Abstract
Objective
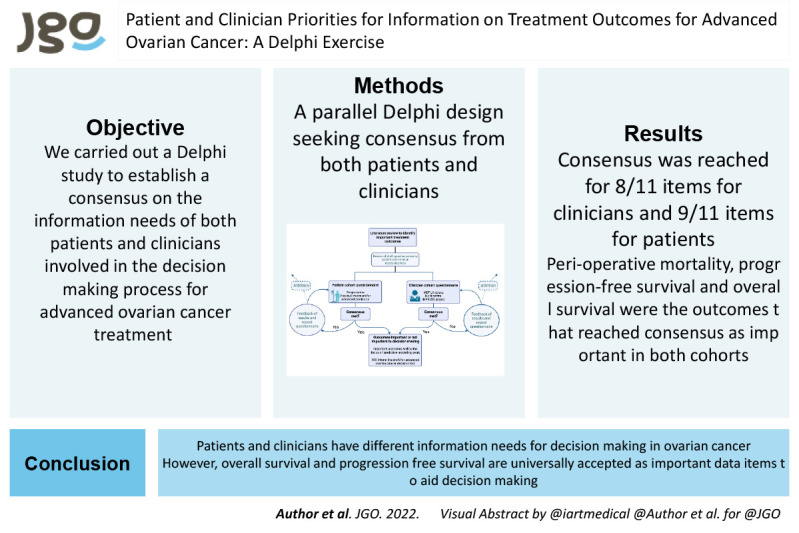
Patients with advanced ovarian cancer face a range of treatment options, and there is unwarranted variation in treatment decision-making between UK providers. Decision support tools that produce data on treatment outcomes as a function of individual patient characteristics, would help both patients and clinicians to make informed, preference- and values-based choices. However, data on treatment outcomes to include in such tools are lacking.
Methods
Following a literature review, a questionnaire was designed for use in a Delphi process to establish which treatment outcomes are important to both patients and clinicians in decision-making for treatment for advanced ovarian cancer. Patient and clinician panels were established.
Results
Following 2 Delphi rounds, consensus was achieved for 7/11 items in the patient panel and 8/11 items in the clinician panel. Consensus across both panels was achieved for inclusion of both overall survival and progression free survival as important items in the decision-making process, although there remained differences of opinion as to whether these should be presented as relative or absolute values.
Conclusion
Information needs for treatment decision-making in ovarian cancer differ between and within patient and clinician groups. Whilst overall survival and progression free survival are universally accepted as important data items, decision support tools will need to be nuanced to allow presentation of a range of outcomes and associated probabilities, and in a range of formats, that can be tailored to the preferences of clinician and patients.
Keywords: Ovarian Cancer, Shared Decision Making, Patient Preference
Synopsis
Patients and clinicians have different information needs for decision-making in ovarian cancer. Clinicians require more information items than patients. Overall survival, progression free survival, and perioperative mortality are items important to both groups.
Graphical Abstract
INTRODUCTION
Ovarian cancer remains one of the most lethal gynecological cancers [1]. Advances in maintenance therapies, and improvements in the management of relapsed disease, have improved outcomes; nevertheless, the primary management of advanced ovarian cancer remains the largest determinant of overall outcome [2].
The primary treatment for advanced disease is a combination of surgery and chemotherapy. Two large randomized controlled trials have shown that neo-adjuvant chemotherapy followed by interval debulking surgery is non-inferior to the traditional treatment of primary surgery followed by chemotherapy [3,4]. It is widely accepted however, that the 2 treatment arms differ in their adverse effects/risks, benefits and consequences in both the short- and long-term [5]. Furthermore, large population level audits have shown that many patients are not offered surgery and up to 28 percent of patients do not receive any anti-cancer therapy at all [6]. An audit of UK practice shows that there is a significant unwarranted variation between providers with respect to the likelihood of offering treatment and the rates of treatment for the range of available options for treatment [6]. This is of concern when considering the importance of optimal primary treatment on outcomes.
To improve quality of care (patient experience, safety and effectiveness) and reduce unwarranted variation in treatment, clinicians would benefit from decision support (based on individual differential effectiveness of available treatments for individual patients) [7]. In turn, clinicians can use the outputs of this personalized decision support (PDS) to communicate personalized data (based on patient preferences for information) on different balances of benefits and risks of treatments to a patient in a shared decision-making (SDM) discussion, which would to enable both clinicians and patients to make an informed, evidence-based decisions to determine an optimal, treatment plan (informed by the preferences and values of patients). Decision support for clinicians can range from aggregate level of event rates reported in randomized controlled trials or registries and conventional nomograms, through to artificial-intelligence-driven algorithms, which can generate individual predictions for outcomes as a function of individual patient characteristics. SDM is an established process comprising 3 primary elements: recognizing and acknowledging that a decision is required; knowing and understanding the best available evidence; and incorporating the patient’s values and preferences into the decision [8].
Both PDS and SDM require knowledge of the preferences and values, from the perspective of clinicians and patients, on treatment outcomes that are important for making decisions about the available options for treatment. This is described as the first design step of the International Patient Decision Aids Standards Process [9].
Previous qualitative work has identified information preferences of ovarian cancer patients [10,11,12], but there have been no attempts to compare these with clinician preferences for information needs around treatment decision-making.
We therefore carried out a Delphi study to establish a consensus on the information needs of both patients and clinicians involved in the decision-making process for advanced ovarian cancer treatment. Such information will be critical to the development of PDS and SDM in this decision-making context.
Delphi methodology is a well-established tool that seeks to gain consensus between participants. Initially developed in industry, it has been adapted for use in healthcare, either seeking consensus from professionals in the process of guideline development, or gaining consensus from patients when formulating patient report outcome measures [13]. However, it has rarely been used to gain consensus on an issue from the perspective of both clinicians and patients.
We used a modified Delphi methodology which comprises an initial literature review, followed by iterative rounds of questionnaires, interspersed with feedback on results to participants, until consensus was reached.
MATERIALS AND METHODS
1. Questionnaire design
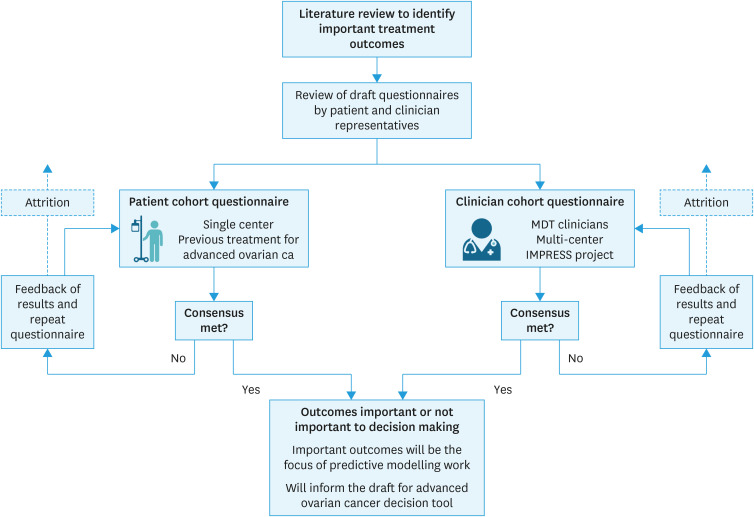
An overview of the methodology for this study is shown in Fig. 1. Following accepted modified Delphi methodology, a rapid literature review was conducted to identify the treatment outcomes for use in the Delphi exercise (the propositions). The search strategy was focused on “patient preferences in ovarian cancer,” and expanded to include preferences for recurrent cancer treatment. The search terms “priorities,” “communication,” “shared decision” and “ovarian cancer” were used, with citation review of pertinent papers. All outcomes relevant to patient treatment decision-making or preferences for treatment were extracted.
Fig. 1. A graphical representation of the modified Delphi method methodology used.
The list of outcomes extracted from the literature was made available to an expert committee to inform the design of the questionnaire for use in the Delphi process. For each treatment outcome item in the questionnaire, respondents were asked to rate them on a 4-point rating scale ranging from: ‘it would not affect my decision’; ‘it would affect my decision a little’; ‘it would affect my decision quite a lot’; and ‘it would very much affect my decision.’ A copy of the questionnaire is available in (Data S1).
Opportunities for panellist to provide free text responses were provided in order to inform subsequent Delphi rounds.
The questionnaire was reviewed by an independent clinician, a specialist nurse and a patient representative to maximise readability, clarity, and as a sense check. The same questions were used for the clinician questionnaire, but the wording for the clinician and patient versions of the questionnaire was amended to maximise comprehensibility and engagement in the study. The decision to design the questionnaire principally for the patient panel and modify for a clinical audience was intentional; patients are the central focus of the SDM process, and their informational requirements are of primary concern in the design of decision support and SDM.
2. Panel generation
The patient panel was identified from a comprehensive database of patients undergoing treatment for advanced ovarian cancer at one center in the UK. Eligible patients had undergone primary treatment between 1/1/2018 and 31/12/2020 (ensuring that their first treatment cycle was complete), aged 18+ years, had previously received surgery for a diagnosis of high grade epithelial cancer; and capability to read English. Patients being treated on an “end of life care pathway” were excluded.
Invitation letters and a project information leaflet were sent to patients. This was followed by a telephone call. Those that consented to participate completed a paper-based questionnaire or an internet-based form via an email link. One follow up telephone call was made to non-responding panellists to increase response rate.
The clinician panel was generated from staff involved in an ongoing, multi-center project involving 6 gynecology cancer centers around England (IMPRESS project). The project leads for each center were asked to disseminate an electronic questionnaire link to the following clinicians: gynecology oncology surgeons; non surgical oncologists that care for ovarian cancer patients; and gynecology cancer specialist nurses. E-mail reminders were sent to maximise response rate.
An arbitrary target of 25 responses per panel was set, thus ensuring that sufficient responses were received to deliver the breadth of opinion required but still have a likelihood of reaching consensus [14]. A period of 4 weeks was allocated for panellists to respond to each round.
3. Delphi process
The final questionnaire was disseminated to both panels as above.
The results for round 1 were analyzed as binary responses. The responses ‘it would not affect my decision’ and ‘it would affect my decision a little’ were classified as not being an important outcome; whereas, responses ‘it would affect my decision quite a lot’ and ‘it would very much affect my decision’ were classified as an important outcome. The results were assessed for consensus across all panellists, and then for consensus with-in each panel. Consensus was predefined as 70% agreement on classification of important outcomes in line with published guidance [15].
Once results were collated, the expert panel was reconvened to plan the second Delphi round. All outcomes for which consensus were reached were excluded from round 2. Some of the questions and response options were altered based on panellist feedback in round 1. An additional question was added to investigate whether any items should be positively rejected, that is, a patient would definitely not want to receive information about a specific treatment outcome.
The round 2 questionnaire was disseminated to panellists who took part in round 1, aiming to achieve a minimum of 67% response rate to maintain integrity of the results [14].
The results from the second round were analyzed using the same methodology described for round 1.
Advice was sought from the local Research and Innovation team. As this work was deemed to be patient and participant involvement and engagement, formal ethical approval was not required according to UK Health Research Authority algorithm.
RESULTS
1. Literature review
A literature search for studies reporting on patient preferences in ovarian cancer treatment identified 6 relevant papers; 3 studies explored patient preferences in the primary treatment setting [11,16,17], whilst 3 explored patient priorities when managing recurrent disease [10,12,18]. These studies identified 6 treatment outcomes of importance to patients. Following input from a multidisciplinary review team and patient advisor, 4 more outcomes were added to this list. These 11 outcomes (Table 1) were then incorporated into the questionnaire design.
Table 1. The outcomes identified from a literature review for inclusion in the Delphi questionnaires.
| Outcome |
|---|
| Extent of surgery |
| The likelihood of stoma formation during surgery |
| The likelihood of surgery successfully removing all of the cancer |
| The likelihood of being readmitted to hospital in the 2 wk following surgery |
| The likelihood of experiencing complications as a result surgery |
| The likelihood of death as a result of surgery |
| The expected stay in hospital after surgery |
| The likelihood of being admitted to intensive care/high dependency unit |
| The likelihood of needing to be discharged to somewhere other than home (for example intermediate care or residential care, either as a temporary or permanent measure) |
| The length of time following treatment with-out the cancer coming back or growing |
| Life-expectancy following treatment |
2. Delphi panellists
One hundred three patients were assessed for inclusion and 51 met the eligibility criteria. Of these, 42 were contactable and 29 agreed to participate as a member of the patient panel. For the clinical panel, 21 responses were received. Table 2 shows the demographics of both panels.
Table 2. The participants for the first round of Delphi questionnaires.
| Variables | Values | ||
|---|---|---|---|
| Patient cohort (n=22) | |||
| Age (yr) | 68.5 (41–84) | ||
| Histology | 1/22 (4.5) | ||
| Clear cell | 21/22 (95.5) | ||
| High grade serous | |||
| FIGO stage at diagnosis | |||
| III | 15 (68.1) | ||
| IV | 7 (31.8) | ||
| Surgery | |||
| Primary surgery | 10 (45.5) | ||
| Interval debulking | 12 (54.5) | ||
| Time since diagnosis (mo) | 19.5 (13.9–25.2) | ||
| Clinician cohort (n=21) | |||
| Role | |||
| Gynecological oncological surgeon | 13 (61.9) | ||
| Medical oncologist | 5 (23.8) | ||
| Gynecology specialist trainee | 1 (4.7) | ||
| Cancer specialist nurse | 2 (9.5) | ||
Values are presented as median (range) or number (%).
FIGO, International Federation of Gynecology and Obstetrics.
3. Analysis of round 1 results and design of round 2 questionnaire
Table 3 shows the response rates for each panel in each round of the questionnaire. In the first round, 22/29 (76%) of the patient panel responded to the questionnaire.
Table 3. Summary of the items in each round of questionnaires, the number of people invited and the response rate.
| Variables | Round 1 | Round 2 | ||
|---|---|---|---|---|
| Patient cohort | Clinician cohort | Patient cohort | Clinician cohort | |
| Items in the questionnaire | 11 | 11 | 7 | 4 |
| No. of people invited to partake | 29 | NA | 22 | 21 |
| Responses received | 22 | 21 | 16 | 14 |
| Response rate (%) | 76 | NA | 73 | 67 |
NA, not available.
Amongst the 22 patients who responded in round 1, consensus was achieved for 4/11 (36%) items, including “readmission rate,” “perioperative mortality,” “length of hospital stay” and “likelihood of intensive treatment unit admission.” For the 21 members of the clinician cohort who responded, consensus was achieved for 8/11 (73%) of the items (Table 4). Overall, consensus was achieved between both panels for only one item, namely perioperative mortality.
Table 4. A summary out the results for rounds 1 and 2 of the Delphi questionnaires.
| Outcome of interest | Cohort | Round 1 | Round 2 | Overall result | ||
|---|---|---|---|---|---|---|
| Proportion that felt this outcome was important in decision making | Consensus met? | Proportion that felt this outcome was important in decision making | Consensus met? | |||
| Extent of surgery | Patient | 7/22 (31.8%) | No | 4/16 (25%) | Yes; unimportant outcome | Consensus in both cohorts; discordant views |
| Clinician | 19/21 (90.5%) | Yes; important outcome | ||||
| The likelihood of stoma formation during surgery | Patient | 10/22 (45.5%) | No | 4/16 (25%) | Yes; unimportant outcome | Consensus in both cohorts; discordant views |
| Clinician | 20/21 (95.2%) | Yes; important outcome | ||||
| The likelihood of surgery successfully removing all of the cancer | Patient | 10/22 (45.5%) | No | 7/16 (43.75%) | No | Consensus in clinicians (important) |
| Clinician | 21/21 (100%) | Yes; important outcome | No consensus in patients | |||
| The likelihood of being readmitted to hospital in the 2 weeks following surgery | Patient | 6/22 (27.3%) | Yes; unimportant outcome | Consensus in patients (unimportant) | ||
| Clinician | 13/21 (61.9%) | No | 7/14 (50%) | No | No consensus in clinicians | |
| The likelihood of experiencing complications as a result surgery | Patient | 9/22 (40.9%) | No | 4/16 (25%) | Yes; unimportant outcome | Consensus in patients (unimportant) |
| Clinician | 20/21 (95.2%) | Yes; important outcome | No consensus in clinicians | |||
| The likelihood of death as a result of surgery | Patient | 16/22 (72.7%) | Yes; important outcome | Consensus in both cohorts (important) | ||
| Clinician | 20/21 (95.2%) | Yes; important outcome | ||||
| The expected stay in hospital after surgery | Patient | 3/22 (13.6%) | Yes; unimportant outcome | Consensus in patients (unimportant) | ||
| Clinician | 9/21 (42.9%) | No | 5/14 (35.7%) | No | No consensus in clinicians | |
| The likelihood of being admitted to intensive care/high dependency unit | Patient | 6/22 (27.6%) | Yes; unimportant outcome | Consensus in patients (unimportant) | ||
| Clinician | 11/21 (52.4%) | No | 8/14 (57.1%) | No | No consensus in clinicians | |
| The likelihood of needing to be discharged to somewhere other than home | Patient | 12/22 (54.5%) | No | 8/16 (50%) | No | Consensus in clinicians (important) |
| Clinician | 19/21 (90.5%) | Yes; important outcome | No consensus in patients | |||
| The length of time following treatment with-out the cancer coming back or growing | Patient | 13/22 (59.1%) | No | Absolute PFS 8/15 (53.3%) | Consensus in clinicians | |
| Relative PFS 4/15 (26.7%) | Consensus in patients they would want to know this outcome | |||||
| Do not want to know 3 (20%) | ||||||
| Clinician | 21/21 (100%) | Yes; important outcome | ||||
| Life-expectancy following treatment | Patient | 13/22 (59.1%) | No | Absolute OS 10/15 (66.7%) | Consensus in clinicians | |
| Relative OS 2/15 (13.3%) | Consensus in patients they would want to know this outcome | |||||
| Do not want to know 3/15 (20%) | ||||||
| Clinician | 21/21 (100%) | Yes; important outcome | ||||
Analysis was conducted in each cohort of each round, with a predefined threshold for consensus of 70%.
OS, overall survival; PFS, progression-free survival.
Review of the free-text responses from the patient panel highlighted concerns about communicating survival outcomes: ‘I would both want to know and not want to know,’ ‘Communicating survival at this stage may not be useful.’ This prompted us to reconsider the phrasing of the survival and progression free survival for the patient questionnaire in round 2; we offered the options of ‘I would want to know absolute survival,’ ‘I would want to know the relative survival of the 2 options’ or ‘I would not want to know this outcome.’ A majority of the patient panellists stated they did want survival information. This suggests overall consensus for this item although there was no overall preference stated for presentations of these data using relative versus absolute terms.
The second Delphi round questionnaire only contained outcomes that did not meet consensus in the first round, and was therefore specifically tailored to each panel. The patient questionnaire in round 2 included feedback from round 1, and panellists were asked to rate the importance of each item. This is the traditional format. For the clinician questionnaire however, a decision was made to feed back the responses from the patient panel in round 1 for each treatment outcome, to inform the development of a patient decision aid.
4. Analysis of round 2 results
In the second round, 16/22 (73%) of the patient panel (Table 3). Consensus was achieved for 3/5 (60%) of the treatment outcomes items, including “extent of surgery,” “likelihood of stoma formation” and “likelihood of surgical complications” (Table 4). For the 14/21 (67%) members of the clinician panel who responded, no further consensus was achieved (Table 4). In contrast to the patient panel, we were unable to keep attrition in the clinician cohort to 30% or less despite several reminders.
As consensus in the patient panel had been reached for all but 2 outcomes, and there had been no increase in consensus from the second round for the clinician panel. Consequently, a third round was not required.
5. Overall interpretation of responses
Overall, the clinician panel reached consensus sooner (8/11 outcomes) in the first round than the patient cohort (4/11 outcomes). After 2 rounds however, there was no further increase in consensus in the clinician group but in the patient cohort, consensus was reached in 9 out of 11 outcomes.
Peri-operative mortality, progression-free survival and overall survival were the outcomes that reached consensus as important in both cohorts. The extent of surgery, the need for a stoma and the risk of surgical complications met consensus in both groups, but the clinician group agreed these should be important to decision-making whereas the patient group felt these were unimportant. Clinicians also felt that surgical cytoreduction and the risk of discharge to a place other than home were important, whereas these did not meet consensus in the patient group. Length of stay, high dependency unit admission and the chance of hospital readmission did not reach consensus in the clinician group but did reach consensus in the patient group and were agreed to be unimportant in decision-making. Although some items were deemed unimportant for the purposes of decision-making, no items were identified that patients would definitely want to be omitted in a decision-making tool.
DISCUSSION
This work provides valuable insight into what information on clinical outcomes for ovarian cancer that patients and clinicians consider to be important for decision-making about treatment. Although other recent studies have used the Delphi process to gain consensus for clinicians managing ovarian cancer [19], ours is the first study to combine opinions from patients and clinicians in the development of a decision-making tool for women with advanced ovarian cancer. The contrast in the results between the 2 panels was marked, which highlights the importance of involving both patients and clinicians in consensus exercises to inform the development of tools to support evidence-based, preference-based decision-making.
An interesting and unexpected finding is that clinicians appear to have greater decision support needs than patients. Whilst patients reported only 3 outcomes to be important, clinicians reported 8 to be important. The 3 outcomes prioritised by patients (survival, progression free survival and peri-operative mortality) were also important to clinicians. Three of the outcomes that clinicians considered important were considered to be unimportant by patients. These were the risk of stoma formation, the extent of the surgery and the risk of post operative complications. Future patient engagement work will explore whether these outcomes are useful to guide patient’s expectations of recovery, even if these do not affect their treatment decisions. These findings also highlight that outputs of decision support for use in SDM discussions with patients would benefit from being tailored to show outcomes that patients consider to be important. It will be interesting to see if these findings are validated when tested in other jurisdictions and health care settings.
How to communicate a patient’s predicted survival was a key consideration in the design of this study. Previous, heterogeneous, studies have shown that between 59%–91% of patients wish to be given prognostic information at diagnosis, but none of these studies included women with advanced ovarian cancer [20]. In this study we have shown that there is consensus that survival is an important item to be discussed although there remain differences of opinion as to whether this should be presented using relative or absolute figures. This is despite recommendations (based on a substantial body of research), indicating that presenting relative data should be avoided.
The clinician panel reached consensus that survival was important after one round, although some clinicians expressed the sentiment that it was not useful to discuss prognosis at this stage in treatment. This is in keeping with work that shows that although some patients will want to receive information on prognosis, they prefer their physician to start this conversation. Conversely clinicians often avoid giving this information due to uncertainty, preferring to wait for patients to ask [21]. To aid decision-making, information regarding predicted survival should be available for patients and clinicians to use, but how this is presented and utilised will need to be personalized in terms of patient preferences and values.
A strength of our study is the inclusion of both patients and clinicians. SDM involves at least 2 parties and it is thus imperative that both stakeholders receive information that is needed for them to arrive at the optimal decision. Although numbers were low, we included the full range of clinicians involved in decision-making, including specialist nurses. Family members are important, but were not able to include family members in this study.
In summary, we have shown that information needs on outcomes for treatment decision-making in ovarian cancer, differ between and within patient and clinician groups. Whilst overall survival and progression free survival are universally accepted as important, decision support tools, which will be essential to promote compliance with these findings, will need to be nuanced to allow presentation of a range of treatment outcomes and associated probabilities, and in a range of formats, which can be tailored to the needs and preferences of clinician and patients.
Footnotes
Conflict of Interest: Richard Edmondson has received honoraria from GSK. No other author declares a conflict of interest.
- Conceptualization: B.K., E.R.
- Data curation: B.K., A.H., M.J., H.C., E.R.
- Formal analysis: B.K., E.R.
- Investigation: M.J., H.C.
- Methodology: B.K., A.H., H.C., F.D.
- Project administration: E.R.
- Supervision: F.D., E.R.
- Writing - original draft: B.K., E.R.
- Writing - review & editing: B.K., A.H., M.J., H.C., F.D.
SUPPLEMENTARY MATERIAL
Survey
References
- 1.Cancer Research UK. Ovarian cancer statistics. London: Cancer Research UK; 2021. [Google Scholar]
- 2.Lheureux S, Gourley C, Vergote I, Oza AM. Epithelial ovarian cancer. Lancet. 2019;393:1240–1253. doi: 10.1016/S0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 3.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 4.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 5.Coleridge SL, Bryant A, Kehoe S, Morrison J. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst Rev. 2021;2:CD005343. doi: 10.1002/14651858.CD005343.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.British Gynaecological Cancer Society. Ovarian cancer audit feasibility pilot. London: British Gynaecological Cancer Society; 2020. [Google Scholar]
- 7.Morrison J. Neoadjuvant chemotherapy for ovarian cancer: avoiding ‘needless hurt’? BJOG. 2023;130:1589–1590. doi: 10.1111/1471-0528.17627. [DOI] [PubMed] [Google Scholar]
- 8.Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27:1361–1367. doi: 10.1007/s11606-012-2077-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S2. doi: 10.1186/1472-6947-13-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, Berchuck A, Valea FA, Lee PS, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120:3651–3659. doi: 10.1002/cncr.28940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havrilesky LJ, Yang JC, Lee PS, Secord AA, Ehrisman JA, Davidson B, et al. Patient preferences for attributes of primary surgical debulking versus neoadjuvant chemotherapy for treatment of newly diagnosed ovarian cancer. Cancer. 2019;125:4399–4406. doi: 10.1002/cncr.32447. [DOI] [PubMed] [Google Scholar]
- 12.Donovan KA, Greene PG, Shuster JL, Jr, Partridge EE, Tucker DC. Treatment preferences in recurrent ovarian cancer. Gynecol Oncol. 2002;86:200–211. doi: 10.1006/gyno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 13.Shearsmith L, Kennedy F, Lindner OC, Velikova G. Delphi survey to inform patient-reported symptom monitoring after ovarian cancer treatment. J Patient Rep Outcomes. 2020;4:71. doi: 10.1186/s41687-020-00237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keeney S, Hasson F, McKenna H. The Delphi technique in nursing and health research. Hoboken, NY: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 15.Humphrey-Murto S, Varpio L, Gonsalves C, Wood TJ. Using consensus group methods such as Delphi and Nominal Group in medical education research. Med Teach. 2017;39:14–19. doi: 10.1080/0142159X.2017.1245856. [DOI] [PubMed] [Google Scholar]
- 16.Stewart DE, Wong F, Cheung AM, Dancey J, Meana M, Cameron JI, et al. Information needs and decisional preferences among women with ovarian cancer. Gynecol Oncol. 2000;77:357–361. doi: 10.1006/gyno.2000.5799. [DOI] [PubMed] [Google Scholar]
- 17.den Ouden JE. Development of a decision aid for primary treatment of patients with advanced-stage ovarian cancer. Int J Gynecol Cancer. 2020;30:837–844. doi: 10.1136/ijgc-2019-001095. [DOI] [PubMed] [Google Scholar]
- 18.PEBC’s Ovarian Oncology Guidelines Group. A systematic review of patient values, preferences and expectations for the treatment of recurrent ovarian cancer. Gynecol Oncol. 2017;146:392–398. doi: 10.1016/j.ygyno.2017.05.039. [DOI] [PubMed] [Google Scholar]
- 19.Redondo A, Barretina P, Pérez-Fidalgo A, Rubio MJ, González-Martín A. Controversies in the treatment of advanced ovarian cancer in the PARP inhibitors era: a Delphi consensus. J Gynecol Oncol. 2023;34:e57. doi: 10.3802/jgo.2023.34.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagerty RG, Butow PN, Ellis PA, Lobb EA, Pendlebury S, Leighl N, et al. Cancer patient preferences for communication of prognosis in the metastatic setting. J Clin Oncol. 2004;22:1721–1730. doi: 10.1200/JCO.2004.04.095. [DOI] [PubMed] [Google Scholar]
- 21.Step MM, Ray EB. Patient perceptions of oncologist-patient communication about prognosis: changes from initial diagnosis to cancer recurrence. Health Commun. 2011;26:48–58. doi: 10.1080/10410236.2011.527621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Survey