Abstract
Objective
To compare the efficacy of the levonorgestrel intrauterine system (LNG-IUS) versus megestrol acetate (MA) in inducing complete regression among women with atypical endometrial hyperplasia (AEH) who declined hysterectomy.
Methods
In this single-center, open-label randomized controlled trial, we included 148 women with AEH who declined hysterectomy. We randomized participants to receive either daily oral MA 160 mg (n=74) or apply LNG-IUS (n=74) and scheduled their follow-up by endometrial sampling at 3, 6, 9, 12, 18, and 24 months. The success rate and duration until complete regression were the primary outcomes.
Results
The mean duration until complete regression was 5.52 months (95% confidence interval [CI]=4.85–6.18) for the LNG-IUS group versus 6.87 months (95% CI=6.09–7.64) for the megestrol group (log-rank test p-value=0.011). The cumulative regression rate after 12 months was 91.9% with the LNG-IUS versus 77% with MA (p=0.026). Weight gain in the MA group vs LNG-IUS group after one year (4.7±4 kg vs. 2.7±2.6 kg, 95% CI=0.89–3.12; p=0.001) and after two years of therapy (7.8±5.1 kg vs. 4.1±2.9 kg, 95% CI=2.29–5.06; p<0.001).
Conclusion
Compared to MA, the LNG-IUS was more efficacious in treating AEH in women who declined hysterectomy, especially those with moderate/severe obesity, with fewer adverse effects and less weight gain. Extending therapy to 12 months for persistent cases would improve regression rates with reasonable safety. Alternate hysteroscopic and office sampling seemed convenient for follow-up.
Trial Registration
ClinicalTrials.gov Identifier: NCT04385667
Keywords: Intrauterine Devices, Progesterone-Releasing; Megestrol Acetate; Atypical Endometrial Hyperplasia; Uterine Bleeding
Synopsis
The levonorgestrel intrauterine system was more effective than megestrol acetate in treating atypical endometrial hyperplasia in women who declined hysterectomy, especially those with moderate/severe obesity, with fewer adverse effects. Prolonging the therapy to 12 months for persistent cases seemed safe and improved the complete regression rate.
INTRODUCTION
Endometrial hyperplasia is an uneven proliferation of the endometrium with an increased gland-to-stroma ratio compared to the normal proliferative endometrium [1]. It is a precursor for endometrial carcinoma, especially when atypia is present. Simple or complex atypical endometrial hyperplasia (AEH) is associated with a higher incidence of malignant progression in 15%–75% [2] or concurrent malignancy in approximately one-third of cases [3].
Hysterectomy is the recommended treatment for AEH, especially for postmenopausal women. Although this approach saves women from the risk of disease progression and hidden concurrent malignancy, it would be unsuitable for premenopausal women seeking fertility preservation. This group of women who wish to have children is steadily expanding because of the current tendency to delay first conception [4]. These women retaining their uteri for future fertility would try child-bearing at least once before opting for a hysterectomy. In addition, intraoperative and postoperative risks are predicted in such a population with a higher prevalence of diabetes mellitus, obesity, and metabolic syndrome [5,6].
In recent years, conservative strategies have indicated considerable feasibility and safety as fertility-sparing options [7]. Progestagen therapy is one option used to treat selected women with endometrial cancer and AEH who desire to preserve fertility or have severe medical comorbidities, precluding immediate surgery. The megestrol acetate (MA) and levonorgestrel intrauterine system (LNG-IUS) are the most commonly used progestagen regimens [8,9,10].
LNG-IUS would provide better compliance to therapy as a single application and is predicted to be associated with fewer adverse effects due to lower serum progestagen levels. However, there is limited clinical evidence to prove its efficacy and safety as a conservative treatment for AEH [11].
The study’s main goal was to compare the efficacy of the LNG-IUS and MA regarding the ability and duration to produce complete regression for cases with AEH. Secondary goals included a comparison of the incidence of failure rate, recurrence rate, risk of excessive weight gain, and thromboembolic and metabolic complications.
MATERIALS AND METHODS
1. Study setting
Our study was an open-label superior randomized controlled trial primarily comparing LNG-IUS to MA regarding their efficacy in achieving complete regression of AEH. Before the start of recruitment, we obtained institutional approval from the Institutional Review Board of the Faculty of Medicine, Zagazig University, Zagazig, Egypt (registration number: ZU-IRB# 4119/13-06-2018, renewed 13-06-2020). We registered the trial at ClinicalTrials.gov with registration number NCT04385667 (09/05/2020). The study setting was the Obstetrics & Gynecology Department of Zagazig University Hospitals, Zagazig, Egypt. It is the leading health facility that serves Egypt’s third most populous governorate (Sharqia Governorate, 8 million population). The Obstetrics & Gynecology Department has a subspecialized gynecologic oncology unit and receives referrals from different facilities in the governorate and the neighboring governorates.
We recruited women with a tissue diagnosis of simple or complex hyperplasia with atypia and declining hysterectomy as the standard disease management and requested conservative management either for fertility preservation or to avoid surgical intervention starting May 20, 2020. All women received detailed counseling that the ideal treatment for their cases was hysterectomy and that we cannot exclude the possibility of progression to or coexistence of endometrial cancer. We also counseled them regarding the beneficial value, disadvantages, and risks of both LNG-IUS and MA. Then, we obtained written informed consent for the refusal of hysterectomy and their acceptance to participate in the study.
The inclusion criteria were women with a confirmed tissue diagnosis of AEH who were willing to preserve their uterus and accept both methods of planned hormonal therapy. The exclusion criteria were patients with evidence of concurrent endometrial cancer and simple or complex hyperplasia without atypia.
2. Sample size calculation
We calculated the sample size using G power software version 3.10 (Heinrich-Heine-Universität Düsseldorf Impressum Kontakt Druckversion) and an online sample size calculator (https://clincalc.com/stats/samplesize.aspx). Gallos et al. [12] reported an atypical hyperplasia regression rate of 90% with LNG-IUS compared to 69% with oral progestagen therapy. We estimated the least required sample size to detect this difference: 114–128 women divided into both groups, with an alpha error of 0.05, a power of 80%, and a one-to-one allocation ratio. The authors collected 148 cases to afford the possibility of a 10% dropout of participants.
We produced the simple random sequence using the random sequence generator method on the website https://www.random.org/sequences, with a one-to-one allocation ratio into two separate columns for either group. The randomization/allocation process was concealed using sequentially sealed opaque envelopes. We labeled each envelope with a serial number. Inside, it had a card mentioning the type of intervention. Once we obtained allocation, we did not change it. We documented the patient’s identification on the intervention card for follow-up.
Women in the LNG-IUS arm had an IUS releasing 20 µg of levonorgestrel daily (Mirena®; Bayer Schering Pharma AG, Berlin, Germany). The MA arm received oral MA (Bausch & Lomb, Kingston, UK) 160 mg daily in divided doses.
We scheduled endometrial sampling for all study patients 3, 6, 9, 12, 18, and 24 months after the start of allocated therapy to evaluate the response [1]. Samples taken at 6, 12, and 24 months of therapy were under hysteroscopic guidance. Multiple specimens were taken from different aspects of the uterine cavity, and the LNG-IUS was left in place for women in this arm. We took the remaining scheduled samples (at 3, 9, and 18 months) using a Pipelle endometrial sampler. With negative pressure and opening against the endometrium, we moved the Pipelle multiple times circularly. While maintaining negative pressure, we removed the Pipelle from the uterine cavity. We repeated this process more than once to ensure a sufficient specimen.
An expert pathologist examined all slides to monitor the response to therapy over surveillance. We defined the response with every examination as complete regression (complete absence of atypia and hyperplasia), partial regression, persistence (no evidence of regression or progression), progression to endometrial cancer, and recurrence (recurrence of AEH after complete regression). In addition, serial transvaginal ultrasound follow-up was performed at every visit to evaluate the endometrial thickness as part of the follow-up.
We collected participants’ demographic data, including age, parity, history of infertility, pretherapy weight and body mass index, menopausal state, and symptomatic bleeding. We also collected the primary and secondary study outcomes, including regression rate, duration until regression, persistence rate, recurrence rate, time until recurrence, and serial endometrial thickness. Finally, we searched for the side effects of therapy, including one- and two-year posttherapy body weight and body mass index (BMI) changes, thromboembolism, breakthrough bleeding, uncontrolled diabetes, and hypertension.
3. Statistical methods
Statistical analysis was performed using IBM SPSS version 25 (IBM Corp., Armonk, NY, USA). We adopted an intention-to-treat analysis of the data of all study participants. Kolmogorov–Smirnov and Shapiro–Wilk tests were used to test the normality of the distribution of the numerical data. Normally distributed data are presented as the mean ± standard deviation, and between-group differences were calculated using independent samples t tests. Skewed data are presented as the median and interquartile range, and between-group differences were calculated nonparametrically using the Mann–Whitney U test. Categorical data are presented as numbers and percentages (%), and between-group differences were calculated using the χ2 test or Fisher’s exact test. A Kaplan–Meier plot was used to detect the survival difference between both groups. A p-value below 0.05 was considered significant. We performed χ2 post hoc testing after Bonferroni adjustment to estimate the significance of the difference between the glycemic and blood pressure control levels for both groups [13].
RESULTS
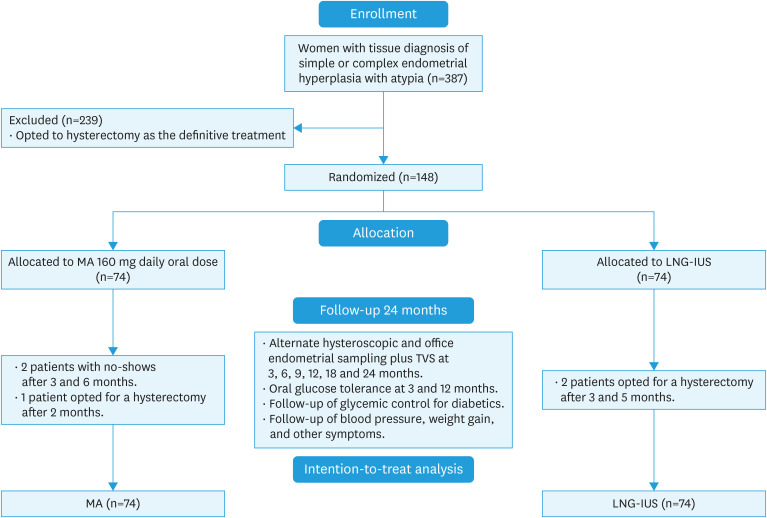
We recruited 148 women with AEH equally distributed to both arms of the study. Seventy-four patients received MA therapy, while the remaining seventy-four had LNG-IUS application. After the start of the study, 5 participants dropped out of the follow-up. Two women had no show in the megestrol arm after three and 6 months of follow-up, and one opted for hysterectomy after two months. At the same time, two participants in the LNG-IUS arm changed their minds and opted for a hysterectomy three and 5 months after the start of therapy. The remaining participants completed their 24 months of regular follow-up (Fig. 1).
Fig. 1. CONSORT flow diagram for trial recruitment.
LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate; TVS, transvaginal ultrasound.
Both groups were comparable regarding their demographic data, including age, weight, BMI, parity, menstrual status, and comorbidities in the form of diabetes mellitus and hypertension. Most of the study population was premenopausal and was comparably distributed to both groups. Similarly, the study population tended to have moderate obesity (BMI, 34.1±4.3 kg/m2) (Table 1).
Table 1. Patient demographic data.
| Characteristics | MA group (n=74) | LNG-IUS group (n=74) | p-value | |
|---|---|---|---|---|
| Age (yr) | 46.1±4.3 | 46.1±4.1 | 0.984 | |
| Age group | 0.675 | |||
| ≥40 and <45 years | 33 (44.6) | 32 (43.2) | ||
| ≥45 and <50 years | 31 (41.9) | 35 (47.3) | ||
| ≥50 years | 10 (13.5) | 7 (9.5) | ||
| Weight (kg) | 91.8±7.9 | 91.1±5.6 | 0.529 | |
| BMI (kg/m2) | 34.3 (26.3–46.1) | 33.9 (28–46.5) | 0.570 | |
| Degree of obesity | 0.258 | |||
| Overweight (BMI ≥18 and <25) | 15 (20.3) | 12 (16.2) | ||
| Grade I obesity (BMI ≥25 and <30) | 30 (40.5) | 42 (56.8) | ||
| Grade II obesity (BMI ≥30 and <35) | 18 (24.3) | 13 (17.6) | ||
| Grade III obesity (BMI ≥35) | 11 (14.9) | 7 (9.5) | ||
| Parity | 2 (0–6) | 3 (0–7) | 0.106 | |
| Abnormal uterine bleeding | 60 (81.1) | 58 (78.4) | 0.838 | |
| Infertility | 14 (18.9) | 12 (16.2) | 0.666 | |
| Menstrual status | 0.848 | |||
| Premenopause | 57 (77.0) | 55 (74.3) | ||
| Postmenopause | 17 (23.0) | 19 (25.7) | ||
| Diabetes mellitus | 18 (24.3) | 20 (27.0) | 0.851 | |
| Hypertension | 8 (10.8) | 9 (12.2) | 0.797 | |
| Pretreatment endometrial thickness | 14.4±3.1 | 13.2±2.3 | 0.013* | |
Data are presented as number (percentage), the mean ± standard deviation, and median (interquartile range) as appropriate.
BMI, body mass index; LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate.
*Statistically significant.
The mean duration until achieving complete regression was 5.52 months (95% confidence interval [CI]=4.85–6.18) for the LNG-IUS group versus 6.87 months (95% CI=6.09–7.64) for the megestrol group (log-rank test p-value=0.011) (Table 2), with a higher rate in the LNG-IUS group than in the megestrol group after six months (73% vs. 52.7%, p=0.036). After nine months of therapy, the response rates were 85.1% and 68.9%, respectively (p=0.055). The best outcome was achieved after the completion of twelve months of therapy, with a significantly higher complete regression rate in the LNG-IUS group than in the megestrol group (91.9% vs. 77%, p=0.026). A single patient (on the LNG-IUS arm) progressed to have endometrioid adenocarcinoma at 6 months sampling and opted for a hysterectomy (Table 3). Patients with persistent AEH who completed 12 months of therapy were counseled regarding the failure of treatment and had a hysterectomy (4 vs. 14), with a total hysterectomy rate for the study population of 6 versus 15 (p=0.033) (Table 4). After achieving complete regression, none of the patients developed recurrence of hyperplasia until 24 months of therapy during follow-up.
Table 2. Kaplan-Meier survival curve comparing time to regression of atypical endometrial hyperplasia in response to both methods of progestational therapy.
| Progestin type | Means for regression time | Log rank (Mantel-Cox) p-value | |||
|---|---|---|---|---|---|
| Estimate (mo) | SE | 95% CI | |||
| Lower bound | Upper bound | ||||
| MA | 6.865 | 0.395 | 6.090 | 7.639 | 0.011* |
| LNG-IUS | 5.519 | 0.339 | 4.854 | 6.184 | |
| Overall | 6.176 | 0.264 | 5.658 | 6.694 | |
LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate; SE, standard error.
*Statistically significant.
Table 3. Histopathological and ultrasound response to progestational therapy.
| Timing and sampling method | Cumulative histopathological response during the follow-up sampling | p-value | Follow-up endometrial thickness (mm) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Complete regression | Persistence | Progression | ||||||||
| Megestrol | LNG-IUS | Megestrol | LNG-IUS | Megestrol | LNG-IUS | Megestrol | LNG-IUS | |||
| 3 mo (Pipelle) | 17 (23) | 32 (43.2) | 56 (75.7) | 41 (55.4) | 0 (0.0) | 0 (0.0) | 0.032* | 11 (8–16) | 10 (6–15) | 0.003* |
| (Dropped† 1+1) | ||||||||||
| 6 mo (Hysteroscopic guided) | 39 (52.7) | 54 (73.0) | 33 (44.6) | 17 (23.0) | 0 (0.0) | 1 (1.4) | 0.036* | 8 (5–15) | 6 (4–10) | <0.001* |
| (Dropped† 2+2) | ||||||||||
| 9 mo (Pipelle) | 51 (68.9) | 63 (85.1) | 20 (27) | 8 (10.8) | 0 (0.0) | 1 (1.4) | 0.055 | 8 (5–13) | 6.5 (4–9) | <0.001* |
| (Dropped† 3+2) | ||||||||||
| 12 mo (Hysteroscopic guided) | 57 (77) | 68 (91.9) | 14 (18.9) | 3 (4.1) | 0 (0.0) | 1 (1.4) | 0.026* | 7 (4–12) | 5 (4–8) | <0.001* |
| (Dropped† 3+2) | ||||||||||
| 18 mo (Pipelle) | 57 (77) | 68 (91.9) | 14 (18.9) | 3 (4.1) | 0 (0.0) | 1 (1.4) | 0.026* | 6 (4–9) | 5 (4–8) | <0.001* |
| (Dropped† 3+2) | ||||||||||
| 24 mo (Hysteroscopic guided) | 57 (77) | 68 (91.9) | 14 (18.9) | 3 (4.1) | 0 (0.0) | 1 (1.4) | 0.026* | 5 (4–8) | 5 (4–8) | <0.001* |
| (Dropped† 3+2) | ||||||||||
Data are presented as numbers (percentage).
LNG-IUS, levonorgestrel intrauterine system.
*Statistically significant.
†Dropped: The number of patients dropped out of follow-up (Megestrol + LNG-IUS, respectively) due to opting for a hysterectomy or a no-show.
Table 4. Complications and morbidities associated with progestational therapy.
| Variables | MA group (n=74) | LNG-IUS group (n=74) | p-value | ||
|---|---|---|---|---|---|
| Hysterectomy (due to persistence/progression) | 15 (20.3) | 6 (8.1) | 0.033* | ||
| Post-hysterectomy histopathological result | |||||
| Normal | 1 | 0 | |||
| Atypical hyperplasia | 15 | 5 | |||
| Endometrial carcinoma | 0 | 1 | |||
| Weight after 12 months | 96.9±8.3 | 93.8±5.5 | 0.009* | ||
| Weight after 24 months | 100±8.7 | 95.2±5.1 | <0.001* | ||
| Weight gain after 12 months | 4.7±4 | 2.7±2.6 | 0.001* | ||
| Weight gain after 24 months | 7.8±5.1 | 4.1±2.9 | <0.001* | ||
| Glycemic control at 3 months of therapy | 0.003* | Post-hoc | |||
| Normal | 43 (58.1) | 53 (71.6) | |||
| Controlled pretherapy diabetes | 6 (8.1) | 15 (20.3) | 0.15 | ||
| Poorly controlled pretherapy diabetes | 8 (10.8) | 2 (2.7) | 0.32 | ||
| New onset glucose intolerance | 11 (14.9) | 2 (2.7) | 0.03* | ||
| New-onset diabetes mellitus | 3 (4.1) | 0 (0.0) | 0.31 | ||
| Glycemic control at 12 months of therapy | 0.006* | Post-hoc | |||
| Normal | 40 (54.1) | 50 (67.6) | |||
| Controlled pretherapy diabetes | 7 (9.5) | 15 (20.3) | 0.28 | ||
| Poorly controlled pretherapy diabetes | 7 (9.5) | 2 (2.7) | 0.33 | ||
| New onset glucose intolerance | 9 (12.2) | 5 (6.8) | 0.25 | ||
| New-onset diabetes mellitus | 8 (10.8) | 0 (0.0) | 0.01* | ||
| Blood pressure at 6 months of therapy | 0.030* | Post-hoc | |||
| Normal | 58 (78.4) | 63 (85.1) | |||
| Controlled pretherapy hypertension | 3 (4.1) | 8 (10.8) | 0.36 | ||
| Poorly controlled pretherapy hypertension | 3 (4.1) | 1 (1.4) | 0.91 | ||
| New onset hypertension | 7 (9.5) | 0 (0.0) | 0.02* | ||
| Blood pressure at 12 months of therapy | 0.068 | Post-hoc | |||
| Normal | 56 (75.7) | 60 (81.1) | |||
| Controlled pretherapy hypertension | 2 (2.7) | 8 (10.8) | 0.16 | ||
| Poorly controlled pretherapy hypertension | 4 (5.4) | 1 (1.4) | 0.50 | ||
| New onset hypertension | 9 (12.2) | 3 (4.1) | 0.20 | ||
| Breakthrough bleeding | 7 (9.5) | 16 (21.6) | 0.119 | ||
| Thromboembolic disorders | 1 (1.4) | 0 (0.0) | 0.541 | ||
Data are presented as number (percentage) or mean ± standard deviation.
LNG-IUS, levonorgestrel intrauterine system; MA, megestrol acetate.
*Statistically significant.
We studied the possible relationship between obesity and the response rate to therapy. The degree of obesity negatively impacted the complete regression rate. These findings were for the overall treatment outcome (96.3% for overweight, 90.3% for grade I, 74.2% for grade II, and 61.1% for grade III obesity, respectively, p=0.002) and MA arm (93.3%, 86.7%, 66.7%, and 45.5%, respectively, p=0.016). In contrast, this impact was less evident in the LNG-IUS arm (100%, 92.9%, 84.6%, and 85.7%, respectively, p=0.058). The LNG-IUS maintained reasonably high efficacy for women with moderate and severe obesity (Table 5).
Table 5. Comparison of response rate to therapy according to women’s BMI category.
| Response rate after 12 months of therapy | Category according to BMI at the start of therapy | p-value | ||||
|---|---|---|---|---|---|---|
| Over-weight | Grade I obesity | Grade II obesity | Grade III obesity | |||
| The overall response to therapy | 0.002* | |||||
| Complete regression (n=125) | 26 (96.3) | 65 (90.3) | 23 (74.2) | 11 (61.1) | ||
| Persistence (n=17) | 0 (0.0) | 4 (5.6) | 7 (22.6) | 6 (33.3) | ||
| Progression (n=1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (5.6) | ||
| Response to Megestrol acetate | 0.016* | |||||
| Complete regression (n=57) | 14 (93.3) | 26 (86.7) | 12 (66.7) | 5 (45.5) | ||
| Persistence (n=14) | 0 (0.0) | 3 (10.0) | 5 (27.8) | 6 (54.5) | ||
| Progression (n=0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Response to LNG-IUS | 0.058 | |||||
| Complete regression (n=68) | 12 (100) | 39 (92.9) | 11 (84.6) | 6 (85.7) | ||
| Persistence (n=3) | 0 (0.0) | 1 (2.4) | 2 (15.4) | 0 (0.0) | ||
| Progression (n=1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (14.3) | ||
BMI, body mass index; LNG-IUS, levonorgestrel intrauterine system.
*Statistically significant.
Women who received megestrol therapy had significantly higher weight gain than the LNG-IUS group after 1 year (4.7±4 kg vs. 2.7±2.6 kg, 95% CI=0.89–3.12; p=0.001) and after completing 2 years of therapy (7.8±5.1 kg vs. 4.1±2.9 kg, 95% CI=2.29–5.06; p<0.001) (Table 4).
Regarding follow-up of glycemic state, women on MA were more prone to poor control of diabetes, new onset glucose intolerance, and diabetes mellitus compared to the LNG-IUS group at 3 and 12 months of therapy. Similarly, the MA group showed a higher incidence of new-onset hypertension at 6 months of treatment than the LNG-IUS group (7 vs. 0, p=0.030). With time, new-onset hypertension was also reported in the LNG-IUS group at 12 months of therapy but still occurred less frequently than in the MA group (3 vs. 9, p=0.068) (Table 4).
Breakthrough bleeding was reported more frequently in women on LNG-IUS than in those on MA (21.6% vs. 9.5%, p=0.119). Only one patient receiving megestrol was affected by deep venous thrombosis in the lower limb and received anticoagulation therapy (Table 4).
DISCUSSION
The definitive treatment for AEH is a hysterectomy with or without oophorectomy [1,14]. Given the high risk of concurrence of or progression to cancer endometrium in non-responders, conservative treatment is a medical challenge with ethical considerations. Some affected women would decline hysterectomy due to an intense desire to preserve their fertility or are unfit for major operative intervention for medical or previous surgical reasons.
This randomized controlled trial has provided good evidence of the efficacy of progestagen therapy in treating AEH and compared 2 standard methods of its administration. One day, if we find predictive biomarkers to detect the risk of poor response and progression tendency, this therapy might be a valid alternative for all women wishing to preserve their uteri, not only for an exceptional group [15].
The median duration until complete regression was six months for both therapy methods. LNG-IUS had a significantly higher success rate than MA during all the study phases (73% vs. 52.7% and 85.1% vs. 68.9% at 6 and 9 months, respectively) until the results plateaued at 12 months, with a final success rate of 91.9% versus 77%, respectively (p=0.026). None of the responders developed a recurrence of AEH during the follow-up (Table 3). Previous studies also elicited this higher response rate to LNG-IUS at varying levels. Gallos et al. [16] reported a pooled complete regression rate with LNG-IUS of 90% versus 69% with oral progestagen in their meta-analysis. Leone Roberti Maggiore et al. [17] found complete regression in 89.3% of women with AEH on LNG-IUS, with a median time to regression of 6.7 months. Pal et al. [18] reported complete regression at 6 months on the LNG-IUS of 80% (95% CI=52–96). A prospective cohort study by Novikova et al. [19] included 228 women with AEH and reported a complete regression rate of 98% with LNG-IUS compared to 87% with medroxyprogesterone acetate. The higher bioavailability of levonorgestrel to the endometrium could explain the considerably higher efficacy of LNG-IUS reported by us and others. The device steadily provides local levonorgestrel with a concentration multiple times that of oral therapy with minimal systemic concentrations [20,21].
While prolonging the therapy for up to 12 months for non-responders improved the complete regression rate and still seems reasonable and safe, one of the participants in the LNG-IUS group (1.4%) had evidence of progression to endometrioid adenocarcinoma grade II during sampling at 6 months of follow-up. The myometrial invasion was less than 50%, and a radical hysterectomy with lymphadenectomy was performed. Doherty et al. [3] reported in their meta-analysis a concurrent endometrial cancer with atypical hyperplasia of 32.6% (95% CI=24.1%–42.4%) with an annual incidence rate of progression to cancer of 8.2% (95% CI=3.9%–17.3%). Despite our low incidence of progression, AEH remains a premalignant lesion that we should manage cautiously, especially with the lack of markers to predict the tendency for progression or to distinguish atypia from low-grade malignancy [3,15].
Our follow-up during the study was by endometrial sampling plus transvaginal ultrasound every three months during the first year of therapy and every 6 months during the following year. This frequency aligns with the sampling frequency of 3–6 months reported in multiple previous studies [5,17,22,23]. We used hysteroscopic-guided endometrial biopsies alternating with Pipelle office sampling to maintain accuracy at a reasonable cost. Hysteroscopy provides precise and targeted sampling even with focal lesions and LNG-IUS in place and was the preferred method in multiple previous studies [5,17,19,22,24,25]. On the other hand, an expert gynecologist performed office sampling using a Pipelle sampler, which was more convenient to patients, with minimal discomfort and cost. Multiple previous studies reported a reasonable accuracy of Pipelle sampling, especially with atypical hyperplasia, with a sensitivity of 70%–97.8% compared to hysteroscopy/dilatation and curettage [22,23,26,27,28,29]. Conversely, some questioned the efficacy of office sampling given the small percentage of sampled endometrial surface, especially in women with thinned-out endometrium or focal endometrial lesions [22,27,30]. The posthysterectomy histological assessment of the nonresponders was concordant with the preoperative sampling in 13/14 (92.9%). The remaining patient specimen showed simple hyperplasia without evidence of atypia. This result is reassuring that our strategy of follow-up sampling was convenient.
The degree of obesity negatively impacted the response rate. Women with grade III obesity in the MA group reported the lowest complete regression rate (45.5%). LNG-IUS was more effective in inducing complete regression for women of the same category (85.7%) (Table 4). This finding suggests that the LNG-IUS might be more convenient for morbidly obese women. Obesity and metabolic syndrome are substantial risk factors for endometrial hyperplasia and malignancy by various mechanisms, including hyperestrogenism, insulin resistance, and different growth factors [31]. Ding et al. [5] found metabolic syndrome predisposing to a delayed regression time (odds ratio=3.1; 95% CI=1.0–5.2; p=0.005). Graul et al. [32] reported an increased risk of progression with a rising BMI. However, a better response to therapy was reported by Mandelbaum et al. [33] in class III obese women compared to nonobese women.
Regarding the side effects of therapy, the mean weight gain in the MA group was significantly greater than that in the LNG-IUS group at 1 year (4.7 vs. 2.7 kg, p=0.001) and 2 years of treatment (7.8 vs. 4.1 kg, p<0.001), respectively. Our study population’s weight gain in both groups was greater than the reported weight gain in most available studies. Cholakian et al. [8] reported a mean weight gain of 2.95 kg with MA versus 0.5 kg with LNG-IUS after 1 year. Park et al. [34] reported a mean weight gain of 1.2 kg after 18 months of oral progestagens, 13.6% had a weight gain of >1 kg per month, and 4.5% had a weight gain of ≥2 kg per month. However, Güven et al. [35] also reported that the median weight gain on MA after 3 months was 4 kg. Similarly, Novikova et al. [19] reported weight gain exceeding 5 kg in 36% of women on medroxyprogesterone acetate compared to 12% of women on LNG-IUS. Other studies reported weight gain of 0.7–2.9 kg after one year on LNG-IUS as a contraceptive device [36,37,38]. The variance in weight gain between our population and other previous studies may be explained by racial differences, lifestyle, dietary habits, and high basal BMI.
It is worth mentioning that MA was associated with less control of diabetes and hypertension in affected women. Women in the megestrol group also had a higher incidence of new-onset glucose intolerance, diabetes mellitus, and hypertension. We could partly explain this finding by the high basal BMI, weight gain, and increased appetite induced by MA [39].
The study’s strengths include the randomized controlled design with a powered sample size for the primary outcome and intention-to-treat analysis. This trial is one of the few registered trials comparing LNG-IUS to MA in treating AEH. We delineated explicit inclusion and exclusion criteria, a clear duration of therapy, a cutoff time until treatment failure, and a convenient strategy for sampling and other outcome follow-ups. The lack of a comprehensive survey of other adverse effects, such as mood changes, headache, and sexual dysfunction, limits our study. Additionally, we did not study the relationship between items of metabolic syndrome, the risk of AEH, and the impact of selected therapy. Although the study lacks a long-term recurrence rate, progression rate, and conception rate follow-up, the authors plan to conduct a posttrial follow-up study to assess the therapy’s long-term efficacy.
Progestational therapy effectively induced complete regression in 84.5% of women with AEH who initially declined hysterectomy. Compared to oral MA, the LNG-IUS showed significantly higher efficacy in inducing complete regression (91.9% vs. 77%), especially in morbidly obese women. It was associated with substantially less weight gain and better blood pressure and glycemic control. It is more convenient with better compliance being a single application during the whole therapy without requiring daily oral doses.
Extending conservative therapy until 12 months will improve the complete regression rate in persistent atypical hyperplasia cases with reasonable safety, given that timely and cautious follow-up is maintained. The alternation between hysteroscopic-guided and Pipelle office endometrial sampling seems to be an adequate and balanced follow-up strategy with high accuracy and affordable costs.
The long-term efficacy of therapy and optimal therapy duration still need further assessment. The future challenge in this aspect is to find reliable markers to predict the tendency for progression. Then, the LNG-IUS would be a reasonable treatment option for all women with AEH.
Compared to MA, the LNG-IUS was more efficacious in treating AEH in women who declined hysterectomy, especially in morbidly obese women, with fewer adverse effects. Extending therapy for 12 months to persistent cases would improve regression rates with reasonable safety. We can alternate between hysteroscopic and office sampling for follow-up.
ACKNOWLEDGEMENTS
The authors thank all trial participants who agreed to be enrolled. We are indebted to the respected colleague physicians and nursing staff in the Obstetrics & Gynecology Department of Zagazig University Hospitals, Zagazig, Egypt, for their endless support and cooperation during the recruitment and follow-up of the trial population.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: A.A.A., A.H.
- Data curation: A.A.A.
- Formal analysis: H.O.A., A.H.
- Investigation: A.A.A., H.O.A.
- Methodology: A.A.A., A.H.
- Software: A.H.
- Supervision: A.H.
- Validation: H.O.A.
- Writing - original draft: A.H.
- Writing - review & editing: A.A.A., A.H.
References
- 1.Royal College of Obstetricians and Gynaecologists (RCOG); British Society for Gynaecological Endoscopy (BSGE) Management of Endometrial Hyperplasia (Green-top Guideline No. 67) [Internet] London: RCOG; 2016. [cited 2023 Sep 27]. Available from: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/management-of-endometrial-hyperplasia-green-top-guideline-no-67/ [Google Scholar]
- 2.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–412. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Doherty MT, Sanni OB, Coleman HG, Cardwell CR, McCluggage WG, Quinn D, et al. Concurrent and future risk of endometrial cancer in women with endometrial hyperplasia: a systematic review and meta-analysis. PLoS One. 2020;15:e0232231. doi: 10.1371/journal.pone.0232231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou R, Yang Y, Lu Q, Wang J, Miao Y, Wang S, et al. Prognostic factors of oncological and reproductive outcomes in fertility-sparing treatment of complex atypical hyperplasia and low-grade endometrial cancer using oral progestin in Chinese patients. Gynecol Oncol. 2015;139:424–428. doi: 10.1016/j.ygyno.2015.09.078. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Fan Y, Li X, Wang Y, Wang J, Tian L. Metabolic syndrome is an independent risk factor for time to complete remission of fertility-sparing treatment in atypical endometrial hyperplasia and early endometrial carcinoma patients. Reprod Biol Endocrinol. 2022;20:134. doi: 10.1186/s12958-022-01006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suidan RS, He W, Sun CC, Zhao H, Fleming ND, Ramirez PT, et al. Impact of body mass index and operative approach on surgical morbidity and costs in women with endometrial carcinoma and hyperplasia. Gynecol Oncol. 2017;145:55–60. doi: 10.1016/j.ygyno.2017.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corzo C, Barrientos Santillan N, Westin SN, Ramirez PT. Updates on conservative management of endometrial cancer. J Minim Invasive Gynecol. 2018;25:308–313. doi: 10.1016/j.jmig.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Cholakian D, Hacker K, Fader AN, Gehrig PA, Tanner EJ., 3rd Effect of oral versus intrauterine progestins on weight in women undergoing fertility preserving therapy for complex atypical hyperplasia or endometrial cancer. Gynecol Oncol. 2016;140:234–238. doi: 10.1016/j.ygyno.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Buttini MJ, Jordan SJ, Webb PM. The effect of the levonorgestrel releasing intrauterine system on endometrial hyperplasia: an Australian study and systematic review. Aust N Z J Obstet Gynaecol. 2009;49:316–322. doi: 10.1111/j.1479-828X.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 10.Baker J, Obermair A, Gebski V, Janda M. Efficacy of oral or intrauterine device-delivered progestin in patients with complex endometrial hyperplasia with atypia or early endometrial adenocarcinoma: a meta-analysis and systematic review of the literature. Gynecol Oncol. 2012;125:263–270. doi: 10.1016/j.ygyno.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Chen X. The current situation of the levonorgestrel intrauterine system (LNG-IUS) in conservative treatment for patients with early-stage endometrial cancer and atypical hyperplasia. J Gynecol Oncol. 2019;30:e79. doi: 10.3802/jgo.2019.30.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallos ID, Ganesan R, Gupta JK. Prediction of regression and relapse of endometrial hyperplasia with conservative therapy. Obstet Gynecol. 2013;121:1165–1171. doi: 10.1097/AOG.0b013e31828cb563. [DOI] [PubMed] [Google Scholar]
- 13.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: post hoc and planned comparison procedures. J Exp Educ. 1995;64:79–93. [Google Scholar]
- 14.Auclair MH, Yong PJ, Salvador S, Thurston J, Colgan TT, Sebastianelli A. Guideline No. 390-classification and management of endometrial hyperplasia. J Obstet Gynaecol Can. 2019;41:1789–1800. doi: 10.1016/j.jogc.2019.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Dore M, Filoche S, Danielson K, Henry C. Efficacy of the LNG-IUS for treatment of endometrial hyperplasia and early stage endometrial cancer: can biomarkers predict response? Gynecol Oncol Rep. 2021;36:100732. doi: 10.1016/j.gore.2021.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallos ID, Shehmar M, Thangaratinam S, Papapostolou TK, Coomarasamy A, Gupta JK. Oral progestogens vs levonorgestrel-releasing intrauterine system for endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:547.e1–547.10. doi: 10.1016/j.ajog.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Leone Roberti Maggiore U, Martinelli F, Dondi G, Bogani G, Chiappa V, Evangelista MT, et al. Efficacy and fertility outcomes of levonorgestrel-releasing intra-uterine system treatment for patients with atypical complex hyperplasia or endometrial cancer: a retrospective study. J Gynecol Oncol. 2019;30:e57. doi: 10.3802/jgo.2019.30.e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal N, Broaddus RR, Urbauer DL, Balakrishnan N, Milbourne A, Schmeler KM, et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet Gynecol. 2018;131:109–116. doi: 10.1097/AOG.0000000000002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novikova OV, Nosov VB, Panov VA, Novikova EG, Krasnopolskaya KV, Andreeva YY, et al. Live births and maintenance with levonorgestrel IUD improve disease-free survival after fertility-sparing treatment of atypical hyperplasia and early endometrial cancer. Gynecol Oncol. 2021;161:152–159. doi: 10.1016/j.ygyno.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson CG, Haukkamaa M, Vierola H, Luukkainen T. Tissue concentrations of levonorgestrel in women using a levonorgestrel-releasing IUD. Clin Endocrinol (Oxf) 1982;17:529–536. doi: 10.1111/j.1365-2265.1982.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 21.Sitruk-Ware R, Inki P. The levonorgestrel intrauterine system: long-term contraception and therapeutic effects. Womens Health (Lond Engl) 2005;1:171–182. doi: 10.2217/17455057.1.2.171. [DOI] [PubMed] [Google Scholar]
- 22.Won S, Kim MK, Seong SJ. Fertility-sparing treatment in women with endometrial cancer. Clin Exp Reprod Med. 2020;47:237–244. doi: 10.5653/cerm.2020.03629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudesia R, Singer T, Caputo TA, Holcomb KM, Kligman I, Rosenwaks Z, et al. Reproductive and oncologic outcomes after progestin therapy for endometrial complex atypical hyperplasia or carcinoma. Am J Obstet Gynecol. 2014;210:255.e1–255.e4. doi: 10.1016/j.ajog.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Nappi L, Angioni S, De Feo V, Greco P, Stabile G, Greco F, et al. Diagnostic accuracy of hysteroscopy vs dilation and curettage (D&C) for atypical endometrial hyperplasia in patients performing hysterectomy or serial follow-up. Clin Exp Obstet Gynecol. 2022;49:24. [Google Scholar]
- 25.Yang B, Xu Y, Zhu Q, Xie L, Shan W, Ning C, et al. Treatment efficiency of comprehensive hysteroscopic evaluation and lesion resection combined with progestin therapy in young women with endometrial atypical hyperplasia and endometrial cancer. Gynecol Oncol. 2019;153:55–62. doi: 10.1016/j.ygyno.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 26.van Hanegem N, Prins MM, Bongers MY, Opmeer BC, Sahota DS, Mol BW, et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2016;197:147–155. doi: 10.1016/j.ejogrb.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Huang GS, Gebb JS, Einstein MH, Shahabi S, Novetsky AP, Goldberg GL. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am J Obstet Gynecol. 2007;196:243.e1–243.e5. doi: 10.1016/j.ajog.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Demirkiran F, Yavuz E, Erenel H, Bese T, Arvas M, Sanioglu C. Which is the best technique for endometrial sampling? Aspiration (pipelle) versus dilatation and curettage (D&C) Arch Gynecol Obstet. 2012;286:1277–1282. doi: 10.1007/s00404-012-2438-8. [DOI] [PubMed] [Google Scholar]
- 29.Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial hyperplasia. Acta Obstet Gynecol Scand. 2001;80:784–793. doi: 10.1034/j.1600-0412.2001.080009784.x. [DOI] [PubMed] [Google Scholar]
- 30.Kim MK, Seong SJ, Song T, Kim ML, Yoon BS, Jun HS, et al. Comparison of dilatation & curettage and endometrial aspiration biopsy accuracy in patients treated with high-dose oral progestin plus levonorgestrel intrauterine system for early-stage endometrial cancer. Gynecol Oncol. 2013;130:470–473. doi: 10.1016/j.ygyno.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Mackintosh ML, Crosbie EJ. Obesity-driven endometrial cancer: is weight loss the answer? BJOG. 2013;120:791–794. doi: 10.1111/1471-0528.12106. [DOI] [PubMed] [Google Scholar]
- 32.Graul A, Wilson E, Ko E, Haggerty AF, Reed H, Koelper N, et al. Conservative management of endometrial hyperplasia or carcinoma with the levonorgestrel intrauterine system may be less effective in morbidly obese patients. Gynecol Oncol Rep. 2018;26:45–48. doi: 10.1016/j.gore.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandelbaum RS, Ciccone MA, Nusbaum DJ, Khoshchehreh M, Purswani H, Morocco EB, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1–103.13. doi: 10.1016/j.ajog.2019.12.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JY, Seong SJ, Kim TJ, Kim JW, Bae DS, Nam JH. Significance of body weight change during fertility-sparing progestin therapy in young women with early endometrial cancer. Gynecol Oncol. 2017;146:39–43. doi: 10.1016/j.ygyno.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Güven M, Dikmen Y, Terek MC, Özsaran AA, İtil IM, Erhan Y. Metabolic effects associated with high-dose continuous megestrol acetate administration in the treatment of endometrial pathology. Arch Gynecol Obstet. 2001;265:183–186. doi: 10.1007/s004040000154. [DOI] [PubMed] [Google Scholar]
- 36.Modesto W, de Nazaré Silva dos Santos P, Correia VM, Borges L, Bahamondes L. Weight variation in users of depot-medroxyprogesterone acetate, the levonorgestrel-releasing intrauterine system and a copper intrauterine device for up to ten years of use. Eur J Contracept Reprod Health Care. 2015;20:57–63. doi: 10.3109/13625187.2014.951433. [DOI] [PubMed] [Google Scholar]
- 37.Vickery Z, Madden T, Zhao Q, Secura GM, Allsworth JE, Peipert JF. Weight change at 12 months in users of three progestin-only contraceptive methods. Contraception. 2013;88:503–508. doi: 10.1016/j.contraception.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dal’Ava N, Bahamondes L, Bahamondes MV, de Oliveira Santos A, Monteiro I. Body weight and composition in users of levonorgestrel-releasing intrauterine system. Contraception. 2012;86:350–353. doi: 10.1016/j.contraception.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, Gonzalvez Perales JL, Bort-Martí S. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev. 2013;2013:CD004310. doi: 10.1002/14651858.CD004310.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]