Abstract
Objective
To evaluate the long-term efficacy and safety of niraparib in Japanese women with heavily pretreated ovarian cancer.
Methods
This was the follow-up analysis of a phase 2, multicenter, open-label, single-arm study in Japanese women with homologous recombination-deficient, platinum-sensitive, relapsed, high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who had completed 3–4 lines of chemotherapy and were poly(ADP-ribose) polymerase inhibitor naïve. Participants received niraparib (starting dose, 300 mg) once daily in continuous 28-day cycles until objective disease progression, unacceptable toxicity, or consent withdrawal. The primary endpoint was confirmed objective response rate (ORR), as assessed using Response Evaluation Criteria in Solid Tumors version 1.1. Safety evaluations included treatment-emergent adverse events (TEAEs).
Results
20 patients were enrolled in the study and included in both efficacy and safety analyses. Median total study duration was 759.5 days. Median dose intensity was 201.3 mg/day. Confirmed ORR was 60.0% (90% confidence interval [CI]=39.4–78.3); 2 patients had complete response and 10 patients had partial response. Median duration of response was 9.9 months (95% CI=3.9–26.9) and the disease control rate was 90.0% (95% CI=68.3–98.8). The most common TEAEs were anemia (n=15), nausea (n=12), and decreased platelet count (n=11). TEAEs leading to study drug dose reduction, interruption, or discontinuation were reported in 16 (80.0%), 15 (75.0%), and 2 patients (10.0%), respectively.
Conclusion
The long-term efficacy and safety profile of niraparib was consistent with previous findings in the equivalent population in non-Japanese patients. No new safety signals were identified.
Trial Registration
ClinicalTrials.gov Identifier: NCT03759600
Keywords: Clinical Trial, Phase II; Poly(ADP-ribose) Polymerase Inhibitors; Ovarian Cancer
Synopsis
We analyzed the long-term safety and efficacy of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. The objective response rate was 60.0% and no new safety signals were identified in this follow-up study. Efficacy and safety were consistent with previous findings in non-Japanese patients.
INTRODUCTION
Ovarian cancer is associated with high morbidity and mortality and is increasing in incidence in Japan [1]. Patients with advanced ovarian cancer have limited options for late-line therapy and median overall survival (OS) after third-line treatment is 5–9 months [2]. Poly(ADP-ribose) polymerase (PARP) is a DNA-binding enzyme that plays a key role in single-strand break (SSB) DNA repair [3]. Homologous recombination-deficient (HRd) cancer cells, such as those with BRCA1/2 mutations, rely on intact SSB pathways to repair DNA damage and maintain cell viability. PARP inhibitors are a class of anticancer agents that exploit this synthetic lethality and induce tumor cell death by both inhibiting the catalytic activity of PARP and trapping it at the site of DNA damage [3]. Niraparib, an oral, highly selective PARP inhibitor [4], has been shown to be clinically efficacious in treating ovarian cancer in the NOVA [5], PRIMA [6], and QUADRA [2] clinical trials. It has marketing approval in several countries and territories, including Europe, the USA, and Japan. In Japan, niraparib is indicated for maintenance treatment of ovarian cancer after initial chemotherapy, maintenance treatment of platinum-sensitive recurrent ovarian cancer, and treatment of platinum-sensitive recurrent ovarian cancer with homologous recombination deficiency [7].
In a single-arm, open-label, phase 2 trial conducted in the USA and Canada (QUADRA), niraparib met its primary endpoint, with an objective response rate (ORR), as assessed by the investigator, of 27.7% (95% confidence interval [CI]=15.6–42.6; p<0.001) in platinum-sensitive, HRd-positive, PARP inhibitor-naive women with heavily pretreated ovarian cancer (n=47) [2]. In addition to this primary analysis population, QUADRA also included patients with platinum-resistant or refractory ovarian cancer, HRd-negative tumors, and both mutated BRCA and wild-type BRCA tumors. Exploratory analyses found that the clinical benefit of niraparib extended to patients beyond the primary group, including those with wild-type BRCA disease [2]. Based on the results of QUADRA, niraparib was also approved in the USA for the treatment of adults with advanced ovarian, fallopian tube, or primary peritoneal cancer who have received ≥3 previous chemotherapy regimens and who have HRd-positive cancer; however, this indication was voluntarily withdrawn by GSK in September 2022. This decision was made in consultation with the US Food and Drug Administration (FDA) and based on a totality of information on PARP inhibitors in the late-line treatment setting in ovarian cancer. A potential detrimental effect on OS had been observed with other PARP inhibitors in 2 independent randomized, active-controlled clinical trials conducted in women with BRCA-mutated, advanced ovarian cancer who had received ≥3 previous chemotherapy regimens [8,9]. Given the design of the QUADRA trial (single arm, uncontrolled), no comparative OS information can be obtained from the study, and it is difficult to assess any potential effect of niraparib on time-to-event endpoints. Unlike in the USA, niraparib retains this indication in Japan, although it is limited to patients sensitive to platinum-based chemotherapy. The American Society of Clinical Oncology (ASCO) also updated its guidelines for ovarian cancer to no longer recommend routinely offering niraparib to patients [10]. However, the guidelines also state that evidence on PARP inhibitor use in this setting is evolving and data continue to emerge. Thus, it is becoming increasingly important to collect further data on the use of PARP inhibitors in ovarian cancer.
Niraparib-2002 was a phase 2 study designed to evaluate the efficacy and safety of niraparib in a population of Japanese women equivalent to the primary analysis population in QUADRA. The study enrolled 20 patients, all with HRd-positive tumors and 65.0% of whom had BRCA-mutated tumors [11]. Primary data (median study duration, 120.5 days) have already been reported [11]. The confirmed ORR in the full analysis set (FAS) was 35.0% (7/20 patients; 1 had complete response [CR] and 6 had partial response [PR]), similar to that in the QUADRA study, and the disease control rate (DCR) was 90.0%. No new safety signals were identified and the short-term safety profile of niraparib in Japanese patients was considered acceptable and consistent with previous experience in non-Japanese patients [11]. Here, we report long-term follow-up data for Niraparib-2002.
MATERIALS AND METHODS
1. Study design and treatment
This was a phase 2, multicenter, open-label, single-arm study to evaluate the safety and efficacy of niraparib in heavily pretreated Japanese patients with advanced, relapsed, high-grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer (ClinicalTrials.gov: NCT03759600). The study design and patient inclusion and exclusion criteria have been described in detail previously [11]. Briefly, eligible participants were Japanese women ≥20 years old, with HRd-positive, relapsed, high-grade, serous epithelial ovarian, fallopian tube, or primary peritoneal cancer with recurrent disease, who had completed 3 or 4 previous chemotherapy regimens and were platinum-sensitive to the last platinum-based therapy. Patients had to have ≥1 measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and an Eastern Cooperative Oncology Group performance status of 0 or 1. HRd testing was performed on tumor tissue using the Myriad myChoice® HRD diagnostic test (Myriad Genetics), which uses next generation sequencing to assess genomic instability and detect rearrangements and variants in the BRCA1/2 genes. Individuals who had received other PARP inhibitors were excluded from participation.
The study was conducted at 17 sites in Japan, with data for the final analysis collected between December 26, 2018 and December 6, 2021. Participants received niraparib 300 mg (3×100 mg hard capsules) orally once daily, in continuous 28-day cycles until objective disease progression, unacceptable toxicity, or withdrawal of consent, as described previously [11]. The starting dose was set at 300 mg for consistency with the 300 mg starting dose of the QUADRA trial [2]. The choice of 300 mg as the starting dose was also based on the maximum dose at which tolerability was observed in Japanese patients and was agreed upon with the Pharmaceuticals and Medical Devices Agency. Clinic visits were conducted weekly during cycle 1 and then approximately every 4 weeks for subsequent cycles. Dose interruption of up to 28 days and dose reduction of up to 100 mg per day were permitted for any toxicity deemed intolerable to the patient. Patients discontinued niraparib if dose interruption did not resolve toxicity completely or to grade 1 toxicity during the 28-day dose interruption period and/or the patient had already undergone the maximum dose reductions. Dose intensity (mg/day) was calculated as the sum of the total daily dose ingested divided by the overall treatment exposure.
All patients provided written informed consent before participating. The study was conducted in accordance with the Declaration of Helsinki and the International Council on Harmonisation Tripartite Guideline on the ethical principles of Good Clinical Practice. The clinical study protocol, investigator’s brochure, a sample informed consent form, and other study-related documents were reviewed and approved by the local or central Institutional Review Boards of all study sites.
2. Outcomes
Tumor assessment was performed using computed tomography or magnetic resonance imaging of the abdomen/pelvis and at clinically indicated areas. Imaging was performed at baseline, at the end of every 2 cycles until cycle 6, then at the end of every 3 cycles until cycle 39, and at the end of every 6 cycles until progression. Patients were classified as CR, PR, progressive disease (PD) or stable disease (SD) as assessed by the investigator using RECIST version 1.1. The primary endpoint was the confirmed ORR, defined as the proportion of patients classified as CR or PR. Secondary efficacy endpoints were duration of response (DOR), DCR (proportion of patients with CR, PR, or SD), progression-free survival (PFS), and OS. DOR was defined as the time from the earliest date of initial response (confirmed CR or PR) until the first date of radiological PD or death by any cause. PFS was defined as the time from the first dose of niraparib until the first date of documented disease progression as determined by RECIST version 1.1, clinical criteria, or death by any cause. OS was defined as the time from the first dose of niraparib to death by any cause. Secondary safety endpoints were incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, severe (grade ≥3 according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03) TEAEs, and TEAEs that led to study drug dose reduction, interruption, or discontinuation.
3. Statistics
A sample size of 16 patients was planned for this study, with data from some patients anticipated to be non-evaluable (NE). A total of 14 enrolled patients was considered to provide ≥80% power to detect an ORR ≥29% when testing a null hypothesis of ORR ≤5% at a 1-sided significance level of 5% (binomial test). The FAS (efficacy analyses) included all patients who received ≥1 dose of study drug and had measurable disease at baseline. The safety analysis set (SAS) included all patients who received ≥1 dose of study drug. For analysis of the primary endpoint, ORR and its 2-sided 90% CI were calculated based on binomial distribution. DCR and its 2-sided 95% CI were also calculated based on binomial distribution. PFS and OS were analyzed using the Kaplan–Meier method to provide quartiles and progression/survival rate at specified points with 2-sided 95% CI. DOR was analyzed in the same way, using the population who responded to niraparib. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Patient disposition and baseline characteristics
In total, 20 patients were enrolled in the study and included in both the FAS and SAS. Baseline characteristics have been described previously [11] and are shown in Table 1. At data cutoff, 17 patients had discontinued treatment, either owing to PD (n=15) or an adverse event (n=2). Median (range) total study duration and overall treatment exposure were 759.5 (237–1010) days and 254.5 (14–1,033) days, respectively. The median niraparib dose intensity was 201.3 mg/day and the median relative dose intensity was 67.1%.
Table 1. Demographics and baseline clinical characteristics.
| Characteristics | Niraparib 300 mg (n=20) | |
|---|---|---|
| Age (yr) | 62.0 (47.0–85.0) | |
| Weight (kg) | 54.5 (36.4–80.2) | |
| <58 | 14 (70.0) | |
| ≥58, <77 | 5 (25.0) | |
| ≥77 | 1 (5.0) | |
| Mean±standard deviation | 53.7±9.7 | |
| Primary tumor site | ||
| Ovarian | 13 (65.0) | |
| Primary peritoneal | 5 (25.0) | |
| Fallopian tube | 2 (10.0) | |
| ECOG status | ||
| 0 | 15 (75.0) | |
| 1 | 5 (25.0) | |
| Tumor BRCA1/2 mutation status | ||
| Negative | 6 (30.0) | |
| Positive | 13 (65.0) | |
| Unknown | 1 (5.0) | |
| Cancer stage (FIGO) at initial diagnosis | ||
| IA | 1 (5.0) | |
| IC | 1 (5.0) | |
| IIB | 1 (5.0) | |
| IIC | 1 (5.0) | |
| IIIA | 1 (5.0) | |
| IIIC | 12 (60.0) | |
| IV | 3 (15.0) | |
| Number of previous chemotherapy lines | ||
| 3 | 12 (60.0) | |
| 4 | 8 (40.0) | |
| Type of previous chemotherapy | ||
| Taxane | 20 (100.0) | |
| Bevacizumab | 10 (50.0) | |
| Doxorubicin | 9 (45.0) | |
| Gemcitabine | 11 (55.0) | |
| Liposomal doxorubicin | 0 | |
| Best response to most recent platinum therapy | ||
| CR | 9 (45.0) | |
| PR | 8 (40.0) | |
| SD | 2 (10.0) | |
| Unknown | 1 (5.0) | |
Values are presented as median (min–max) or number (%) unless otherwise indicated. Table adapted from Okamoto et al. [11].
CR, complete response; ECOG, Eastern Cooperative Oncology Group; FIGO, International Federation of Gynecology and Obstetrics; PR, partial response; SD, stable disease.
2. Primary endpoint
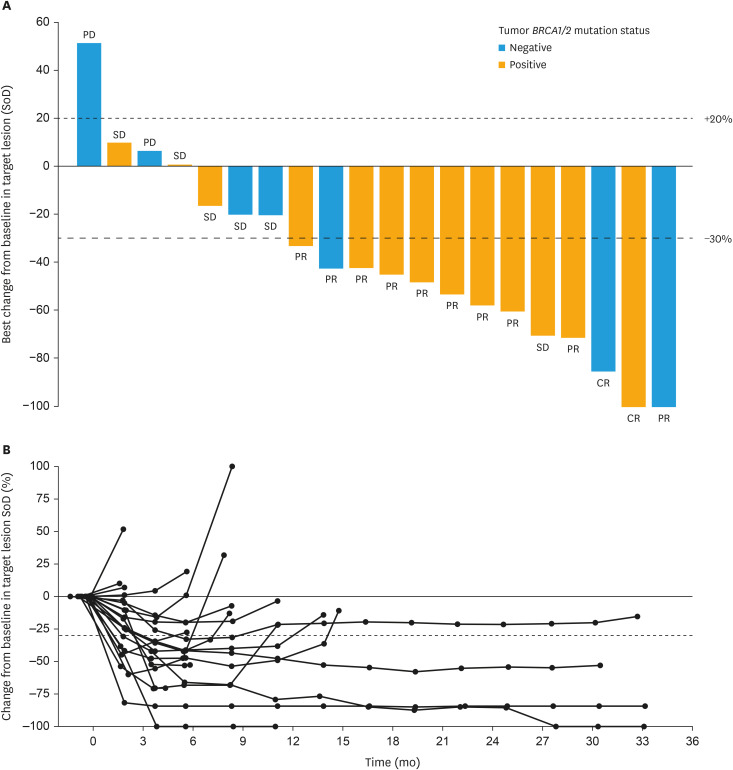
Confirmed ORR in the FAS was 60.0% (90% CI=39.4–78.3). Out of 12 responders, 2 patients (10.0%) had CR and 10 patients (50.0%) had PR. In the remaining patients, the responses observed were SD (n=6) and PD (n=2) (Fig. 1A). In patients who had BRCA1/2 mutations (n=13), the ORR was 69.2% (90% CI=42.7–88.7), whereas in patients without BRCA1/2 mutations (n=7, includes 1 patient with unknown mutation status) the ORR was 42.9% (90% CI=12.9–77.5). The ORR in patients aged 18 to <65 years (n=11) was 45.5% (90% CI=20.0–72.9) and the ORR in patients aged ≥65 years (n=9) was 77.8% (90% CI=45.0–95.9). Patients for whom the primary tumor site was ovarian (n=13), primary peritoneal (n=5), and fallopian tube (n=2) had ORRs of 61.5% (90% CI=35.5–83.4), 60.0% (90% CI=18.9–92.4), and 50.0% (90% CI=2.5–97.5), respectively. Changes from baseline in target lesion size are shown for each patient in Fig. 1B.
Fig. 1. Tumor responses. (A) Waterfall plot of ORR in the FAS. Includes all patients who had evaluable post-baseline target lesion(s). Tumor BRCA1/2 mutation status is dichotomized as ‘positive’ or ‘negative’ (includes ‘unknown’). The individual bars show the reduction rate at the time when the sum of the target lesion size decreased the most after starting administration of niraparib for each patient. The best overall response based on RECIST v1.1 shown at the end of each bar takes into account not only changes in the size of target lesions, but also evaluation of non-target lesions and the presence or absence of new lesions. (B) Change from baseline in target lesion size over time.
CR, complete response; FAS, full analysis set; ORR, objective response rate; PD, progressive disease; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SoD, sum of diameters.
3. Secondary endpoints
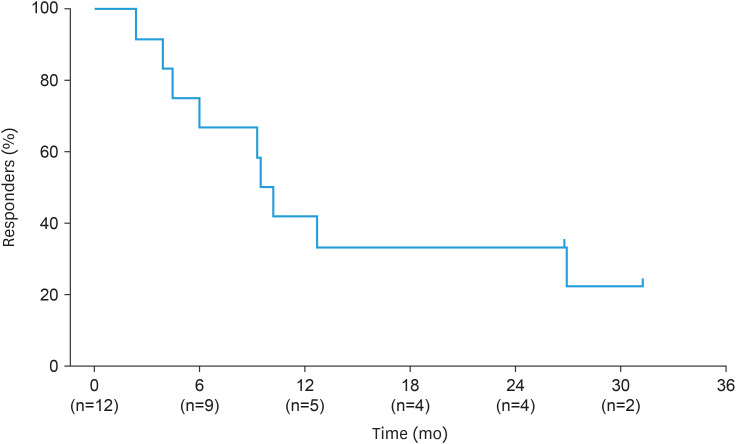
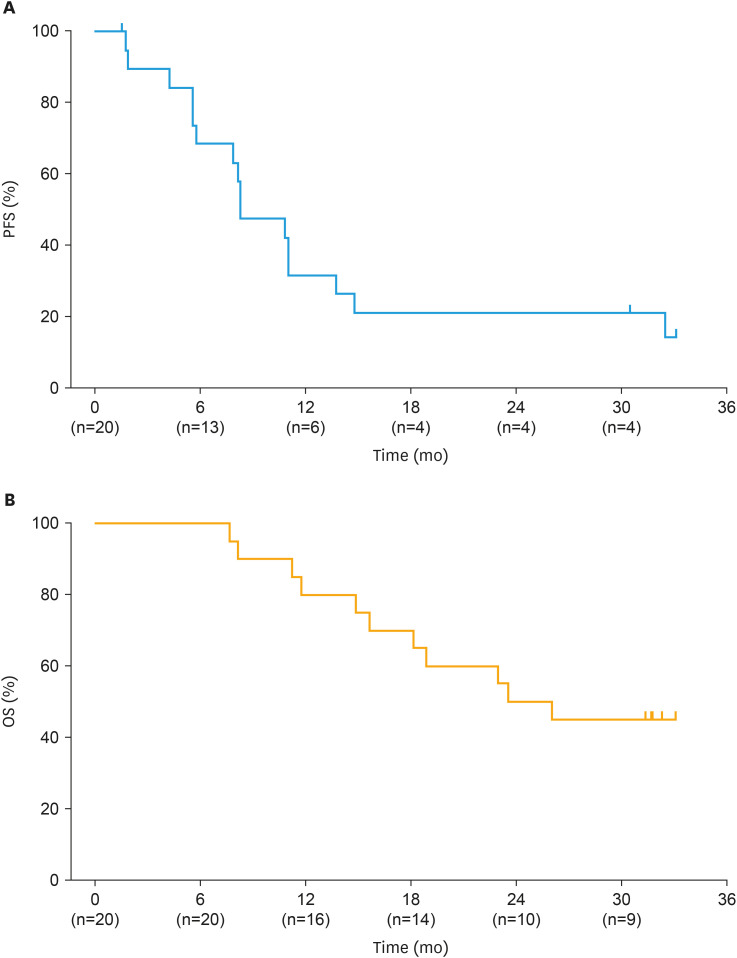
Median DOR in ORR responders (n=12) was 9.9 months (95% CI=3.9–26.9) across a median follow-up period of 31.3 months (Fig. 2). DCR in the FAS was 90.0% (95% CI=68.3–98.8). Kaplan–Meier plots for PFS and OS in the FAS are shown in Fig. 3. Median PFS was 8.3 months (95% CI=5.6–13.8), with 16 patients (80.0%) experiencing disease progression and 4 patients censored (either at last assessment [n=3] or last assessment before new anticancer therapy [n=1]). At data cutoff, median OS was 24.9 months (95% CI=14.9–NE); 9 patients were censored at their last known alive date and 11 patients had died. The median follow-up periods for PFS and OS were 33.1 months and 31.8 months, respectively.
Fig. 2. Kaplan–Meier plot of duration of response in responders.
Fig. 3. Kaplan–Meier plot of (A) PFS and (B) OS in the FAS.
FAS, full analysis set; OS, overall survival; PFS, progression-free survival.
All patients experienced ≥1 TEAE during the study (Table 2). Of the 286 TEAEs that occurred, 202 were considered treatment-related. The most common TEAEs were anemia, nausea, and decreased platelet count. The most common TEAEs leading to study drug dose reduction (experienced by ≥10% of patients) were anemia (n=11, 55.0%), decreased platelet count (n=8, 40.0%), decreased neutrophil count (n=3, 15.0%), nausea (n=2, 10.0%), and vomiting (n=2, 10.0%). TEAEs leading to study drug interruption experienced by ≥10% of patients were anemia (n=10, 50.0%), decreased platelet count (n=9, 45.0%), decreased neutrophil count (n=4, 20.0%), and decreased white blood cell count (n=2, 10.0%). TEAEs leading to study drug discontinuation were each only reported in 1 patient. Serious TEAEs were recorded for 8 patients (40.0%). TEAEs rated as Grade ≥3 severity, and serious TEAEs experienced by ≥10% of patients are shown in Table 3. No TEAEs leading to death were reported.
Table 2. Summary of TEAEs.
| Adverse events | Niraparib 300 mg (n=20) | ||
|---|---|---|---|
| Any TEAEs | 20 (100.0) | ||
| Related to study drug | 20 (100.0) | ||
| Leading to study drug dose reduction | 16 (80.0) | ||
| Leading to study drug interruption | 15 (75.0) | ||
| Leading to study drug discontinuation | 2 (10.0) | ||
| Serious TEAEs | 8 (40.0) | ||
| Related to study drug | 4 (20.0) | ||
| TEAEs observed in ≥20% of patients | |||
| Blood and lymphatic system disorders | 15 (75.0) | ||
| Anemia | 15 (75.0) | ||
| Cardiac disorders | 4 (20.0) | ||
| Palpitations | 4 (20.0) | ||
| Gastrointestinal disorders | 20 (100.0) | ||
| Nausea | 12 (60.0) | ||
| Constipation | 9 (45.0) | ||
| Vomiting | 8 (40.0) | ||
| Stomatitis | 5 (25.0) | ||
| General disorders and administration site conditions | 7 (35.0) | ||
| Malaise | 6 (30.0) | ||
| Infections and infestations | 9 (45.0) | ||
| Investigations | 17 (85.0) | ||
| Platelet count decreased | 11 (55.0) | ||
| Neutrophil count decreased | 6 (30.0) | ||
| White blood cell count decreased | 6 (30.0) | ||
| Blood creatinine increased | 4 (20.0) | ||
| Weight decreased | 4 (20.0) | ||
| Metabolism and nutrition disorders | 5 (25.0) | ||
| Decreased appetite | 5 (25.0) | ||
| Musculoskeletal and connective tissue disorders | 8 (40.0) | ||
| Nervous system disorders | 14 (70.0) | ||
| Headache | 7 (35.0) | ||
| Dysgeusia | 4 (20.0) | ||
| Respiratory, thoracic, and mediastinal disorders | 9 (45.0) | ||
| Skin and subcutaneous tissue disorders | 8 (40.0) | ||
| Vascular disorders | 8 (40.0) | ||
| Hypertension | 6 (30.0) | ||
Values are presented as number (%) of patients. TEAEs were defined as adverse events that occurred after administration of the first dose of study drug and are shown by MedDRA System Organ Class and Preferred Term.
MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
Table 3. Grade ≥3 TEAEs and serious TEAEs with ≥10% incidence.
| Adverse events | Niraparib 300 mg (n=20) | |
|---|---|---|
| Grade ≥3 TEAEs | 17 (85.0) | |
| Anemia | 11 (55.0) | |
| Platelet count decreased | 6 (30.0) | |
| Neutrophil count decreased | 4 (20.0) | |
| Lymphocyte count decreased | 2 (10.0) | |
| Weight decreased | 2 (10.0) | |
| White blood cell count decreased | 2 (10.0) | |
| Serious TEAEs observed in ≥10% of patients | 8 (40.0) | |
| Anemia | 2 (10.0) | |
| Platelet count decreased | 2 (10.0) | |
Values are presented as number (%) of patients. TEAEs were defined as adverse events that occurred after administration of the first dose of study drug and are shown by MedDRA Preferred Term.
MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse event.
DISCUSSION
Patients with ovarian cancer who have received ≥3 lines of chemotherapy have high unmet medical needs. These patients typically have few late-line treatment options available to them and low (<10%) overall response to therapy [2]. This long-term follow-up of a phase 2, multicenter, open-label, single-arm study (Niraparib-2002) investigated the efficacy and safety of niraparib in 20 heavily pretreated Japanese women with relapsed, HRd-positive, high-grade, serous epithelial ovarian, fallopian tube, or primary peritoneal cancer. Study participants were selected to represent a similar patient population to the non-Japanese primary analysis population in the QUADRA study [2,11].
The primary endpoint of confirmed ORR, as assessed by the investigator using RECIST version 1.1, was 60.0% at final data cutoff (median study duration, 759.5 days), compared with 35.0% in the initial report (median study duration, 120.5 days) [11] and 27.7% in QUADRA [2]. This apparent improvement in ORR compared with the initial report may be related to the timing of the initial data cutoff, which was executed very early in order to accelerate regulatory approval. At the time of data cutoff for the initial report, only 2 radiological tumor assessments (cycle 3 and cycle 5) at most had been performed for each patient after initiation of niraparib. Seven patients who had an initial response at cycle 3 were confirmed to have a lasting response at cycle 5 and were reported as responders. There were, however, several patients for whom a response was observed for the first time at cycle 5 or later, and therefore confirmation of response occurred after the data cutoff for the initial report and they were counted as non-responders. In this follow-up, 5 additional responders were reported versus the initial report, 4 of whom had their first response at cycle 5 and one who had their first response at cycle 6. Responders included patients with and without BRCA1/2 mutations. Median DOR, which was not evaluable in the initial analysis, was 9.9 months, with the disease controlled for about 2 years in 4 patients. DCR was 90.0% and the median PFS and OS in this study were 8.3 months and 24.9 months, respectively. Together, these findings indicate clinically meaningful efficacy of niraparib in Japanese patients with ovarian cancer, particularly given the late-line setting.
The safety profile of niraparib in this study was consistent with the initial report [11], as well as with previous studies of niraparib in Japanese and non-Japanese patients [2,12]. The most common TEAEs, experienced by ≥30% of patients, were anemia, nausea, decreased platelet count, constipation, vomiting, headache, malaise, decreased neutrophil count, decreased white blood cell count, and hypertension. Participants initially received niraparib 300 mg once daily; following dose modification, the median niraparib dose intensity was 201.3 mg/day. This is in line with the most commonly administered dose of 200 mg after initial dose adjustment in the pivotal phase 3 NOVA trial [13]. The niraparib dosing approved in Japan is an individualized starting dose determined by body weight and platelet count, rather than the fixed starting dose used in the QUADRA study and Niraparib-2002.
Overall, the efficacy and safety profile of niraparib in Japanese patients was considered similar to results in the equivalent non-Japanese population in the QUADRA study [2]. As found in QUADRA, clinical benefit was observed in patients with and without BRCA mutations, providing further evidence of clinical utility in heavily pretreated patients with HRd-positive ovarian cancer beyond those with BRCA mutations.
PARP inhibitors are widely used in clinical practice in Japan and are recommended by the Japanese guidelines for use in first-line maintenance [14]. However, there is also a role for PARP inhibitor treatment in a later-line setting, given the unmet needs of patients who are ineligible to receive chemotherapy owing to past platinum hypersensitivity reactions. ASCO guidelines recommend that treatment decisions for late-line therapy are based on a thorough risk–benefit assessment in individual patients, taking into account a comprehensive understanding of available data, the patient’s condition, and their preferences [10]. Niraparib is the only approved PARP inhibitor in these later treatment lines in Japan, and this study is one of very few clinical trials in Japanese patients receiving late-line treatment. In addition to Niraparib-2002, clinical trials investigating PARP inhibitor combination or rechallenge are also taking place in platinum-sensitive patients. Thus, data from this study will support physicians and patients in making treatment decisions for late-line therapy.
Based on the unmet needs of heavily pretreated patients, the efficacy data in Japanese patients and the different dosage to the US label (which may lead to a different risk–benefit profile in Japanese clinical practice to that seen in the QUADRA study), the Japan Society of Gynecologic Oncology requested that the Ministry of Health, Labour and Welfare maintain the indication for niraparib in this setting in Japan, contrary to the decision of the FDA, under the condition that patients are well informed about the benefits and risks, as well as the FDA decision. Data from this present study provide further support for the clinical utility of niraparib in Japan. However, given the different niraparib indications and dosing in Japan compared with the USA, as well as the rarity of the late-line patient population, it is still important to generate real-world data on niraparib use in Japan. The efficacy and safety of niraparib in Japanese patients will be further investigated in an ongoing observational study (UMIN Clinical Trial Registry: JGOG3031).
This trial has several limitations typically associated with local phase 2 oncology studies, including the lack of a comparator arm and a small sample size. However, the lower limit of the 90% CI of the primary endpoint in this study (39.4%) was higher than the threshold response (5%) based on historical cases, which suggests that data were collected from enough patients to be able to draw meaningful conclusions.
In conclusion, the final results of this phase 2 study demonstrate efficacy of niraparib in Japanese women with heavily pretreated ovarian cancer and support our initial report, which had similar findings to those seen in the equivalent population of non-Japanese patients. The long-term safety profile of niraparib in Japanese patients was considered acceptable and consistent with previous studies, with no new safety signals identified.
ACKNOWLEDGEMENTS
The authors thank the patients, their families and caregivers, and all of the investigators and their team members at each study site. Medical writing support was provided by Sarah Graham, PhD and Jennifer Hung, PhD of Oxford PharmaGenesis, Melbourne, Australia and funded by Takeda Pharmaceutical Company Ltd in accordance with Good Publication Practice (GPP 2022) guidelines (www.ismpp.org/gpp-2022).
Footnotes
Funding: This study was funded by Takeda Pharmaceutical Company Ltd.
Presentation: Content included in this manuscript has been previously presented online at the 64th Annual Congress of the Japan Society of Obstetrics and Gynecology, July 14–16, 2022, Abstract HS-024.
Conflict of Interest: Daisuke Aoki declares the receipt of consulting fees from AstraZeneca, Chugai, MSD, and Takeda Pharmaceutical Company Ltd, and honoraria from AstraZeneca, Chugai, MSD, Myriad Genetics, and Takeda Pharmaceutical Company Ltd. Tsutomu Tabata declares the receipt of lecture fees from AstraZeneca, Chugai, and Takeda Pharmaceutical Company Ltd. Satoshi Yanagida, Toshiaki Nakamura and Hidekatsu Nakai declare the receipt of lecture fees from Takeda Pharmaceutical Company Ltd. Kenichi Harano declares the receipt of grants from AstraZeneca, Chugai, Daiichi Sankyo, MSD, and Takeda Pharmaceutical Company Ltd, and speaker fees from Astra Zeneca, Chugai, Eisai, MSD, Taiho, and Takeda Pharmaceutical Company Ltd. Kenichi Harano also declares an advisory role for AstraZeneca, Chugai, Taiho and Takeda Pharmaceutical Company Ltd, and receipt of institutional research funding from Takeda Pharmaceutical Company Ltd. Kosei Hasegawa declares the receipt of research grants from Daiichi Sankyo, Eisai, MSD, and Takeda Pharmaceutical Company Ltd, honoraria from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, Genmab, Kaken, Kyowa Kirin, MSD, Sanofi, and Takeda Pharmaceutical Company Ltd, and travel expenses from Regeneron. Kosei Hasegawa is also on the advisory board of Chugai, Eisai, Genmab, MSD, Roche, Sanofi, and Takeda Pharmaceutical Company Ltd. Shinichi Komiyama declares the receipt of consulting fees from AstraZeneca, Chugai, Daiichi Sankyo, Eisai, and MSD, and honoraria from AstraZeneca, Chugai, Eisai, and Takeda Pharmaceutical Company Ltd. Kazuhiro Takehara declares the receipt of speaker bureaus fees from AstraZeneca, MSD, and Takeda Pharmaceutical Company Ltd, and manuscript writing fees from MSD. Yoichi Kase and Ai Kato are employees of, and hold stocks in Takeda Pharmaceutical Company Ltd. Shuuji Sumino and Junpei Soeda are employees of Takeda Pharmaceutical Company Ltd. Ajit Suri is an employee of Millennium Pharmaceuticals, part of Takeda, and holds stocks in Takeda Pharmaceutical Company Ltd. Aikou Okamoto declares the receipt of honoraria from ASKA Pharmaceutical Co., Ltd, AstraZeneca, Bayer Holding Ltd, Chugai, Covidien Japan Inc., Eisai, Fuji Pharma Co., Ltd, Johnson & Johnson K.K., Kaken Pharmaceutical Co., Ltd, Kissei Pharmaceutical Co., Ltd, MSD, Mochida Pharmaceutical Co., Ltd, Myriad Genetics G.K., Otsuka Pharmaceutical Co., Ltd, Sanofi, Takeda Pharmaceutical Company Ltd, and Zeria Pharmaceutical Co., Ltd. Aikou Okamoto also declares the receipt of institutional grants from ASKA Pharmaceutical Co., Ltd, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Fuji Pharma Co., Ltd, Gyne Mom Co., Ltd, Kaken Pharmaceutical Co., Ltd, Linical Co., Ltd, Meiji Holdings Co., Ltd, Merck BioPharma Japan, Mochida Pharmaceutical Co., Ltd, MSD, Nippon Shinyaku Co., Ltd, Taiho Pharmaceutical Co., Ltd, and Tsumura & Co. Other authors have no conflict of interest to disclose.
Data Availability: The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
- Conceptualization: K.Y.
- Formal analysis: A.D., T.T., Y.S, N.T., K.E., H.J., 1H.K., 2H.K., H.T., 3H.K., K.S., M.M., 1N.H., 2N.H., S.J., T.K., T.M., Y.Y., K.Y., S.S., S.J., K.A, S.A., O.A., S.T.
- Funding acquisition: K.Y.
- Investigation: A.D., T.T., Y.S, N.T., K.E., H.J., 1H.K., 2H.K., H.T., 3H.K., K.S., M.M., 1N.H., 2N.H., S.J., T.K., T.M., Y.Y., K.Y., S.S., S.J., K.A, S.A., O.A., S.T.
- Methodology: K.Y., S.S., S.A.
- Resources: K.Y.
- Supervision: K.Y., S.T.
- Validation: K.Y., S.S.
- Writing - review & editing: A.D., T.T., Y.S, N.T., K.E., H.J., 1H.K., 2H.K., H.T., 3H.K., K.S., M.M., 1N.H., 2N.H., S.J., T.K., T.M., Y.Y., K.Y., S.S., S.J., K.A, S.A., O.A., S.T.
1H.K., Kenichi Harano; 2H.K., Kosei Hasegawa; 3H.K., Kensuke Hori.
1N.H., Hidekatsu Nakai; 2N.H., Hiroko Nakamura.
References
- 1.Yamagami W, Nagase S, Takahashi F, Ino K, Hachisuga T, Aoki D, et al. Clinical statistics of gynecologic cancers in Japan. J Gynecol Oncol. 2017;28:e32. doi: 10.3802/jgo.2017.28.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF, et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:636–648. doi: 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed] [Google Scholar]
- 3.Yi M, Dong B, Qin S, Chu Q, Wu K, Luo S. Advances and perspectives of PARP inhibitors. Exp Hematol Oncol. 2019;8:29. doi: 10.1186/s40164-019-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachdev E, Tabatabai R, Roy V, Rimel BJ, Mita MM. PARP inhibition in cancer: an update on clinical development. Target Oncol. 2019;14:657–679. doi: 10.1007/s11523-019-00680-2. [DOI] [PubMed] [Google Scholar]
- 5.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375:2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 6.González-Martín A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391–2402. doi: 10.1056/NEJMoa1910962. [DOI] [PubMed] [Google Scholar]
- 7.Pharmaceuticals and Medical Devices Agency. Review report (niraparib) [Internet] Tokyo: Pharmaceuticals and Medical Devices Agency; 2020. [cited 2024 Feb 11]. Available from: https://www.pmda.go.jp/files/000245811.pdf. [Google Scholar]
- 8.Leath C, 3rd, Scambia G, Villalobos R, Colombo N, Cibula D, Bidziński M, et al. Late-breaking abstract presentation: overall survival by number of prior lines of chemotherapy in patients with BRCA-mutated platinum-sensitive relapsed ovarian cancer receiving olaparib treatment or non-platinum chemotherapy in SOLO3. Int J Gynecol Cancer. 2022;32:A1. [Google Scholar]
- 9.Oza AM, Lisyanskaya AS, Fedenko AA, de Melo AC, Shparik Y, Bondarenko I, et al. Overall survival results from ARIEL4: a phase III study assessing rucaparib vs chemotherapy in patients with advanced, relapsed ovarian carcinoma and a deleterious BRCA1/2 mutation. Ann Oncol. 2022;33:S780. [Google Scholar]
- 10.Tew WP, Lacchetti C, Kohn EC PARP Inhibitors in the Management of Ovarian Cancer Guideline Expert Panel. Poly(ADP-Ribose) polymerase inhibitors in the management of ovarian cancer: ASCO guideline rapid recommendation update. J Clin Oncol. 2022;40:3878–3881. doi: 10.1200/JCO.22.01934. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto A, Kondo E, Nakamura T, Yanagida S, Hamanishi J, Harano K, et al. Phase 2 single-arm study on the efficacy and safety of niraparib in Japanese patients with heavily pretreated, homologous recombination-deficient ovarian cancer. J Gynecol Oncol. 2021;32:e16. doi: 10.3802/jgo.2021.32.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takehara K, Matsumoto T, Hamanishi J, Hasegawa K, Matsuura M, Miura K, et al. Phase 2 single-arm study on the safety of maintenance niraparib in Japanese patients with platinum-sensitive relapsed ovarian cancer. J Gynecol Oncol. 2021;32:e21. doi: 10.3802/jgo.2021.32.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berek JS, Matulonis UA, Peen U, Ghatage P, Mahner S, Redondo A, et al. Safety and dose modification for patients receiving niraparib. Ann Oncol. 2018;29:1784–1792. doi: 10.1093/annonc/mdy181. [DOI] [PubMed] [Google Scholar]
- 14.Japan Society of Gynecologic Oncology. The 2020 Japan Society of Gynecologic Oncology guidelines for the treatment of ovarian cancer, fallopian tube cancer, and primary peritoneal cancer. Update CQ12, 13, 25 [Internet] Tokyo: Japan Society of Gynecologic Oncology; 2021. [cited 2024 Jun 6]. Available from: https://jsgo.or.jp/guideline/ransou/2021/pdf/ov2020update_english.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]