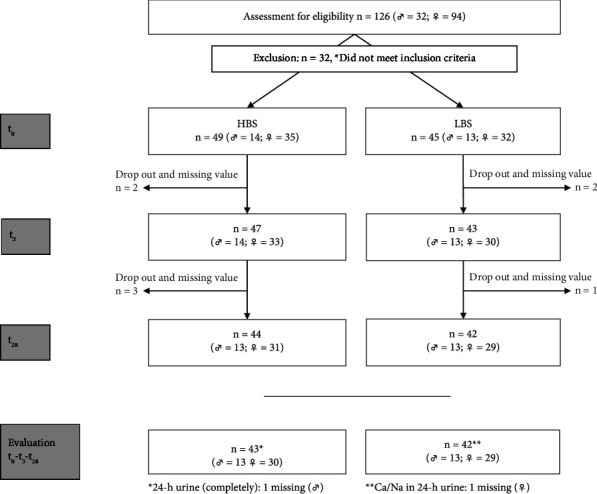
Flowchart of the study.
General baseline characteristics of both intervention groups are presented in Table 2. At baseline, 59 women (69.4%) and 26 men (30.6%) aged 53 ± 10 years were included in the study. There were no significant differences in anthropometric parameters between the two intervention groups, and these parameters did not change significantly from the beginning to the end of the intervention. Participants maintained their physical activity levels during the intervention period.
Table 2.
Baseline (t0) characteristics of the study groups.
| Parameter | HBS group n = 43 | LBS group n = 42 | p value∗ |
|---|---|---|---|
| Women/Men | 30/13 | 29/13 | 0.943 |
| Age (years) | 55 (17) | 55 (10) | 0.725 |
| Body weight (kg) | 70.5 (19.3) | 69.8 (17.9) | 0.582 |
| Height (m) | 1.70 (0.12) | 1.70 (0.10) | 0.287 |
| BMI (kg/m2) | 23.8 (4.51) | 24.6 (5.63) | 0.529 |
| WC (cm) | 85.0 (14.0) | 83.5 (18.0) | 0.519 |
| HC (cm) | 99.0 (12.0) | 101 (9.00) | 0.748 |
| WHR | 0.83 (0.09) | 0.85 (0.11) | 0.547 |
Data are shown as median (IQR). HBS = high bicarbonate and sodium, LBS = low bicarbonate and sodium. BMI = body mass index, HC = hip circumference, WC = waist circumference, WHR = waist-to-hip ratio. ∗Distribution of gender between groups was analyzed using the Chi-Quadrat test. All other group differences were assessed using unpaired t-test/Welch-test, or Mann–Whitney U test.
3.2. Dietary Intake of PRAL-Related Nutrients and Dietary Acid Load
Intake of macronutrients as well as PRAL-related micronutrients is summarized in Table 3. In both intervention groups, the intake of macronutrients did not change significantly between baseline (t0) and final examination (t28), with no significant interaction between time and group. Moreover, there were no significant changes in micronutrient intake with respect to calcium, magnesium, and phosphorus intake in both intervention groups, with no significant interaction between time and group. On the contrary, the intake of sodium, chloride, and potassium differed between baseline (t0) and final examination (t28), depending on the intervention group. In the HBS group, sodium and chloride intake was significantly higher at the end of the study (t28) compared to baseline (t0), as a result of test water consumption (p < 0.001 and p=0.001, respectively). In the LBS group, there were no significant changes in sodium intake, but chloride intake was significantly lower at the end of the intervention (t28) (p=0.025). Consequently, there was a significant change in salt consumption (NaCl) in both groups, but showing opposite directions. While the consumption of NaCl in the HBS group was significantly higher at the end of the intervention (t28) compared to baseline (t0) (p=0.001), it was significantly lower in the LBS group at the end of the intervention (t28) (p=0.025). Furthermore, potassium intake in the LBS group was significantly lower at the end of the intervention (t28) compared to baseline (t0) (p=0.002).
Table 3.
Dietary energy and nutrient intake calculated from 3-day dietary records at the beginning (t0) and at the end of the intervention (t28) as well as absolute changes (∆).
| Parameter | Group | Time | Change | p value (time × group)∗ | p value (time)∗ | |
|---|---|---|---|---|---|---|
| t 0 | t 28 | ∆ 28-0 | ||||
| Fiber (g/d) | HBS | 23.4 (14.7) | 25.5 (10.2) | 1.53 (7.73) | 0.441 | 0.452 |
| LBS | 19.8 (13.4) | 20.9 (11.1) | −0.85 (8.97) | 0.593 | ||
|
| ||||||
| Protein (g/d) | HBS | 83.0 (29.3) | 80.4 (24.5) | −1.76 (25.9) | 0.330 | 0.832 |
| LBS | 77.5 (32.4) | 70.9 (28.0) | −4.71 (29.2) | 0.109 | ||
|
| ||||||
| Fat (g/d) | HBS | 99.0 (53.8) | 101 (30.0) | −1.85 (42.6) | 0.284 | 0.928 |
| LBS | 89.4 (35.6) | 82.8 (49.6) | −4.27 (39.9) | 0.160 | ||
|
| ||||||
| Carbohydrates (g/d) | HBS | 234 (91.9) | 228 (77.3) | 15.0 (75.2) | 0.922 | 0.646 |
| LBS | 266.4 (79.9) | 202.9 (87.2) | −2.76 (78.1) | 0.365 | ||
|
| ||||||
| Energy (kJ/d) | HBS | 9687 (3093) | 9525 (2454) | −249 (2648) | 0.414 | 0.552 |
| LBS | 9436 (2957) | 8629 (3319) | −550 (3234) | 0.120 | ||
|
| ||||||
| Calcium (mg/d) | HBS | 932 (515) | 1002 (423) | −24.2 (352) | 0.820 | 0.938 |
| LBS | 862 (396) | 860 (488) | 49.0 (393) | 0.861 | ||
|
| ||||||
| Chloride (mg/d) | HBS | 4240 (1556) | 4952 (1720) | 709 (1472) | <0.001 | 0.001 |
| LBS | 4128 (2097) | 3240 (1568) | −386 (1706) | 0.025 | ||
|
| ||||||
| Potassium (mg/d) | HBS | 3286 (771) | 3444 (930) | 214 (1064) | 0.002 | 0.303 |
| LBS | 3411 (1650) | 2713 (1183) | −582 (1070) | 0.002 | ||
|
| ||||||
| Magnesium (mg/d) | HBS | 378 (164) | 383 (135) | −11.6 (133) | 0.581 | 0.626 |
| LBS | 378 (171) | 343 (155) | −32.4 (158) | 0.218 | ||
|
| ||||||
| Sodium (mg/d) | HBS | 2706 (884) | 5880 (1207) | 3078 (1073) | <0.001 | <0.001 |
| LBS | 2601 (1155) | 2249 (958) | −146 (1457) | 0.128 | ||
|
| ||||||
| Phosphorus (mg/d) | HBS | 1429 (631) | 1377 (457) | −43.0 (415) | 0.838 | 0.809 |
| LBS | 1304 (482) | 1197 (493) | −26.5 (517) | 0.507 | ||
|
| ||||||
| NaCl (mg/d) | HBS | 7066 (2593) | 8254 (2867) | 1182 (2453) | <0.001 | 0.001 |
| LBS | 6880 (3495) | 5400 (2613) | −643 (2844) | 0.025 | ||
|
| ||||||
| PRAL1995 (mEq/d) | HBS | 3.89 (26.35) | −107 (35.2) | −110 (29.1) | <0.001 | <0.001 |
| LBS | −0.89 (19.14) | 1.23 (14.32) | 2.82 (22.18) | 0.200 | ||
Data are shown as median (IQR). HBS = high bicarbonate and sodium, LBS = low bicarbonate and sodium. PRAL = potential renal acid load. ∗Time × group interactions were analyzed using two-way repeated measures ANOVA. ∗∗Differences between t0 and t28 within each group were analyzed using paired t-Test and Wilcoxon-Test. The bold values are significant at p <0.05.
Moreover, PRAL values calculated from dietary protocols (PRAL1995) showed a significant reduction of dietary acid load in the HBS water group (p < 0.001) and no change in the LBS water group (p=0.200).
3.3. Bicarbonate and Salt Intake from Mineral Water
The two different mineral waters provided different amounts of minerals. While the HBS group consumed a high amount of additional bicarbonate, sodium, and chloride from the test water, the additional amounts of minerals from the consumed water in the LBS group were very low. While participants in the HBS group consumed an additional 6552 mg/d of bicarbonate, participants in the LBS group consumed an additional 342 mg/d of bicarbonate from the study water (1,500 mL). The consumption of 1,500 mL HBS water led to an additional intake of 2.56 grams of sodium and 0.48 grams of chloride per day, while the consumption of LBS water led to an additional intake of 0.013 grams of sodium and 0.017 grams of chloride per day. Despite the different amounts of sodium and chloride, blood pressure did not change differently between the two water groups (data published elsewhere) [20]. With the exception of potassium and sulfate, which were present in higher concentrations in HBS mineral water (Table 1), no other constituent of the two mineral waters led to substantial different ingested amounts in minerals.
3.4. Effect of Mineral Water on Urine Volume and Urinary Acid-Base Parameters
Urine volume did not change differently between the water groups (p=0.176). Both groups showed an increase in urine volume of about 400 mL within the first 3 days of water consumption (p=0.002 and p < 0.001 for HBS and LBS group, respectively) followed by stable urine volume until the end of the intervention (t28) (p=1.000 and p=0.222 for HBS and LBS group, respectively). Consequently, long-term assessments also showed an increase in urine volume (p < 0.001 and p < 0.012 for HBS and LBS group, respectively). There was no significant difference in urine volume between groups at any time point (data not shown).
Daily mineral water consumption in the HBS group was 1,625 (214) mL of HBS mineral water, while LBS mineral water consumption in the LBS group was 1,643 (286) mL. At the end of the intervention, fluid intake and urinary excretion was balanced in both groups. Participants in the HBS group consumed 2,704 (1,100) mL of main fluids (beverages + soups), while the LBS group consumed 2,605 (822) mL. As shown in Table 4, urinary output was 2,687 (1,081) mL and 2,704 (1,021) mL, respectively. A comparison of fluid intake and urine volume showed no significant differences within both groups (pHBS=0.671, pLBS=0.512).
Table 4.
Urine analysis and fluid consumption (main fluids) at the beginning of the study (t0), at the interim examination (t3), and at the end of the intervention (t28) as well as absolute changes (∆).
| Parameter | Group | Time | p value (time × group)∗ | p value (time)∗ | Change | |||
|---|---|---|---|---|---|---|---|---|
| t 0 | t 3 | t 28 | ∆ 3-0 | ∆ 28-0 | ||||
| Fluid consumption (mL) | HBS | 2300 (1065)c | — | 2704 (1100)c | 0.255 | <0.001 | — | 520 (810) |
| LBS | 2260 (920)c | — | 2605 (822)c | 0.010 | — | 175 (1175) | ||
|
| ||||||||
| Urine volume (mL) | HBS | 2341 (1221)a,c | 2742 (879)a | 2687 (1081)c | 0.176 | <0.001 | 395 (815) | 365 (962) |
| LBS | 2193 (1098)a,c | 2685 (951)a | 2704 (1021)c | <0.001 | 402 (943) | 363 (1214) | ||
|
| ||||||||
| pH (24-hour urine) | HBS | 6.17 (0.66)a,c | 7.28 (0.22)a | 7.23 (0.32)c | <0.001 † | <0.001 † | 1.15 (0.77) | 1.15 (0.65) |
| LBS | 5.98 (0.88) | 6.00 (0.66) | 5.92 (0.68) | 0.710 | 0.07 (0.96) | −0.02 (0.53) | ||
|
| ||||||||
| pH (spontaneous urine) | HBS | 5.66 (0.58)a,c | 7.07 (0.53)a | 6.90 (0.71)c | <0.001 | <0.001 | 1.36 (0.88) | 0.97 (0.78) |
| LBS | 5.68 (0.99) | 5.65 (0.86) | 5.53 (0.91) | 0.922 | −0.05 (0.74) | −0.04 (0.92) | ||
|
| ||||||||
| TA (mmol/L) | HBS | 7.78 (13.85)a,c | 0.00 (0.00)a | 0.00 (0.00)c | <0.001 † | <0.001 † | −7.78 (13.85) | −7.78 (13.10) |
| LBS | 9.20 (11.75)a | 6.95 (5.35)a | 8.33 (6.75) | 0.152 | −2.43 (10.35) | −0.68 (10.70) | ||
|
| ||||||||
| HCO3− (mmol/L) | HBS | 8.25 (4.00)a,c | 24.73 (9.00)a | 24.50 (12.53)c | <0.001 † | <0.001 | 16.48 (10.33) | 13.68 (16.90) |
| LBS | 6.83 (6.15)a | 6.18 (2.65)a | 6.18 (2.25) | 0.004 | −1.43 (2.50) | −0.74 (3.37) | ||
|
| ||||||||
| NH4+ (mmol/L) | HBS | 22.15 (12.85)a,c | 10.70 (5.00)a | 11.50 (5.60)c | <0.001 | <0.001 | −9.68 (12.95) | −9.81 (12.73) |
| LBS | 20.45 (11.48)a,c | 17.25 (6.70)a | 17.05 (8.57)c | <0.001 | −3.94 (8.55) | −2.68 (11.80) | ||
|
| ||||||||
| NAE (mEq/d) | HBS | 22.44 (23.80)a,c | −15.40 (11.40)a | −14.25 (15.00)c | <0.001 † | <0.001 † | −37.29 (23.25) | −36.72 (20.57) |
| LBS | 22.28 (19.00)a | 17.80 (9.65)a | 18.53 (13.20) | 0.011 † | −5.68 (17.00) | −3.81 (18.15) | ||
Data are shown as median (IQR). HBS = high bicarbonate and sodium, LBS = low bicarbonate and sodium. TA = titratable acids, HCO3− = bicarbonate in urine, NH4+ = ammonium, NAE = net acid excretion. ∗Time × group interactions were analyzed using two-way repeated measures ANOVA. ∗∗Differences over the intervention time within each group were assessed using repeated measures ANOVA. †Greenhouse–Geisser or Huynh–Feldt adjustment was used to correct for violations of sphericity. a = sign. Differences between t0 and t3 (short term), b = sign. Differences between t3 and t28 (follow-up), c = sign. Differences between t0 and t28 (long-term). The bold values are significant at p <0.05.
As shown in Table 4, the effect of mineral water consumption on urinary acid-base parameters was substantial, with significant effects were primarily evident in the HBS group.
All urinary acid-base parameters showed significant interactions between time and group (all p < 0.001), indicating different effects of water consumption in the two intervention groups. While the consumption of HBS water resulted in significant changes in all urinary acid-base parameters (all p < 0.001), the consumption of LBS water significantly affected only a few parameters.
Specifically, in the HBS group, urinary pH (24-hour urine and spontaneous urine) and HCO3− increased significantly within the first 3 days of water consumption (all p < 0.001), followed by a stable high level until the end of the intervention (t28) (p24−h urin=1.000, pspot urine=0.097, pHCO3−=1.000). Consequently, long-term consumption of HBS water also resulted in significant increases in urine pH (24-hour urine and spontaneous urine) and HCO3− (all p < 0.001) of approximately the same magnitude as in the short-term period (Figure 2). In contrast, in the LBS group, HCO3− decreased slightly within the first 3 days of water consumption (p=0.009), followed by a stable period until the end of the intervention (t28) (p=0.805). However, long-term consumption of LBS water showed no significant effect (p=0.092).
Figure 2.
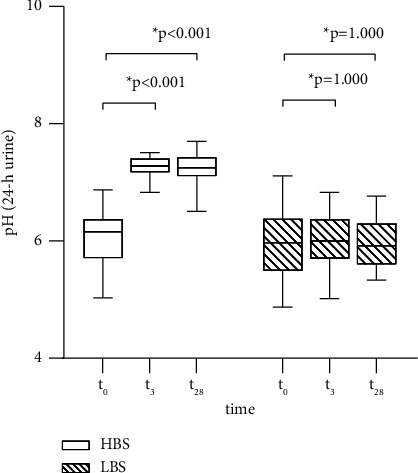
pH of 24-hour urine at the beginning of the study (t0), at the interim examination (t3), and at the end of the intervention (t28).
Moreover, TA and NH4+ decreased significantly within the first 3 days of water consumption in both intervention groups (HBS: both p < 0.001; LBS: pTA=p=0.028, pNH4+ < 0.001), followed by almost stable levels until the end of the intervention (t28) (HBS: pTA=1.000, pNH4+=0.131; LBS: pTA=0.129, pNH4+=0.830). However, the decrease in both TA and NH4+ was significantly greater in the HBS group compared to the LBS group. In the HBS group, TA levels were almost negligible, while NH4+ was reduced by half. Again, long-term consumption of HBS water resulted in significant reductions of TA and NH4+ (both p < 0.001) of approximately the same magnitude as in the short-term period. In contrast, only NH4+ was significantly reduced in the LBS group after long-term consumption of mineral water (p=0.023). However, these declines were smaller than those in the HBS group. In addition, TA in the LBS group showed no significant changes over the long-term period of consumption (p=0.520).
Finally, this resulted in a significant interaction between time and group for NAE (p < 0.001), indicating different effects of water consumption in the two water groups (Figure 3). Nevertheless, in both intervention groups, NAE significantly decreased within the first 3 days of water consumption (p < 0.001 and p=0.018 for HBS and LBS group, respectively). However, the reductions were more pronounced in the HBS group than in the LBS group. From the interim examination (t3) until the end of the intervention (t28), both groups showed a stable NAE (p=0.875 and p=0.377 for HBS and LBS group, respectively). Moreover, long-term consumption of both mineral waters showed a decline in NAE levels, which were only significant in the HBS group (p < 0.001 and p=0.257 for HBS and LBS group, respectively).
Figure 3.
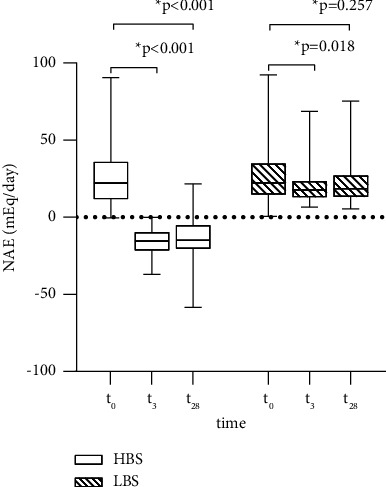
NAE at the beginning of the study (t0), at the interim examination (t3), and at the end of the intervention (t28).
3.5. Effect of Mineral Water on Blood Parameters of Acid-Base-Status
As shown in Table 5, the effect of mineral water consumption on venous blood gas parameters was modest, with significant effects observed primarily in the HBS group.
Table 5.
Blood gas analysis at the beginning of the study (t0), at the interim examination (t3), and at the end of the intervention (t28) as well as absolute changes (∆).
| Parameter | Group | Time | p value (time × group)∗ | p value (time)∗ | Change | |||
|---|---|---|---|---|---|---|---|---|
| t 0 | t 3 | t 28 | ∆ 3-0 | ∆ 28-0 | ||||
| pH | HBS | 7.381 (0.035) | 7.396 (0.048)b | 7.382 (0.045)b | 0.145 | <0.001 | 0.01 (0.04) | −0.01 (0.03) |
| LBS | 7.397 (0.036)c | 7.383 (0.039) | 7.374 (0.037)c | 0.027 | 0.00 (0.04) | 0.00 (0.04) | ||
|
| ||||||||
| HCO3− (mmol/L) | HBS | 25.6 (2.70)a,c | 26.00 (2.60)a | 25.80 (3.20)c | 0.001 | <0.001 | 0.60 (2.50) | 0.90 (2.20) |
| LBS | 25.6 (2.50) | 25.00 (2.10) | 25.10 (1.50) | 0.428 | −0.30 (1.70) | −0.40 (1.80) | ||
|
| ||||||||
| BE (b) (mmHg) | HBS | 0.20 (2.30)a,c | 0.90 (2.60)a,b | 0.80 (2.00)b,c | <0.001 | <0.001 | 0.80 (1.50) | 0.70 (1.70) |
| LBS | 0.20 (1.40) | −0.15 (2.00) | −0.05 (1.30) | 0.146 | −0.15 (1.30) | −0.45 (1.80) | ||
|
| ||||||||
| pCO2 (mmHg) | HBS | 43.40 (7.70)c | 43.40 (8.00) | 43.60 (9.10)c | 0.303 | 0.016 | 1.20 (6.80) | 1.90 (5.40) |
| LBS | 41.85 (7.40) | 41.10 (5.50) | 43.00 (5.90) | 0.132 | −0.70 (6.40) | −0.15 (5.10) | ||
Data are shown as median (IQR). HBS = high bicarbonate and sodium, LBS = low bicarbonate and sodium. BE = base excess, HCO3− = bicarbonate in venous blood, pCO2 = partial pressure of carbon dioxide in venous blood. ∗Time × group interactions were analyzed using two-way repeated measures ANOVA. ∗∗Differences over the intervention time within each group were assessed with repeated measurements ANOVA. a = sign. Differences between t0 and t3 (short term), b = sign. Differences between t3 and t28 (follow-up), c = sign. Differences between t0 and t28 (long term). The bold values are significant at p <0.05.
In blood samples, pH and pCO2 did not show a significant interaction between time and group (p=0.145 and p=0.303, respectively), indicating similar effects of water consumption in the two intervention groups. However, pH in the HBS group tended to increase during the initial 3 days of water consumption (p=0.068) and significantly decreased from the interim examination (t3) to the end of the intervention (t28) (p < 0.001); no long-term changes were observed (p=0.427). In the LBS group, only long-term changes were significant (p=0.036), showing a decrease in pH. For pCO2, the HBS group showed a slight but nonsignificant increase during the first 3 days of water consumption (p=0.807) and from the interim (t3) examination to the end of the intervention (t28) (p=0.167), with a significant increase during the long-term consumption (p=0.027). In the LBS group there were no significant changes.
Moreover, for HCO3− and BE significant time and group interactions were shown (p=0.001 and p < 0.001, respectively), indicating different effects of water consumption. Differences were significant only in the HBS group (both p < 0.001). Specifically, HCO3− and BE levels significantly increased during the initial 3 days of water consumption (p=0.004), followed by a slight, but nonsignificant decrease of HCO3− (p=1.000) and a significant decrease of BE (p=0.015) until the end of the intervention (t28). Long-term consumption of the HBS water also led to a significant increase in HCO3− and BE levels (p=0.011 and p=0.030, respectively).
4. Discussion
In this study, the daily consumption of 1500–2000 mL of bicarbonate- and sodium-rich mineral water led to significant positive changes in urinary and blood acid-base parameters. On the contrary, drinking the LBS water showed only slight effects. The favorable outcomes of consuming high-bicarbonate-sodium (HBS) water were observed after a short-term consumption period (3 days) and maintained until the end of the study (28 days). Throughout the follow-up period, the majority of parameters in both blood and urine remained stable, maintaining similar levels to those observed after the initial short-term intervention.
Considering that the diet remained unchanged, except for the main beverages, it is reasonable to conclude that the observed differences are not due to alterations in dietary intake. Instead, these differences can be exclusively attributed to the impact of the mineral water.
The long-term results of the present study are consistent with previous mineral water studies showing positive effects on acid-base balance after consumption of bicarbonate-rich mineral water [10, 13, 14, 21–26]. Except one study, which evaluated effects on several acid-base parameters in blood and urine samples [10], most of the conducted mineral water studies concentrated on blood and/or urinary pH. Blood gas parameters and net acid excretion (NAE) are not commonly assessed in studies involving mineral water, with only three exceptions [10, 13, 25] having delved into this area. Nonetheless, several studies have highlighted the favorable impacts of HCO3− supplementation on blood HCO3− levels [27–31], blood BE [30], and urinary NAE [32, 33]. Moreover, short-term HCO3− supplementation studies have demonstrated positive effects on urinary pH [34, 35].
4.1. Effects on Urine Volume and Urinary Acid-Base Parameters
In the current study, urine volume increased significantly in both intervention groups. At the end of the study, both groups showed a neutral fluid balance, with no significant differences between the main fluid intake and urinary fluid excretion. However, considering that the body eliminates water not solely through urine but also via skin, feces, and respiration [36], a slightly negative fluid balance could be assumed according to the presented data. Nevertheless, fluids may have been balanced, because measured fluid intake just covered beverages and fluid intake from soups. Additional fluid intake from fruits, legumes, and other foods was not evaluated, leading to a lower reported fluid intake than actually consumed. Moreover, there were no differences in urine volume between the two study groups at all time points. This indicated that both study groups underwent comparable dilution effects due to urine volume. The findings align with previous mineral water studies, where significant increases have been observed in response to specific daily intakes ranging from 1,500 ml to 2,000 ml of mineral water [10, 14, 22–24]. With the exception of one study, the magnitudes of increase did not diverge between intervention groups within each study. In the mentioned study, the group consuming bicarbonate-rich mineral water displayed an elevation in urine volume during the initial 4 weeks, followed by a reduction in the magnitude of increase almost returning to baseline levels. Conversely, the control groups exhibited minor declines in volume during the initial 8 weeks, followed by a slight increase by the end of the intervention [23]. In addition, only one study showed a reduction in urine volume after the consumption of alkalizing mineral water [13].
Furthermore, impacts on urinary acid-base parameters include changes in pH (24-hour urine and spontaneous urine), TA, HCO3−, and NH4+. The latter three parameters can be summarized as urinary NAE, serving as an indicator for dietary acid load [37]. As mentioned above, urinary parameters were strongly influenced by the consumption of HBS water within this study. The very high HCO3− intake through HBS water resulted in an excess of HCO3− being excreted via urine. The buffering effect was substantial; TA became virtually undetectable. All effects were highly significant in the short-term and in the long-term analysis. For pH in 24-hour urine, these findings are consistent with the results of several previous mineral water studies [10, 13, 14, 21–26]. In the previous studies, pH in 24-hour urine increased by about 0.29 to 0.80 points (mean), whereas the consumption of HBS water resulted in an even more pronounced pH increase of about 1.19 points (mean). Concerning spontaneous urine, only a single prior mineral water study has examined the effect on pH, demonstrating comparatively smaller positive effects than those seen after HBS water consumption [10]. Regarding acid-base parameters determined by titration, there are only a few previous studies evaluating the impact on these parameters in mineral water studies [10, 14, 22], all showing similar effects. In the largest study conducted with 129 healthy participants, three different bicarbonate-rich mineral waters with different PRAL values (MBMP water, HBLP water, and MBLP water) were tested against low-bicarbonate mineral water with a high PRAL value (LBHP water) [10]. While MBMP water was characterized by a moderate HCO3− content and a medium PRAL value, HBLP water had a high HCO3− content and a low PRAL value, and MBPL water had a medium HCO3− content and a low PRAL value. In this study, the consumption of the bicarbonate-rich mineral waters with different PRAL values resulted in favorable effects on urinary TA, HCO3−, NH4+, and NAE compared to the low-bicarbonate mineral water with a high PRAL value. Nonetheless, some distinctions emerged among the three bicarbonate-rich mineral waters used in this study. The positive changes in urinary parameters were more pronounced in the groups drinking bicarbonate-rich mineral water with a low PRAL value (HBLP and MBLP water). The researchers concluded that alkalinity (PRAL value in mineral water) has a greater influence on acid-base parameters than HCO3− content [10]. Under the same presumption, it was anticipated that the acid-base balance in the current study would be more profoundly affected by HBS water consumption in the current study. This was attributed to its higher alkalinity (PRAL −63.07), resulting from a high sodium content. In fact, although the effects on urinary parameters showed the same direction in both studies, the effects of HBS water consumption surpassed those exhibited by the waters investigated by Wasserfurth et al. [10].
It is noteworthy that baseline NAE was lower than expected. Typically, individuals following an omnivorous Western diet display a NAE ranging between 70 and 100 mEq/day [38]. In contrast, in both intervention groups, NAE was about 27 mEq/day at baseline. There are two explanations for such a low NAE. Firstly, the study population may have been more health conscious than the general population. They may have consumed a more healthy and therefore less acidic diet than the average population. Lower NAE values have already been reported with a plant-based diet [39]. Moreover, there are several studies that have reported similar baseline NAE values as reported in the current study following omnivorous diets [10, 40–42]. Secondly, urinary bicarbonate, a component of NAE calculations, may have been somewhat overestimated by titration of HCO3− [17]. Considering the initial low NAE levels and the substantial buffering impact on urinary acid-base parameters, the substantial reduction in NAE to negative values is not surprising. The negative NAE is consistent with a previous study showing that certain diets can lead to negative values [37].
Despite the direct influence of diet on NAE, several hormones are involved in the regulation of renal acid-base excretion. Higher levels of aldosterone, angiotensin II, endothelin, parathyroid hormone, and glucocorticoids are linked to higher renal acid excretion. Moreover, metabolic acidosis has been shown to increase hormonal activity [43]. Therefore, the correction of the slightly low-grade metabolic acidosis may have involved a change in hormonal regulation leading to a change in TA, HCO3−, and NH4+excretion, possibly caused by the mineral water consumption. Nevertheless, this is speculative, as only aldosterone has been examined in the present study. As previously reported, the consumption of bicarbonate- and sodium-rich mineral water in this study resulted in a significant decrease in urinary aldosterone [20], which may have contributed to a lower NAE.
4.2. Effects on Venous Acid-Base Blood Gas Parameters
Effects on acid-base blood gas parameters include pH, BE, HCO3−, and pCO2. This is the first study showing beneficial effects of short-term and long-term consumption of mineral water very high in bicarbonate and high in sodium on venous blood gas parameters.
As mentioned above, long-term effects have already been demonstrated in previous studies [10, 13, 25] and could be supported by this study results. In line with our findings, the study carried out by Wasserfurth et al. [10] similarly indicated no noteworthy shifts in venous blood pH after a 4-week consumption of three different bicarbonate-rich mineral waters possessing different PRAL values. Nevertheless, in the study presented here, pH values in blood tended to increase over the first three intervention days. This may also have occurred in the Wasserfurth study, but it could not be determined due to the long-term design of the study. Moreover, the short-term results of the current study are similar to the results of a previous intervention study with an alkaline mineral water (pH 10). In that study, the consumption of mineral water resulted in a significant increase in blood pH after a rather short duration of 2 weeks [13]. However, capillary blood was used. Although arterial blood analyses are required for the evaluation of respiratory conditions, metabolic disturbances can also be adequately measured in venous blood samples [44, 45]. Venous pH, bicarbonate, and base excess closely align with arterial values. However, the agreement between arterial and venous pCO2 is inconsistent [44]. Under normal physiological conditions, blood pH is kept in a narrow range between 7.35 and 7.45 (arterial pH) to maintain physiological processes in the body, mediated by intracellular and interstitial pH (4.8). The varying effects of bicarbonate-rich mineral water on blood pH in the short term and long term may be the result of efficient buffering systems to maintain a stable blood pH. Although drinking mineral water can lead to a transient pH increase, buffering systems react sequentially to keep it within the normal range over the long term.
In addition, within the scope of this study, there was a noteworthy increase in HCO3− and BE during the initial 3-day phase of mineral water consumption and these elevated concentrations remained until the end of the intervention. This illustrates that the impact of consuming mineral water on blood acid-base balance becomes apparent shortly after the first consumption and continues to endure over a long period of time, without any habituation effects. These findings align with the outcomes of the study carried out by Wasserfurth et al. [10]. In that research, noteworthy impacts on HCO3− and BE were exclusively observed in the subset consuming mineral water with a moderately high HCO3− content and a low PRAL value (MBLP water, PRAL = −22.1). However, the group consuming bicarbonate-rich mineral water with a low PRAL (HBLP water, PRAL = −19.3) also displayed a tendency towards an increase in these parameters. Notably, no distinctions were evident for the group consuming bicarbonate-rich mineral water with a moderate PRAL value (MBMP water, PRAL = −10.8). The authors conclude that alkalinity may be more important than bicarbonate in regulating acid-base balance in blood. Based on this assumption and considering that the PRAL value of the water consumed in this current study (HBS water, PRAL = −63.07) was even lower than the PRAL value of the HBLP water in Wasserfurth's study, a greater impact on blood gas parameters was anticipated in the present study. Nevertheless, the effect on HCO3− and BE was slightly lower with HBS water than the effect of low-PRAL mineral water in Wasserfurth's study (MBLP and HBLP group).
Although pCO2 significantly increased from the beginning of the intervention to the end, changes were marginal following HBS water consumption. In contrast, the changes on pCO2 in the MBHP group were more pronounced in the mineral water study by Wasserfurth et al. [10].
4.3. Effects on Blood Pressure
Both mineral waters contained different amounts of sodium. The evaluation of the changes in blood pressure due to mineral water consumption has been published elsewhere [20]. For the sake of completeness, the blood pressure results are also mentioned here. Despite the different amounts of sodium, blood pressure did not change differently between the two water groups. Furthermore, no differences were found between men and women, or between younger (<50 years) and older (≥50 years) participants in either intervention group. This is in line with the results of other studies in which the consumption of bicarbonate- and sodium-rich mineral water did not lead to blood pressure changes [26, 46–48].
4.4. Implications for Human Health
Urine pH of healthy subjects ranges usually between 5.8 and 6.8, depending on the consumed diet [49]. It has been shown that vegans have a higher urine pH (6.7) than omnivores (6.2) [50]. This is in line with our findings in the LBS group, where urine pH was about 6 and did not change significantly over the intervention period. Thus, mineral water consumption did not modify acid-base balance in this group. Moreover, NAE was only slightly positive in this group, indicating a moderate acidic diet. Since included subjects were without impaired kidney function, it is reasonable to assume that the excess acid from the diet would have been excreted without negative effects on human health. However, a lower urine pH value, associated with a higher NAE, can promote the formation of certain kidney stones [51]. In addition, higher NAE values are associated with inflammatory processes in the kidneys [52].
Base supplements and bicarbonate-rich mineral waters are commonly used for the modulation of urine composition. Their alkalizing effect has been proven in several studies until now [10, 14, 22]. Therefore, the most obvious impact on human health relates to the modulation of kidney stone risk (calcium oxalate stones, uric acid stones) due to changes in urinary pH. While a low urine pH favors crystallization, an elevated urine pH counteracts crystallization [49, 53]. Moreover, bicarbonate-rich mineral water has been shown to improve urinary inhibitors for crystallization, mainly citrate and magnesium [49]. Accordingly, the consumption of bicarbonate-rich mineral water has a positive effect on the risk of calcium oxalate stones and uric acid stones due to its urine alkalizing effect and changes in urine composition.
In addition, as demonstrated by the lowering of NAE, bicarbonate-rich mineral water has a positive impact on acid-base-balance [10]. Therefore, the negative effects of an acidic diet can be mitigated by its intake. On the one hand, a high intake of acid-producing precursors has been shown to negatively affect bones [54]. On the other hand, the intake of bicarbonate-rich mineral water has been shown to lower bone resorption [14, 25, 55]. Thus, it could be assumed that the consumption of bicarbonate-rich mineral water has a positive effect on bones due to its improvement in acid-base balance.
4.5. Strengths and Limitations
Within the context of this study, there are some strengths and limitations that should be mentioned. One limitation is the absence of blinding in the study design which may have an influence on the study outcome. Since the taste of the water alone indicated the group assignment, blinding was not feasible. Moreover, the lack of meal standardization should be recognized as a possible source of influence on the study outcomes. The inclusion of such standardized meals would have been beneficial to increase the robustness of our results. This approach would have taken into account the different effects of different meals on urinary acid excretion, thus providing more reliable results. Furthermore, it would have been beneficial to include additional control groups consuming mineral water high in sodium alone. This would have allowed for a more precise evaluation of the individual effects of HCO3− and PRAL of the mineral water and improved the study's ability to make specific statements regarding their respective impacts.
Conversely, it is important to acknowledge the strengths of this study. A strength is the sample size, surpassing previous studies that examined similar effects with smaller participant groups. This increased sample size enhances the study's statistical power, making it more likely to detect true effects and enabling more precise estimates of the observed effects. Furthermore, assessing effects at multiple time points provides the opportunity to capture progression over time and uncover potential habituation effects. Another advantage of the study is the extensive data collection on various parameters, including blood gas and urinary parameters, providing a comprehensive view of potential effects.
5. Conclusion
In conclusion, this study highlights the significant and beneficial effects of mineral water consumption, characterized by a very high bicarbonate and high sodium content (HBS water) content, on acid-base parameters. In particular, the consumption of 1,500 to 2,000 mL mineral water led to a significant reduction in urinary NAE, as reflected by decreased levels of TA and NH4+, with a concomitant increase in urinary HCO3− excretion. A significant increase in urinary pH was also observed. Moreover, blood gas parameters were positively affected, although to a lesser extent. Taken together, these results emphasize that the consumption of mineral water with high HCO3− and sodium concentrations exerts a beneficial effect on the acid-base balance in humans. These effects became apparent shortly after the beginning of consumption and showed no signs of habituation. Moreover, no adverse effects on human health have been observed.
Further research should address the different effects on acid-base parameters of consuming mineral water with a very low PRAL and simultaneous low bicarbonate content compared to a bicarbonate-rich mineral water. This research is warranted to isolate the potential effects of the co-occurrence of high HCO3− content and low PRAL.
Acknowledgments
The authors would like to express their gratitude to the participants and to everyone who helped, in any way, to make this study possible. The study was funded in parts by SNC Neptune, France. Study realization, data analysis, interpretation, and writing of the manuscript were undertaken independently from the sponsor. The publication of this article was funded by the Open Access Fund of Leibniz University Hanover. Open Access funding enabled and organized by Projekt DEAL.
Data Availability
The data used to support the findings of this study are made available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
In accordance with established guidelines for reporting clinical trials, the CONSORT (Consolidated Standards of Reporting Trials) checklist was utilized to ensure transparency and completeness in the presentation of trial methodology and results. To check this list, please see Supplementary S1.
References
- 1.Vos T., Lim S. S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet . 2020;396(10258):1204–1222. doi: 10.1016/s0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Who. Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2019 . Geneva, Switzerland: World Health Organization; 2019. [Google Scholar]
- 3.Jayedi A., Shab-Bidar S. Dietary acid load and risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective observational studies. Clinical Nutrition ESPEN . 2018;23:10–18. doi: 10.1016/j.clnesp.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Osuna-Padilla I. A., Leal-Escobar G., Garza-García C. A., Rodríguez-Castellanos F. E. Dietary acid load: mechanisms and evidence of its health repercussions. Nefrologia . 2019;39(4):343–354. doi: 10.1016/j.nefroe.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffari H., Siassi F., Guilani B., Askari M., Azadbakht L. Association of dietary acid-base load and psychological disorders among Iranian women: a cross-sectional study. Complementary Therapies in Medicine . 2020;53 doi: 10.1016/j.ctim.2020.102503.102503 [DOI] [PubMed] [Google Scholar]
- 6.Robey I. F. Examining the relationship between diet-induced acidosis and cancer. Nutrition and Metabolism . 2012;9(1):p. 72. doi: 10.1186/1743-7075-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehghan P., Abbasalizad Farhangi M. Dietary acid load, blood pressure, fasting blood sugar and biomarkers of insulin resistance among adults: findings from an updated systematic review and meta‐analysis. International Journal of Clinical Practice . 2020;74(4):p. e13471. doi: 10.1111/ijcp.13471. [DOI] [PubMed] [Google Scholar]
- 8.Quade B. N., Parker M. D., Occhipinti R. The therapeutic importance of acid-base balance. Biochemical Pharmacology . 2021;183 doi: 10.1016/j.bcp.2020.114278.114278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goraya N., Simoni J., Jo C. H., Wesson D. E. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clinical Journal of the American Society of Nephrology . 2013;8(3):371–381. doi: 10.2215/cjn.02430312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wasserfurth P., Schneider I., Ströhle A., Nebl J., Bitterlich N., Hahn A. Effects of mineral waters on acid-base status in healthy adults: results of a randomized trial. Food & Nutrition Research . 2019;63(0) doi: 10.29219/fnr.v63.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller A., Zimmermann-Klemd A. M., Lederer A. K., et al. A vegan diet is associated with a significant reduction in dietary acid load: post hoc analysis of a randomized controlled trial in healthy individuals. International Journal of Environmental Research and Public Health . 2021;18(19):p. 9998. doi: 10.3390/ijerph18199998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frassetto L., Remer T., Banerjee T. Dietary contributions to metabolic acidosis. Advances in Chronic Kidney Disease . 2022;29(4):373–380. doi: 10.1053/j.ackd.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Heil D. P. Acid-base balance and hydration status following consumption of mineral-based alkaline bottled water. Journal of the International Society of Sports Nutrition . 2010;7(1):p. 29. doi: 10.1186/1550-2783-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wynn E., Krieg M. A., Aeschlimann J. M., Burckhardt P. Alkaline mineral water lowers bone resorption even in calcium sufficiency. Bone . 2009;44(1):120–124. doi: 10.1016/j.bone.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Koeppen B. M. The kidney and acid-base regulation. Advances in Physiology Education . 2009;33(4):275–281. doi: 10.1152/advan.00054.2009. [DOI] [PubMed] [Google Scholar]
- 16.Hamm L. L., Nakhoul N., Hering-Smith K. S. Acid-base homeostasis. Clinical Journal of the American Society of Nephrology . 2015;10(12):2232–2242. doi: 10.2215/cjn.07400715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lüthy. Three-step acid-base titration in urine. Medicina & Laboratorio . 1977;30(7) [Google Scholar]
- 18.Remer T., Manz F. Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association . 1995;95(7):791–797. doi: 10.1016/s0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 19.Bortz J. Statistics: For human and social scientists . Berlin, Germany: Springer; 2005. [Google Scholar]
- 20.Mansouri K., Greupner T., Hahn A. Blood pressure stability and plasma aldosterone reduction: the effects of a sodium and bicarbonate-rich water- A randomized controlled intervention study. Blood Pressure . 2024;33(1) doi: 10.1080/08037051.2023.2291411.2291411 [DOI] [PubMed] [Google Scholar]
- 21.Keßler T., Hesse A. Cross-over study of the influence of bicarbonate-rich mineral water on urinary composition in comparison with sodium potassium citrate in healthy male subjects. British Journal of Nutrition . 2000;84(6):865–871. doi: 10.1017/s0007114500002488. [DOI] [PubMed] [Google Scholar]
- 22.Siener R., Jahnen A., Hesse A. Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. European Journal of Clinical Nutrition . 2004;58(2):270–276. doi: 10.1038/sj.ejcn.1601778. [DOI] [PubMed] [Google Scholar]
- 23.Lu Y., Sundaram P., Li H., Chong T. W. The effects of drinking bicarbonate-rich mineral water in calcium oxalate stone formers: an open label prospective randomized controlled study in an Asian cohort. International Urology and Nephrology . 2022;54(9):2133–2140. doi: 10.1007/s11255-022-03256-8. [DOI] [PubMed] [Google Scholar]
- 24.Karagülle O., Smorag U., Candir F., et al. Clinical study on the effect of mineral waters containing bicarbonate on the risk of urinary stone formation in patients with multiple episodes of CaOx-urolithiasis. World Journal of Urology . 2007;25(3):315–323. doi: 10.1007/s00345-007-0144-0. [DOI] [PubMed] [Google Scholar]
- 25.Roux S., Baudoin C., Boute D., Brazier M., De La Guéronniere V., De Vernejoul M. C. Biological effects of drinking-water mineral composition on calcium balance and bone remodeling markers. The Journal of Nutrition, Health & Aging . 2004;8(5):380–384. [PubMed] [Google Scholar]
- 26.Toxqui L., Vaquero M. P. An intervention with mineral water decreases cardiometabolic risk biomarkers. A crossover, randomised, controlled trial with two mineral waters. Moderately Hypercholesterolaemic Adults . 2016;12 doi: 10.3390/nu8070400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.König D., Muser K., Dickhuth H. H., Berg A., Deibert P. Effect of a supplement rich in alkaline minerals on acid-base balance in humans. Nutrition Journal . 2009;8(1):p. 23. doi: 10.1186/1475-2891-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bovée D. M., Roksnoer L. C. W., Van Kooten C., et al. Effect of sodium bicarbonate supplementation on the renin-angiotensin system in patients with chronic kidney disease and acidosis: a randomized clinical trial. Journal of Nephrology . 2021;34(5):1737–1745. doi: 10.1007/s40620-020-00944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boschmann M., Kaiser N., Klasen A., et al. Effects of dietary protein-load and alkaline supplementation on acid–base balance and glucose metabolism in healthy elderly. European Journal of Clinical Nutrition . 2020;74(S1):48–56. doi: 10.1038/s41430-020-0695-3. [DOI] [PubMed] [Google Scholar]
- 30.Limmer M., De Marées M., Platen P. Effects of daily ingestion of sodium bicarbonate on acid-base status and anaerobic performance during an altitude sojourn at high altitude: a randomized controlled trial. Journal of the International Society of Sports Nutrition . 2020;17(1):p. 22. doi: 10.1186/s12970-020-00351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Qiu J., Yi L., Hou Z., Benardot D., Cao W. Effect of sodium bicarbonate ingestion during 6 weeks of HIIT on anaerobic performance of college students. Journal of the International Society of Sports Nutrition . 2019;16(1):p. 18. doi: 10.1186/s12970-019-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurer M., Riesen W., Muser J., Hulter H. N., Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. American Journal of Physiology- Renal Physiology . 2003;284(1):F32–F40. doi: 10.1152/ajprenal.00212.2002. [DOI] [PubMed] [Google Scholar]
- 33.Tyson C. C., Luciano A., Modliszewski J. L., Corcoran D. L., Bain J. R., Muehlbauer M., et al. Effect of bicarbonate on net acid excretion, blood pressure, and metabolism in patients with and without ckd: the acid base compensation in ckd study. American Journal of Kidney Diseases . 2022;78 doi: 10.1053/j.ajkd.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xue W., Cheng J., Zhao J., Wang L., Peng A., Liu X. Comparison potassium sodium hydrogen citrate with sodium bicarbonate in urine alkalization: a prospective crossover-controlled trial. International Urology and Nephrology . 2022;55(1):61–68. doi: 10.1007/s11255-022-03387-y. [DOI] [PubMed] [Google Scholar]
- 35.Cohen B., Laish I., Brosh-Nissimov T., et al. Efficacy of urine alkalinization by oral administration of sodium bicarbonate: a prospective open-label trial. The American Journal of Emergency Medicine . 2013;31(12):1703–1706. doi: 10.1016/j.ajem.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Iversen P. O., Fogelholm M. Fluid and water balance: a scoping review for the Nordic Nutrition Recommendations 2023. Food & Nutrition Research . 2023;67 doi: 10.29219/fnr.v67.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frassetto L., Shi L., Schloetter M., Sebastian A., Remer T. Established dietary estimates of net acid production do not predict measured net acid excretion in patients with Type 2 diabetes on Paleolithic–Hunter–Gatherer-type diets. European Journal of Clinical Nutrition . 2013;67(9):899–903. doi: 10.1038/ejcn.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poupin N., Calvez J., Lassale C., Chesneau C., Tomé D. Impact of the diet on net endogenous acid production and acid–base balance. Clinical Nutrition . 2012;31(3):313–321. doi: 10.1016/j.clnu.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Ausman L. M., Oliver L. M., Goldin B. R., Woods M. N., Gorbach S. L., Dwyer J. T. Estimated net acid excretion inversely correlates with urine pH in vegans, lacto-ovo vegetarians, and omnivores. Journal of Renal Nutrition . 2008;18(5):456–465. doi: 10.1053/j.jrn.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Dawson-Hughes B., Castaneda-Sceppa C., Harris S. S., et al. Impact of supplementation with bicarbonate on lower-extremity muscle performance in older men and women. Osteoporosis International . 2010;21(7):1171–1179. doi: 10.1007/s00198-009-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jehle S., Hulter H. N., Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. Journal of Clinical Endocrinology & Metabolism . 2013;98(1):207–217. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 42.Shea M. K., Gilhooly C. H., Dawson-Hughes B. Food groups associated with measured net acid excretion in community-dwelling older adults. European Journal of Clinical Nutrition . 2017;71(3):420–424. doi: 10.1038/ejcn.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagami G. T., Kraut J. A. The role of the endocrine system in the regulation of acid–base balance by the kidney and the progression of chronic kidney disease. International Journal of Molecular Sciences . 2024;25(4):p. 2420. doi: 10.3390/ijms25042420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelly A. Review article: can venous blood gas analysis replace arterial in emergency medical care. Emergency Medicine Australasia . 2010;22(6):493–498. doi: 10.1111/j.1742-6723.2010.01344.x. [DOI] [PubMed] [Google Scholar]
- 45.Saberian L., Sharif M., Aarabi M., Broumand B., Shafiee M. A. Arterial versus venous blood gas analysis comparisons, appropriateness, and alternatives in different acid/base clinical settings: a systematic review. Cureus . 2023;15(7) doi: 10.7759/cureus.41707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zair Y., Kasbi-Chadli F., Housez B., et al. Effect of a high bicarbonate mineral water on fasting and postprandial lipemia in moderately hypercholesterolemic subjects: a pilot study. Lipids in Health and Disease . 2013;12(1):p. 105. doi: 10.1186/1476-511x-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dore M. P. Health properties of the italian san martino ® mineral-rich water: a self-controlled pilot study. Biomedicine \& Pharmacotherapy . 2021;138 doi: 10.1016/j.biopha.2021.111509. [DOI] [PubMed] [Google Scholar]
- 48.Schoppen S., Pérez-Granados A. M., Vaquero M. P., et al. A sodium-rich carbonated mineral water reduces cardiovascular risk in postmenopausal women. The Journal of Nutrition . 2004;134(5):1058–1063. doi: 10.1093/jn/134.5.1058. [DOI] [PubMed] [Google Scholar]
- 49.Gundermann G., Hoffmann H., Gutenbrunner C. Drinking- but what?- Medicinal waters in urinary stone metaphylaxis. Ernährung & Medizin . 2004;19(4):178–183. doi: 10.1055/s-2004-837280. [DOI] [Google Scholar]
- 50.Penczynski K. J., Remer T., Menzel J., Abraham K., Weikert C. Urinary potential renal acid load (uPRAL) among vegans versus omnivores and its association with bone health in the cross-sectional risks and benefits of a vegan diet study. Nutrients . 2022;14(21):p. 4468. doi: 10.3390/nu14214468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siener R. Can the manipulation of urinary pH by beverages assist with the prevention of stone recurrence? Urolithiasis . 2016;44(1):51–56. doi: 10.1007/s00240-015-0844-7. [DOI] [PubMed] [Google Scholar]
- 52.Derakhshandeh-Rishehri S. M., Franco L. P., Hua Y., et al. Higher renal net acid excretion, but not higher phosphate excretion, during childhood and adolescence associates with the circulating renal tubular injury marker interleukin-18 in adulthood. International Journal of Molecular Sciences . 2024;25(3):p. 1408. doi: 10.3390/ijms25031408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siener R. Nutrition and kidney stone disease. Nutrients . 2021;13(6):p. 1917. doi: 10.3390/nu13061917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carnauba R., Baptistella A., Paschoal V., Hübscher G. Diet-Induced low-grade metabolic acidosis and clinical outcomes: a review. Nutrients . 2017;9(6):p. 538. doi: 10.3390/nu9060538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burckhardt P. The effect of the alkali load of mineral water on bone metabolism: interventional studies. The Journal of Nutrition . 2008;138(2):435S–437S. doi: 10.1093/jn/138.2.435s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In accordance with established guidelines for reporting clinical trials, the CONSORT (Consolidated Standards of Reporting Trials) checklist was utilized to ensure transparency and completeness in the presentation of trial methodology and results. To check this list, please see Supplementary S1.
Data Availability Statement
The data used to support the findings of this study are made available from the corresponding author upon reasonable request.


